Back to Journals » Clinical Ophthalmology » Volume 17
Ocular Surface Disease Related to the Inflammatory and Non-Inflammatory Phases of Thyroid Eye Disease
Authors Riguetto CM, Barbosa EB, Atihe CC, Reis F, Alves M , Zantut-Wittmann DE
Received 10 August 2023
Accepted for publication 2 November 2023
Published 15 November 2023 Volume 2023:17 Pages 3465—3475
DOI https://doi.org/10.2147/OPTH.S430861
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Cinthia Minatel Riguetto,1,* Eduardo Buzolin Barbosa,2,* Camila Cristina Atihe,2 Fabiano Reis,3 Mônica Alves,2 Denise Engelbrecht Zantut-Wittmann1
1Endocrinology Division, Department of Internal Medicine, Faculty of Medical Sciences, University of Campinas, São Paulo, Brazil; 2Department of Ophthalmology and Otorhinolaryngology, Faculty of Medical Sciences, University of Campinas, São Paulo, Brazil; 3Department of Radiology, Faculty of Medical Sciences, University of Campinas, São Paulo, Brazil
*These authors contributed equally to this work
Correspondence: Denise Engelbrecht Zantut-Wittmann, Endocrinology Division, Department of Internal Medicine, Faculty of Medical Sciences – University of Campinas, Rua Tessália Vieira de Camargo, 126, Campinas, São Paulo, 13084-971, Brazil, Tel +55 19 32894107, Email [email protected]
Purpose: This study evaluated the ocular surface disease (OSD), especially dry eye disease (DED) parameters by combining qualitative and quantitative tools, including tear matrix metalloproteinase 9 (MMP-9), in patients with Graves’ disease (GD) with and without Thyroid eye disease (TED).
Patients and Methods: A total of 17 active TED, 16 inactive TED, 16 GD without ophthalmopathy, and 16 healthy controls were included. All patients were assessed with CAS, ophthalmometry, qualitative tear MMP-9, Ocular Surface Disease Index (OSDI), ocular surface staining, Schirmer test, meibography, tear meniscus height, conjunctival hyperemia, and non-invasive tear film break-up time. Patients were classified into three subtypes of DED: aqueous tear deficiency, meibomian gland dysfunction (MGD) and mixed dry eye.
Results: Inactive TED was shown to be an associated factor with DED (odds ratio 14, confidence interval 2.24– 87.24, p=0.0047), and presented more DED than healthy controls (87.5% versus 33.3%, p=0.0113). MGD was also more prevalent among these subjects than in healthy control (62.5% versus 6.7%; p=0.0273). No significant differences were found in other ophthalmological parameters, except for more intense conjunctival redness among active TED than GD without ophthalmopathy (p=0.0214). Qualitative MMP-9 test was more frequently positive in both eyes among active TED than in other groups (p < 0.0001).
Conclusion: Patients with GD were symptomatic and presented a high prevalence of ocular surface changes and DED, particularly the subgroup with inactive TED. Tear MMP-9 detection was associated with active TED suggesting a relationship between ocular surface changes and the initial inflammatory phase of ophthalmopathy.
Keywords: Graves’ ophthalmopathy, thyroid eye disease, ocular surface disease, dry eye, MMP-9
Introduction
Graves’ ophthalmopathy, also called Thyroid eye disease (TED), is the most frequent extrathyroidal manifestation of Graves’ disease (GD), affecting about 50% of patients. A majority will also experience exophthalmos and symptoms related to ocular surface disease (OSD), mostly dry eye disease (DED). Previous studies have reported that 65–85% of patients with TED present DED based on qualitative or quantitative ocular surface assessments.1–4
Some underlying mechanisms, such as ocular surface exposure secondary to proptosis and eyelid retraction, impaired Bell’s phenomenon, reduced tear production by the lacrimal glands (LGs), and dysfunction in the meibomian glands (MGs) play an essential role in TED.5–7 Thyroid-stimulating hormone receptors are also expressed in LGs, probably reducing tear production, and increasing tear film osmolarity.8,9 Hyperosmolarity may stimulate the expression of cytokines, including interleukins, tumor necrosis factor α (TNF-α), and matrix metalloproteinases (MMPs).10,11 Further, tear proteins have also been demonstrated to be altered, reflected by the upregulation of proteins involved in inflammatory processes and downregulation of protective proteins in patients with TED.12,13
MMPs are involved in inflammation, migration, differentiation, angiogenesis, and fibrosis, leading to extracellular matrix (ECM) remodeling.14 The imbalance between MMPs and their counteracting tissue inhibitors of metalloproteinases (TIMPs) has also been studied in other conditions, including cardiovascular diseases, cancer, wound healing, and rheumatoid arthritis (RA).15–19 Among MMPs, MMP-9 has been linked to DED, inflammation, and TED.20,21 MMP-9 has been assessed in tear samples from patients using a qualitative point-of-care immunoassay, which detects concentrations ≥ 40 ng/mL. Qualitative tear MMP-9 has shown high sensitivity and specificity to diagnose DED, also correlating with the severity of the disease.22
To the best of our knowledge, no previous studies have assessed OSD and DED by combining qualitative and quantitative ocular surface tools, including qualitative tear MMP-9 in patients with GD. Herein, we investigated OSD among patients with GD without apparent ophthalmopathy and those with ophthalmopathy in active or chronic and fibrosis phases of inflammation.
