Back to Journals » Clinical Ophthalmology » Volume 17
Ocular Survival Following Intravitreal Melphalan as Adjuvant Treatment for Vitreous Retinoblastoma Seeding
Authors Alahmadi G , Maktabi AMY, Sesma G , Almesfer S
Received 22 April 2023
Accepted for publication 20 June 2023
Published 22 June 2023 Volume 2023:17 Pages 1789—1800
DOI https://doi.org/10.2147/OPTH.S417370
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Ghaida Alahmadi,1 Azza MY Maktabi,2 Gorka Sesma,1 Saleh Almesfer1
1Pediatric Ophthalmology and Strabismus Division, King Khaled Eye Specialist Hospital, Riyadh, Saudi Arabia; 2Pathology and Laboratory Medicine Department, King Khaled Eye Specialist Hospital, Riyadh, Saudi Arabia
Correspondence: Gorka Sesma, Pediatric Ophthalmology and Strabismus Division, King Khaled Eye Specialist Hospital, Al Urubah Branche Road, West Building 2 nd Floor, Riyadh, 11462, Saudi Arabia, Tel +966114849700, Fax +966114821908, Email [email protected]
Purpose: To evaluate the efficacy of intravitreal chemotherapy for vitreous seeding in patients with retinoblastoma (Rb).
Design: Retrospective, single-arm cohort study.
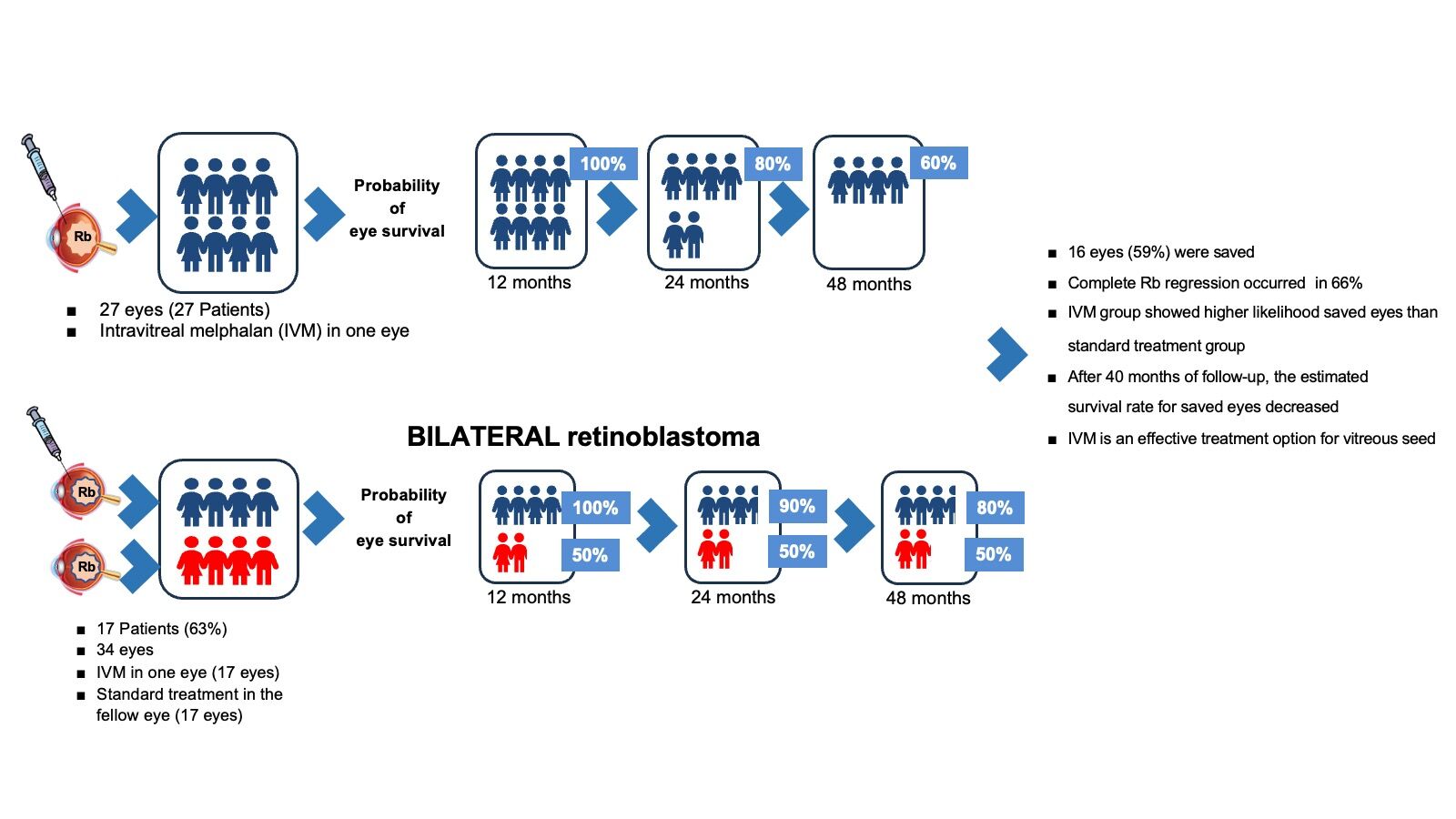
Methods: This study was conducted at a tertiary eye center. Between 2013 and 2021, 27 patients (27 eyes) with vitreous Rb receiving adjuvant intravitreal melphalan (IVM) as secondary/salvage treatment in one eye were included. Patients who were unable to follow-up or treated elsewhere were excluded. Survival analysis was performed to assess the incidence of enucleation in the melphalan-treated group, as well as in bilateral cases with eyes receiving melphalan and those receiving standard treatment, consisting of chemotherapy, thermotherapy, and enucleation according to the disease stage.
Results: The median (interquartile range) follow-up time was 65 months (range, 34– 83 months). Seventeen patients (63%) had bilateral disease. Sixteen eyes (59%) were saved. The Kaplan–Meier survival estimates for eyes receiving melphalan were 100% at 1 year (95% confidence interval [CI]:11.2– 14.3), 75% (95% CI:14.2– 48.9) at 3 years, and 50% at 5 years. Melphalan-treated patients with bilateral disease showed a significantly higher number of saved eyes than the standard treatment group (P=0.002). Tumor recurrence was the primary cause of enucleation, accounting for 36% of the cases. In the vitreous hemorrhage group, the odds of enucleation were 13 times higher (95% CI:1.04– 165.28) than in the group without this condition.
Conclusion: IVM is an effective treatment option for vitreous seeds. After 3 years of follow-up, the estimated survival rate for saved eyes decreased, and vitreous hemorrhage significantly increased the likelihood of enucleation. Further studies are required to determine the precise effects of IVM.
Keywords: retinoblastoma, melphalan, intravitreal chemotherapy, ocular oncology, intraocular tumors
Graphical Abstract:
Plain Language Summary
