Back to Journals » Eye and Brain » Volume 15
Ocular Myasthenia Gravis: A Current Overview
Authors Behbehani R
Received 31 October 2022
Accepted for publication 24 January 2023
Published 5 February 2023 Volume 2023:15 Pages 1—13
DOI https://doi.org/10.2147/EB.S389629
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Margaret Wong-Riley
Raed Behbehani
Neuroophthalmology Unit, Ibn Sina Hospital, Kuwait City, Kuwait
Correspondence: Raed Behbehani, Ibn Sina Hospital, P.O Box 1180, Tel +965 2224 2999, Fax +965 2249 2406, Email [email protected]
Abstract: Ocular myasthenia gravis (OMG) is a neuromuscular disease characterized by autoantibody production against post-synaptic proteins in the neuromuscular junction. The pathophysiological auto-immune mechanisms of myasthenia are diverse, and this is governed primarily by the type of autoantibody production. The diagnosis of OMG relies mainly on clinical assessment, the use of serological antibody assays for acetylcholine receptors (AchR), muscle-specific tyrosine kinase (MusK), and low-density lipoprotein 4 (LPR4). Other autoantibodies against post-synaptic proteins, such as cortactin and agrin, have been detected; however, their diagnostic value and pathogenic effect are not yet clearly defined. Clinical tests such as the ice test and electrophysiologic tests, particularly single-fiber electromyography, have a valuable role in diagnosis. The treatment of OMG is primarily through cholinesterase inhibitors (pyridostigmine), and steroids are frequently required in cases of ophthalmoplegia. Other immunosuppressive therapies include antimetabolites (azathioprine, mycophenolate mofetil, methotrexate) and biological agents such as B-cell depleting agents (Rituximab) and complement inhibitors (eculizumab). Evidence is scarce on the effect of immunosuppressive therapy on altering the natural course of OMG. Clinicians must be vigilant of a myasthenic syndrome in patients using immune-check inhibitors. Reliable and consistent biomarkers are required to assess disease severity and response to therapy to optimize the management of OMG. The purpose of this review is to summarize the current trends and the latest developments in diagnosing and treating OMG.
Keywords: ocular myasthenia, anti-acetyl-choline receptor antibody, anti-MuSK antibodies, generalized myasthenia
Introduction
Myasthenia gravis (MG) is an autoimmune disease caused by antibodies directed at acetylcholine receptors or functionally related proteins in the postsynaptic membrane at the neuromuscular junction. The overall incidence of OMG based on a population study is 1.13 per 100,000 per year, while studies focusing on MG have reported a range of incidence from 0.17 to 7 per 100,000 per year.1 Almost 50% of myasthenia patients present initially with only ocular symptoms at presentation.2
Ocular manifestations of myasthenia are common and occur in 15–50% of cases, most frequently as fluctuating ptosis, diplopia, and orbicularis weakness.3–5 Ocular myasthenia gravis (OMG) is characterized by almost exclusively ocular symptoms and can evolve into generalized myasthenia gravis (GMG) in about 20–60% of cases.3,5 There are current challenges in managing OMG due to the lack of evidence-based guidelines and standard diagnostic criteria. The diagnosis is based on a combination of relevant symptoms and signs and a positive test for specific autoantibodies. This review discusses the clinical presentation of OMG and the recent updates in diagnostic tests, including serological tests and biomarkers. We will also review updates on the natural course of OMG and prognostic factors which can predict the generalization of OMG into GMG.
Pathophysiology
Myasthenia gravis is an autoimmune disease characterized by the production of autoantibodies that target the neuromuscular junction. Autoantibodies against the nicotinic acetylcholine receptor (AchR) are detected in approximately 85% of patients with generalized myasthenia. In the remaining 15% of patients, other autoantibodies against muscle-specific kinase (MuSK) or lipoprotein receptor-related protein 4 (LRP4) are detected.