Materials and Methods
Study Design and Patients
Forty-nine consecutive patients with active GD and 16 healthy controls with similar age and sex, were included in this cross-sectional clinic-based study. Participants were recruited from a joint endocrinology and ophthalmology outpatient clinic between August 2019 and March 2020. Subjects with active and inactive ophthalmopathy and simultaneous onset of thyroid disease were included. In total, 65 subjects were recruited and divided into four groups according to clinical characteristics and the presence of ophthalmopathy. Basically, 17 patients with active inflammatory ophthalmopathy (CAS ≥ 3/7), 16 patients with inactive ophthalmopathy (CAS < 3), 16 patients with GD and without apparent ophthalmopathy, and 16 healthy euthyroid individuals from an iodine-sufficient area. All the subjects with active and inactive TED presented mild or moderate eye disease. Subjects with severe or sight-threatening TED were excluded due to likely requiring immunosuppression or surgical intervention. Exclusion criteria were acute infectious or inflammatory disease, hypothyroidism, subjects on or previous corticosteroid use (intravenous, oral, or ocular), subjects using eyedrops or ointments, previous orbital radiotherapy, and other acute or chronic OSD.
The study was approved by the Institutional Ethics Committee of the institution (CAAE: 92689218.8.0000.5404) and was conducted in accordance with the Declaration of Helsinki and current legislation on clinical research. Written informed consent was obtained from participants to participate in the study.
Clinical Assessment
Diagnosis of GD and Biochemical Assessment
The diagnosis of GD was established by an endocrinologist based on clinical characteristics and biochemical data. All biochemical data were measured by electrochemiluminescence immunoassay before the assessments, which included serum thyroid stimulating hormone (TSH) [reference values (RV) 0.30–4.2 uUI/mL], free thyroxine (fT4) (RV 0.9–1.7 ng/dL), free triiodothyronine (fT3) (RV 0.2–0.4
Presence and Severity of Ophthalmopathy
Clinical eye evaluation defined the presence of ophthalmopathy, and degree of inflammation based on the clinical activity score.23 The exclusion of orbital involvement was based on clinical characteristics suggested by EUGOGO23 in addition to the patient’s perspective of any changes in their eyes and previous photographs. CAS was calculated from 7 items, assigning 1 point for alteration presented: spontaneous orbital pain, gaze-evoked orbital pain, eyelid swelling, eyelid erythema, conjunctival redness, chemosis, and inflammation of caruncle or plica. Active inflammation in TED was indicated by a CAS of 3 or higher.23 Ophthalmometry was assessed with an ophthalmometer that measured the distance in millimeters between the outer corner of the eye and the cornea.
Tear MMP-9
Extracellular MMP-9 levels were measured using the InflammaDry strip test (Rapid Pathogen Screening, Inc, Sarasota, FL, USA). Positive results indicate that tear fluid MMP-9 levels were > 40ng/mL. The tear MMP-9 immunoassay was performed by a single investigator according to the manufacturer’s instructions. The tear sample collection started lowering the eyelid to expose the palpebral conjunctiva and dabbing the sampling fleece in 6–8 locations in the bulbar conjunctiva and fornix. The fleece glistered after saturation with tear film, and the sample collector was then transferred to the test cassette body. The absorbent tip was immersed into the buffer vial for a minimum of 20 seconds and laid flat on a horizontal surface for 10 minutes. Two different lines appeared in the result window: the control (blue) and the result (red) line. A positive result showed both blue and red lines, while a negative result showed only a blue line. A test was only considered valid if a blue line appeared.
