Back to Journals » Journal of Pain Research » Volume 13
Operation, Effectiveness, and Limitations of Continuous Serratus Anterior Plane Blocks for Thoracoscopic Surgery in Adults
Authors Yang X , Gu H, Hu J , Wang S, Wei X, Shu S, Zhou W, Tao C, Wang D, Chai X
Received 5 June 2020
Accepted for publication 11 August 2020
Published 28 September 2020 Volume 2020:13 Pages 2401—2410
DOI https://doi.org/10.2147/JPR.S264139
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Robert B. Raffa
Xin-lu Yang,1,* Hai Gu,1,* Ji-cheng Hu,1 Sheng Wang,1 Xin Wei,1 Shu-hua Shu,1 Wei-de Zhou,1 Chun-rong Tao,2 Di Wang,1 Xiao-qing Chai1
1Department of Anesthesiology, Pain Clinic, First Affiliated Hospital, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, Anhui, People’s Republic of China; 2Department of Neurology, First Affiliated Hospital, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, Anhui, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Di Wang; Xiao-qing Chai
Department of Anesthesiology, First Affiliated Hospital, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei 230001, People’s Republic of China
Tel/Fax +86-551-62283963
; +86-551-62283912
Email [email protected]; [email protected]
Purpose: This randomized, double-blind study evaluated the effectiveness and limitations of continuous serratus anterior plane block (cSAPB) by comparing the effect of cSAPB to patient-controlled intravenous analgesia (PCIA) on postoperative acute pain after thoracoscopic surgery in adults.
Patients and Methods: Sixty-six patients who underwent elective video-assisted thoracoscopic surgery (VATS) were randomly allocated to cSAPB or PCIA groups (n=33 per group) after surgery. For the cSAPB group, patients were treated by an initial does of 20 mL ropivacaine (0.375%), followed by continuous infusion at a rate of 5 mL/h of ropivacaine (0.2%) and a patient-controlled bolus of 5 mL ropivacaine (0.2%). PCIA started with an initial does of 0.03 μg/kg sufentanil, followed by a basal infusion of 0.03 μg/kg/h sufentanil and a patient-controlled bolus of 0.03 μg/kg sufentanil. Visual analog scale (VAS) and other items were examined postoperatively. The area under the curve of VAS-time (AUCVAS-time) at rest and on coughing in the first 24 hours postoperatively were primary outcomes.
Results: At the first 24 hours postoperatively, patients in the cSAPB group exhibited a smaller AUCVAS-time at rest (44.0± 17.1 vs 68.9± 11.8 cm·h, P< 0.001) and AUCVAS-time on coughing (67.1± 8.8 vs 78.0± 12.5 cm·h, P< 0.001) compared with those in the PCIA group. The differences in means of VAS score at rest were more than 1.0 cm between the two groups, however, on coughing they were less than 1.0 cm at each observation point. Additionally, patients in the cSAPB group had a longer time to first patient-controlled bolus (15.8± 7.6 vs 10.6± 8.6 hours, P=0.011). Furthermore, a higher rank of satisfaction was recorded with patients in the cSAPB group.
Conclusion: cSAPB using PCA devices might be superior to traditional intravenous continuous analgesia, particularly with an advantage of pain relief at rest following VATS operation. Meanwhile, cSAPB lacks a satisfactory analgesic effect on cough.
Keywords: patient-controlled regional analgesia, continuous regional analgesia, continuous peripheral nerve blocks, postoperative acute pain, video-assisted thoracoscopic surgery
Introduction
Postoperative acute pain after thoracoscopic surgery is common and severe, which is a high-risk factor for developing chronic post-surgical pain (CPSP) and postoperative pulmonary complications (PPCs).1 Patient-controlled intravenous analgesia (PCIA), which primarily employs intravenous administration of opioids, is a common and convenient approach for postoperative analgesia in the clinic. Some studies, particularly in thoracic surgery, have indicated that the traditional approach of PCIA is associated with inadequate pain relief and significant side-effects (eg, respiratory depression, nausea/vomiting, and pruritus), resulting in additional needs for analgesia and dissatisfaction.2,3 However, the perioperative multimodal treatment (eg, regional anesthetic blockade and systemic administration of opioid analgesics and/or NSAIDs) is effective in management of acute post-thoracotomy pain.
