Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 13
One21: A Novel, Customizable Injection Protocol for Treatment of the Forehead with IncobotulinumtoxinA
Authors de Sanctis Pecora C
Received 6 November 2019
Accepted for publication 21 December 2019
Published 5 February 2020 Volume 2020:13 Pages 127—136
DOI https://doi.org/10.2147/CCID.S237519
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Video abstract presented by Carla de Sanctis Pecora.
Views: 10335
Carla de Sanctis Pecora
Dermatologie-Clínica, Cirurgia, Cosmiatria e Laser, São Paulo, Brazil
Correspondence: Carla de Sanctis Pecora Avenida Ibirapuera, 2907, Conj. 901, Moema, São Paulo, SP CEP: 04029-200, Brazil
Tel/Fax +55 11 50420871
Email [email protected]
Abstract: It is well known that incobotulinumtoxinA (INCO) is an effective approved treatment for dynamic wrinkles of the upper face caused by the action of mimetic muscles over time. In doing so, it is important to maintain a balance between muscle groups and a natural facial appearance. Patients differ enormously in their facial anatomy regarding structure and function, both within and between genders, ethnicities, and age. Therefore, treating all patients with the same injection pattern and the same doses can result in undesired outcomes. There is a need for a tailored approach to achieve optimal results, as well as to increase patient satisfaction. With this in mind, the novel one21 injection technique which allows for individualized treatment has been developed for the treatment of horizontal forehead lines using INCO, resulting in a positive impact on eyebrow position and shape. This technique is the next step in a customized approach, giving natural-looking results and high patient satisfaction.
Keywords: incobotulinumtoxinA, botulinum toxin, injection technique, one21, dynamic wrinkles, upper face
Introduction
Injection with botulinum toxin is the most popular non-surgical aesthetic intervention worldwide1,2 with the upper face being the most requested and most commonly treated area.3 Currently, several botulinum toxin type A formulations are approved for aesthetic use. The most widely used of these include onabotulinumtoxinA (ONA; Botox®/Vistabel®, Allergan Inc., Irvine, CA, USA), abobotulinumtoxinA (ABO; Dysport®/Azzalure®, Ipsen, Paris, France), and incobotulinumtoxinA (INCO; Xeomin®/Bocouture®, Merz Pharmaceuticals GmbH, Frankfurt am Main, Germany). Though all these formulations have the same mode of action and each contain the core 150 kDa neurotoxin, INCO remains the only formulation which is purified to contain just the required therapeutic component and is free from unnecessary bacterial proteins.4
The understanding of an individual’s muscle anatomy and muscle contraction pattern is considered a key element in determining the appropriate neuromodulator treatment for forehead lines.3,10 Abramo et al classified four distinct anatomical shapes of the frontalis muscle.11 In Type I, “full shape”, the muscle covers the entire forehead, including the central part, giving rise to parallel wrinkles that extend continuously throughout the forehead (Figure 1A). Type II is described as “V-shape” and is formed by two bands separated by aponeurotic tissue (Figure 1B). In Type III, the “central” form, the aponeurotic tissue is located laterally, and the muscle is in the central part of the forehead, leading to a column of lines in the central part of the forehead, with the lateral part of the eyebrow dropped (Figure 1C). Type IV, the “lateral” form, is characterized by 2 bands placed laterally in the forehead with a large portion of aponeurotic tissue in the central part (Figure 1D).
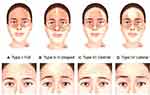 |
Figure 1 Variation in frontalis anatomy and corresponding forehead line patterns.11 Artwork by Rodrigo Tonan. |
Based on anatomical findings and the shape of the forehead lines, Moqadam et al9 related the “full shape” of the frontalis to straight parallel lines and the “V-shaped” distribution to wavy horizontal forehead lines. A more obtuse muscle fascicle angle of the frontalis muscle correlated with wavy forehead lines, whereas a more acute muscle fascicle angle correlated to straighter forehead lines. Based on these findings, conclusions can be drawn about the shape and distribution of the frontalis muscle by assessing the appearance of the patient’s forehead lines during consultation.