This study examined the effectiveness of intraocular chemotherapy for the treatment of eye cancer. The researchers studied 27 patients with vitreous seeding in their eyes due to retinoblastoma. The patients received a chemotherapy called intravitreal melphalan in one eye. The researchers wanted to determine how many eyes were saved, and how many eyes needed to be removed. They found that 59% of the eyes were saved, but the number decreased after three years. Researchers also found that bleeding inside the eye made it more likely that the eye required removal. This study shows that intravitreal chemotherapy can be a good treatment for this type of eye cancer, but more research is needed to better understand this.
Introduction
Retinoblastoma (Rb) is the most common primary intraocular malignancy diagnosed in childhood.1 The clinical approach to treating Rb aims to save lives, preserve the eye and vision, and avoid enucleation.2
External beam radiation has been used to treat patients with vitreous seeding, but one of the adverse effects of this treatment modality is an increased rate of second primary malignancies.3 Intravenous chemotherapy (IVC) and intra-arterial chemotherapy (IAC) improve Rb control and eyeball salvage in these patients,4,5 but the most challenging aspect of Rb treatment is the control of vitreous and subretinal seeds. The location of vitreous seeds away from the blood supply makes them less susceptible to IVC and IAC.6 IAC can control vitreous seeds in 67% of patients with Rb and 82% with subretinal seeds. Since their original ground-breaking report, Kaneko and Suzuki (2003) have demonstrated the efficacy of intravitreal melphalan (IVM) in Rb by using experimental models.7
Intravitreal chemotherapy has emerged as a useful treatment for vitreous seeding in patients with Rb. Ocular survival in the presence of refractory vitreous seeding was dramatically improved by using IVM. This drug has been shown to control non-vitreal refractory diseases in eyes previously treated with IAC. However, it may also increase the risk of retinal toxicity.6
Munier et al (2012) and other investigators8,9 report on the efficacy of IVM with ocular survival rates of more than 80% for the eye. Complications after IVM treatment are rare, and the risk can be minimized using careful injection techniques and conventional dosing regimens.10
Our hospital is the referral center for all Rb cases in the Kingdom of Saudi Arabia and many Middle Eastern countries. We collaborated with King Faisal Specialist Hospital and the Research Center to provide appropriate treatment to patients. An average of 27 new patients with Rb come to us annually, with an estimated incidence of 0.79 new cases per a population of 1 million.11
One of the motivations for this study was the work of a popular article by Munier et al8 on the use of IVM in patients with Rb and vitreous seeds. Our goal was to evaluate the incidence of enucleation as the primary outcome in patients with Rb who underwent IVM. Second, we studied the efficacy, clinical results, and complications of this drug, which has been used as a treatment option at our center since 2013. This is a recurring theme in the literature and is critical for saving lives, vision, and eyes.
