Back to Journals » Clinical Ophthalmology » Volume 17
One Year Results of Faricimab for Aflibercept-Resistant Diabetic Macular Edema
Authors Rush RB
Received 4 June 2023
Accepted for publication 9 August 2023
Published 16 August 2023 Volume 2023:17 Pages 2397—2403
DOI https://doi.org/10.2147/OPTH.S424314
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Ryan B Rush1– 3
1Panhandle Eye Group, Amarillo, TX, USA; 2Southwest Retina Specialists, Amarillo, TX, USA; 3Department of Surgery, Texas Tech University Health Science Center, Amarillo, TX, USA
Correspondence: Ryan B Rush, Southwest Retina Specialists, 7411 Wallace Blvd, Amarillo, TX, 79106, USA, Tel +1 806 351-1870, Email [email protected]
Purpose: To assess the 12 month outcomes of intravitreal faricimab (IVF) in treatment-resistant diabetic macular edema (DME) recalcitrant to intravitreal aflibercept (IVA).
Methods: This study was undertaken as a retrospective interventional case series of DME subjects receiving care at a single private practice facility. Subjects at baseline had undergone ≥ 8 IVA injections over the previous 12 months, ≥ 4 IVA injections over the previous 6 months, had an optical coherence tomography (OCT)-measured central macular thickness (CMT) of ≥ 320 microns, and had observable edema on OCT. The baseline visit for this study’s purpose was considered the examination in which the subject was changed from IVA to IVF. Subjects were managed with a treat-and-extend (TAE) protocol and followed over 12 months from baseline.
Results: A total of 51 eyes of 51 subjects were analyzed. There were 39.2% (20/51) of patients who reached a treatment interval of ≥ 8 weeks and had a fluid-free macula on OCT at 12 months. The CMT on OCT of the patient population reduced from 400.2 (385.3– 415.3) microns at baseline to 340.6 (324.3– 356.9) microns at 12 months (p< 0.01). There were 21.6% (11/51) of patients who improved ≥ 3 lines of Snellen visual acuity at 12 months. The visual acuity of the overall study population improved from 0.60 (0.54– 0.66) logMAR (Snellen 20/80) at baseline to 0.47 (0.41– 0.53) logMAR (Snellen 20/59) at 12 months (p< 0.01).
Conclusion: A longer treatment interval and improved functional and anatomical outcomes at 12 months may be attained in a clinically significant minority of aflibercept-resistant DME patients after changing to IVF when a TAE protocol is employed. Specialists may consider IVF whenever resistance to IVA is experienced in this patient population.
Keywords: faricimab, treatment-resistant, diabetic macular edema, recalcitrance
Introduction
Vascular endothelial growth factor (VEGF) inhibition is the standard-of-care management strategy for diabetic macular edema (DME).1,2 However, the need for frequent treatments and insufficient response continue to encumber a certain subset of patients with DME. About 20% of patients with DME have persistent macular fluid on optical coherence tomography (OCT) despite monthly anti-VEGF treatments over a period of 36 months.3 As a result, targeted therapies to other mediators involved in angiogenesis and leakage of the retinal vasculature besides VEGF have recently been developed.
Faricimab (Roche/Genentech; Basel, Switzerland) is a targeted antibody therapy that binds with high affinity both to VEGF-A and Angiopoietin-2 (Ang-2). Faricimab gained Food and Drug Administration (FDA) approval for the indication of DME in early 2022 based on the results of the key clinical trials, YOSEMITE and RHINE.4 Subjects treated with intravitreal faricimab (IVF) demonstrated non-inferior functional outcomes at a fixed treatment interval of 8 weeks or according to a personalized treat-and-extend (TAE) protocol compared to subjects who were treated with intravitreal aflibercept (Eylea/Regeneron; NY, USA) (IVA) at a fixed 8 week treatment interval.4 However, a noteworthy limitation in the design of YOSEMITE and RHINE is that these trials did not permit subjects randomized to treatment with IVA to extend past an 8-week treatment interval irrespective of the subject’s clinical course, while subjects randomized to treatment with IVF were permitted to follow a prespecified TAE schedule. As a result, subjects who underwent IVF at an interval of ≥12 weeks could not be compared to subjects who underwent IVA at an interval of ≥12 weeks. Additionally the results of YOSEMITE and RHINE may only be applied to treatment-naïve subjects with DME and are not generalizable to the overall population with DME since subjects formerly treated with other anti-VEGF medications were specifically excluded. The short-term (4 month) outcomes of switching subjects with DME from IVA to IVF when treatment-recalcitrance was encountered has been previously reported by the author.5 The author in this study reports the 12-month outcomes in this same aflibercept-resistant DME population using a TAE protocol in a real-world setting.