Low-density lipoprotein receptor-related protein 4 (LRP4) is a membrane protein that serves as a receptor for agrin in the neuromuscular junction. The binding of agrin to LRP4 enables the activation of MuSK, resulting in the phosphorylation of cortactin, which mediates the clustering of AChR. This AchR clustering results in high receptor availability to synaptic acetylcholine, facilitating muscle excitability.6
Many studies have established that these autoantibodies have a pathogenic effect.7 In a small fraction of patients, no autoantibodies are seen and therefore designated seronegative myasthenia. The pathophysiology of congenital myasthenia syndromes, unlike adult and juvenile myasthenia, is not auto-immune and involves structural or functional, presynaptic or postsynaptic abnormalities that lead to inadequate release of acetylcholine or AchR dysfunction and will not be discussed in this review.8
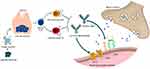
Anti-AchR antibodies are of the IgG1, IgG2, and IgG3 subclass derived from long-lived plasma cells and are produced by B cells in the hyperplastic thymus and other tissue compartments, such as the bone marrow.9 (Figure 1) They exert their pathologic effect at the neuromuscular junctions through different mechanisms. They can block AchR either at the receptor or in its proximity at the neuromuscular junction and therefore block acetylcholine binding. They can also cross-link AchR through bivalent binding with the two binding sites of the antibody, which will lead to the internalization of the AchR and diminish the number of receptors at the neuromuscular junction. Finally, they can activate the complement pathway, leading to the formation of the membrane attack complex.10 Complement-mediated damage will result in reduced postsynaptic junctional folds, effacement of AchR clustering and voltage-gated sodium channels from the membrane, and increased synaptic distance.11
Anti-MuSK antibodies are targeted against Musk, a transmembrane kinase that sends signals to induce the clustering of AchR. (Figure 1) Anti-MuSK belongs to the IgG4 subclass produced mainly by short-lived plasmablasts and is, therefore, less effective in activating the complement pathway.12 Anti-MuSK autoantibodies in their normal divalent status (two identical Fab regions) will induce phosphorylation. This will disrupt the interaction between LPR4 and MuSK, which is necessary for AchR clustering.13 Another pathophysiologic effect of Anti-MsSK autoantibodies is through what is known as “Fab-exchange”. This is a process peculiar to IgG4 subclass antibodies, in which antibodies can dissociate to produce two identical half molecules, recombining to produce antibodies with two different Fab regions.14 Experimental models have shown that these functionally monovalent autoantibodies block MuSK and LRP4 interaction, leading to a reduction in AchR clustering.15
Compared to what is known about AchR and MuSK-mediated MG, less is known about the pathophysiology of LPR4-mediated MG and seronegative MG. LPR4 antibodies belong mainly to the IgG1 subclass, which can also activate the complement pathway and disrupt the Agrin-LRP4 signaling.16 Other autoantibodies, including agrin, collagen Q, cortactin, and the voltage-gated potassium channel, Kv1.4. However, the pathogenic mechanisms of these autoantibodies have yet to be fully elucidated.17–20
The susceptibility of extraocular muscles in OMG can be attributed to several physiologic characteristics of the post-synaptic endplate; (1) the high-frequency firing of twitch motor units, (2) lower density of AchR at extraocular muscles twitch synapses as a result of a lower number of secondary synaptic folds (3) less acetylcholine released at extraocular muscle twitch synapses, and (4) a high number of multiterminal fibers which have one en plaque and several en-grappe synapses.21
Clinical Features
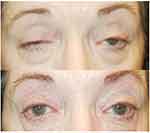
OMG typically presents with either ptosis or diplopia due to ophthalmoplegia. (Figures 2 and 3) The disease’s hallmark is fatigability and variability, which can often be observed and confirmed during the clinical examination. Ptosis can be unilateral or bilateral; the upper eyelid elevator muscle will exhibit fatigability with sustained upgaze. Enhanced ptosis, also known as the “curtain effect”, is when one eyelid is lifted, and the contralateral eyelid develops ptosis or worsening of pre-existing ptosis. This sign is due to Hering’s law of equal innervation that yoke muscles acting in the same direction are innervated equally. The “Cogan’s lid twitch” is a more striking clinical sign in which the upper eyelid shoots upward excessively and appears to “twitch” for a brief moment after sustained downward gaze exposing the upper limbus before returning to resting position. This sign has been attributed to fatigue, increased gain, and rapid muscle recovery. It is reported to be 99% specific and 75% sensitive, with and false positive rate of 1% in the diagnosis of OMG.22
Another modification of this sign is the “forced eyelid closure test”, in which the patient is asked to squeeze his eyes shut for 5–10 seconds and then open his eyes. The patient is then asked to fixate on a target in the primary position, and an upward overshoot of the eyelid followed by a downward drift constitutes a positive test. Gerling et al found this sign to be 94% sensitive and 91% specific for OMG.23 Orbicularis weakness is another distinctive sign for OMG and, in some cases, can lead to incomplete eyelid closure in what is known as the “Peek-sign”.24
Ophthalmoplegia in OMG can mimic any ocular motility disorder, including thyroid eye disease, cranial nerve palsy, decompensated phorias, chronic progressive external ophthalmoplegia, and unilateral or bilateral inter-nuclear ophthalmoplegia. Testing the pupils is helpful since it is not involved in myasthenia. However, a careful clinical examination can demonstrate the variability of orthoptic measurements, which helps differentiate OMG from other ocular motility disorders. Patients may show intrasaccadic fatigue, a decline in the saccadic velocity during a long saccade. The ocular deviation in OMG is classically incomitant (unequal in different gaze positions). In contrast, comitant deviation is thought to represent congenital phorias or central etiology such as brain stem or cerebellar lesions. Nevertheless, comitant ocular deviation or deviations changing from comitant to incomitant and vice versa have been reported in about 25% of OMG.25,26 Pseudo-internuclear ophthalmoplegia (unilateral or bilateral), pseudo-one-and-a-half syndrome, complete external ophthalmoplegia, divergence and convergence paresis, and double elevator palsy have all been described in OMG.27,28 The clinical diagnosis can be challenging and often delayed, particularly in the absence of ptosis. Since OMG can mimic various ocular motility disorders, some of which may be life-threatening, MRI brain imaging may be necessary in case of diagnostic uncertainty. Meanwhile, the differential diagnosis of isolated ptosis can be congenital ptosis, Horner’s syndrome, early chronic progressive external ophthalmoplegia, Botulinum toxin injection, and levator dehiscence (involutional) ptosis.