According to the American Pain Society guidelines, the use of continuous, rather than single-injection, peripheral regional analgesic techniques is strongly recommended for postoperative pain management, as it can offer more prolonged duration of analgesia.4–6 Thoracic epidural analgesia (TEA) and paravertebral blocks (PVBs) was the earliest regional anesthetic techniques for the thoracic surgery.7 Due to the side-effects and the fact that it is challenging to perform, TEA may not be the best choice in the management of postoperative pain. Bleeding in a loose space limits the value of PVB in patients being treated with anticoagulants or antiplatelet agents.8 Besides, it is not an easy technique to place and fix the catheter for continuous analgesia in PVB.
Serratus anterior plane block (SAPB), which is easy-to-perform, has been indicated to provide regional analgesia by blocking the lateral cutaneous branches of the intercostal nerves.9 The continuous SAPB (cSAPB), in which a catheter is placed and connected to a patient-controlled device for continuous peripheral nerve blocks (CPNB), has recently started to be discussed10–12. But the results of previous research seemed inconsistent with the effects of using cSAPB in our own clinical work. This trial, therefore, modified the blind method and statistical analyzed pain scores at cough and rest to evaluate the effectiveness and limitations of continuous serratus anterior plane block (cSAPB) by comparing the effect of cSAPB and PCIA after thoracoscopic surgery up to the first and the second 24 hours. We speculate that cSAPB has improved postoperative analgesia compared with PCIA, but we do not know whether it can achieve satisfactory analgesia.
Patients and Methods
Trial Design
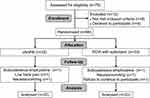
This is a prospective, randomized, double-blind and controlled trial implemented in First Affiliated Hospital, University of Science and Technology of China (USTC). From April to June 2019, 66 patients who were scheduled for elective thoracoscopic segmentectomy or lobectomy were recruited. Figure 1 shows the flow diagram of participant recruitment.
Participants
Participants meeting the following inclusion criteria were recruited: aged 18–65 years, body mass index (BMI) 18.5–25 kg/m2, both genders, American Society of Anesthesiologists (ASA) class I–III, voluntary participation with an ability to precisely complete a pain assessment. The exclusion criteria included: 1) coagulation dysfunction; 2) systemic or puncture site infection; 3) local anesthetic allergy; 4) Peripheral nervous system disease or SAPB-impacted area nerve damage; 5) impairment with severe heart (New York Heart Association classes III–IV), lung (forced expiratory volume in 1 second <50% of the predicted values), kidney, or liver functions; 6) chronic pain history or on lasting analgesic therapy before the surgery; 7) other circumstances under which the patients to be ineligible for this study considered by the investigators. Such as, the incision site located beyond the area SAPB impacted (ie, the area bounded by the anterior axillary line and posterior axillary line), VATS intraoperatively converted to open thoracotomy procedure.
General Anesthesia
No premedication was used prior to surgery. The ECG, heart rate (HR), mean arterial pressure (MAP), pulse oxygen saturation (SpO2), end-tidal carbon dioxide partial pressure (ETCO2), temperature, and bispectral index (BIS) were monitored before anesthesia by multi-function monitor and BIS monitor. General anesthesia was initiated with intravenous 0.05 mg/kg midazolam, 2 mg/kg propofol, 0.4 μg/kg sufentanil, and 1.0 mg/kg rocuronium. Intubation was performed using a double-lumen tube. An additional 0.15 μg/kg of sufentanil was given before incision. Subsequently, the intraoperative anesthesia was maintained with a continuous infusion of propofol (4–8 mg/kg/h) and remifentanil (0.05–0.2 μg/kg/min) in order to achieve a target BIS value between 40–50. Cisatracurium was administered intermittently as required during the surgery. All patients were mechanically ventilated to maintain around 35–45 mmHg of ETCO2. All surgical operations were performed by the same surgeon team, without any additional administration of local anesthetic by surgeons. When the incision began to be stitched, 40 mg of parecoxib and 0.1 μg/kg of sufentanil was intravenously given. At the end of surgery, randomization and treatment by a independent team were commenced prior to the patients being extubated and fully conscious.