Ophthalmological Examination
All participants underwent a detailed ocular anamnesis, including the Ocular Surface Disease Index (OSDI) questionnaire, assessing symptoms, functional limitations, and environmental factors. The total score of the OSDI ranges from 0 to 100, and values below 12 are considered normal. A score of 13–20, 23–32, and ≥ 33 reflect mild, moderate, and severe disease, respectively.24
The ocular surface was assessed by Keratograph 5M (OCULUS Optikgerate GmbH, Wetzlar, Germany), a non-invasive equipment developed to evaluate tear film and ocular surface through an objective process and photo documentation. The parameters analyzed included meibography (assess the morphology of MGs), tear meniscus height (TMH), non-invasive tear film break-up time (NITBUT) (assess tear stability with the time taken from a blink to the appearance of the first dry spot on the cornea), and conjunctival hyperemia graded from 0 to 3 (0 – absent; 1 – mild; 2 – moderate; 3 – severe). Ocular surface staining was assessed with sodic fluorescein, lissamine, and Schirmer test without anesthesia. All procedures were performed by the same ophthalmologist following a pre-established protocol.25
Classification of Ocular Surface and Tear Film Dysfunction
The OSD was classified according to the Tear Film and Ocular Surface Society guidelines, Dry Eye Workshop II (24) and the International Workshop on Meibomian Gland Dysfunction.26 The two main subtypes of dry eye, aqueous tear deficient (ATD) and evaporative dry eye (EDE), were discriminated based on the ocular surface parameters findings. Patients with OSDI score ≥ 13 and NITBUT < 10s or corneal staining > 5 spots or conjunctival staining > 3 were considered to have dry eye. Those diagnosed with dry eye that had TMH less than 0.2mm were subclassified as ATD, while those with meiboscore grade ≥ 1 were subclassified as MGD and EDE. Patients who met both criteria were classified as having a mixed form of DED.
Statistical Analysis
Statistical analyses were carried out in the Statistical Analysis System (SAS) - System for Windows, version 9.4. SAS Institute Inc., 2002–2012, Cary, NC, USA. Data are shown as mean ± SD. Descriptive analysis with frequency tables for categorical variables and position and dispersion measurements for continuous variables. The Chi-square or Fisher’s exact tests were used to compare proportions. The Mann–Whitney and Kruskal–Wallis tests were applied to compare continuous measures between 2 and 3 groups, respectively, followed by the Dunn test to locate differences. To assess the relationship between numerical variables, Spearman’s linear correlation coefficient was used. Factors associated with OSD/DED were assessed using logistic regression analysis. The significance level was set at p < 0.05.
Results
Demographic Data
Sixty-five individuals were enrolled, of which 17 GD with active ophthalmopathy, 16 GD with inactive ophthalmopathy, 16 GD without ophthalmopathy, and 16 healthy controls. Groups were similar in sex, age, smoking habits, comorbidities, treatment, TSH and fT4 at evaluation. There was no difference regarding thyroid disease duration, TgAb, TPOAb, TRAb, and types of treatment among patients with GD. Healthy controls presented a lower fT3 than GD patients without ophthalmopathy. Table 1 summarizes the demographic and clinical variables of the cohort.
 |
Table 1 Demographic Data from Patients with Active and Inactive TED, without TED and Healthy Controls |
Clinical Assessment
Subjects with active ophthalmopathy presented higher CAS than those with inactive ophthalmopathy (3.36 versus 0.88, p<0.001). The ophthalmometry was higher in patients with active TED than GD without ophthalmopathy and healthy controls, inactive TED than GD without ophthalmopathy, and GD without ophthalmopathy than healthy controls (p<0.0001). MMP-9 test was more frequently positive in both eyes among individuals with active TED than in other groups (p < 0.0001). Both sides reported similar percentages of positive and negative results (Table 2).