Methods
This single-arm retrospective cohort study was conducted in 2022 within the Division of Pediatric Ophthalmology at King Khaled Eye Specialist Hospital (KKESH) in Riyadh, Saudi Arabia. As a referral facility, the hospital provides care for children with Rb. This study included 27 patients diagnosed with Rb who received IVM and had vitreous seeding without an active tumor in one eye between May 2013 and November 2021. All patients were followed up for at least six months after the last injection. We excluded patients for whom follow-up was impossible and those treated outside our institution.
This study was approved by the Institutional Ethics and Research Committee of KKESH (R – 22014). This study conforms to the tenets of the Declaration of Helsinki. Written informed consent was obtained from both parents of the patients. Possible complications including ocular and extraocular risks are explained.
All patients were examined by an expert team under general anesthesia. A portable slit-lamp examination of the anterior segment with measurement of intraocular pressure and a 360-degree fundus examination with scleral depression by indirect ophthalmoscopy were performed. Drawings of the anterior segment and the ocular fundus were obtained for documentation. Retcam3® (Natus Medical Inc., Pleasanton, CA, USA) was used for fundus photography. All injections were performed by the same ophthalmologist (SAM) using an ophthalmic microscope. Melphalan hydrochloride (Alkeran®, GSK plc, Middlesex, UK) was prepared at the hospital (30 μg/0.1 mL). Vitreous injections were performed using a 30-gauge needle through the pars plana, 2.75–3.0 mm from the limbus, depending on the patient’s age. After the melphalan injection, cryotherapy was applied to the injection site to keep the needle in the ice ball while still in the eye. During the first freeze, the needle was pulled through the ice ball. Three freeze-thaw cryotherapy cycles were performed. The eye was then gently moved back and forth using forceps to distribute the drug into the vitreous. Indirect ophthalmoscopy was performed to examine the ocular fundus after injection. At the end of the procedure, neomycin, polymyxin B sulfate, and dexamethasone (Maxitrol®; Alcon Labs. Inc., TX, USA) and eye ointments were applied.
The Trakcare® (InterSystems Corp., Cambridge, MA, USA) electronic medical record system was used to obtain patients’ demographic data, including age at presentation and sex. Tumor characteristics were recorded at the initial presentation, including the Rb classification, stage according to the International Classification of Intraocular Rb, and tumor laterality.12 Injection data included the date of the first IVM injection, number of injections administered, date of each injection, interval between injections, therapeutic indication for each injection, and type of response to each injection. The treatment methods before and after the injection were recorded. At the time of IVM injection, tumor characteristics were recorded, including solid primary tumor status, pattern, and placement of subretinal and vitreous seeds according to the quadrant of the eye. Retinal camera images were used to record, analyze, compare, and follow the fundus findings during the follow-up period. The vitreous seed pattern was divided into type I (dust), type II (sphere), and type III (clouds).13 Regression of vitreous Rb seeding was defined as the reduction or disappearance of vitreous seeds due to treatment. The response of vitreous seeds to IVM was classified as type 0 (complete disappearance of seeds), type I (calcified seeds), or type II (amorphous seeds), according to the guidelines published by Mounir.13,14 If some of the vitreous seed patterns were present, we assumed the viability of vitreous seeding. Recurrence refers to the reappearance of cancer cells in the vitreous after initial treatment and regression.