Interventions
Patients in group cSAPB received a continuous fascia plane block, according to the following protocol.
- At the end of surgery, anesthesia was still sustained for the patients receiving cSAPB by using a continuous infusion of propofol (4 mg/kg/h). The patients remained in the lateral position.
- After sterilization of the injection site on the lateral chest wall, the muscles overlying the fourth to sixth ribs between the anterior axillary line, and posterior axillary line were identified by the ultrasound (Navis, Wisonic, Shenzhen, China) with a linear transducer (4–15 Hz, L15-4B). The serratus anterior and latissimus dorsi muscles were easily identified above the ribs.
- The needle was sometimes placed on the fourth or sixth rib, not only restricted to the fifth rib in the mid-axillary line, to avoid disturbing the surgical incision. The disposable spinal-epidural anesthesia kit AS-E/S (Tuoren, China) was used for following the block procedure.
- When the probe was placed in a coronal plane, the epidural needle (Tuoren, China) with a size of 1.6 (outer diameter) × 80 mm was inserted using an in-plane approach. The needle was introduced in caudal-cephalad direction until beneath the serratus anterior muscle. When the needle reached the surface of the rib, 3 mL of saline was injected to test the location of the needle tip and open the potential interfacial space between the rib and the serratus anterior muscle.
- Afterwards, an epidural catheter with a size of 0.5 (inner diameter) × 113 mm (Tuoren) was passed through the needle, and the needle was removed subsequently, leaving 4.5 cm of catheter inside the serratus anterior muscle plane. After a confirming negative aspiration, a bolus of 20 mL of 0.375% ropivacaine was administrated beneath the serratus anterior muscle.
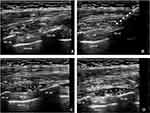
- The ultrasound confirmed that injected local anesthetic liquid distribute adequately into the fascial plane between the serratus anterior muscle and the external intercostal muscle (Figure 2).
- PCA pump was connected to the catheter prior to commencement of continuous administration. Patients were treated with continuous infusion through the PCA device in which a background infusion at a rate of 5 mL/h of 0.2% ropivacaine as well as a patient-controlled bolus of 5 mL 0.2% ropivacaine with a 30 minutes lockout.
In the PCIA group, intravenous administration of sufentanil using a PCA device started with an initial dose of 0.03 µg/kg sufentanil, followed by a background infusion of 0.03 µg/kg/h sufentanil and patient-controlled bolus of 0.03 µg/kg sufentanil with a lockout interval of 15 minutes.
In order to achieve double blindness, patients in both groups were connected to the No.1 catheter with the above-mentioned PCA pump and, at the same time, they received a mock infusion device with the same appearance and connected to the No.2 catheter. The No.2 catheter in the PCIA group was taped to the skin of the chest wall and covered with the same bandage as the cSAPB. In the cSAPB group, the No.2 catheter was connected to the intravenous indwelling needle.
Postoperatively, NSAIDs flurbiprofen 40 mg was administrated every 12 hours for both groups. When the pain score at rest was ≥4 or on patient request, additional analgesia of intravenous tramadol (50 mg) was provided.
Outcomes
After the patients were extubated and fully conscious, assessment of pain intensity was taken. Pain intensity at rest and on coughing were measured by an independent staff member using a visual analog scale (VAS) of 0–10 (0=no pain, 10=worst pain imaginable). VAS pain scores were examined at 1, 2, 6, 12, 24, 36, and 48 hours postoperatively. The area under the curve of VAS-time (AUCVAS-time) was calculated to represent a cumulative pain intensity.13
The first primary outcome AUCVAS-time at rest and the second primary outcome AUCVAS-time on coughing were measured over the first 24 hours after surgery. In terms of the secondary outcomes, we recorded AUCVAS-time at rest and on coughing over the second 24 hours after surgery, the differences in means of VAS score over 24 hours after surgery, the time to first patients-controlled bolus, the press counts of patients-controlled bolus, and the incidence of additional analgesia. Respiratory depression was defined as a respiratory rate of <8 breaths per minute and oxygen saturation either below 92% or a decrease of more than 5% from baseline in patients with a baseline of SPO2 <90%.14 The number of patients with respiratory depression and nausea/vomiting within 6–48 hours after surgery was recorded. Participants ranked their satisfaction using a 5-point scale, from “highly unsatisfied” to “highly satisfied”. Some perioperative data, such as total propofol dose, total remifentanil dose, surgical type, operation duration, and chest tubes number were also collected.
