Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Open Pilot Study on the Rejuvenation Effect of Absorbable Threads in the Genital Area
Authors Poleva I , Markova N, Sulamanidze M
Received 2 May 2023
Accepted for publication 1 August 2023
Published 16 August 2023 Volume 2023:16 Pages 2237—2248
DOI https://doi.org/10.2147/CCID.S416232
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Irina Poleva,1,* Natalia Markova,2,* Marlen Sulamanidze3,*
1UNICAM, School of Pharmaceutical and Health Sciences, Camerino, Italy; 2Department of Dermatology, Medical Esthetic CRO, Prague, Czech Republic; 3Department of Plastic and Aesthetic Surgery, Total Charm, Tbilisi, Georgia
*These authors contributed equally to this work
Correspondence: Irina Poleva, Tel +393388473415, Email [email protected]
Purpose: We propose a new method of genital rejuvenation based on absorbable thread insertion. In this study, we aimed to assess the safety and efficacy of particular absorbable threads made of P (LA/CL), the so-called Nano Spring 7, for vulvar rejuvenation.
Patients and Methods: The study was conducted in two parts: the first by anatomical dissection and the second by clinical study. The first part of the study clarified safety and efficacy of thread insertion in this anatomical area. During the second part, 19 patients underwent Nano Spring 7 absorbable thread insertion in the subcutaneous layer of the labia majora to improve esthetic parameters and were followed up after 7, 30, 90, and 180 days. We evaluated outcomes using four different patients’ questionnaires and one investigator’s questionnaire.
Results: The anatomical dissection defined the correct anatomical layer of threads implantation and the subcutaneous structures allowing for the thread anchoring. All the patients completed the study. The patients’ and investigators’ subjective evaluations during follow-up and at the end of the study were very positive. All the patients showed a decrease in discomfort sensations related to the labia majora conditions and aesthetic improvement in the vulvar area and recommended the treatment to their friends.
Conclusion: The use of absorbable threads is an innovative, safe minimally invasive approach to genital rejuvenation.
Keywords: genital rejuvenation, threads in polylactic acid, non-surgical rejuvenation, threads in spring shape
Introduction
In the past decade, attention to the genital area has increased in terms of rejuvenation and beautification. Vaginal rejuvenation market growth is predicted to increase by 33.7% through 2026.1
The patient concerns are as follows: shape and size of the labia minora and majora, skin turgor, wrinkles, dryness, pigmentation, and discomfort during sexual intercourse.
There are non-surgical methods available for correcting or improving specific concerns related to the vulva. These methods encompass the following: 1. Topical medications: Certain topical medications, such as estrogen creams, may be prescribed to address specific vulvar concerns, particularly those related to hormonal imbalances or menopause; 2. Laser therapy: Laser treatments can be used to address various vulvar conditions, including skin discoloration, scarring, or certain types of skin conditions like lichen sclerosus. The laser stimulates collagen production and promotes tissue regeneration; 3. Radiofrequency treatments: Similarly to laser therapy, radiofrequency treatments use targeted heat energy to tighten and rejuvenate the skin, promoting collagen production and improving the appearance and texture of the vulvar area; 4. Platelet-rich plasma (PRP) injections: PRP therapy involves injecting concentrated platelets from the patient’s own blood into the vulvar area to stimulate tissue regeneration and promote healing. It can be used to address issues such as thinning tissues, scarring, or mild to moderate. 5. Filler injections for volume replacement.
All the aforementioned treatments, except for filler injections, contribute to varying degrees of skin regeneration and colour enhancement without altering the shape and firmness of the vulva. Furthermore, these treatments typically require multiple sessions to achieve noticeable results. In the case of filler injections, they are primarily used to increase volume without making significant alterations to the vulva’s other characteristics, such as skin quality, colour, or shape.
Surgical correction of the vulvar area could provide an important improvement. However, women take into consideration potential surgical correction complications, including pain, bleeding, infection, scarring, adhesions, altered sensation, dyspareunia, and the need for reoperation;2 hence, requests for non-surgical correction have increased considerably.
Although filler injections, peelings, and laser treatments have been widely used in the genital area with variable results,3 not always satisfactory.