 |
Table 2 Clinic Assessment of the Eyes and Tears MMP-9 in Patients with Active and Inactive TED, without TED and Healthy Controls |
Ophthalmological Assessment
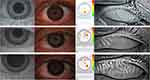
OSD symptoms, assessed through OSDI, were higher among active and inactive TED than in healthy controls (43.37 and 36.89 versus 16.02, p=0.0037), although only the inactive TED group presented higher rates of DED compared to healthy individuals (87.5% versus 33.3%, p=0.0113). Regarding the DED subclassification, MGD and EDE were the most common causes of DED among these patients with inactive TED, also being different only from healthy control (62.5% versus 6.7%; p=0.0273). Active TED patients presented no statistical difference among MGD (37.5%), ATD (12.5%) and MDE (18.8%). Patients without apparent ophthalmopathy had similar rates of MGD (13.3%), ATD (13.3%), and MDE (20%). We did not find differences regarding other ophthalmological parameters, except for more intense redness in active TED than GD without ophthalmopathy (2.02 versus 1.36, p=0.0214) (Table 3). Figure 1 displays examples of the ocular surface and tear film main findings in active TED patients’ cohort.
 |
Table 3 Ophthalmological Assessment in Patients with Active and Inactive TED, without TED and Healthy Controls |
Ocular Surface Disease
There were no differences between the presence of dry eye regarding age at the evaluation, sex, smoking habits, TSH, fT4, fT3, TRAb, and CAS. OSDI was higher in patients diagnosed with DED (active TED, p=0.0414; without TED, p=0.0014; and healthy control, p=0.0026). P-value was not calculated in the inactive group because only two patients did not have DED. Patients with active TED who underwent orbital decompression surgery had less DED (p=0.0128). Tear film stability evaluated by TBUT was more prolonged in active TED without DED (p=0.0326), which was not seen among patients without TED and healthy control. The p-value was not calculated in the inactive group because only two patients did not have DED. MMP-9 was more frequently positive among patients with active and inactive TED with DED, however, we could not calculate the p-value due to the number of patients. The same was not seen among GD without ophthalmopathy and healthy controls. Most patients without ophthalmopathy or DED had a higher frequency of positive MMP-9 (62.5%) and a greater ophthalmometry (p=0.0304) (Table 4).
 |
Table 4 Dry Eye Disease Between Groups of Patients with Graves’ Disease Regarding the Presence of Thyroid Eye Disease |
Spearman Correlation
The correlation between exophthalmometry and OSDI was analyzed in all groups, as well as between CAS, OSDI and redness among patients with active and inactive TED, however, none was found to be significant. No correlation was also found between TSH, fT4, and fT3 at evaluation and redness among all groups. (Data not shown).
Factors Associated with DED
By univariate logistic regression analysis, inactive TED was a factor associated with DED (odds ratio 14, confidence interval 2.24–87.24, p=0.0047). Age at assessment and age at diagnosis of GD, sex, smoking, as well as individuals in the control group, patients with GD without ophthalmopathy and with active TED were evaluated without showing statistical significance (data not shown). It was not possible to perform multivariate logistic regression analysis.
Discussion
This study investigated the presence of OSD through a comprehensive qualitative evaluation in GD patients with ophthalmopathy in different phases of inflammation and without this manifestation. A high prevalence of DED was demonstrated among the patients with GD, particularly in the group with inactive TED, reaching up to 87.5% of the subjects analyzed. In fact, inactive TED was a factor associated with DED. Moreover, the qualitative tear MMP-9 test was more frequently positive among individuals with active TED than in other groups, possibly establishing a link between MMP-9 and ocular soft tissue inflammation.
DED is an important multifactorial OSD associated with ocular discomfort that significantly impairs the quality of life and daily activities, especially in groups where other risk factors might be present such as ageing, sex, hormone dysfunctions, environmental exposures, and lifestyle conditions.24 GD patients are at increased risk of DED, as shown by our and other studies.1–3 We found a high prevalence of DED in all subjects with GD, especially among patients with inactive ophthalmopathy, representing the chronic and fibrotic phases of TED. On the other hand, the prevalence of DED was also elevated among patients without ophthalmopathy and with active TED, reaching 68.8% and 46.7%, respectively.
In the context of thyroid disease, this study remarks the noteworthy findings related to alterations in ocular surface and the broad presentation of diagnostic tests, ocular symptoms measurements, and possible markers of DED subtypes in affected patients. The findings revealed a high prevalence of symptoms reported through the application of the OSDI questionnaires shedding light on the potential impact on patients’ quality of life. Additionally, there were observed correlations and disparities in the ocular surface set of tests, such as the conjunctival hyperemia scores, indicative of ocular surface inflammation and increased Schirmer test results, suggesting a potential compensatory reflex mechanism, respectively. These findings underscore the importance of assessing ocular surface parameters in a comprehensive way and managing ocular surface health in thyroid disease patients to improve their overall well-being. Of note, the quantification of ocular symptoms in thyroid disease patients holds paramount significance. By systematically assessing and quantifying symptoms, healthcare providers gain valuable insights into the extent of ocular discomfort and impairment experienced by these individuals. Such evaluation not only aids in the early detection and diagnosis of DED, but also provides a basis for monitoring treatment effectiveness over time. Furthermore, it highlights the profound impact that thyroid disease can have on ocular health and underscores the importance of tailored interventions to improve the overall quality of life for affected patients.