Complete regression of all vitreous seeds without recurrence was considered therapeutic success, whereas persistence or recurrence of viable vitreous seeds was considered therapeutic failure.
Ocular survival, treatment duration, and the time of the first observed response were recorded. Retinal toxicity was classified as grade I (less than 2 hours of salt-and-pepper retinopathy in the peripheral retina and anterior to or at the level of the equator), grade II (more than 2 hours of retinopathy), grade III (retinopathy extending posteriorly but not affecting the macula), grade IV (retinopathy affecting the macula [maculopathy]), and grade V (extensive pan-retinopathy with concomitant optic retinopathy).15 Both intraocular and extraocular problems were recorded.
Statistical Analyses
The variables were entered into an Excel® spreadsheet (Microsoft Corp., Redmond, WA, USA) and coded. After consistency and frequency checks, they were transferred to Stata® version 14 (Stata Corp., College Station, TX, USA) for statistical analysis. Categorical variables are presented as numbers and percentages, and continuous variables are summarized using medians and interquartile ranges (IQRs) for nonparametric data. Fisher’s exact test was used to compare results between subgroups. A logistic regression model adjusted for age at diagnosis, number of injections, retinoblastoma stage, systemic chemotherapy, thermotherapy, IAC, cryotherapy before and after IVM, retinal toxicity, vitreous hemorrhage, and retinal detachment was used to measure the risk factors affecting enucleation. The relative risk of enucleation was measured as an odds ratio (OR) with a 95% confidence interval (CI) and a P-value <0.05, which was considered statistically significant. We used Kaplan–Meier survival analysis to evaluate enucleation events in the melphalan-treated group. The same analysis was performed for patients with bilateral disease, comparing the eyes receiving melphalan with the contralateral eyes receiving standard treatment, using the Wilcoxon–Breslow method.
Results
This study included 27 patients (27 eyes) with Rb and vitreous seeds who received IVM in one eye. Eighteen patients (67%) were men. Ten patients (37%) had unilateral Rb, while 17 (63%) had bilateral disease, including one patient with trilateral Rb. The median (IQR) age of the patients was 7 (range, 6–8) years and the age at diagnosis was 15 (9–34) months. The first injection was administered 12 months (6–19 months) after birth. Patients received a median (IQR) of two (1–3) melphalan injections, with a period of 32 (17–48) months between diagnosis and the first injection. The median follow-up was 65 (34–83) months. Most clinical findings, procedures, and complications are summarized in Table 1.
 |
Table 1 Characteristics and Treatment Outcomes in Patients with Retinoblastoma Who Received Intravitreal Melphalan |
After the first injection, twenty-five (93%) patients showed a reduction in the size of the spheres, clouds, or amount of dust in the vitreous, the most common type 0 regression pattern. Complete regression of vitreous seeding occurred in 18 patients (66%) after the second injection (Figure 1).
Therapeutic failure was observed in five (18%) patients; complete regression did not occur in three patients, while two patients had a recurrence of vitreous seeds after regression.
Eleven (44%) patients had some degree of retinal toxicity, and 9 (33%) had grade I toxicity (Figure 2).
Sixteen eyes (59%) of our patients were saved. The leading cause of enucleation was tumor recurrence (n=4, 36%), followed by tumor persistence and vitreous seeding in 3 patients (27%), tumor persistence in 2 patients (18%), vitreous seeding in 1 patient (9%), and tumor persistence with recurrence of vitreous seeding (9%).
Enucleation was associated with vitreous hemorrhage (P=0.006). Enucleation was not significantly related to sex, laterality, retinal toxicity, pre- or post-injection laser and cryotherapy, pupillary synechiae, seed pattern, seed location, anterior segment seeds, or pre-injection carboplatin. Retinal toxicity was not associated with the number of injections (P=0.08) or enucleation (P=0.80). The association of vitreous seed pattern and location with response to IVM injection was not demonstrated (P=0.20 and P=0.46, respectively).