Clinical observations of skin movement utilizing skin displacement vector analyses revealed a bi-directional movement of the skin of the forehead.12 Eyebrow elevation seems to be responsible for the cranial movement of the skin of the lower forehead whereas hairline depression seems to be responsible for the caudal movement of the skin of the upper forehead. These two movements seem to converge at a non-mobile horizontal forehead line at approximate 60% relative to the total forehead height, irrespective of gender and ethnicity. These clinical observations suggest that while individual differences in frontalis anatomy must be taken into account prior to treatment with neuromodulators, the C-line represents a commonality across patient types. By minimizing the number of neuromodulator units injected below this static, non-mobile forehead line, the risk of brow ptosis could potentially be mitigated.
Beyond differences in muscle distribution, the overall shape of the forehead can differ from patient to patient. Differences in the shape of the skull can also be observed between ethnicities. Furthermore, differences between genders must be taken into account. Men have a larger skull than women, with a larger forehead and prominent supraorbital ridges. The glabella is wider in men, and the brows are more prominent and straight, and positioned lower than in women.13 Additionally, the aging process affects each patient differently. Flattening of the forehead, atrophy of the subcutaneous forehead fat pads and loss of skin elasticity leads to accentuation of bone projections. Eyelid drooping can also be observed due to an increase in orbit diameter through bone atrophy, with some patients exhibiting eyebrow ptosis already in their twenties.14–16 All these features may influence the appearance of the upper face and should be considered as part of a personalized botulinum toxin treatment approach.
The shape and position of the eyebrows are key elements of upper facial beauty and are determining factors in the expression of emotion, masculinity, femininity, personality, and state of mind.17–20 Few facial features are as powerful as the eyebrows, and studies have shown that when viewing a face, people spend the most time looking at the periocular region.21 The concept of brow beauty continues to evolve over time, as fashion and beauty trends change.22 Each individual’s brow aesthetics are unique and contribute significantly to the overall balance of the face.13 Inappropriate injection of botulinum toxin in the forehead may lead to unpredictable and unattractive results, including a frozen appearance, eyebrow ptosis or brow asymmetry, especially in females with low and flat eyebrows. Consequently, there is a need for a customizable but predictable treatment approach for shaping and positioning the eyebrows. The technique presented here can be customized to each patient’s particular brow aesthetic, which to the author’s knowledge has not been addressed in customized injection protocols to date. The results achieved are natural-looking, predictable and reproducible.
A recent consensus outlined the need for a tailored approach to the treatment of the upper face with botulinum toxin for optimal results and patient satisfaction. A series of individualized injection protocols were recommended, based on major parameters that differ between patients, with the division of the forehead into 12 zones to assist assessment. However, to the author’s knowledge, a single, clearly defined protocol that is adapted to each patient is not presented in the currently published literature. Building on these consensus recommendations and the initial development of this technique by Dr. Phillip Levy5 to include treatment of the entire upper face, a practical, dynamic and straightforward methodology that can be easily customized to give predictable and precise results has been developed and is presented here. The one21 technique is based on each patient’s unique functional anatomy, with 21 potential injection sites across the forehead and a customizable dosing scheme.
Materials and Methods
Patients are considered eligible for this technique once they present forehead wrinkles, and there is a need to treat at least one of the brow depressors in order to balance the position and the shape of the eyebrows. For injection, a 100 U vial of INCO is reconstituted with 2 mL of 0.9% sterile, non-preserved saline. The ability to precisely inject 0.5 U is crucial. The total number of units and total volume injected varies per patient according to the dosing scheme described in the following section.
The technique consists of a three-step protocol:
- Assessment of the functional anatomy unique to each patient.
- Bespoke evaluation of the patient’s desired outcome.