The etiopathogenesis of DED in TED is not entirely known, but some underlying mechanisms have been proposed. Ocular surface exposure secondary to proptosis, eyelid retraction, widening of the palpebral fissure and incomplete eyelid closure seems to be the leading cause. Any abnormality in the function of the eyelids results in increased tear evaporation and the formation of dry spots, due to epithelial disruption.7 Although our study has not found a clear association between ophthalmometry and DED, we found that patients with active TED who underwent orbital decompression surgery had less DED, probably due to lower ocular surface exposure. Additionally, the degree of tear film evaporation is closely related to ocular surface exposure and damage.7,27,28
The reduced tear production, possibly associated with TSHR expressed in LGs, is another critical mechanism involved in the pathogenesis of DED in TED.8 Moreover, LGs also express somatostatin receptors and were found to be enlarged in imaging exams.29–31 The combination of these features may result in LGs impairment, hence, aqueous deficiency. Interestingly, our study found a small percentage of patients with DED secondary to ATD among all groups, suggesting that the tear volume was not associated with the presence of ophthalmopathy, both in the inflammatory activity and fibrotic phases. Indeed, it is relevant to consider the compensatory phases of DED, where some reflex response of tearing is stimulated to compensate tear film imbalance and ocular surface inflammation.
MGD also plays an essential role in this process, since indicates eyelid gland dysfunction that impairs the production of the lipid layer of the tears, leading to EDE. Likewise other authors32–36, our study demonstrated that the main cause of DED among the subjects with inactive TED was MGD. The function and morphology of the MGs have been investigated in previous studies, and most found a higher prevalence of obstructive MGD but mild or no loss of MGs.34,36 Furthermore, it has been shown a particular involvement of the upper eyelids.32,36 The mechanisms behind the MGD in subjects with TED are not entirely elucidated. Still, evidence suggests that the incomplete blinking resulting from proptosis and palpebral fissure height lead to obstruction of Meibomian glands.32–37 Kim et al 34 found an association between CAS and loss of MGs structure, possibly explained by ocular inflammation causing ocular surface and morphological changes. Our study did not find an association between symptoms of dry eye and proptosis or inflammation among patients with active and inactive TED, which could be explained by the relatively low number of patients in each group compared to the study previously mentioned.
Although the link between soft tissue inflammation and change of ocular surface was not clearly demonstrated in our study, 16 out of 17 patients in the active TED group had positive MMP-9 tests, even the ones without DED, suggesting a relationship between ocular inflammation and MMP-9. Elevated levels of active MMP-2 and MMP-9 in tear fluid and cornea epithelium have also been reported among patients with recurrent corneal ulceration, possibly modifying the cornea barrier function and increasing cornea epithelial permeability.38
MMPs are considered serum markers of fibrosis as they play a key role in extracellular matrix remodeling. MMPs break down ECM while TIMPs regulate MMPs activity preventing inadequate ECM deposition and degradation. The fine balance between MMPs and their counteracting TIMPs, essential to maintain adequate ECM remodeling and disposal, may be impaired in both acute and chronic phases of ophthalmopathy.14,15 Mysliwiec et al21 assessed serum MMP-2, MMP-9, and TIMP-1 in patients with active TED before and after GCS therapy. There were no changes in MMP-2 and TIMP-1, but MMP-9 levels were significantly lower after GCS administration. Similarly, Kapelko-Slowik et al20 evaluated serum MMP-9, MMP-2, TIMP1 and TIMP-2 among patients with and without TED. All levels were elevated in all patients than in healthy subjects, but only MMP-9 was able to differentiate between patients with and without TED.