Sample Capacity
Difference of 1.0–1.3 cm of VAS means between both groups for single measurement was previously reported to be clinically significant.13 In our preliminary study, a difference of 27.26 cm·h of AUCVAS-time means in rest pain between groups was estimated (P<0.001) and was considered clinically meaningful also. Meanwhile, a markedly smaller difference of 4.53 cm·h of AUCVAS-time means for cough pain (P=0.003) was reported with only statistical significance. The difference of AUCVAS-time of cough pain, not AUCVAS-time of rest pain, between groups was therefore employed to ensure an adequate power for sample size calculation. As a SD of 4.6 cm·h of means, a minimum sample size of 24 patients for each group was estimated, with a one-sided type I error rate of 0.05 and a power of 0.9. A total of 33 patients for each group was finally included in view of potential dropout.
Randomization
Eligible patients were randomly allocated to either cSAPB or PCIA group before leaving the operation room. PCIA is the control group in the present study. An allocation sequence was created by a computer-generated list. Allocation concealment was implemented by using sequentially numbered, opaque, sealed envelopes. Block randomization was performed with a 1:1 allocation ratio by randomly selected block sizes of 4 and 6. The investigator who analyzed the data was not informed of the group assignment.
Statistical Methods
Continuous variables were displayed as mean (SD) or, if skewed distribution, as median (IQR), and categorical variables as percentages. For comparisons of sociodemographic and surgical characteristics between groups, continuous variables were examined using the independent t-test or Mann–Whitney U-test, and categorical variables with χ2 test or Fisher exact test. The descriptive information of VAS scores at rest and on coughing at individual time points between groups was displayed (Figure 3). In addition, the survival analysis was used to examine the time to first bolus (Figure 4), which was reckoned as the censored data when the patients-controlled bolus did not occur within 24 hours after surgery. All statistical analyses were performed using SAS version 9.4 software (SAS, USA). All statistical tests were two-tailed, and a P-value<0.05 was considered significant.
Results
Seventy-eight patients were assessed for eligibility. Sixty-six patients were randomized in the present study. Six patients were not accomplished because of: 1) pain occurred out of SAPB-impacted area, due to massive subcutaneous surgical emphysema (n=1 per group), 2) lower back pain caused by urinary stone disease (n=1 for cSAPB), 3) unacceptable nausea/vomiting (n=1 per group), and 4) refusal of continuing to participate (n=1 for PCIA). The results were analyzed on an intention-to-treat basis. In order to keep all subjects in both groups at the end of the trial consistent with the beginning, the missing data of the six uncompleted patients were treated by carrying forward the last observation to the end point. The final analysis included all 66 patients (33 per group). Baseline characteristics are shown in Table 1.
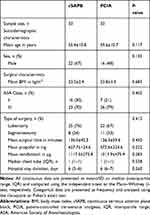 |
Table 1 Demographic and Surgical Characteristics of Participating Patients (n=66) |
Comparisons of postoperative pain intensity as measured by AUCVAS-time and the differences in means of VAS score are shown in Table 2. During the first 24 hours after surgery, the cSAPB group had smaller AUCVAS-time at rest (44.0±17.1 vs 68.9±11.8 cm·h, P<0.001) and AUCVAS-time on coughing (67.1±8.8 vs 78.0±12.5 cm·h, P<0.001) compared with the PCIA group. Thereafter, AUCVAS-time at rest (38.0±16.1 vs 60.4±13.2 cm·h, P<0.001) and AUCVAS-time on coughing (65.5±14.3 vs 75.1±13.3 cm·h, P=0.006) following cSAPB decreased during the second 24 hours after surgery, and still remained statistically significant compared with the PCIA group. The differences in means of VAS score at rest were more than 1.0 cm between two groups, however, on coughing were less than 1.0 cm at each observation point in 48 ouhrs postoperatively. Furthermore, other secondary outcomes are shown in Table 3. The cSAPB group had a longer time to first bolus (15.8±7.6 vs 10.6±8.6 hours, P=0.011) than patients in the PCIA group during the first 24 hours. The rate of nausea/vomiting in the cSAPB group was lower (6% vs 36%, P=0.003) compared with the PCIA group. There was no significant difference in respiratory depression between the two groups. The higher satisfaction was ranked with the cSAPB group. The incidence of additional analgesia, as well as bolus press counts, were not significant between both groups.