Herein, we proposed a new method of genital rejuvenation based on absorbable thread insertion. Absorbable threads have a reliable reputation in the esthetic market. In particular, absorbable threads made of Poly(l-lactide-co-ε-caprolactone) copolymer (P(LA/CL)) have been extensively used for nonsurgical facelifts and biostimulation of various parts of the body, such as the inner thighs, arms, and abdomen.
PCL is a well-known synthetic polymer that is biocompatible and biodegradable and has been used in medicine for more than 20 years.4,5 Its ability to degrade slowly over a long period of time (approximately 1 year) makes it suitable as a drug delivery system. In fact, it has been approved by the Food and Drug Administration for this function. PLLA is also a biocompatible and biodegradable synthetic polymer known to be one of the most powerful collagen production stimulators to date,6 it’s also approved by FDA for the esthetic proposals. The combination of these two substances creates an innovative Poly(l-lactide-co-ε-caprolactone) copolymer (P(LA/CL). The P (LA/CL) threads have a strong and resistant core structure and can stimulate collagen production over a long period of thread absorption (1 year).7 Thus, we investigated the use of these threads in the correction of age-related signs in the vulvar area for a long-lasting rejuvenating effect.
Materials and Methods
The study was made in 2 parts:
The first part consisted in anatomical dissection to obtain data on the topographic location of implanted threads and additional clarification of the subtleties of the topographic anatomy of this zone and determine the optimally safe layer for implantation of spiral threads, as well as to verify the possibility of using spring rings as a fixing apparatus of the thread which has no cogs. The study was approved by the Ethical Committee of the Faculty of Medicine of the Comenius University in Bratislava and conducted. All rights to research and its results are reserved. The study was accompanied by compliance with all legal aspects of the use of cadaveric material.
The second part consisted in clinical use of the threads clinical to assess the effectiveness of the technique.
The clinical part of the study was carried out in two medical centers, one in Rome, Italy, and the second in Prague, Czech Republic. A particular type of absorbable threads were used in our study: so-called NanoSpring 7 (NS7) threads. NS7 threads have no barbs; they have a spiral-shaped structure and are charged on the blunt-tip 7-cm-long needle guide. The composition of the threads is peculiar, they are made of a copolymer of PCL and PLLA. The three-dimensional spiral copolymer has different effects:
- The threads are elastic due to the elasticity of the spring; thus, they do not create a rigid, fixed structure under the skin.
- They simultaneously maintain a suitable shape owing to the three-dimensional structure.
- They provide long-lasting strong biostimulation due to the slow release of PLLA.
There were 19 female patients in this study with a mean age of 51.5 years (range, 42–62).
The inclusion criteria were as follows:
- Fine wrinkles in the vulvar area.
- Loss of tone, mild ptosis, and other esthetic defects of the labia majora (LM).
- Loss of volume of LM.
- Loss of shape of the vulvar area.
The exclusion criteria were as follows:
- Allergy to suture material and lidocaine-containing medicine.
- Previously implanted permanent fillers.
- The tendency to develop keloid or hypertrophic scars.
- The active phase of inflammatory diseases.
- Acute stage of chronic diseases.
- Autoimmune connective tissue disease.
- Use of anticoagulants and antiplatelet medicine.
- Coagulopathy.
- Hypertension not medically compensated.
- Neurological and mental disorders in the acute stage.
- Dysmorphophobia.
- Inadequate or disproportionately high expectations from the procedure.
- Pregnancy and breastfeeding.
- Active cancer and chemotherapy.
- Hepatitis B, C, and/or human immunodeficiency virus infection in the acute period.
- Porphyria.
- Acute genital herpes.
- Acute colpitis or vaginitis.
- Venereal diseases.
- Menstruation days.
- Refusal to consent to having photos taken and being filmed, fill out questionnaires, sign an informed consent to carry out the procedure, and/or participate in the study.
All the patients provided written informed consent before participating in the study including publication of anonymized responses.
The study was conducted in accordance with the guidelines laid down in the Declaration of Helsinki in 2013.