Methods
This retrospective case series was administered according to the principles of the Helsinki Declaration and was compliant with the Health Insurance Portability and Accountability Act of 1996. Research approval was acquired from the Panhandle Eye Group Institutional Review Board (IORG0009239; IRB00011013-13). The requirement for informed consent was waived because the patient information was collected retrospectively and all identifying patient data was omitted. The research was taken from patients receiving treatment at a single private practice facility in Amarillo, TX from February 2022 until June 2023.
Table 1 exhibits the study’s inclusion and exclusion criteria. The baseline evaluation for the purpose of this research project was the appointment in which the change from IVA to IVF was made. The TAE protocol employed in this study has been previously reported by the authors5 and similar protocols have been widely adopted by other specialists for the management of DME.6,7 In short, patients at baseline received a loading dose of IVF consisting of monthly (28–34 days) treatments for a minimum of three injections. After the effect of the third injection of IVF was assessed, patients would be extended if all identifiable retinal edema on central subfoveal OCT cross-sectional view was resolved. IVF injections continued monthly until the resolution of the edema on OCT was confirmed; patients continued on monthly injections indefinitely if the edema was unable to be cleared. Following the resolution of the retinal fluid on OCT, the interval of treatment was extended out at intervals of 1–2 weeks until the edema on OCT recurred and the treatment interval would then be adjusted accordingly in order to obtain a fluid-free retina again. The Heidelberg Spectralis system (Heidelberg Engineering, Heidelberg, Germany) was the OCT platform utilized during the study. Baseline and 12 month follow-up OCT images were evaluated by two masked retina specialists for the appearance of intraretinal and/or subretinal edema. If and when a disagreement arose between OCT reviewers, a third masked retina specialist made the final determination. If both eyes of the same patient met the study’s inclusion/exclusion criteria, simple randomization (a random number generating program) selected which eye would be included in the analysis.
 |
Table 1 Faricimab for Aflibercept-Resistant Diabetic Macular Edema. Inclusion and Exclusion Criteria |
Principal Outcomes and Statistical Analysis
The primary outcome of the study was the percentage of patients who reached a treatment interval of ≥8 weeks and maintained a fluid-free macula on OCT at the study’s end (12 months). The secondary outcome was the percentage of patients who improved ≥3 lines of Snellen visual acuity at the study’s end (12 months). The JMP 17 (SAS Institute; USA) software was utilized to perform the statistical analysis. Snellen visual acuity was changed into logMAR for analysis of the data. Statistical significance was measured at the alpha level of <0.05.
Results
A total of 51 eyes of 51 subjects were included in the study’s analysis. The agreement rate was 92.2% (47/51) between the two OCT reviewers. The baseline characteristics of the research population have been presented in Table 2.
 |
Table 2 Faricimab for Aflibercept-Resistant Diabetic Macular Edema. Baseline Characteristics of the Research Population. Means with (95% Confidence Intervals) |
There were 39.2% (20/51) of patients who reached a treatment interval of ≥8 weeks and had a fluid-free macula on OCT at 12 months. There were 11.8% (6/51) of patients at a 12 week treatment interval, 39.2% (20/51) of patients at a treatment interval between 5–7 weeks, and 21.6% (11/51) of subjects still at a 4 week treatment interval at the study’s end (12 months). Of the 11 subjects still at a 4 week treatment interval at 12 months, only 18.2% (2/11) had a fluid-free macula on OCT. The average treatment interval at the study’s end was 6.9 (6.1–7.6) weeks. The CMT on OCT of the patient population reduced from 400.2 (385.3–415.3) microns at baseline to 340.6 (324.3–356.9) microns at 12 months (p<0.01). Figure 1 exhibits the CMT on OCT distributions from baseline to 4 months to 12 months. As exhibited, the mean 4 month CMT on OCT was similar to the 12 month value and significantly reduced compared to baseline. When the CMT on OCT reduced by ≥50 microns after the first three injections of IVF, 82.4% (14/17) of patients achieved a treatment interval of ≥8 weeks and had a fluid-free macula on OCT at 12 months.