Increased MMP activity, predominantly MMP-2 and MMP-9, is also present in other conditions such as RA and psoriatic arthritis (PA), as well as diabetic foot ulcers, contributing to persistent inflammation and poor healing.17,18,39,40 Giannelli et al19 investigated the expression of MMP-2, MMP-9, TIMP-1 and TIMP-9, in serum and synovial fluid of patients with RA and PA. They found dramatically elevated levels of MMP-9 in the active and latent forms of both diseases, while MMP-2 was only detected in the latent form. We hypothesized that a similar process occurs in GD due to the high positivity rates of MMP-9 in our subjects with GD, regardless of the presence of DED.
One of the limitations of our study is the small number of patients in each group, which might be why we could not find a connection between DED and inflammation. Also, lid width, including upper-lower lid retraction and lid closure measurements were not performed once the ocular evaluation was focused on the ocular surface parameters. Still, we were able to compare patients in two different phases of the inflammatory process, active and non-active ophthalmopathy, along with a group without apparent ophthalmopathy and healthy control. In addition, our patients underwent a thorough ophthalmological assessment to detect abnormalities of the ocular surface, tear film, symptoms quantification and MMP-9 as a possible biomarker. Distinct subtypes of DED were evaluated, as well as the main ocular surface and tear parameters.
We suggest that the assessment of the ocular surface and severity of dry eye should be performed with the classic evaluation of eye involvement in all patients with GD, regardless of the presence of ophthalmopathy, given the high rates of DED. By providing a detailed evaluation of the ocular surface parameters along with symptom quantification, this study emphasized awareness about the impact of TED on the ocular surface and diagnostic pathways that could be helpful. Once identified, proper therapeutic strategies may be prescribed to improve comfort and avoid complications. Patients should be educated about the main aspects of OSD and how to improve comfort with tear replacement and environmental exposure control, such as visual display use, pollution, low humidity, and air-conditioning.
Conclusion
Patients with GD were symptomatic and presented a high prevalence of ocular surface changes and DED, particularly the subgroup with inactive TED, adding another burden to their already compromised quality of life. Among all subgroups of TED markedly high scores of ocular surface symptoms were found. OSD was also more prevalent among patients with inactive TED, mainly due to MGD, indicating dysfunction in marginal eyelid glands that produce the tear lipid layer. Tear MMP-9 detection was associated with active TED suggesting a possible relationship between ocular surface changes and the initial inflammatory phase of ophthalmopathy. Further studies are needed to investigate the relationship between orbital soft tissue inflammation and change in the ocular surface.
Acknowledgments
We acknowledge all patients who voluntarily participated in our study. We also acknowledge the financial support from FAEPEX (Research Support Fund of University of Campinas) number 92867-20. DEZ-W had a National Council of Technological and Scientific Development Scholarship (CNPq) (303068/2021-3).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Rana HS, Akella SS, Clabeaux CE, Skurski ZP, Aakalu VK. Ocular surface disease in thyroid eye disease: a narrative review. Ocul Surf. 2022;24:67–73. doi:10.1016/j.jtos.2022.02.001
2. Achtsidis V, Tentolouris N, Theodoropoulou S, et al. Dry eye in Graves ophthalmopathy: correlation with corneal hypoesthesia. Eur J Ophthalmol. 2013;23(4):473–479. doi:10.5301/ejo.5000259