Diagnosis
Clinical Tests
The ice test is performed by placing surgical gloves filled with crushed ice over the ptosis eyelid in unilateral ptosis or both eyelids in bilateral ptosis for 2 minutes. An unequivocal improvement of the ptosis has to be observed for the test to be positive. The ice test is 90% sensitive and 100% specific in OMG and is more useful in the setting of ptosis but less sensitive in ophthalmoplegia.29 The sleep and rest tests are similar tests based on muscles’ fatigue with exertion as their recovery after rest. Odel et al found that ptosis and diplopia improve after 30 minutes of rest and worsen again within 30 to 5 minutes.30 The sleep and rest test are specific but not completely sensitive, as some OMG patients fail to respond.
Pharmacologic Tests
The edrophonium (cholinesterase inhibitor) test was used historically to diagnose OMG, but newer diagnostic modalities have primarily supplanted it. The test has low sensitivity (60%) in cases of OMG and low specificity, as it has been reported in other conditions such as multiple sclerosis, brain stem glioma, Guillain Barre syndrome, pituitary tumors, and even ischemic cranial neuropathy.4,31,32 Edrophonium is a short-acting acetylcholinesterase inhibitor with an onset of action within 10 to 30 seconds of intravenous administration, noted by improvement in muscle function. A starting dose of 2 mg is given; if no positive response occurs after 45 seconds, an additional 2 mg is administered up to a maximum of 10 mg until a positive result is observed. The drawback of the test is its predisposition to subjective interpretation like any clinical test and its potential association with significant systemic side effects, including bradycardia, syncope, and cholinergic crisis. More commonly, oral pyridostigmine is used in a therapeutic trial in cases of suspected OMG, especially in seronegative cases, and thereby indirectly as a diagnostic test by testing pharmacologic response to treatment.
Serological Tests
Anti-Acetylcholine Receptor (AchR) Antibodies
Anti-acetylcholine receptor (AchR) antibodies are the most frequently used serological test in diagnosing OMG. Although the AchR antibody assay is very sensitive (80–90%) in GMG, it is classically thought to be less sensitive in OMG, with reported seropositivity ranging between 40–70%.5,33 However, in recent years, higher sensitivity of the assay was reported in OMG. Peeler et al found a sensitivity of 70.9% in a study of 223 OMG subjects. In comparison, Chung et al reported a sensitivity of 86.7% after they assessed 114 cases referred for suspected OMG patients.34,35 The increased sensitivity of AchR antibody assay has been attributed to the increased sensitivity of radio-immunoassays the availability of the newer cell-based assays, which are more likely to be positive in “seronegative OMG”.36 Some authors have also proposed that the increased seropositivity rate of AchR antibody assays is due to the increased prevalence of late-onset GMG (between 50–70 years) over the last two decades.37 In a cohort of 133 patients with OMG, AchR antibody assay was positive in 65%, and the seropositivity increased with later onset, peaking in patients older than 70.37 Other studies have also shown a recent shift towards a late-onset disease with male predominance in OMG and an association between older age and increased AchR antibody positivity with conversion to GMG.1,34,38,39
There are three types of AchR antibodies; binding, modulating and blocking. The binding AchR antibody is the most useful for diagnostic testing because of its higher seropositivity. Nevertheless, testing for modulating AchR antibodies leads to slightly increased test sensitivity, while blocking AchR antibodies is of lower clinical utility as they are not detected in isolation.40
Anti-Muscle Specific Kinase (Musk) Antibodies
Anti-Musk antibodies have been initially reported in 38–54% of patients who were seronegative for AchR antibodies, but rarely in the “pure” form of OMG.41,42 Although early reports of Anti-Musk-positive myasthenia are classically characterized by bulbar, neck, and respiratory involvement, recent reports have shown that ocular manifestations were observed in 50–96.4% and were the presenting symptoms in 58.5%.43
Other Autoantibodies (Low-Density Lipoprotein Receptor-Related Protein 4, Cortactin, Agrin)
In cases of double-seronegative myasthenia gravis (dsNMG), in which both AchR and MuSK antibodies are negative, low-density lipoprotein receptor-related protein 4 (LRP4) and other autoantibodies to other components of the post-synaptic apparatus are assumed to be present and should be tested for.44 The frequency of positive LPR4 in dsNMG ranges from 2–50% across different studies.16