The unadjusted and adjusted logistic regression models (Table 2) showed a statistically significant difference in the probability of enucleation between patients who had vitreous hemorrhage and those who did not (OR=13.1, 95% CI:1.0–165.3). The model failed to demonstrate the same association between enucleation probability and age, stage of the disease, retinal detachment, number of injections, retinal toxicity, and other clinical variables.
 |
Table 2 Univariate and Multivariate Logistic Regression Analyses of Factors Associated with Enucleation in Retinoblastoma Patients Receiving Intravitreal Melphalan |
Table 3 illustrates the frequency and percentage distribution of these eyes according to the disease stage. Of the 27 eyes that received IVM, the median (IQR) stage of Rb was in group D (groups C and D). For bilateral Rb, the median (IQR) Rb stage was group D (group C, group D) in patients who received IVM, and group D (group B-group E) in patients who did not receive IVM. Nine contralateral eyes were enucleated and received standard treatment, compared with five eyes treated with IVM.
 |
Table 3 Distribution of the Stage Disease in Eyes Affected with Unilateral and Bilateral Retinoblastoma (ICRB)** |
The Kaplan–Meier curve (Figure 3) shows an estimated survival for eyes receiving IVM of 100% at 12 months (95% CI:11.2–14.3), 90% at 16 months (95% CI:11.2–26.7); and 80% at 25 months (95% CI:11.2–45.2). After 43 months, the survival rate decreased to 75% (95% CI:14.2–48.9) and reached 50% after 65 months.
Seventeen of the 27 patients had bilateral disease. These patients received adjuvant IVM in one eye, and the contralateral eye was treated with conventional therapy without IVM. The results of the survival analysis of these patients are summarized in Figure 4. Eyes that received IVM had an estimated survival rate of 100% at 21 months (95% CI:20.7–26.8), 90% at 28 months (95% CI:20.7–48.9), 80% at 45 months (95% CI:20.8–48.2), and 67% at 49 months. In the contralateral eye group that did not receive IVM, 48% of eyes were saved after 33 months. There was a statistically significant difference between the two groups (P<0.001), with a higher probability of eye preservation in the IVM group.
Discussion
This is the first study in Saudi Arabia to describe intravitreal melphalan as a therapy for the vitreous seeding of Rb. According to Saudi census data,11 the ratio of boys to girls is 1.31, which probably explains why most of our patients were boys, although there were no statistically significant differences between the sexes in terms of eyes saved (P=0.69).
It has been reported that eyes with cloud seed morphology received significantly more injections,16,17 and spherical seeds responded best to injections of melphalan.18 However, our results did not show this association (P=0.23). In most cases, if the patient responded, it occurred after the second injection and was unrelated to the vitreous seed type.
Various adverse effects of IVM have been reported, including cataracts, vitreous hemorrhage, hypotony, phthisis bulbi, salt-and-pepper retinopathy, anterior segment toxicity, conjunctival chemosis, iris heterochromia, posterior synechiae, anterior uveitis, optic disc edema, and hemorrhagic retinal necrosis.5,19,20 We found that four patients had cataracts. Three of these four patients received transpupillary thermotherapy, which has been reported to cause cataracts.21 Our results are comparable to those of other study series. Surgical techniques, drug toxicity, and cryotherapy should be considered.
In their study, Berry et al discovered a retinal toxicity rate of 91% in 40% of patients with grade III or higher retinal toxicity.22 In another study, Berry et al describe retinal toxicity by arguing that the more pigmented the eye, the higher the risk of IVM toxicity, although not all highly pigmented eyes exhibit toxicity. Therefore, it is difficult to predict whether toxicity will occur, especially higher-grade toxicity.16 They also to highlight that the risk of toxicity is lower in eyes that receive fewer injections and a lower cumulative dose of melphalan. Two-thirds of our patients received one or two injections, which may explain the low rate of retinal toxicity. Retinal toxicity was marginally significantly associated with the number of injections. Additionally, our results did not show that the extent of retinal toxicity was associated with a higher risk of enucleation or tumor recurrence. Optical coherence tomography angiography and electroretinograms may be helpful tools for evaluating the effects of IVM therapy on the choriocapillaris and retina.2,23
Therefore, it is essential to understand the melphalan injection techniques to reduce metastasis. The injection site should be carefully selected, and anti-reflux measures should be taken.8 Intraocular melphalan showed minimal ocular toxicity in Caucasian populations in Europe and North America after an injected dose of 20–-30 micrograms, calculated based on patient age.4,13,24
In our series, the eye salvage rate was 59%, which is lower than those reported by Munier et al,8,15 Ghassemi and Shields,5 Shields et al,4 Ji et al,25 Yousef,17 and Kaneko,7 who reported rates of 87%, 83%, 100%, 84%, 81%, and 68%, respectively. The advanced stage of our patient and long follow-up period may explain our results. Most studies showed promising results after a short time.8,26 Survival probabilities for the eyes were similar to those of other studies: over 95% in the first 12 months and over 75% in the first 24 months.1,2,4,17,22,27 However, after 40 months of follow-up, we found a decrease in the probability of ocular survival at 54%. Shield et al published similar results but pointed out the need for a more extended follow-up period to determine the long-term stability of their results.4 Berry et al followed group D patients for 6 years, and the rate of enucleated eyes increased after 36 months.26 We obtained similar results to those of Berry et al, González et al, and Ancona-Lezama et al, and followed our patients for a median (IQR) of 65 (34–83) months.1,3
It is important to note that while previous studies25 have shown that eyes with dust morphology have the best response to intravitreal melphalan and those with cloud morphology have the lowest response, we were unable to establish a statistically significant link between vitreous seed pattern and intravitreal melphalan response.