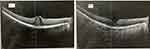
There were 21.6% (11/51) of patients who improved ≥3 lines of Snellen visual acuity at 12 months. Of the 11 patients who improved ≥3 lines of Snellen visual acuity at 12 months, 90.1% (10/11) of patients were able to maintain a treatment interval ≥8 weeks and have a fluid-free macula on OCT at 12 months. Of the 11 subjects who improved ≥3 lines of Snellen visual acuity at 12 months, 72.3% (8/11) of patients achieved the improvement of the ≥3 lines of Snellen visual acuity after the first three injections of IVF. The visual acuity of the overall study population improved from 0.60 (0.54–0.66) logMAR (Snellen 20/80) at baseline to 0.47 (0.41–0.53) logMAR (Snellen 20/59) at 12 months (p<0.01). Figure 2 exhibits the research population’s visual acuity from baseline to 4 months to 12 months. As exhibited, the mean 4 month visual acuity was about half way between the baseline and 12 month values. A case example of a patient from the study population has been exhibited in Figure 3.
Discussion
Studies have reported an assortment of treatment schedules prior to considering a subject to be with recalcitrant DME, including three consecutive monthly anti-VEGF treatments,8 four successive anti-VEGF injections,9 or a minimum of six consecutive anti-VEGF treatments.10 However, a subset of patients with DME may need 6–12 months of continuous treatment in order to resolve the fluid on OCT11 so the author only included patients in this study who had undergone at least eight aflibercept injections over 12 months to make sure the patient with DME was truly recalcitrant to aflibercept treatment. Recurring anti-VEGF treatments over long time periods may produce immunoreactivity and tachyphylaxis with reduced therapeutic effect,12 and changing to another anti-VEGF medication may sometimes overcome this issue.13 Aflibercept became a treatment option in patients with DME recalcitrant to other anti-VEGF agents since its coming to market more than a decade ago. Since then the benefits of aflibercept in this patient population has been well-established.14,15 However, little direction presently exists on how to proceed when aflibercept-resistance is observed. Unfortunately persistent DME despite monthly aflibercept injections has been reported to occur in 32% of patients at 24 weeks and 44% of those same patients still on aflibercept after 24 months had persistent DME.16
To the awareness of the author, this is the first study to report 12 month outcomes in DME patients changed to faricimab secondary to aflibercept recalcitrance. Functional and anatomical responses were assessed by measuring visual acuity and CMT on OCT before and after switching to faricimab from aflibercept, and these assessments directly affected the interval of treatment per the TAE protocol utilized in this study. In the DME patients meeting the study’s primary outcome (approximately 40%), fewer treatments could be administered over 12 months without compromising the functional and/or anatomical outcomes of the patient. Of special note, the mean decrease in CMT on OCT was manifested by 4 months and remained stable throughout the study period of 12 months, whereas the mean improvement in visual acuity at 4 months was approximately half the value obtained at 12 months, suggesting visual acuity may gradually improve over 12 months in this study population. This study utilized a real-world TAE protocol typical to what most specialists who treat DME are familiar with, thereby allowing this study’s findings to be clinically meaningful to other physicians considering faricimab when aflibercept-recalcitrance is experienced.