- Customized injection with the one21 technique
Assessment
A careful assessment must be performed in order to accomplish the best aesthetic outcomes; taking into account the individual anatomy of each patient, muscle function and habitual facial movements. The mass and strength of the muscles should also be considered. The forehead assessment should include an evaluation of the muscles at rest and at maximum contraction, while observing the patient speaking and performing specific facial movements.23 Patterns of aging, skin elasticity, surface landmarks, volume loss in the temples and forehead, position of the eyebrow, and the presence of excess skin in the upper and lower eyelids must also be assessed.14 INCO dosing and injection site distribution must reflect variations in muscle pattern, size, mass and dynamic movement to ensure optimal results. It is important to respect gender-, cultural- and ethnic-specific features of attractiveness, and to understand the patient’s goals and aesthetic self-perception.23
Photographic documentation is mandatory before any aesthetic treatment and should be shown to the patient and used as part of the pre-treatment assessment and communication process.23 Visual scales are useful in establishing realistic patient expectations and they can help in the discussion of the treatment plan by providing an objective view of relevant facial landmarks, aging signs, and any pre-existing asymmetry.23 The Merz Aesthetic Scales (MAS) are validated aesthetic grading scales which can be used to evaluate brow position and the severity of forehead lines, both at rest and in the dynamic position.24,25
For the assessment, the assessor and patient should sit eye-to-eye across from one another. Here, it is important to observe and respect functional anatomy during maximum contraction. Particular attention should be taken to detect any static lines at rest, any asymmetries, and any eyebrow and/or eyelid ptosis.14 A unique and individualized frontalis muscle pattern can be identified by observing the distribution and shape of the forehead lines in the status of maximum contraction. It is crucial to evaluate the influence of the depressors in the positioning and shape of the eyebrows. In order to identify the relevant anatomical landmarks, two key components must be assessed:
- Anatomical: determined by the position of the mid-pupillary line, medial canthus, lateral canthus and medial facial line.
- Functional: determined by the distribution and shape of the patient’s forehead lines at rest and in motion.
The anatomically defined components are marked by seven vertical lines on the patient’s forehead (Figure 2). The first step consists of determining the position of the mid-pupillary lines. Two lines should be drawn vertically down the forehead to mark the position of each mid-pupillary line. Two additional lines at the lateral canthus and two additional lines at the medial canthus should then be added. The final vertical line should be drawn along the medial facial line.
 |
Figure 2 Anatomically defined components of the one21 assessment grid. |
The functionally defined components are indicated by three horizontal lines on the patient’s forehead (Figure 3). The first to be determined is the lowest horizontal forehead line, or inferior limit line. The line may be continuous, or in some cases also medially discontinued. Injections should not be administered below this line, to avoid the risk of brow ptosis. The second horizontal line to be determined corresponds to the uppermost forehead line. Finally, the third line should be drawn horizontally, equidistant from and parallel to the upper and lower forehead lines (Figure 3, green line). It is important to note that this line does not necessarily correspond to the middle of the forehead or to the middle horizontal forehead wrinkle, but is always an equal distance between the uppermost horizontal line and the lowest horizontal line or inferior limit line. The patient may have horizontal forehead wrinkles that lie between the lines of the treatment grid, depending on their individual anatomy.
Clinical observations revealed that this third horizontal line is located at approximately 60% of the total forehead height and corresponds to a non-mobile area of the forehead where the bi-directional movement of the forehead skin converges (= line of convergence; C-Line) (Figure 4). To achieve the best possible results using the one21 technique, dosing assessment should begin with this intermediate non-mobile forehead line (= C-Line), referred to here as the green line. The highest number of units will typically be injected along this line. When injecting in the inferior limit line, care should be taken to minimize the number of units and injection points used, in order to positively impact the shape and positioning of the brow, while abating the risk of brow ptosis.