Procedure
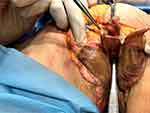
All the patients underwent thread insertion after clinical examination. The thread insertion was carried out after disinfection of the anatomical area using povidone-iodine (Betadine solution; Avrio Health L.P., Stamford, CT, USA). Anesthesia infiltration was performed with 2–3 mL of 1% lidocaine for each LM using a 9 cm-long 22-G cannula. Two to three entry points for each LM were created using 18-G needle. The position of the entry points has been chosen in accordance with the anatomical presentation: in 9 patients in the perineal area, in the other 10 patients in the pubic area. Ten threads were inserted into the LM (five threads for each LM) in the superficial subcutaneous layer (Figure 1A and B).
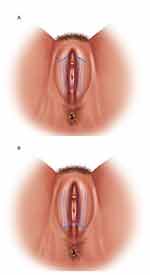 |
Figure 1 Scheme of tread implantation from (A) Perineal entry point and from (B) Pubic entry point. |
All included patients were given recommendation of sexual abstinence for 2 weeks after the procedure. These recommendations are given to the patients in order to avoid any prolongation of post-procedure recovery (inflammatory) symptoms such as swelling, pain, discomfort, bruising after threads insertion. No additional specific precautions for sexual activity needed after 2 weeks of procedure if no complications observed during the first 2 weeks.
The patients were followed up at 7, 30, 90, and 180 days.
Methods of Evaluation
Four questionnaires for the subjective evaluation were administered to all the patients:
The questions were as follows:
- I feel shy about having sex due to the appearance of my LM.
- The skin of the LM looks too dark.
- The skin is too dry.
- The skin has wrinkles.
- Loss of skin tone.
- I feel uncomfortable due to the excessive rubbing of the LM on my underwear.
- LM does not sufficiently cover the vaginal opening.
- LM does not look symmetrical enough.
- The skin of the LM is dry and prone to small fissures.
- I do not like the shape of my LM.
- I am physically uncomfortable due to excessive friction on the LM during sex.
- If the procedure was comfortable (5 = intolerable; 4 = very uncomfortable; 3 = moderately uncomfortable; 2 = slightly uncomfortable; and 1 = totally comfortable).
- If the patient felt any pain (5 = intolerable; 4 = intense; 3 = moderate; 2 = slight; and 1 = no pain at all).
- The duration of the procedure (5 = too long; 4 = very long; 3 = moderately long; 2 = consistent with expectations; and 1 = very short, I expected a longer procedure).
The patients were asked to express their personal observations from the procedure as well.
Results
Results of Anatomical Dissection
The main purpose of the dissection was to verify the main anatomical structures of the anterior perineum in women and the region of the labia majora. Determine the anatomical structures involved in aesthetic correction with threads in spring form, show anatomical safety and determine the safe layer for thread implantation.
Anatomical dissections were performed on 3 female bodies, age range from 75 to 83 years. The dissections were carried out based on the Anatomical Institute of the Faculty of Medicine of the Comenius University in Bratislava (Slovakia). All research rights and results are reserved. The study was accompanied by compliance with all legal aspects of the use of cadaver material.
Labia Majora
The labia majora, a rounded paired skin fold 7–10 cm long and 2–5 cm wide, are formed by the adipose tissue, the outer side of which is covered with skin, the inner side is covered with mucous membrane. The labia majora are formed by two folds of skin containing fatty tissue, sebaceous and sweat glands, connected with each other by an anterior and posterior commissure and separated by a genital slit. Unlike the mons pubis, there is no division into superficial and deep subcutaneous fat in the labia majora (Figures 2 and 3). In the thickness of the lower third of the labia majora there are large glands of the vestibule (the Bartholin glands), the alkaline secretion of which moisturizes the vaginal opening and dilutes the seminal fluid. The excretory ducts of these glands open in the groove between the labia minora and the hymen or its remnants. This zone is well supplied with blood from the internal pudendal arterial system, the main trunk of which is located deep enough, which must be taken into consideration when correcting them, choosing, if possible, a more superficial layer of the subcutaneous fat.
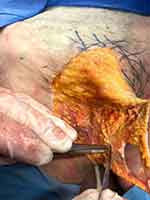 |
Figure 3 The labia majora from anatomical dissection: subcutaneous fatty tissue, dermis and epidermis of the labia majora. Septal intersections of the subcutaneous fatty tissue of the labia majora. |
The subcutaneous fat is organized in lobules which represent a group of fat cells enclosed within a small thin membrane, fibrous septum. These anatomical formation, fibrous septa, represent the anchoring structures for the threads NS7 (Figure 4).