It is now recognized that DME is driven both by the VEGF and angiopoietin-Tie2 pathways, including a synergistic interplay between such pathways.17 Activation of the Tie2 pathway results in a signaling sequence that promotes blood vessel stability and reduces inflammation and vascular permeability. Since faricimab is a bispecific monoclonal antibody that has different light chains in each fragment antigen-binding region with the possibility of binding two separate targets, it may simultaneously and independently neutralize VEGF and Ang-2, a hypothetical advantage over aflibercept and the other anti-VEGF agents presently available.18,19 While only about 40% of patients could be extended out to 8 weeks or beyond and just about 22% of patients improved ≥3 lines of Snellen visual acuity at 12 months, every patient included in this study had been receiving active treatment for ≥12 months and on average had received about 14 anti-VEGF injections prior to their switch to faricimab. Focusing on this point in particular, it may be inferred that the absence of a clinically meaningful treatment response in the majority of the patients following the switch to faricimab may be the result of enduring retinal damage and photoreceptor loss prior to the switch in anti-VEGF agents. Research in this patient population does suggest that long-term visual outcomes are best when all residual retinal edema is resolved.20 Be this the situation, a shorter delay before changing to faricimab may have resulted in better outcomes in our aflibercept-resistant DME population. Nevertheless, in the 40% of aflibercept-resistant DME patients who were able to extend to 8 weeks or beyond between faricimab treatments utilizing the TAE protocol employed in this study, all relevant parties benefited: the patient (less physician visits and treatments), the physician (more time to care for other patients requiring treatment), and society (lower cost burden on the insurance system).
Limitations of this research include its retrospective design, its absence of a control group, and the application of Snellen visual acuity converted into logMAR units rather than ETDRS scoring. Strengths of this research comprise the moderately large patient numbers included considering faricimab’s newness, the study’s relatively long period of follow-up (12 months) considering faricimab’s newness, and the application of a TAE protocol familiar to most physicians who treat this patient population in a real-world environment. In close, a clinically meaningful minority of aflibercept-resistant DME patients who switch to faricimab may attain longer treatment intervals without compromising anatomical and/or visual outcomes after 12 months when a real-world TAE protocol is utilized. Additional studies are warranted to corroborate these findings.
Abbreviations
TAE, treat-and-extend; OCT, optical coherence tomography; VA, visual acuity; DME, diabetic macular edema; VEGF, vascular endothelial growth factor; CMT, central macular thickness.
Ethics and Consent Statements
The study was approved by the Panhandle Eye Group Institutional Review Board (IORG0009239; IRB00011013-013) in accordance with the Ethical Standards laid down in the Declaration of Helsinki. Informed consent from study participants was waived because this was a retrospective study.
Consent for Publication
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author Contributions
The author made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The author reports no conflicts of interest in this work.
References
1. Wells JA, Glassman AR, Ayala AR, et al.; Diabetic Retinopathy Clinical Research Network. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372(13):1193–1203.
2. Elman MJ, Aiello LP, Beck RW, et al.; Diabetic Retinopathy Clinical Research Network. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117(6):1064–1077 e35. doi:10.1016/j.ophtha.2010.02.031
3. Bressler SB, Ayala AR, Bressler NM, et al. Persistent macular thickening after ranibizumab treatment for diabetic macular edema with vision impairment. JAMA Ophthalmol. 2016;134(3):278–285. doi:10.1001/jamaophthalmol.2015.5346
4. Wykoff CC, Abreu F, Adamis AP, et al. Efficacy, durability, and safety of intravitreal faricimab with extended dosing up to every 16 weeks in patients with diabetic macular oedema (YOSEMITE and RHINE): two randomised, double-masked, Phase 3 trials. Lancet. 2022;399(10326):741–755. doi:10.1016/S0140-6736(22)00018-6
5. Rush RB, Rush SW. Faricimab for treatment-resistant diabetic macular edema. Clin Ophthalmol. 2022;16:2797–2801. doi:10.2147/OPTH.S381503
6. Sarohia GS, Nanji K, Khan M, et al. Treat-and-extend versus alternate dosing strategies with anti-vascular endothelial growth factor agents to treat center involving diabetic macular edema: a systematic review and meta-analysis of 2346 eyes. Surv Ophthalmol. 2022;67(5):1346–1363. doi:10.1016/j.survophthal.2022.04.003
7. Chujo S, Sugimoto M, Sasaki T, et al. Comparison of 2-year outcomes between intravitreal ranibizumab and intravitreal aflibercept for diabetic macular edema with “Treat-and-Extend” regimen-its usefulness and problems. J Clin Med. 2020;9(9):2848. doi:10.3390/jcm9092848
8. Choi MY, Jee D, Kwon JW, Vavvas DG. Characteristics of diabetic macular edema patients refractory to anti-VEGF treatments and a dexamethasone implant. PLoS One. 2019;14(9):e0222364. doi:10.1371/journal.pone.0222364
9. Rahimy E, Shahlaee A, Khan MA, et al. Conversion to aflibercept after prior anti-VEGF therapy for persistent diabetic macular edema. Am J Ophthalmol. 2016;164:118–127.