3. Kashkouli MB, Alemzadeh SA, Aghaei H, et al. Subjective versus objective dry eye disease in patients with moderate-severe thyroid eye disease. Article Ocular Surface. 2018;16(4):458–462. doi:10.1016/j.jtos.2018.07.003
4. Bartley GB, Fatourechi V, Kadrmas EF, et al. Long-term follow-up of Graves ophthalmopathy in an incidence cohort. Ophthalmology. 1996;103(6):958–962. doi:10.1016/s0161-6420(96)30579-4
5. Selter JH, Gire AI, Sikder S. The relationship between Graves’ ophthalmopathy and dry eye syndrome. Clin Ophthalmol. 2015;9:57–62. doi:10.2147/OPTH.S76583
6. Ismailova DS, Fedorov AA, Grusha YO. Ocular surface changes in thyroid eye disease. Orbit. 2013;32(2):87–90. doi:10.3109/01676830.2013.764440
7. Gilbard JP, Farris RL. Ocular surface drying and tear film osmolarity in thyroid eye disease. Acta Ophthalmol. 1983;61(1):108–116. doi:10.1111/j.1755-3768.1983.tb01401.x
8. Eckstein AK, Finkenrath A, Heiligenhaus A, et al. Dry eye syndrome in thyroid-associated ophthalmopathy: lacrimal expression of TSH receptor suggests involvement of TSHR-specific autoantibodies. Acta Ophthalmol Scand. 2004;82(3 Pt 1):291–297. doi:10.1111/j.1395-3907.2004.00268.x
9. Lacheta D, Miskiewicz P, Gluszko A, et al. Immunological aspects of graves’ ophthalmopathy. Biomed Res Int. 2019;2019:7453260. doi:10.1155/2019/7453260
10. Luo L, Li DQ, Doshi A, Farley W, Corrales RM, Pflugfelder SC. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci. 2004;45(12):4293–4301. doi:10.1167/iovs.03-1145
11. Gurdal C, Genc I, Sarac O, Gonul I, Takmaz T, Can I. Topical cyclosporine in thyroid orbitopathy-related dry eye: clinical findings, conjunctival epithelial apoptosis, and MMP-9 expression. Curr Eye Res. 2010;35(9):771–777. doi:10.3109/02713683.2010.490320
12. Matheis N, Okrojek R, Grus FH, Kahaly GJ. Proteomics of tear fluid in thyroid-associated orbitopathy. Thyroid. 2012;22(10):1039–1045. doi:10.1089/thy.2012.0119
13. Matheis N, Grus FH, Breitenfeld M, et al. Proteomics differentiate between thyroid-associated orbitopathy and dry eye syndrome. Invest Ophthalmol Vis Sci. 2015;56(4):2649–2656. doi:10.1167/iovs.15-16699
14. Nissinen L, Kahari VM. Matrix metalloproteinases in inflammation. Biochim Biophys Acta. 2014;1840(8):2571–2580. doi:10.1016/j.bbagen.2014.03.007
15. Le NT, Xue M, Castelnoble LA, Jackson CJ. The dual personalities of matrix metalloproteinases in inflammation. Front Biosci. 2007;12:1475–1487. doi:10.2741/2161
16. Medeiros NI, Gomes JAS, Fiuza JA, et al. MMP-2 and MMP-9 plasma levels are potential biomarkers for indeterminate and cardiac clinical forms progression in chronic Chagas disease. Sci Rep. 2019;9(1):14170. doi:10.1038/s41598-019-50791-z
17. Yager DR, Zhang LY, Liang HX, Diegelmann RF, Cohen IK. Wound fluids from human pressure ulcers contain elevated matrix metalloproteinase levels and activity compared to surgical wound fluids. J Invest Dermatol. 1996;107(5):743–748. doi:10.1111/1523-1747.ep12365637
18. Lobmann R, Ambrosch A, Schultz G, Waldmann K, Schiweck S, Lehnert H. Expression of matrix-metalloproteinases and their inhibitors in the wounds of diabetic and non-diabetic patients. Diabetologia. 2002;45(7):1011–1016. doi:10.1007/s00125-002-0868-8
19. Giannelli G, Erriquez R, Iannone F, Marinosci F, Lapadula G, Antonaci S. MMP-2, MMP-9, TIMP-1 and TIMP-2 levels in patients with rheumatoid arthritis and psoriatic arthritis. Clin Exp Rheumatol. 2004;22(3):335–338.
20. Kapelko-Slowik K, Slowik M, Szalinski M, et al. Elevated serum concentrations of metalloproteinases (MMP-2, MMP-9) and their inhibitors (TIMP-1, TIMP-2) in patients with Graves’ orbitopathy. Adv Clin Exp Med. 2018;27(1):99–103. doi:10.17219/acem/68991
21. Mysliwiec J, Adamczyk M, Pawlowski P, Nikolajuk A, Gorska M. Serum gelatinases (MMP-2 and MMP-9) and VCAM-1 as a guideline in a therapeutic approach in Graves’ ophthalmopathy. Endokrynol Pol. 2007;58(2):105–109.
