Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
One Case of Tuberculosis-Like Leprosy with a Type I Leprosy Reaction
Authors Li J, Wang CH, Yu DH, He Q, He W
Received 23 May 2023
Accepted for publication 20 August 2023
Published 18 September 2023 Volume 2023:16 Pages 2517—2523
DOI https://doi.org/10.2147/CCID.S421159
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Jie Li,1,* Chun-Hong Wang,2,* De-Hou Yu,2 Qin He,2 Wei He1
1Department of Dermatology, GuiQian International General Hospital, Guiyang, Guizhou, 550018, People’s Republic of China; 2Department of Dermatology, The Affiliated Hospital of Guizhou University, Guiyang, Guizhou, 550001, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qin He, Department of Dermatology, The Affiliated Hospital of Guizhou University, No. 28, Guiyi Street, Guiyang, Guizhou, 550001, People’s Republic of China, Tel +86 18585037228, Email [email protected] Wei He, Department of Dermatology, GuiQian International General Hospital, No. 1, Dongfeng Street, Wudang District, Guiyang, Guizhou, 550018, People’s Republic of China, Email [email protected]
Abstract: Leprosy is a chronic infectious disease primarily affecting the skin and peripheral nerves and is caused by Mycobacterium leprae. Although effective control measures have significantly reduced its global incidence in recent years, its insidious onset and diverse skin manifestations pose considerable challenges to early diagnosis, particularly among young medical practitioners. This study reports a case of tuberculoid leprosy accompanied by a type I reaction (T1R) to leprosy, aiming to contribute to the broader understanding and management of the disease. The patient came from a leprosy-endemic region and had a family history of leprosy. They first presented with neuritis, characterised by numbness in the left upper limb, which is an early-stage symptom often overlooked. This case accentuates the importance of comprehensive examination techniques, including bacteriological and histological investigations, ultrasound and magnetic resonance imaging, to identify early nerve damage, which is critical for prompt diagnosis and intervention. According to World Health Organization data, approximately 200,000 new cases of leprosy are reported worldwide each year, with a prevalence rate of 0.2 cases per 10,000 individuals. The disease exhibits two clinical forms based on the host’s immune response: tuberculoid leprosy in a well-immunised population and lepromatous leprosy in a poorly immunised host. The patient in this study demonstrated signs of tuberculoid leprosy, marked by isolated skin papules and plaques, and a T1R, a tissue-destructive, immune-driven inflammatory process. This case underscores the need for ongoing education and updated diagnostic tools to facilitate the early detection of leprosy, particularly in endemic areas. Moreover, attention must be given to the comprehensive care of patients, encompassing both physical and psychological aspects, to improve their quality of life and mitigate social discrimination and prejudice.
Keywords: leprosy, tuberculoid leprosy, leprosy reaction
Introduction
Leprosy is a chronic infectious disease induced by attacks on the skin and peripheral nerves by Mycobacterium leprae.1 Despite being curable, the World Health Organization reported over 200,000 new leprosy cases in 2019. In the early stages of leprosy, signs and symptoms are often absent or minimal, and they do not surface until the bacteria have multiplied to a significant number.2 This makes early detection and timely diagnosis of leprosy challenging.
The disease is primarily prevalent in tropical and subtropical regions, with a significant number of cases in India, Brazil, and Indonesia.3 Due to active prevention and treatment efforts in recent years, the disease incidence has seen a significant decline worldwide. However, due to the insidious nature of leprosy and the diversity of its skin lesions, the disease often remains underdiagnosed, especially among young physicians with limited exposure to this disease.4
Leprosy presents in a spectrum of clinical forms that vary based on the host’s immune response. At one end of the spectrum, Tuberculoid leprosy (TT) presents with few well-demarcated skin lesions, strong cell-mediated immunity, and fewer bacilli. On the other end, Lepromatous leprosy (LL) is characterized by numerous skin lesions, weak cell-mediated immunity, and a high bacterial load. In between, there are Borderline forms of leprosy, such as Borderline Tuberculoid (BT) leprosy, which is characterized by an intermediate immune response.3
In this report, we present a case of a tuberculosis-like leprosy with a type I leprosy reaction in order to provide a reference for the diagnosis and treatment of such cases.