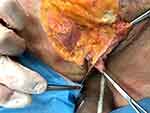 |
Figure 4 A thread-spring implanted in the subcutaneous fatty tissue near its upper layer and fixed by its rings in the septal intersections of the subcutaneous fatty tissue of the labia majora. |
The safest and most suitable level of thread implantation is in the superficial subcutaneous layer because there are no structures which could be altered or hurt during the thread implantation. It was also confirmed that the implantation of NS7 in the upper layer of subcutaneous fat closer to the mesh layer of the skin provides fixation of threads due to the presence of the coils, which guarantee their reliable fixation in tissues (fibrous interlobular septa) and prevention of their possible migration.
Results of Clinical Evaluation
All the patients completed the procedure and did not experience any side effects except some swelling immediately after the procedure due to anesthesia infiltration and the trauma of thread insertion.
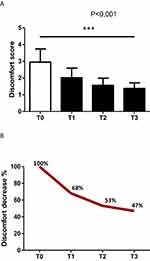
The first questionnaire, “Patient’s evaluation of LM conditions before the treatment and 30, 90, and 180 days after”, showed that 100% of the patients were satisfied with the results of the treatment. They found that the LM conditions gradually improved from T0 to T3, with a consequent decrease in discomfort from baseline (score 2.95) to T3 (score 1.38) (Figure 5A).
This questionnaire showed that the average condition of discomfort decreased by 32% at T1, by 47% at T2, and by 53% at T3 (Figure 5B). All the patients felt happier with the appearance of their LM (less dry, fewer wrinkles, more turgid, better closure of the vaginal opening, more symmetrical appearance, and better shape). Statistical evaluation of the data using a t-test showed the statistical significance of the differences between T0 and T3 (p < 0.001).
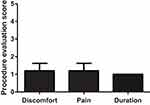
The second questionnaire, “5-point questionnaire concerning the procedure itself”, showed that the average score of discomfort varied from 1.2 points to 1.0 points (mean, 1.13), indicating that the treatment with NS7 was carried out comfortably without pain (except some pain during anesthesia infiltration) and was very short (Figure 6).
The personal observations of the patients were as follows:
- “I was afraid to perform the treatment in the genital area since it is my first time, and the area is very sensitive. I was surprised by the absence of pain and how quickly the doctor inserted the threads”.
- “It was surprising that the entire procedure was completed in less than 15 minutes”.
- “The only unpleasant sensation was the anesthesia infiltration, with no other discomfort at all”.
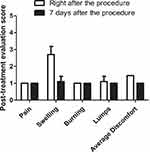
The results of the third questionnaire, “5-point questionnaire on the post-treatment period”, (administered immediately after and 7 days after the procedure) showed very few unpleasant sensations immediately after the procedure, with an average discomfort score of 1.45, and the total disappearance of any sensation 7 days after the treatment (Figure 7). In some cases, slight to moderate swelling was observed immediately after the procedure (score: 2.7), which was due to anesthesia infiltration and trauma associated with thread insertion.
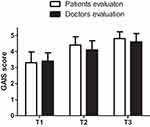
The results of the GAIS questionnaire are represented in Figure 8. All the patients were satisfied with the results of the procedure. The mean patient satisfaction score was 3.3 at T1, 4.4 at T2, and 4.8 at T3. This means that all the patients were satisfied by T3. The doctor’s scores were similar: 3.4 at T1, 4.1 at T2, and 4.6 at T3.
Some of the clinical results are illustrated in Figure 9A and B.
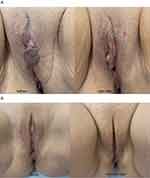 |
Figure 9 (A) Before and right after threads implantation from the pubic entry point. (B) Before and after 6 months after threads implantation. |
Discussion
Following patients’ requests, companies try to find new minimally invasive treatments for genital rejuvenation. This is the first pilot study to evaluate the safety and effectiveness of NS7 absorbable thread insertion for LM rejuvenation.