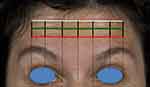 |
Figure 3 Functionally defined components of the one21 assessment grid. Red = inferior limit line. Green = intermediate line corresponding to the C-line. |
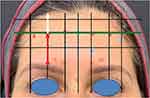 |
Figure 4 Position of the intermediate line relative to the total forehead height. Red = 45 mm. White = 28 mm. Green = intermediate line at 60% of the total height. |
The final assessment outcome gives rise to an assessment grid with 21 intersecting points. These points of intersection should be clearly marked on the patient (Figure 5). The corresponding gridlines can be subsequently erased if desired. Each of these intersecting points corresponds to a potential injection site and has a different effect on the treatment outcome. The dosing of each point can be tailored accordingly, for a fully customized approach.
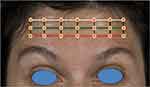 |
Figure 5 Final assessment grid with 21 potential injection sites. |
Bespoke Evaluation of the Patient’s Desired Outcome
Once the 21 potential injection points are identified, the patient’s individual desires must be catered for. Gender, ethnicity and age must be taken into account, considering that shape and position of the eyebrows play a role in femininity and masculinity13 and that the concept of eyebrow beauty changes over time.22 Using a mirror, the patient is encouraged to analyze the natural shape of their eyebrows. The practitioner should point out any asymmetries or eyebrow ptosis to the patient. While having the patient fully relax and fully contract the frontalis muscle, the effect of paralysis is simulated by application of varying pressure with one finger, allowing the patient to clearly observe the differences in the mirror. Finally, through palpation, the natural eyebrow shape should be preserved at maximum contraction and at rest. This allows the patient to visualize the differences between a paralyzed or natural look, enabling them to make an informed decision about their desired treatment outcome.
Customized Injection with the One21 Technique
To assist in defining the distribution of injection points and doses, it is important to gently palpate the forehead while the patient contracts the frontalis, in order to feel the presence or absence of the muscle in each particular area, as well as the strength of the muscle. The dosing assessment should begin with the green horizontal line, which is equidistant to the uppermost and lowest horizontal forehead line and corresponds to the C-line. Here, the highest dose of neuromodulator will typically be required. For dose determination, three different degrees of pressure should be used to simulate the effect of paralysis, and the effect on the appearance of the forehead lines and the changes in the eyebrow shape and position observed. The appropriate dose per injection site can be defined based on the force of the muscle and the degree to which each particular segment should be blocked. The following dosing scheme can be used for guidance:
- 2 U INCO: strong segment of frontalis.
- 1 U INCO: moderately strong segment of frontalis.
- 0.5 U INCO: weak segment of frontalis.
- 0 U INCO: no muscle force palpated; no toxin needed.
The scale of doses can be modified if the patient is a male with a very strong frontalis muscle, using 0 U–1 U–2 U–3 U in place of the above. Each of the 21 potential injection sites can be marked in a different color to indicate the dosage required, to assist in application.
Positioning and Shaping of Eyebrows
To shape the eyebrows, injection should take place along the lowest horizontal forehead line, or inferior limit line (Figure 6). This line falls below the C-line and as such, care should be taken to minimize the total dose of neuromodulator injected along this line to ensure optimal outcomes. To arch and laterally raise the eyebrow, inject medially to the mid-pupillary line. If the patient desires a more flattened eyebrow, the point intersecting with the mid-pupillary line should be injected. To drop the eyebrow, inject laterally to the mid-pupillary line. If the goal is a soft arching effect of the brow, this can be achieved using different doses in each injection site, creating a gradient; injecting higher doses medially and lower ones laterally. It is mandatory to treat the depressor muscles when using this technique, in order to create a balance that will allow the brow to raise.
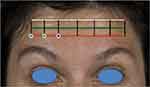 |
Figure 6 Eyebrow positioning with the one21 technique. Blue = drop eyebrow. Green = flattened eyebrow. Purple = laterally arch and raised eyebrow. |
Results
In order to accurately demonstrate the use of the one21 technique, results for an actual patient are presented here. The patient has provided written informed consent for the publication of images and the use of information. Ethical approval was not required.
A 36-year-old female was injected with INCO for the treatment of horizontal frown lines. Her desire was to erase her forehead wrinkles, but primarily to correct asymmetry in the positioning of her eyebrows and to laterally arch the brow. She was previously treated with ABO on four occasions and reported experiencing a “frozen” face. Achieving a natural look was, therefore, a high priority for this patient.