 |
Table 2 Outcomes Regarding Pain Management of Patients (n=66) |
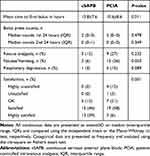 |
Table 3 Other Secondary Outcomes of Patients (n=66) |
Discussion
Since SAPB was firstly reported by Blanco et al, there has been increasing interest in this regional analgesic technique for thoracic surgery which primarily focused on single-injection performed before or after surgery.15–19 Although cSAPB has been reported for the management of acute or chronic pain after thoracoscopic surgery,20–22 we consider that from the perspective of neuroanatomy, there may be some limitations in the application of cSAPB in postoperative analgesia after thoracoscopic surgery. Therefore, we compared the analgesic effect of cSPB with that of PCIA. The main results suggested that cSAPB using a PCA device can provide a better pain relief at rest than PCIA, in terms of a smaller AUCVAS-time and a longer time to first bolus, particularly with a clinically meaningful pain relief at rest following VATS operation. The minimum clinically important difference in VAS score was defined as 1.0 cm for a single measurement,13 so the differences in means of VAS score on coughing between two groups had no clinically meaningful result in our trail. Collectively, cSAPB was more effective with the rest pain, rather than cough pain.
The serratus fascial plane block could be performed at two different interfascial spaces, the first one was superficial to the serratus anterior muscle and the other one was deep underneath the serratus anterior muscle. It’s still controversial so far that which one is better.9,13,23,24 In the present study, we performed SAPB at deep plane based on several considerations. First, performance of deep SAPB was straightforward technically. The needle can be reached directly to the surface of the rib that was clearly identified under ultrasound guidance. Also, local anesthetic was easily administered into the plane beneath the serratus anterior, as it’s not difficult to separate the serratus anterior off from the rib.23 Another consideration relied on a wider spread of local anesthetic following the deep serratus block. Besides blockade of target nerves (including the lateral cutaneous branches of the intercostal nerve, long thoracic nerve, and thoracodorsal nerve), the infiltration of local anesthetic caused by deep serratus block had an additional ability to affect the external intercostal muscles directly adjacent to the local anesthetic. Thus, deep blockade could provide a pain relief with peaceful and quiet respiration in which the external intercostal muscle primarily gets involved.13 Lastly, the catheter was secured steadily below the serratus anterior, as firm fixation of the catheter is one of the keys to success of continuous peripheral regional nerve block. In the preliminary study we already found the catheter located at the same position even with patient’s body-bending.
Wang et al10 found that single-injection serratus plane or thoracic paravertebral block (PVB) could reduce the postoperative opioid consumption and pain scores at the early stage in the patients undergoing uniportal video-assisted thoracoscopic surgery. Serratus plane block is as effective as a thoracic paravertebral block for reducing postoperative pain. But, in their study, they did not classify the rest pain and coughing pain.
Qiu et al11 also confirmed asimilar effect of SAPB and PVB in terms of postoperative acute pain, additional analgesia, postoperative nausea and vomiting, pain assessment at rest and on coughing, without any significant difference. Hanley et al12 found that continuous SAPB provided better postoperative 24-hour NRS assessment of rest pain, coughing pain, and movement pain, compared with continuous PVB. The results of our study show that cSAPB significantly relieved the pain of patients at rest, which is similar to the results of the previous studies. But in our study, of note, cSAPB has no significant advantage over PCIA in the analgesic effect on coughing. We believe that is the limitation of cSAPB. This is very different from Hanley et al’s conclusion.