22. Sambursky R, Davitt WF 3rd, Latkany R, et al. Sensitivity and specificity of a point-of-care matrix metalloproteinase 9 immunoassay for diagnosing inflammation related to dry eye. JAMA Ophthalmol. 2013;131(1):24–28. doi:10.1001/jamaophthalmol.2013.561
23. Bartalena L, Kahaly GJ, Baldeschi L, et al. The 2021 European Group on Graves’ orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves’ orbitopathy. Eur J Endocrinol. 2021;185(4):G43–G67. doi:10.1530/EJE-21-0479
24. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276–283. doi:10.1016/j.jtos.2017.05.008
25. Trindade M, Castro de Vasconcelos J, Ayub G, et al. Ocular manifestations and neuropathy in type 2 diabetes patients with Charcot arthropathy. Front Endocrinol (Lausanne). 2021;12:585823. doi:10.3389/fendo.2021.585823
26. Nichols KK, Foulks GN, Bron AJ, et al. The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci. 2011;52(4):1922–1929. doi:10.1167/iovs.10-6997a
27. Iskeleli G, Karakoc Y, Abdula A. Tear film osmolarity in patients with thyroid ophthalmopathy. Jpn J Ophthalmol. 2008;52(4):323–326. doi:10.1007/s10384-008-0545-7
28. Brasil MV, Brasil OF, Vieira RP, Vaisman M, Amaral Filho OM. Análise do filme lacrimal e sua relação com a largura da fenda palpebral e a exoftalmia na oftalmopatia de Graves [Tear film analysis and its relation with palpebral fissure height and exophthalmos in Graves’ ophthalmopathy]. Arq Bras Oftalmol. 2005;68(5):615–618. Portuguese. doi: 10.1590/s0004-27492005000500007
29. Moncayo R, Baldissera I, Decristoforo C, Kendler D, Donnemiller E. Evaluation of immunological mechanisms mediating thyroid-associated ophthalmopathy by radionuclide imaging using the somatostatin analog 111In-octreotide. Thyroid. 1997;7(1):21–29. doi:10.1089/thy.1997.7.21
30. Chang TC, Huang KM, Chang TJ, Lin SL. Correlation of orbital computed tomography and antibodies in patients with hyperthyroid Graves’ disease. Clin Endocrinol (Oxf). 1990;32(5):551–558. doi:10.1111/j.1365-2265.1990.tb00897.x
31. Harris MA, Realini T, Hogg JP, Sivak-Callcott JA. CT dimensions of the lacrimal gland in Graves orbitopathy. Ophthalmic Plast Reconstr Surg. 2012;28(1):69–72. doi:10.1097/IOP.0b013e31823c4a3a
32. Kim YS, Kwak AY, Lee SY, Yoon JS, Jang SY. Meibomian gland dysfunction in Graves’ orbitopathy. Can J Ophthalmol. 2015;50(4):278–282. doi:10.1016/j.jcjo.2015.05.012
33. Inoue S, Kawashima M, Arita R, Kozaki A, Tsubota K. Investigation of meibomian gland function and dry eye disease in patients with graves’ ophthalmopathy. J Clin Med. 2020;9(9):2814. doi:10.3390/jcm9092814
34. Park J, Baek S. Dry eye syndrome in thyroid eye disease patients: the role of increased incomplete blinking and Meibomian gland loss. Acta Ophthalmol. 2019;97(5):e800–e806. doi:10.1111/aos.14000
35. Yilmaz Tugan B, Ozkan B. Evaluation of meibomian gland loss and ocular surface changes in patients with mild and moderate-to-severe graves’ ophthalmopathy. Semin Ophthalmol. 2022;37(3):271–276. doi:10.1080/08820538.2021.1937662
36. Wang CY, Ho RW, Fang PC, et al. The function and morphology of Meibomian glands in patients with thyroid eye disease: a preliminary study. Article BMC Ophthalmology. 2018;18(9):90. doi:10.1186/s12886-018-0763-9
37. Park J, Kim J, Lee H, Park M, Baek S. Functional and structural evaluation of the meibomian gland using a LipiView interferometer in thyroid eye disease. Can J Ophthalmol. 2018;53(4):373–379. doi:10.1016/j.jcjo.2017.11.006
38. Sakimoto T, Sawa M. Metalloproteinases in corneal diseases: degradation and processing. Cornea. 2012;31(Suppl 1):S50–6. doi:10.1097/ICO.0b013e318269ccd0
39. Trengove NJ, Stacey MC, MacAuley S, et al. Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors. Wound Repair Regen. 1999;7(6):442–452. doi:10.1046/j.1524-475x.1999.00442.x
40. Ladwig GP, Robson MC, Liu R, Kuhn MA, Muir DF, Schultz GS. Ratios of activated matrix metalloproteinase-9 to tissue inhibitor of matrix metalloproteinase-1 in wound fluids are inversely correlated with healing of pressure ulcers. Wound Repair Regen. 2002;10(1):26–37. doi:10.1046/j.1524-475x.2002.10903.x
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.