Assessment began with an analysis of the individual frontalis anatomy. On maximum contraction, forehead lines appeared in a column in the central part of the forehead, indicating the “central” Type III frontalis distribution, with the muscle in the central part of the forehead and aponeurotic tissue lateral (Figure 7).
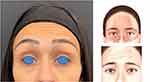 |
Figure 7 Analysis of the patient’s frontalis pattern identified a Type III (central). Artwork by Rodrigo Tonan. |
The strength of the frontalis across the forehead was then evaluated by palpation. The frontalis was overall only moderate in strength, with the central part of the right-hand side being slightly stronger than the left-hand side (Figure 8). Before treatment, the patient presented with moderate forehead lines (Grade 2) at full contraction and mild lines (Grade 1) at rest according to the Merz Aesthetics Scales (MAS) for forehead lines.
This was also apparent when analyzing the eyebrow positioning, where asymmetry between the brows was observed, with the tail of the eyebrows dropped (Figure 9). The patient’s desired outcome was to reduce the appearance of horizontal forehead lines, balance the eyebrow positioning and create an arching effect of the brows laterally.
According to the anatomically- and functionally defined components, the patient’s forehead was divided into an assessment grid with 21 potential injection sites (Figure 10).
 |
Figure 9 Brow asymmetry observed during the assessment. |
 |
Figure 10 Assessment grid according to anatomically- and functionally defined components. |
2 U INCO were administered to those sites where the frontalis was strongest (marked in red, Figure 11) in the upper central part of the forehead. 1 U INCO was injected where the frontalis was weaker (marked in blue, Figure 11) in the central point of the lowest horizontal frown line to create an arching effect of the eyebrow laterally. In the middle horizontal line, 3 points with 1 U each were injected in the central part. 0.5 U INCO was administered on the right-hand side to balance the eyebrow positioning (marked in green, Figure 11). The brow depressors were also treated (Figure 12).
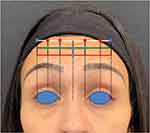 |
Figure 11 Customized dosing scheme across 12 injection sites. Red = 2 U INCO. Blue = 1 U INCO. Green = 0.5 U INCO. Total dose administered: 15.5 U. |
 |
Figure 12 Treatment of the brow depressors. Yellow = 4 U INCO. Red = 2 U. Blue = 1 U. Total dose administered: 22 U. |
The appearance of horizontal forehead lines at maximum contraction was significantly reduced (Figure 13), with a two-point improvement (Grade 2 to Grade 0) on the dynamic MAS and one-point improvement (Grade 1 to Grade 1) on the MAS at rest.
 |
Figure 13 Before treatment (left) and 15 days after treatment (right), dynamic. |
Furthermore, brow asymmetry was successfully treated, giving a balanced and natural look. The dropped tail of the brow was lifted, to give a gentle lateral arching effect (Figures 13 and 14).
 |
Figure 14 Before treatment (left) and 15 days after treatment (right), at rest. |
The patient was highly satisfied with her treatment outcome, in particular with the natural appearance of the results.