As anatomical dissection found, it is possible to implant spiral absorbable threads NS7 in the superficial subcutaneous layer in a safe and effective way. The threads are stable and do not migrate due to the coil’s interaction with interlobular septa while there is evidence on the HA filler migration in different anatomical areas.8,9
NS7 absorbable threads have several advantages, compared with other common treatment methods for this area:
- Only one session is required to achieve the desired result.
- NS7 threads provide better shape without creating unwanted volume.
- The shape of the LM after treatment is elastic and naturally turgid, with no sensation of foreign bodies or a Tindall effect.
- When a certain volume is needed, it is possible to increase the volume moderately by inserting other NS7 threads.
- The biostimulation provided by PLLA in the composition of NS7 threads lasts more than 1 year, contributing to skin rejuvenation and patient satisfaction.
- There is no risk of vascular occlusion.
The purpose of the anatomical dissection was to map anatomical structures relative to underlying and adjacent anatomical structures: bones, ligaments, nerves, vessels, fat bodies and septal bridges in the subcutaneous fat layer. Cadaveric tissues were not assessed in terms of soft tissue quality, skin elasticity, tone, and turgor, which was the subject of clinical investigation using live patients undergoing genital rejuvenation with NS7 absorbable threads.
Upon evaluating the results of the treatment, we observed that the condition of the vulvar area (first questionnaire) improved significantly, with a decrease in uncomfortable sensations and a significant improvement in the esthetic aspect of LM by more than 50% within 6 months. The improvement of skin quality (including dryness) of LM must be related to the known capabilities of P(LA/CL) threads to improve hydration, colour, elasticity of skin.10 LM skin turgor enhancement can be explained as follows: 1) the physical presence of the spiral and flexible threads within the LM creates a defined and turgid shape of the labia. 2) the gradual production of collagen, both at the level of the dermis and in the interlobular septa of the subcutaneous tissue as a result of subcutaneous tissue reaction on P (LA/CL) threads, gives greater elasticity to the tissue.11
All the patients found that the treatment was useful for their psychological and physical comfort. These data confirmed the results of previous studies12–14 on the effects of aesthetic procedures in the genital area to reduce discomfort and increase self-confidence in the female population. In addition to the correction of “abnormalities”, beautification of the normal genital features is requested by modern women.
The patients found that the procedure was painless and surprisingly short. The sensations of pain, swelling, burning, and skin irregularities were minimal immediately after the procedure (the average discomfort score was 1.45 right after the treatment and 1.0 seven days after treatment). This means that not only is the treatment very comfortable and short, but the post-treatment period has no unpleasant phenomena or sensations, hence downtime is minimal. This fact is particularly important considering that this anatomical area is not commonly taken into consideration for the aesthetic proposals, hence, the psychological comfort and the absence of unpleasant sensations are the basis for spreading of the technique among interested population.
The GAIS scale administration to the patients and doctors reveals that the satisfaction with the procedure was very high (the patients’ and doctor’s scores were 4.8 and 4.6, respectively, 180 days after treatment) and that 100% of the patients were satisfied with the treatment.
No complications were observed during the study.
All these data, even though collected subjectively, indicate that genital rejuvenation with NS7 absorbable threads represents a novel, safe, and effective option for selected patients. It significantly decreases the unpleasant sensations of patients and increases their intimate comfort and self-confidence with minimal downtime and without any side effects.
Conclusions
This pilot study showed that genital rejuvenation with the absorbable threadsNS7 is a safe and effective procedure. Based on the observational period of patients in the study, it has been noted that the clinical effect of threads insertion persists for a minimum of 6 months. Further investigation with a larger number of patients should be conducted to confirm our data.
Data Sharing Statement
The data are available on request from corresponding author.
Ethics Statement
The cadaver dissection study was approved by the Ethical Committee of the Faculty of Medicine of the Comenius University in Bratislava. All rights to research and its results are reserved. The study was accompanied by compliance with all legal aspects of the use of cadaveric material. The clinical study has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent Statement
All persons gave their informed consent prior to their inclusion in the study.