Anatomically, SAPB only targets the lateral branch of the intercostal nerves, and does not appear to be equivalent to TEA or PVB blocks of the entire segmental nerve. As the intercostal nerves at the incision sites largely contributed to the pain after thoracoscopic surgery, the intercostal nerve was a potential target for a multi-modal analgesia. Theoretically, fascial plane blocks relied on local anesthetic diffusion across fascial planes and through muscle layers. To determine the extent of injectate spread and nerve involvement, several studies conducted the dissection on cadavers following SAPB.25,26 Mayes et al25 concluded that significant direct intercostal nerve spread is unlikely even deep injectate underneath the serratus anterior muscle, as the latex did not spread to the intercostal nerves on any occasions. In contrast, the latex and dye both did spread to the lateral cutaneous branches of the intercostal nerve on all occasions. Therefore, TEA or PVB offers more adequate and overall pain relief in theory via blockade of the entire segmental nerve, and SAPB could be reasonably expected to be effective for superficial surgery of the lateral thorax.
Consistent with the anatomical basis, we observed that the SAPB was more efficient with the rest pain, not with cough pain. Mechanically, the explanation for advantage of deep SAPB with rest pain seemed to be possible.25,26 The intercostal nerve runs anatomically between the innermost intercostal and internal intercostal, which are functionally essential to the respiration and the efficacy of cough by innervating the corresponding intercostal muscles. The external intercostal muscles are directly adjacent to the serratus anterior muscle, and thus more likely to be fully impacted by the diffusion of local anesthetic. That’s why deep blockade possibly provided pain relief with peaceful and quiet respiration, in which the external intercostal muscle primarily gets involved. In contrast, coughing or forced expiration uses intercostal muscles (innermost and internal) are both deeper and perhaps less likely to be impacted by SAPB, thus it is possible that coughing would still be painful. Another consideration is that cSAPB is unlikely to affect the pleurae, which are richly innervated and likely provide significant pain during cough. Taken together, although there was a statistical difference in terms of cough pain in the present study, SAPB may not have clinical efficacy to active pain due to a lack of an anatomical basis against cough-related pain. Encouragingly, the perioperative experience of patients was likely to be improved as the reduction of postoperative pain following cSAPB, due to the high quality of pulmonary functional exercises and the less incidence of nausea and/or vomiting.
There were some considerations underlying the discordance of our result and Hanley et al’s study. First, the amount of levobupivacaine with the initial dose of 40 mL (2 mg/kg), as well as a sustained dose of 8 mL/h, is far more than the dose of LAs we used. Does this mean a better analgesic effect can be achieved by a large volume of SAPB, and still be safe for the patient? Second, they postulate that secondary spread into communicating interfascial planes may have contributed to the clinical efficacy of the SAPB. The authors believed that this is due to the rhomboid intercostal and subserratus (RISS) plane block, and the deep erector spinae block (ESPB). But this still cannot explain why their continuous SAPB block the sensation of costal pleura. Further cadaveric work is needed to investigate this theory. Last but not the least, their trial included 40 patients, but 10 of them were discharged within the 24–48 hour study timepoint. The authors attempted to overcome the bias caused by a mass of missing data by using an intention to treat (ITT) analysis. But the inaccurate results might influence the interpretation of the trial results.
Our study had several limitations. First, this was a single-center study. Although the same team of performing surgeons and anesthetists ensured the standardization and consistency of the work, the results and conclusions of our trail need to be further confirmed by other centers. Another important limitation is no sensory testing during the operation of fascial plane block as the patients were still not extubated and unconscious. Instead, we confirmed using ultrasound that injection of local anesthetic distributed adequately into the target plane between the serratus anterior muscle and the external intercostal muscle.
Conclusion
The results of this study showed that cSAPB was superior to PCIA for rest pain, as indicated by a smaller AUCVAS-time and a longer time to first patient-controlled bolus. However, this intervention was less effective for cough pain. Further study is necessary to confirm the beneficial effects of this method in a larger sample size. Further cadaver studies are also necessary. In conclusion, cSAPB through a PCA device provided lasting management of postoperative acute pain after thoracoscopic surgery, emerging as a optional candidate for a multi-modal analgesia strategy.
Abbreviations
cSAPB, continuous serratus anterior plane block; PCIA, patient-controlled intravenous analgesia; VATS, video-assisted thoracoscopic surgery; VAS, Visual analog scale; CPSP, chronic post-surgical pain; PPCs, postoperative pulmonary complications; TEA, thoracic epidural analgesia; PVBs, paravertebral blocks; CPNB, continuous peripheral nerve blocks; BMI, body mass index; ASA, American Society of Anesthesiologists; AUCVAS-time, the area under the curve of VAS-time; IQR, interquartile range; Δ, difference in means; LD, latissimus dorsi; SA, serratus anterior; IM, intercostal muscle.