10. Fechter C, Frazier H, Marcus WB, Farooq A, Singh H, Marcus DM. Ranibizumab 0.3 mg for persistent diabetic macular edema after recent, frequent, and chronic bevacizumab: the ROTATE trial. Ophthalmic Surg Lasers Imaging Retina. 2016;47(11):1–18. doi:10.3928/23258160-20161031-07
11. Huang WH, Lai CC, Chuang LH, et al. Foveal microvascular integrity association with anti-VEGF treatment response for diabetic macular edema. Invest Ophthalmol Vis Sci. 2021;62(9):41. doi:10.1167/iovs.62.9.41
12. Bressler NM, Beaulieu WT, Maguire MG, et al.;Diabetic Retinopathy Clinical Research Network. Early response to anti-vascular endothelial growth factor and two-year outcomes among eyes with diabetic macular edema in protocol T. Am J Ophthalmol. 195;2018:93–100. doi:10.1016/j.ajo.2018.07.030
13. Ashraf M, Souka AA, ElKayal H. Short-term effects of early switching to ranibizumab or aflibercept in diabetic macular edema cases with non-response to bevacizumab. Ophthalmic Surg Lasers Imaging Retina. 2017;48(3):230–236. doi:10.3928/23258160-20170301-06
14. Laiginhas R, Silva MI, Rosas V, et al. Aflibercept in diabetic macular edema refractory to previous bevacizumab: outcomes and predictors of success. Graefes Arch Clin Exp Ophthalmol. 2018;256(1):83–89. doi:10.1007/s00417-017-3836-1
15. Bahrami B, Hong T, Schlub TE, Chang AA. Aflibercept for persistent diabetic macular edema: forty-eight-week outcomes. Retina. 2019;39(1):61–68. doi:10.1097/IAE.0000000000002253
16. Bressler NM, Beaulieu WT, Glassman AR, et al. Persistent macular thickening following intravitreous aflibercept, bevacizumab, or ranibizumab for central-involved diabetic macular edema with vision impairment: a secondary analysis of a randomized clinical trial. JAMA Ophthalmol. 2018;136(3):257–269. doi:10.1001/jamaophthalmol.2017.6565
17. Liberski S, Wichrowska M, Kociecki J. Aflibercept versus faricimab in the treatment of neovascular age-related macular degeneration and diabetic macular edema: a review. Int J Mol Sci. 2022;23(16):9424. doi:10.3390/ijms23169424
18. Hussain RM, Neiweem AE, Kansara V, et al. Tie-2/Angiopoietin pathway modulation as a therapeutic strategy for retinal disease. Expert Opin Investig Drugs. 2019;28(10):861–869. doi:10.1080/13543784.2019.1667333
19. Ferro Desideri L, Traverso CE, Nicolò M. The emerging role of the Angiopoietin-Tie pathway as therapeutic target for treating retinal diseases. Expert Opin Ther Targets. 2022;26(2):145–154. doi:10.1080/14728222.2022.2036121
20. Sadda SR, Campbell J, Dugel PU, et al. Relationship between duration and extent of oedema and visual acuity outcome with ranibizumab in diabetic macular oedema: a post hoc analysis of Protocol I data. Eye. 2020;34(3):480–490. doi:10.1038/s41433-019-0522-z
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.