dsNMG with positive LPR4 antibodies is more likely to occur in young females, is frequently mild with isolated ocular symptoms, and typically responds well to pyridostigmine or prednisone.45,46 LPR4 antibodies have also been reported with other neurologic disorders such as polymyositis, neuromyelitis optica, multiple sclerosis, and amyotrophic lateral sclerosis.44 Therefore, clinicians should be cautious as some of these disorders are among the differential diagnoses for OMG.
Cortactin is a protein concentrated at the neuromuscular junction and acts downstream from agrin/LRP4/MuSK, promoting AchR clustering.47 Antibodies to cortactin have been detected in about 20% of patients with dsNMG, but also in cases of myositis and other autoimmune diseases18,48 In a series of 38 dsNMG patients, nine patients (23.7%) were cortactin antibody positive, and six of these patients presented at a young age with predominantly ocular involvement. When adjusted for age, focusing on patients younger than 50, patients with dsNMG and positive cortactin antibodies had a higher frequency of OMG and fewer bulbar signs than AchR- antibody positive myasthenia (at onset: 75.0% vs 30.4%; P = 0.02).49
The different auto-antibodies in dsNMG are therefore associated with the other clinical phenotypes and would account for the lack of response of some patients to specific treatments. In anti-MuSK-positive myasthenia, circulating plasmablasts that produce MuSK-specific autoantibodies express higher levels of CD20 than their tissue resident plasma cell counterparts which produce AchR.50 In keeping with this, MuSK myasthenia responds much better to B-cell-depleting treatment rituximab (anti-CD20) than AchR myasthenia. In a systematic review, 72% of MuSK myasthenia achieved clinical remission compared to 30% of AchR myasthenia (P < 0.001) when treated with rituximab.51
Electrophysiologic Tests
Repetitive nerve stimulation (RNS) will show decremental response cases of GMG. However, it is abnormal in only 30–50% of cases of patients with OMG; therefore, in the absence of generalized symptoms, its diagnostic value is minimal.52 Single-fiber electromyography (SFEMG) is preferred in cases of OMG and is much more sensitive (88–92%).53 Variability in the action potential interval between two muscle fibers in the same motor unit, termed “jitter”, is the critical SFEMG abnormality in myasthenia. The test, however, is time-consuming and requires technical expertise.54,55 It can also be abnormal in pre-synaptic disorders such as Lambert-Eaton myasthenic syndrome and mitochondrial myopathies.56 Therefore, SFEMG is probably more helpful in a seronegative case with a high clinical suspicion of OMG. In a large prospective study of SFEMG of the orbicularis oculi for patients referred for suspected OMG, patients with both ptosis and diplopia were more likely to have an abnormal SFEMG (98%), and patients with isolated diplopia were the least likely (32%). The overall sensitivity was 79%, and the specificity was 80%, with a positive predictive value of 90% and a negative predictive value of 61%.53 Therefore, an abnormal SFEMG helps confirm the diagnosis of OMG, but a negative test does not exclude it. When comparing the sensitivity and specificity of the ice test and SFEMG of the orbicularis, the ice test has a sensitivity of 86% and a specificity of 79%, wheeras SFEMG showed a sensitivity of 94% and a specificity of 79%. When both tests were positive, the sensitivity for OMG diagnosis increased to 82% and the specificity to 92%, with a negative predictive value of 94%. Therefore, when both tests are negative, the diagnosis of OMG becomes extremely unlikely.57
Immune-Check Inhibitor-Related Myasthenia
Immune-check inhibitors (ICI) are novel monoclonal antibodies against programmed cell death protein (PD-1), programmed death ligand-1 (PD-L1), and cytotoxic T-lymphocyte associated protein 4 (CTLA-4). Checkpoint proteins maintain immune homeostasis and prevent autoimmunity. However, they are used by cancer cells to evade normal anti-tumor immune responses. They have been used mainly for treating systemic melanoma, non-small lung cancer, urothelial carcinoma, and renal cell carcinoma.58 ICI stimulates the immune system to react against cancer cells by blocking the negative down-regulation of T cells.59 Commonly used ICI include nivolumab, pembrolizumab (PD1-inhibitor), atezolizumab, durvalumab (PD-L1), and ipilimumab (CTLA-4 inhibitor).