Clinical Data
The patient is a 38-year-old woman from Bijie, Guizhou Province, China. Their left upper extremity has been affected by paralysis for three years, erythema for one year and swelling for half a year. Three years ago, there was no apparent numbness in the upper left limb; the patient gave it no attention, and there were no hospital visits. A year ago, the patient was diagnosed with vasculitis in an outpatient hospital after presenting with erythema on their left upper extremity. Six months ago, the patient’s left upper extremity swelled due to exhaustion, limiting their ability to engage in physical activity. The diagnosis was uncertain, and the oedema subsided after treatment with dexamethasone (the precise dosage is unknown). After the dexamethasone treatment was discontinued, the oedema remained unchanged. The patient was treated in our department so a conclusive diagnosis could be made.
Five years ago, the patient was treated for hepatolithiasis with a cholecystectomy. The patient was born and raised in Bijie, Guizhou Province. One of their relatives died of leprosy; in addition, their aunt’s daughter died of leprosy when she was 18 years old, and their aunt’s son died of leprosy when he was 10 years old, more than two decades ago. Physical examination: The patient’s vital signs were stable, and the system examination revealed no apparent abnormalities. Dermatological findings on the left hand and discoloured distal left forearm revealed oedematous dark erythema. A region of hypopigmentation was observed distal to the left upper arm (Figure 1A–C). Pain and tactile sensation had diminished or vanished in the lesion’s location. The left upper limb was demonstrably weaker than the contralateral side, and the movement was restricted. Mild atrophy of the left thenar muscle and palpable enlargement of the left ulnar nerve were observed. Biopsies of the left wrist and left forearm were obtained. This study was conducted with the approval of the Ethics Committee of Wuhan Central Hospital. The acceptance protocol number in the committee is WHZXKYL2022-073. The patient in the study provided informed consent for her participation and the use of her medical data for research and publication purposes. The results revealed that the epidermis was mildly atrophic and that there was lymphocyte and histiocytic infiltration around the blood vessels and appendages of the dermis and subcutaneous tissues. There was also evidence of partial nerve injury. Negative acid-fast staining was observed (Figure 2A–D). A neurosonography displayed peripheral nerve injury in the left upper extremity (with prominent nerve thickening) (Figure 3A and B).
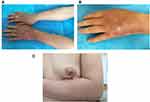 |
Figure 1 Skin lesions on the left arm. (A–C), different directions to show the skin lesions on the left arm. |
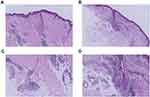 |
Figure 2 Histopathology results of the skin lesions on the left arm. (A and B) HE staining images results of the skin lesions (X 100). (C and D) HE staining results of the skin lesions (X 400). |
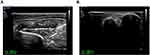 |
Figure 3 Nerve ultrasonic images. (A and B) Respective images for the nerve ultrasonic examination. |
Neuroelectrophysiology: There was nerve injury in the left upper extremity’s periphery. Magnetic resonance imaging (MRI) of the left upper extremity revealed that the ulnar and median nerves had increased signals, indicating nerve enlargement (Figure 4A and B). Diagnosis: Leprosy resembling tuberculosis with a T1R. The patient was administered 30 mg/d of prednisone and received treatment from a multidisciplinary team in conjunction with chemotherapy at a local facility for the prevention of epidemics. After three months of treatment, the daily prednisone dosage was reduced to 10 mg. The hue of the erythema in the left upper extremity became lighter, and the paralysis in the left upper extremity was no longer evident. The patient was still being actively monitored at the time of writing.
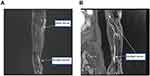 |
Figure 4 MRI of the left arm. (A and B) Respective images of the left arm using MRI examination. |
Following a detailed review of the patient’s symptoms and test results, the patient’s condition was classified as borderline tuberculoid leprosy, according to the Ridley–Jopling classification system.5 This classification system considers clinical, histopathological, microbiological and immunological factors and is widely accepted in the medical community.