Discussion
Neuromodulator treatment of the upper face can be a complex task. There is a huge inter-individual variation in forehead anatomy which must be considered for optimal treatment of forehead lines. Furthermore, each patient has a unique desired outcome and aesthetic preference, particularly with regard to brow aesthetics.22 It is clear that no universal treatment protocol can suit all patients, and a tailored approach is needed to maximize patient satisfaction. Despite this, there is little guidance available in the literature for aesthetic practitioners seeking to develop a customized approach to treatment. A range of different treatment protocols based on common patient scenarios may lack practicability for the physician, as it may not be clear how to classify certain patients and thus decide on an appropriate treatment algorithm. Here, a single, straightforward protocol which can be adapted to each patient’s unique functional anatomy is described. The one21 technique has been designed for use with INCO, due to its high precision and low spread. This technique allows for more precise treatment of the entire frontalis muscle, rather than the usual technique treating 5 injection points in a horizontal line across the mid-forehead, which may not give the best treatment outcome for all patients. Because INCO is used with limited spread, the patient can be injected inferior to the mid-forehead line without the risk of eyebrow ptosis. As data suggest diffusion characteristics may differ between the botulinum toxin preparations, no conclusions can be drawn about the applicability of this technique to other botulinum toxin formulations.6,7,8
The one21 technique is based on a 3-step protocol, involving careful assessment of the individual anatomy of each patient, with its individual function, mass and strength of the frontalis muscle, in both, the static and dynamic states. Guided by anatomical and functional landmarks, a treatment grid with 21 potential injection points individual to each patient can be defined. By palpating each area of the forehead at maximum contraction, the dose of INCO required for each injection point can be determined, allowing for a highly customizable approach. The position of the intermediate line in the treatment grid corresponds approximately to the line at which both directions of movement of the frontalis converge (the C-line) and it is this line that highest number of units of INCO should be injected. It is important to note that not all points in the inferior limit line are typically injected, and where injection is necessary, the dose should typically range between 0.5 U and 1 U per injection site in females, to minimize the risk of brow ptosis.
This technique delivers natural results for the treatment of the upper face, and is customized to each patient’s needs. Using one21, eyebrows can be predictably shaped, minimizing the risk of undesired outcomes which may significantly impact the overall harmony of the face. The technique is associated with high levels of patient satisfaction and provides the physician with a toolkit to meet unique patient demands. The one21 technique may assist both beginner and expert practitioners in achieving natural, customized and highly satisfactory results from neuromodulator treatment of the forehead.
Acknowledgments
The author would like to thank Sebastian Cotofana MD PhD for his input during manuscript preparation and final approval prior to submission. Dr. Phillip Levy is gratefully acknowledged for his work in developing the customized injection protocol on which this technique is based. Medical writing support was provided by Emma Robertson of Merz Pharmaceuticals GmbH.
Disclosure
Dr. Pecora has acted as a consultant and speaker for Merz Pharmaceuticals GmbH and received personal fees from Merz Pharmaceuticals GmbH, outside the submitted work. The author reports no other conflicts of interest in this work.
References
1. ISAPS. International survey on cosmetic procedures performed in 2017; 2017. Available from: https://www.isaps.org/wp-content/uploads/2019/03/ISAPS_2017_International_Study_Cosmetic_Procedures_NEW.pdf.
2. ASAPS. Cosmetic Surgery National Data Bank Statistics for 2018. Available from: https://surgery.org/sites/default/files/ASAPS-Stats2018.pdf. Accessed December 27, 2019.
3. Sundaram H, Signorini M, Liew S, et al. Global aesthetics consensus: botulinum Toxin Type A-evidence-based review, emerging concepts, and consensus recommendations for aesthetic use, including updates on complications. Plast Reconstr Surg. 2016;137(3):518e–529e. doi:10.1097/01.prs.0000475758.63709.23
4. Kerscher M, Wanitphakdeedecha R, Trindade de Almeida A, Maas C, Frevert J. IncobotulinumtoxinA: a highly purified and precisely manufactured Botulinum Neurotoxin Type A. J Drugs Dermatol. 2019;18(1):52–57.
5. Levy PH. Grid21.
6. Kerscher M, Roll S, Becker A, Wigger-Alberti W. Comparison of the spread of three botulinum toxin type A preparations. Arch Dermatol Res. 2012;304(2):155–161. doi:10.1007/s00403-011-1179-z
7. da Costa A, Pegas Pereira ES, Pereira MO, et al. Six-month comparative analysis monitoring the progression of the largest diameter of the sweating inhibition halo of different botulinum toxins Type-A. Aesthet Surg J. 2018;39(9):993–1004.
8. Kerscher M, Maack M, Reuther T, Krueger N. Diffusion characteristics of two different neurotoxins in patients with symmetric forehead lines. J Am Acad Dermatol. 2007;56(2):AB199.