Despite efforts to limit vitreous seeds and the fact that 24 of our patients received intravenous chemotherapy, the leading cause of this decline in survival rate was the reactivation of Rb after 40 months of observation. In a large series, the median time to recurrence after intravenous chemotherapy was reported to be 10–15 months.28,29 It is vital to have a long-term regular follow-up to rule out the possibility of late Rb reactivation. We agree with the term “multi-modal globe salvaging therapy” used by Smith et al to better approach these cases.30 In the bilateral group (Figure 4) patients receiving IVM had a significantly higher likelihood of saving their eyes than those receiving standard treatment. This could be related to the intention to save eyes when the disease is bilateral. Most studies have focused on group D. There are current and promising studies reporting relatively good results after intravitreal melphalan and intraarterial chemotherapy in eyes with group E Rb.25,31,32
Our patients belonged to a heterogeneous group of patients (Table 2). This may have affected the results. Most of the eyes enucleated with bilateral Rb that did not receive IVM were in groups D and E, which implies a more severe disease and a higher probability of enucleation compared with the IVM group, with more cases in groups C and D. The small sample size was a limitation of this study. We believe that bilaterality, Rb stage, desire to preserve vision and eyes, chemotherapy, cryotherapy, thermotherapy, and laser therapy may be confounding factors. Controlled studies are required to precisely determine the effects of IVM. However, these results demonstrate the benefits of IVM injections.
Patients with vitreous hemorrhage were 13 times more likely to undergo enucleation than those without vitreous hemorrhage. Therefore, these results should be interpreted with caution. The wide distribution of the confidence interval could be a consequence of a small sample size, biased sample, or high variability in the retrieved data. This has been reported in many studies, regardless of the presence of vitreous hemorrhage; however, its association with enucleation as a prognostic factor has not been described. Vitreous hemorrhage could prevent proper evaluation of the ocular fundus, which could explain its association with enucleation. This factor should be considered when designing treatment protocols.
Recent presentations of melphalan33 and combinations of melphalan with other drugs, such as topotecan, could improve outcomes,34 but more research is needed. IVM is indicated for the treatment of refractory and recurrent vitreous seeds. The treatment plan for Rb depends on the disease stage and presence of bilaterality. Furthermore, IVM prevents loss of many eyes that would otherwise have been lost.
Conclusions
IVM is an effective treatment option for vitreous seeds. After 40 months of follow-up, the estimated survival rate for saved eyes decreased, and vitreous hemorrhage significantly increased the likelihood of enucleation. Further research is necessary to determine the precise effects of IVM and investigate the improvement of outcomes by melphalan alone and in combination with other drugs.
Funding
There is no funding to report.
Disclosure
None of the authors have any conflicts of interest to disclose.