In this case, our patient demonstrated a series of clinical and pathological characteristics that correspond to either Tuberculoid leprosy (TT) or Borderline tuberculoid (BT) according to the Ridley and Jopling classification system. These features include localized skin lesions with erythema and swelling, loss of sensation in the affected areas, significant thickening of the left ulnar nerve, and pathological changes observed via neurophysiological examination, ultrasound, and MRI that are indicative of peripheral nerve damage. The patient’s negative acid-fast staining result aligns with TT and BT cases, as they typically do not present with a high bacterial load. The term “tuberculosis-like leprosy” is used in our case to denote the tuberculoid forms of leprosy, specifically referring to a potential case of BT Leprosy, which is characterized by stronger cell-mediated immunity, a few well-demarcated skin lesions, and fewer bacilli. In addition, our patient experienced a T1R reaction, also known as a reversal reaction (RR). This is an immunological complication that often occurs in borderline forms of leprosy (including BT), marked by an increase in cellular immune response to Mycobacterium leprae. Therefore, it is appropriate to describe this case as “tuberculosis-like leprosy with a T1R reaction”. We used this terminology in recognition of the clinical and pathological similarities between tuberculoid forms of leprosy and certain aspects of tuberculosis, as well as the potential for diagnostic confusion, particularly in the early stages of the disease.
Discussion
Leprosy is a chronic infectious disease affecting the epidermis and peripheral nerves caused by M. leprae.6 It is now understood that oral, nasal or prolonged skin contact with untreated patients with leprosy is the mode of transmission, but its exact mechanism remains uncertain. The transmission of leprosy is related to the host’s genetic background and immune status.7 It is crucial to emphasise the role of genetic factors and the immune system in the susceptibility to leprosy. Some studies suggest that host genetic factors could significantly affect the susceptibility to M. leprae and the clinical manifestation of the disease.8 Specifically, certain human leukocyte antigen alleles are associated with susceptibility to different forms of leprosy or protection against them.9 This genetic predisposition, coupled with autoimmune status, could have significantly contributed to the patient’s condition. Furthermore, exposure to the bacteria due to living in a leprosy-endemic area and a family history of the disease could have increased the risk of infection.
Leprosy is a chronic condition that does not pose an immediate hazard to an individual’s life. Mycobacterium leprae has a cutaneous and peripheral nerve tropism. The patient in this study initially presented with neuritis (in this case, initial symptoms included paralysis of the left upper extremity). In the later stages of untreated dyskinesia, patients may develop plantar ulcers, lytic bone lesions (nose, phalanges, etc.) and paralysis (ulnar nerve, blepharoptosis). In the early stages of leprosy, known as the indeterminate stage, it is challenging to make a definitive diagnosis due to the presence of multiple clinical symptoms.4 Depending on the host’s immunity, the disease may develop into any other form of leprosy (the definitive form) if this relatively asymptomatic early stage is not clearly defined. The World Health Organization (WHO) classification differentiates between two contradictory clinical patterns: less bacterial leprosy in a well-immunised population and more bacterial leprosy in an inadequately immunised host. This purposefully streamlined classification has proven useful for clinical classification, diagnosis and treatment in contexts with limited resources. In the scientific context, however, the Ridley–Jopling classification has been in use since 1966 and comprises an intermediate stage (borderline type) as well as two polar forms, tuberculous-like leprosy and neoplastic leprosy (LL). The intermediate stage is further subdivided into three subgroups based on their clinical and histological proximity to one of the two polar forms: borderline borderline tuberculosis-like leprosy, intermediate borderline leprosy (BB) and borderline borderline neoplastic leprosy (BL). When the host’s immunity is healthy, leprosy manifests as tuberculosis, with papules and plaque that may manifest as erythema with raised borders and a ring-like appearance. The lesion’s centre is typically characterised by atrophy and hypopigmentation (erythema and hypopigmentation in the patient’s left upper extremity). Due to the small number of cutaneous lesions, tuberculous leprosy is nearly non-infectious. Even at this stage, a crude neurological examination of the affected skin area may reveal abnormalities, including decreased pain and tactile sensation; these neuropathies are frequently asymmetrical (in this case, the ulnar nerve in the left upper extremity was significantly enlarged, with pain and decreased tactile sensation in the lesion area).4 In the early stages of multiflora, lesions (such as BB, BL and LL) are pleomorphic, broadly distributed and without distinct borders. There are also varying degrees of infiltration, frequently accompanied by hair loss and alterations in the mucosa and other organs. Early nerve damage is frequently imperceptible.10
The term “leprosy response” refers to the acute onset of leprosy caused by changes in the host immune response to the M. leprae epitope during the chronic course of the disease.11 These changes are divided into T1Rs and type II responses, also known as erythema nodosum leprosum.12 The T1R is caused by a delayed response that is highly sensitive to M. leprae and primarily affects borderline leprosy.13 This leprosy response is a unique, tissue-destructive and immune-driven inflammatory process. Clinically, T1Rs are characterised by purplish-red dark erythema and oedemas in clinically normal skin with or without neuritis. The histologic changes are usually a mixture of lymphocytes and macrophages (in the study patient’s case, there was swelling dark erythema of the left hand and forearm).