Data Sharing Statement
The authors state that all data in the manuscript are accessible if requested (contact e-mail address [email protected]). The authors verify that all data intended for sharing is deidentified.
Ethics Approval and Informed Consent
The Ethics Committee at the First Affiliated Hospital of USTC approved this prospective trial. Written informed consent was obtained from all patients recruited to the study, in accordance with the code of the Declaration of Helsinki. The trial was registered at the Chinese Clinical Trial Registry (ChiCTR1900022740) on April 24, 2019. This manuscript adheres to the applicable CONSORT guidelines.
Acknowledgements
This study was supported by grant from Anhui Provincial Key Research and Development Project Foundation (No.1804h08020286). Xin-lu Yang and Hai Gu are co-first authors for this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gottschalk A, Cohen SP, Yang S, Ochroch EA. Preventing and treating pain after thoracic surgery. Anesthesiology. 2006;104(3):594–600. doi:10.1097/00000542-200603000-00027
2. Ding W, Chen Y, Li D, et al. Investigation of single-dose thoracic paravertebral analgesia for postoperative pain control after thoracoscopic lobectomy - a randomized controlled trial. Int J Surg. 2018;57:8–14. doi:10.1016/j.ijsu.2018.07.006
3. Jin J, Min S, Chen Q, Zhang D. Patient-controlled intravenous analgesia with tramadol and lornoxicam after thoracotomy: a comparison with patient-controlled epidural analgesia. Medicine (Baltimore). 2019;98(7):e14538. doi:10.1097/MD.0000000000014538
4. Chou R, Gordon DB, de Leon-casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131–157. doi:10.1016/j.jpain.2015.12.008
5. Vorobeichik L, Brull R, Bowry R, Laffey JG, Abdallah FW. Should continuous rather than single-injection interscalene block be routinely offered for major shoulder surgery? A meta-analysis of the analgesic and side-effects profiles. Br J Anaesth. 2018;120(4):679–692. doi:10.1016/j.bja.2017.11.104
6. Maheshwari P, Maheshwari P. Single-injection vs continuous thoracic paravertebral block for postoperative analgesia after mastectomy. J Clin Anesth. 2016;28:90–91. doi:10.1016/j.jclinane.2015.07.022
7. Mehta Y, Arora D, Sharma KK, Mishra Y, Wasir H, Trehan N. Comparison of continuous thoracic epidural and paravertebral block for postoperative analgesia after robotic-assisted coronary artery bypass surgery. Ann Card Anaesth. 2008;11(2):91–96. doi:10.4103/0971-9784.41576
8. Narouze S, Benzon HT, Provenzano D, et al. Interventional spine and pain procedures in patients on antiplatelet and anticoagulant medications (Second Edition): guidelines from the American Society of Regional Anesthesia and Pain Medicine, the European Society of Regional Anaesthesia and Pain Therapy, the American Academy of Pain Medicine, the International Neuromodulation Society, the North American Neuromodulation Society, and the World Institute of Pain. Reg Anesth Pain Med. 2018;43(3):225–262. doi:10.1097/AAP.0000000000000700
9. Blanco R, Parras T, McDonnell JG, Prats-Galino A. Serratus plane block: a novel ultrasound-guided thoracic wall nerve block. Anaesthesia. 2013;68(11):1107–1113. doi:10.1111/anae.12344
10. Wang L, Wang Y, Zhang X, et al. Serratus anterior plane block or thoracic paravertebral block for postoperative pain treatment after uniportal video-assisted thoracoscopic surgery: a retrospective propensity-matched study. J Pain Res. 2019;12:2231–2238. doi:10.2147/JPR.S209012
11. Qiu Y, Wu J, Huang Q, et al. Acute pain after serratus anterior plane or thoracic paravertebral blocks for video-assisted thoracoscopic surgery: a randomised trial. Eur J Anaesthesiol. 2020. doi:10.1097/EJA.0000000000001196