References
1. González ME, Gaviria ML, López M, Escudero PA, Bravo A, Vargas SA. Eye Salvage with Intra-Arterial and Intra-Vitreal Chemotherapy in Patients with Retinoblastoma: 8-Year Single-Institution Experience in Colombia. Ocular Oncol Pathol. 2021;7(3):215–223. doi:10.1159/000511980
2. Abramson DH, Ji X, Francis JH, Catalanotti F, Brodie SE, Habib L. Intravitreal chemotherapy in retinoblastoma: expanded use beyond intravitreal seeds. Br J Ophthalmol. 2019;103(4):488–493. doi:10.1136/bjophthalmol-2018-312037
3. Ancona-Lezama D, Dalvin L, Shields C. Modern treatment of retinoblastoma: a 2020 review. Indian J Ophthalmol. 2020;68(11):2356. doi:10.4103/ijo.IJO_721_20
4. Shields CL, Manjandavida FP, Arepalli S, Kaliki S, Lally SE, Shields JA. Intravitreal melphalan for persistent or recurrent retinoblastoma vitreous seeds: preliminary results. JAMA Ophthalmol. 2014;132(3):319–325. doi:10.1001/jamaophthalmol.2013.7666
5. Ghassemi F, Shields CL. Intravitreal melphalan for refractory or recurrent vitreous seeding from retinoblastoma. Arch Ophthalmol. 2012;130(10):1268. doi:10.1001/archophthalmol.2012.1983
6. Francis JH, Marr BP, Brodie SE, Gobin P, Dunkel IJ, Abramson DH. Intravitreal melphalan as salvage therapy for refractory retinal and subretinal retinoblastoma. Retin Cases Brief Rep. 2016;10(4):357–360. doi:10.1097/ICB.0000000000000262
7. Kaneko A. Eye-preservation treatment of retinoblastoma with vitreous seeding. Jpn J Clin Oncol. 2003;33(12):601–607. doi:10.1093/jjco/hyg113
8. Munier FL, Gaillard MC, Balmer A, et al. Intravitreal chemotherapy for vitreous disease in retinoblastoma revisited: from prohibition to conditional indications. Br J Ophthalmol. 2012;96(8):1078–1083. doi:10.1136/bjophthalmol-2011-301450
9. Shields CL, Fulco EM, Arias JD, et al. Retinoblastoma frontiers with intravenous, intra-arterial, periocular, and intravitreal chemotherapy. Eye. 2013;27(2):253–264. doi:10.1038/eye.2012.175
10. Smith SJ, Smith BD. Evaluating the risk of extraocular tumour spread following intravitreal injection therapy for retinoblastoma: a systematic review. Br J Ophthalmol. 2013;97(10):1231–1236. doi:10.1136/bjophthalmol-2013-303188
11. General Authority for Statistics, Kingdom of Saudi Arabia. Data [Webpage title]. Saudi Census, 2022. Available from: https://www.stats.gov.sa/en/statistics-overview.
12. Shields CL, Mashayekhi A, Au AK, et al. The international classification of retinoblastoma predicts chemoreduction success. Ophthalmology. 2006;113(12):2276–2280. doi:10.1016/j.ophtha.2006.06.018
13. Munier FL. Classification and Management of Seeds in RetinoblastomaEllsworth Lecture Ghent August 24th 2013. Ophthalmic Genet. 2014;35(4):193–207. doi:10.3109/13816810.2014.973045
14. Berry JL, Bechtold M, Shah S, et al. Not all seeds are created equal: seed classification is predictive of outcomes in retinoblastoma. Ophthalmology. 2017;124(12):1817–1825. doi:10.1016/j.ophtha.2017.05.034
15. Munier FL, Beck-Popovic M, Chantada GL, et al. Conservative management of retinoblastoma: challenging orthodoxy without compromising the state of metastatic grace. “Alive, with good vision and no comorbidity. Prog Retin Eye Res. 2019;73:100764. doi:10.1016/j.preteyeres.2019.05.005
16. Berry JL, Kim ME, Pefkianaki M, et al. Intravitreal melphalan for retinoblastoma: the impact of toxicity on recurrence and ultimate globe salvage. Ocul Oncol Pathol. 2020;6(6):388–394. doi:10.1159/000509080
17. Yousef YA, Noureldin AM, Sultan I, et al. Intravitreal melphalan chemotherapy for vitreous seeds in retinoblastoma. J Ophthalmol. 2020;2020:1–7. doi:10.1155/2020/8628525