Type I reactions often occur in patients during the first year of treatment but may also occur before or after treatment. When a T1R occurs, repeated tissue trauma and delayed treatment can result in severe disability.14
In 1981, the WHO introduced a combination of rifampicin, clofazimine and dapsone for the first-line treatment. Minocycline, ofloxacin and clarithromycin are among the drugs used as second-line treatments.15 The advantage of combination therapy is that it prevents the side effects of dapsone and reduces the infection rate and recurrence rate.16 The response to T1R is usually in the first two months of treatment, with a treatment of prednisone (starting at a dose of 40–60 mg/d) that is reduced after symptoms are controlled. For type II leprosy, thalidomide treatment is usually preferred, but sometimes prednisone or clofazimine may be used. The starting dose of thalidomide is typically 100 to 200 mg per day, although it has been prescribed initially at a dose of up to 400 mg per day, which is then discontinued after three to four weeks of treatment.17 The treatment period may be prolonged during recurrence, but the adverse neuropathic and teratogenic effects should be noted.18
The incidence of leprosy has decreased significantly in recent years. As the skin lesions of leprosy are various, only some young dermatologists or non-dermatologists have knowledge of leprosy, which leads to misdiagnosis or missed diagnosis of leprosy in clinical settings.19 This case underscores the importance of improving medical education and training on leprosy, particularly for healthcare professionals working in endemic areas. As an often overlooked yet significant public health concern, understanding of the clinical manifestations, diagnoses and treatments of leprosy should be emphasised in medical education to minimise misdiagnoses or missed diagnoses. Enhanced leprosy surveillance and health education can also help in the early detection and management of the disease, reducing the disease burden.20,21
In the analysis of the patient in this study, it was found that the Ridley–Jopling classification system was particularly useful. Given its comprehensive approach to classifying leprosy based on clinical, histopathological, microbiological and immunological aspects, this study’s researchers were able to gain a more detailed understanding of the patient’s disease. This, in turn, has implications for the treatment approach and prognosis for the patient.
In this case, the patient was identified as having tuberculosis-like leprosy presenting a T1R, an assessment made based on thorough clinical observations, comprehensive histopathological studies and corroborative laboratory investigations. It is crucial to note that the presentation of leprosy can vary, with many cases presenting a challenge in terms of clear classification. Tuberculosis-like leprosy is distinguished by distinct symptoms, as presented in this study’s patient. By contrast, other forms of leprosy, such as neoplastic leprosy, exhibit different clinical manifestations, disease progressions and responses to treatment. Furthermore, the T1R experienced by the patient is a crucial aspect of this case. Type I reactions often occur in patients during the first year of treatment but may also occur before or after treatment, potentially resulting in severe disability. The clinical and histological changes observed in the patient further reinforce this study’s diagnosis. This case is significant because tuberculosis-like leprosy with a T1R is relatively rare/unusual. This unusual presentation of leprosy underscores the need for accurate diagnosis, prompt treatment and ongoing research to improve understanding of the disease’s various manifestations. The insights gained from such cases contribute to a broader medical knowledge base and assist in managing similar future cases.
Conclusion
This study reported a case of tuberculosis-like leprosy with a T1R. When contemplating leprosy in modern medicine, a bacteriological and histological examination is required first. In the early stages of leprosy, only sensory impairment, ultrasonography, MRI and other supplementary examinations can help clinicians comprehend early nerve damage. Despite the advancement of medical technology, there is still no accurate and rapid method for diagnosing leprosy. The future eradication and control of leprosy can be achieved through early diagnosis. In addition, this study’s shared objectives ensure the physical and psychological care of patients with leprosy, enhancing their quality of life and eradicating social discrimination and prejudice.
Data Sharing Statement
All data generated or analysed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Patient Consent for Publication
Accordance with relevant guidelines and regulations, Written informed consent was provided by the patient to have the case details and any accompanying images published.
Funding
This study did not receive any funding in any form.