12. Hanley C, Wall T, Bukowska I, et al. Ultrasound-guided continuous deep serratus anterior plane block versus continuous thoracic paravertebral block for perioperative analgesia in videoscopic-assisted thoracic surgery. Eur J Pain. 2020;24(4):828–838. doi:10.1002/ejp.1533
13. Abdallah FW, Cil T, MacLean D, et al. Too deep or not too deep?: a propensity-matched comparison of the analgesic effects of a superficial versus deep serratus fascial plane block for ambulatory breast cancer surgery. Reg Anesth Pain Med. 2018;43(5):480–487. doi:10.1097/AAP.0000000000000768
14. Savelloni J, Gunter H, Lee KC, et al. Risk of respiratory depression with opioids and concomitant gabapentinoids. J Pain Res. 2017;10:2635–2641. doi:10.2147/JPR.S144963
15. Chu GM, Jarvis GC. Serratus anterior plane block to address postthoracotomy and chest tube-related pain: a report on 3 cases. A a Case Rep. 2017;8(12):322–325. doi:10.1213/XAA.0000000000000502
16. Okmen K, Okmen BM. The efficacy of serratus anterior plane block in analgesia for thoracotomy: a retrospective study. J Anesth. 2017;31(4):579–585. doi:10.1007/s00540-017-2364-9
17. Kim DH, Oh YJ, Lee JG, Ha D, Chang YJ, Kwak HJ. Efficacy of ultrasound-guided serratus plane block on postoperative quality of recovery and analgesia after video-assisted thoracic surgery: a randomized, triple-blind, placebo-controlled study. Anesth Analg. 2018;126(4):1353–1361. doi:10.1213/ANE.0000000000002779
18. Okmen K, Metin Okmen B. Evaluation of the effect of serratus anterior plane block for pain treatment after video-assisted thoracoscopic surgery. Anaesth Crit Care Pain Med. 2018;37(4):349–353. doi:10.1016/j.accpm.2017.09.005
19. Semyonov M, Fedorina E, Grinshpun J, et al. Ultrasound-guided serratus anterior plane block for analgesia after thoracic surgery. J Pain Res. 2019;12:953–960. doi:10.2147/JPR.S191263
20. Khalil AE, Abdallah NM, Bashandy GM, Kaddah TA. Ultrasound-guided serratus anterior plane block versus thoracic epidural analgesia for thoracotomy pain. J Cardiothorac Vasc Anesth. 2017;31(1):152–158. doi:10.1053/j.jvca.2016.08.023
21. Abdallah NM, Bakeer AH, Youssef RB, Zaki HV, Abbas DN. Ultrasound-guided continuous serratus anterior plane block: dexmedetomidine as an adjunctive analgesic with levobupivacaine for post-thoracotomy pain. A prospective randomized controlled study. J Pain Res. 2019;12:1425–1431. doi:10.2147/JPR.S195431
22. Reyad RM, Shaker EH, Ghobrial HZ, et al. The impact of ultrasound-guided continuous serratus anterior plane block versus intravenous patient-controlled analgesia on the incidence and severity of post-thoracotomy pain syndrome: a randomized, controlled study. Eur J Pain. 2020;24(1):159–170. doi:10.1002/ejp.1473
23. Piracha MM, Thorp SL, Puttanniah V, Gulati A. “A Tale of Two Planes”: deep versus superficial serratus plane block for postmastectomy pain syndrome. Reg Anesth Pain Med. 2017;42(2):259–262. doi:10.1097/AAP.0000000000000555
24. Park MH, Kim JA, Ahn HJ, Yang MK, Son HJ, Seong BG. A randomised trial of serratus anterior plane block for analgesia after thoracoscopic surgery. Anaesthesia. 2018;73(10):1260–1264. doi:10.1111/anae.14424
25. Mayes J, Davison E, Panahi P, et al. An anatomical evaluation of the serratus anterior plane block. Anaesthesia. 2016;71(9):1064–1069. doi:10.1111/anae.13549
26. Biswas A, Castanov V, Li Z, et al. Serratus plane block: a cadaveric study to evaluate optimal injectate spread. Reg Anesth Pain Med. 2018;43(8):854–858. doi:10.1097/AAP.0000000000000848
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.