Acknowledgments
Dr. Eliška Kubíková, PhD Surgeon, Professor from the Department of Anatomy, Medical Faculty, Comenius University in Bratislava technically performed anatomical dissections in the first part of the study. This paper was presented at the XVI Kolkhida Congress 2022 (Tbilisi, Georgia) as a presentation with interim findings. The abstract was not published in any digital platform/abstract book.
Funding
No funding was received for this study.
Disclosure
Dr. Irina Poleva is an Aptos company (manufacturer of NS/ threads) trainer. Dr. Natalia Markova is an Aptos company trainer. Dr. Marlen Sulamanidze is the inventor of Aptos technology and co-owner of Aptos company.
References
1. Global Market Insights. Vaginal Rejuvenation Market Size By Treatment (Labioplasty, Vaginoplasty, Hymenoplasty, Perineoplasty, Hoodectomy, g-spot Amplification), By End-use (Hospitals, Plastic Surgery Centers), Industry Analysis Report, Regional Outlook, Application Potential, Price Trends, Competitive Market Share & Forecast, 2020 – 2026 . Available from: https://www.gminsights.com/industry-analysis/vaginal-rejuvenation-market.
2. Wilkie G, Bartz D. Vaginal rejuvenation: a review of female genital cosmetic surgery. Obstet Gynecol Surv. 2018;73:287–292. doi:10.1097/OGX.0000000000000559
3. Vanaman M, Bolton J, Placik O, et al. Emerging trends in nonsurgical female genital rejuvenation. Dermatol Surg. 2016;42(9):1019–1029. PMID: 27153040. doi:10.1097/DSS.0000000000000697
4. Pitt CG, Jeffcoat AR, Zweidinger RA, et al. Sustained drug delivery systems. I. The permeability of poly(epsilon-caprolactone), poly(DL-lactic acid), and their copolymers. J Biomed Mater Res. 1979;13(3):497–507. PMID: 438232. doi:10.1002/jbm.820130313
5. Woodward SC, Brewer PS, Moatamed F, et al. The intracellular degradation of poly(epsilon-caprolactone). J Biomed Mater Res. 1985;19(4):437–444. PMID: 4055826. doi:10.1002/jbm.820190408
6. Turkevych M, Turkevych A, Kadjaya A, et al. Pathomorphological criteria of use efficiency of resorbable and permanent implants in aesthetic medicine and cosmetic dermatology. J Cosmet Dermatol. 2018;17(5):731–735. PMID: 30123967. doi:10.1111/jocd.12737
7. Moyle GJ, Brown S, Lysakova L, et al. Long-term safety and efficacy of poly-L-lactic acid in the treatment of HIV-related facial lipoatrophy. HIV Med. 2006;7(3):181–185. PMID: 16494632. doi:10.1111/j.1468-1293.2006.00342.x
8. Jordan DR, Stoica B. Filler migration: a number of mechanisms to consider. Ophthalmic Plast Reconstr Surg. 2015;31(4):257–262. PMID: 25650796. doi:10.1097/IOP.0000000000000368
9. Chae SY, Lee KC, Jang YH, Lee SJ, Kim DW, Lee WJ. A case of the migration of hyaluronic acid filler from nose to forehead occurring as two sequential soft lumps. Ann Dermatol. 2016;28(5):645–647. PMID: 27746650; PMCID: PMC5064200. doi:10.5021/ad.2016.28.5.645
10. Girgin A. The effectiveness of PLLA/PCL aptos thread on skin quality. Aesthet Med. 2019;5:25.
11. Sulamanidze GM, Sulamanidze MA, Sulamanidze KM, Kajaia AA, Giorgadze SG. The subcutaneous tissue reaction on poly (L-lactide-co-caprolactone) based threads. Int J Clin Expl Dermatol. 2018;3(2). doi:10.33140/IJCED
12. Goodman MP, Placik OJ, Benson RH, et al. A large multicenter outcome study of female genital plastic surgery. J Sex Med. 2010;7(4_Part_1):1565–1577. PMID: 19912495. doi:10.1111/j.1743-6109.2009.01573.x
13. Willis RN, Wong CS, Pai A, et al. Labiaplasty minora reduction. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. PMID: 28846226.
14. Cohen PR. Genital rejuvenation: the next frontier in medical and cosmetic dermatology. Dermatol Online J. 2018;24(9):
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.