Disclosure
The authors had no personal, financial, commercial, or academic conflicts of interest.
References
1. Maymone MBC, Laughter M, Venkatesh S, et al. Leprosy: clinical aspects and diagnostic techniques. J Am Acad Dermatol. 2020;83(1):1–14. doi:10.1016/j.jaad.2019.12.080
2. Maymone MBC, Venkatesh S, Laughter M, et al. Leprosy: treatment and management of complications. J Am Acad Dermatol. 2020;83(1):17–30. doi:10.1016/j.jaad.2019.10.138
3. Makhakhe L. Leprosy review. S Afr Fam Pract. 2021;63(1):e1–e6. doi:10.4102/safp.v63i1.5311
4. Khadilkar SV, Patil SB, Shetty VP. Neuropathies of leprosy. J Neurol Sci. 2021;420:117288. doi:10.1016/j.jns.2020.117288
5. Ridley DS, Jopling WH. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966;34(3):255–273.
6. Mi Z, Liu H, Zhang F. Advances in the Immunology and Genetics of Leprosy. Front Immunol. 2020;11:567. doi:10.3389/fimmu.2020.00567
7. Randhawa A, Kapila R, Schwartz RA. Leprosy: what is new. Int J Dermatol. 2022;61(6):733–738. doi:10.1111/ijd.15998
8. Ebenezer GJ, Scollard DM. Treatment and evaluation advances in leprosy neuropathy. Neurotherapeutics. 2021;18(4):2337–2350. doi:10.1007/s13311-021-01153-z
9. Froes LARJ, Sotto MN, Trindade MAB. Leprosy: clinical and immunopathological characteristics. An Bras Dermatol. 2022;97(3):338–347. doi:10.1016/j.abd.2021.08.006
10. Palit A, Kar HK. Prevention of transmission of leprosy: the current scenario. Indian J Dermatol Venereol Leprol. 2020;86(2):115–123. doi:10.4103/ijdvl.IJDVL_326_19
11. Jesus ILR, Montagner MI, Montagner M, Alves SMC, Delduque MC. Hanseníase e vulnerabilidade: uma revisão de escopo [Leprosy and vulnerability: a scoping review]. Cien Saude Colet. 2023;28(1):143–154. Portuguese. doi:10.1590/1413-81232023281.09722022
12. Sarode G, Sarode S, Anand R, et al. Epidemiological aspects of leprosy. Dis Mon. 2020;66(7):100899. doi:10.1016/j.disamonth.2019.100899
13. Fava VM, Dallmann-Sauer M, Schurr E. Genetics of leprosy: today and beyond. Hum Genet. 2020;139(6–7):835–846. doi:10.1007/s00439-019-02087-5
14. Avanzi C, Singh P, Truman RW, Suffys PN. Molecular epidemiology of leprosy: an update. Infect Genet Evol. 2020;86:104581. doi:10.1016/j.meegid.2020.104581
15. Neema S, Battula S. Lepromatous Leprosy. N Engl J Med. 2022;387(13):1217. doi:10.1056/NEJMicm2202533
16. Deps P, Cruz A. Why we should stop using the word leprosy. Lancet Infect Dis. 2020;20(4):e75–e78. doi:10.1016/S1473-3099(20)30061-X
17. Froes LAR Jr, Trindade MAB, Sotto MN. Immunology of leprosy. Int Rev Immunol. 2022;41(2):72–83. doi:10.1080/08830185.2020.1851370
18. Houghton F, Winterburn M. Leprosy in Nepal: a re-emerging threat. J Public Health Policy. 2021;42(1):176–181. doi:10.1057/s41271-020-00260-z
19. Mushtaq S. Leprosy in the post-elimination phase: so near and yet so far. G Ital Dermatol Venereol. 2020;155(3):269–279. doi:10.23736/S0392-0488.19.06249-7
20. Bala Murugan S, Mahendradas P, Dutta Majumder P, Kamath Y. Ocular leprosy: from bench to bedside. Curr Opin Ophthalmol. 2020;31(6):514–520. doi:10.1097/ICU.0000000000000715
21. Gómez-Cerquera JM, Herrera-Darias S, Domingo-Amela A, Graells-Estrada J. Lepromatous leprosy. Med Clin. 2022;159(11):556. doi:10.1016/j.medcli.2022.07.015
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