9. Moqadam M, Frank K, Handayan C, et al. Understanding the shape of forehead lines. J Drugs Dermatol. 2017;16(5):471–477.
10. Monheit G, Lin X, Nelson D, Kane M. Consideration of muscle mass in glabellar line treatment with botulinum toxin type A. J Drugs Dermatol. 2012;11(9):1041–1045.
11. Abramo AC, Do Amaral TP, Lessio BP, De Lima GA. Anatomy of forehead, glabellar, nasal and orbital muscles, and their correlation with distinctive patterns of skin lines on the upper third of the face: reviewing concepts. Aesthetic Plast Surg. 2016;40(6):962–971. doi:10.1007/s00266-016-0712-z
12. Cotofana S, Freytag DL, Frank K, et al. In Press. 2019.
13. Alex JC. Aesthetic considerations in the elevation of the eyebrow. Facial Plast Surg. 2004;20(3):193–198. doi:10.1055/s-2004-861774
14. de Maio M, Swift A, Signorini M, Fagien S. Aesthetic leaders in facial aesthetics consensus C. Facial assessment and injection guide for botulinum toxin and injectable hyaluronic acid fillers: focus on the upper face. Plast Reconstr Surg. 2017;140(2):265e–276e. doi:10.1097/PRS.0000000000003544
15. Shaw RB, Kahn DM. Aging of the midface bony elements: a three-dimensional computed tomographic study. Plast Reconstr Surg. 2007;119(2):
16. Friedman O. Changes associated with the aging face. Facial Plast Surg Clin North Am. 2005;13(3):371–380. doi:10.1016/j.fsc.2005.04.004
17. Carruthers J, Fagien S, Matarasso SL, Botox Consensus G. Consensus recommendations on the use of botulinum toxin type a in facial aesthetics. Plast Reconstr Surg. 2004;114(6 Suppl):1S–22S. doi:10.1097/01.PRS.0000144795.76040.D3
18. Knoll BI, Attkiss KJ, Persing JA. The influence of forehead, brow, and periorbital aesthetics on perceived expression in the youthful face. Plast Reconstr Surg. 2008;121(5):1793–1802. doi:10.1097/PRS.0b013e31816b13fe
19. Cox SE, Finn JC. Social implications of hyperdynamic facial lines and patient satisfaction outcomes. Int Ophthalmol Clin. 2005;45(3):13–24. doi:10.1097/01.iio.0000167237.49396.7b
20. Lorenc ZP, Smith S, Nestor M, Nelson D, Moradi A. Understanding the functional anatomy of the frontalis and glabellar complex for optimal aesthetic botulinum toxin type A therapy. Aesthetic Plast Surg. 2013;37(5):975–983. doi:10.1007/s00266-013-0178-1
21. Chelnokova O, Laeng B. Three-dimensional information in face recognition: an eye-tracking study. J Vis. 2011;11(13):27. doi:10.1167/11.13.27
22. Griffin GR, Kim JC. Ideal female brow aesthetics. Clin Plast Surg. 2013;40(1):147–155. doi:10.1016/j.cps.2012.07.003
23. Carruthers J, Fournier N, Kerscher M, Ruiz-Avila J, Trindade de Almeida AR, Kaeuper G. The convergence of medicine and neurotoxins: a focus on botulinum toxin type A and its application in aesthetic medicine–a global, evidence-based botulinum toxin consensus education initiative: part II: incorporating botulinum toxin into aesthetic clinical practice. Dermatol Surg. 2013;39(3 Pt 2):510–525. doi:10.1111/dsu.12148
24. Flynn TC, Carruthers A, Carruthers J, et al. Validated assessment scales for the upper face. Dermatol Surg. 2012;38(2Spec No.):309–319. doi:10.1111/j.1524-4725.2011.02248.x
25. Carruthers A, Carruthers J, Hardas B, et al. A validated brow positioning grading scale. Dermatol Surg. 2008;34(Suppl 2):S150–S154. doi:10.1111/j.1524-4725.2008.34363.x
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