18. Kiratli H, İ K, Varan A, Akyüz C. Intravitreal chemotherapy in the management of vitreous disease in retinoblastoma. Eur J Ophthalmol. 2017;27(4):423–427. doi:10.5301/ejo.5000921
19. Francis JH, Abramson DH, Ji X, et al. Risk of extraocular extension in eyes with retinoblastoma receiving intravitreous chemotherapy. JAMA Ophthalmol. 2017;135(12):1426–1429. doi:10.1001/jamaophthalmol.2017.4600
20. Rishi P, Sharma T, Agarwal V, et al. Complications of intravitreal chemotherapy in eyes with retinoblastoma: see editorial on pg. 359. Oph Retina. 2017;1(5):448–450. doi:10.1016/j.oret.2017.03.006
21. Paysse EA, Miller A, Brady McCreery KM, Coats DK. Acquired cataracts after diode laser photocoagulation for threshold retinopathy of prematurity. Ophthalmology. 2002;109(9):1662–1665. doi:10.1016/s0161-6420(02)01169-7
22. Xue K, Ren H, Meng F, Zhang R, Qian J. Ocular toxicity of intravitreal melphalan for retinoblastoma in Chinese patients. BMC Ophthalmol. 2019;19(1):61. doi:10.1186/s12886-019-1059-4
23. Narala R, Kim JW, Lang P, et al. Changes in Retinal Thickness on OCT from Intravitreal Melphalan. Ophthalmology Retina. 2019;3(3):288–289. doi:10.1016/j.oret.2018.09.020
24. Francis JH, Brodie SE, Marr B, Zabor EC, Mondesire-Crump I, Abramson DH. Efficacy and toxicity of intravitreous chemotherapy for retinoblastoma: four-year experience. Ophthalmology. 2017;124(4):488–495. doi:10.1016/j.ophtha.2016.12.015
25. Ji X, Hua P, Li J, Li J, Zhao J, Zhao P. Intravitreal melphalan for vitreous seeds: initial experience in China. J Ophthalmol. 2016;2016:1–7. doi:10.1155/2016/4387286
26. Berry JL, Shah S, Bechtold M, Zolfaghari E, Jubran R, Kim JW. Long-term outcomes of Group D retinoblastoma eyes during the intravitreal melphalan era. Pediatr Blood Cancer. 2017;64(12):e26696. doi:10.1002/pbc.26696
27. Solana-Altabella A, Valero S, Balaguer J, et al. Intravitreal melphalan therapy for vitreous seeds in retinoblastoma: implementation and outcomes of a new chemotherapy protocol. J Oncol Pharm Pract. 2020;26(8):1829–1835. doi:10.1177/1078155220904410
28. Gündüz AK, Mirzayev I, Dinçaslan H, Özalp Ateş FS. Recurrence and new tumor development after frontline intravenous chemotherapy for retinoblastoma: risk factors and treatment results. Eur J Ophthalmol. 2022;32(3):1795–1803. doi:10.1177/11206721211023311
29. Dalvin LA, Bas Z, Tadepalli S, et al. Risk Factors for Tumor Recurrence Following Primary Intravenous Chemotherapy (Chemoreduction) for Retinoblastoma in 869 Eyes of 551 Patients. J Pediatr Ophthalmol Strabismus. 2020;57(4):224–234. doi:10.3928/01913913-20200417-01
30. Smith SJ, Smith BD, Mohney BG. Ocular side effects following intravitreal injection therapy for retinoblastoma: a systematic review. Br J Ophthalmol. 2014;98(3):292–297. doi:10.1136/bjophthalmol-2013-303885
31. Shields CL, Dockery PW, Yaghy A, et al. Intra-arterial chemotherapy for retinoblastoma in 341 consecutive eyes (1292 infusions): comparative analysis of outcomes based on patient age, race, and sex. J AAPOS. 2021;25(3):
32. Mirzayev I, Gündüz AK, Yavuz K, et al. Secondary intra-arterial chemotherapy and/or intravitreal chemotherapy as salvage treatment for retinoblastoma. Eur. J Ophthalmol. 2021;31(5):2692–2698. doi:10.1177/1120672120957587
33. Sims LB, Tyo KM, Stocke S, Mahmoud MY, Ramasubramanian A, Steinbach-Rankins JM. Surface-modified melphalan nanoparticles for intravitreal chemotherapy of retinoblastoma. Invest Ophthalmol Vis Sci. 2019;60(5):1696. doi:10.1167/iovs.18-26251
34. Nadelmann J, Francis JH, Brodie SE, Muca E, Abramson DH. Is intravitreal topotecan toxic to retinal function? Br J Ophthalmol. 2021;105(7):1016–1018. doi:10.1136/bjophthalmol-2020-316588
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