ICI-related myasthenia symptoms typically start following receiving 1–4 treatment cycles, with a median time of 7 days for the development of respiratory failure from the first symptom onset of MG.60 Myopathy is another adverse neurologic reported with ICI and often overlaps with myasthenia. Neuromuscular signs and symptoms associated with anti-PD-1 monoclonal antibodies, include weakness of extremities (78%), oculomotor (48%), respiratory (43%), and bulbar symptoms (35%).61 In a systematic review of ICI-related myasthenia, eight percent had a pre-existing diagnosis of the myasthenia and were in clinical remission, and fifty percent were positive for AchR antibodies, which is lower than typical myasthenia. The clinical course of ICI-related myasthenia is generally more severe, with a myasthenic crisis having a weighted incidence of 46.7% in contrast to idiopathic myasthenia, which has around 15% to 20% lifetime risk of myasthenic crisis.60
Creatinine phosphor kinase (CPK) levels were elevated in (26%) of patients with ICI-related myasthenia cases indicating an associated myositis. In addition, muscle biopsies in some patients showed inflammation with focal inflammatory infiltrates of CD4 and CD8 T cells and CD68-positive macrophages, which suggests a mixed myasthenia-myositis clinical entity in some of these patients.62 Most ICI-related myasthenia cases (77.6%) occurred with anti-PD-1 (eg, pembrolizumab, nivolumab) and anti-PD-L1 (eg, atezolizumab) and less commonly with anti-CTLA-4 (eg, ipilimumab) therapy.63
The prognosis depends on the clinical presentation, as some cases respond to immunosuppressive therapy. In contrast, others can be fatal, particularly if associated with severe cardiac complications such as myocarditis, arrhythmias, and cardiac arrest. An unfavorable outcome, including death, intubation, or tracheostomy, was observed in 21 patients (35%), among which 14 (66.7%) died.64–66 Therefore, the association of myasthenia with myositis in patients using PD-1 inhibitors should alert the clinicians to the risk of cardiac complications and initiate appropriate workup and aggressive immunosuppressive treatment.67 Steroids (oral and intravenous) were the most frequent treatment (91%). Intravenous immunoglobulins and plasma exchange should be given promptly if there is a lack of steroid benefit or severe bulbar or respiratory involvement.61,67
Myasthenia and Covid-19 Infection and Vaccine
There are recent reports of new-onset OMG cases developing shortly after covid-19 infection or after receiving the covid-vaccine.68–72 There are numerous reports that SARS-CoV-2 may trigger a range of neurological autoimmune manifestations through two different mechanisms: inducing hyperstimulation of the innate immune system and, secondly, by inducing hyperstimulation of the innate immune system and molecular mimicry between components of SARS-CoV-2 and structurally similar human peptide protein sequences, such as actin and alpha-myosin.73 mRNA vaccine might induce hyperstimulation of the innate immune system, which would cause causes cytokine production and release of previously existing self-antigens, resulting in the activation of auto-reactive T cells. On the other hand, it is possible that vaccination only unmasks asymptomatic myasthenia in predisposed patients or patients with mild disease. A review of cases with new-onset myasthenia associated with covid-19 showed that the mean age of the patients was 55.9 years (range 21–71). In contrast, the mean latency from Covid-19 infection to the development of myasthenic symptoms was 23 days, and 13 of the 15 cases reported were positive for anti-AchR antibodies (1 of them was positive for both anti-AchR antibodies and anti-titin antibodies) and two patients were positive for anti-MuSK antibodies. Myasthenia manifested as generalized muscle weakness in 11 patients; three presented with pure OMG, and only one presented with oculo-bulbar myasthenia.74
Cases of myasthenia following covid-19 vaccination are much less reported than covid-19 infection, with only 4 cases reported, one of whom had been diagnosed with myasthenia 5 years before vaccination.75 Given the small number of cases reported, there is no conclusive evidence that myasthenia is necessarily a complication of the COVID-19 vaccines, and the benefits of receiving the vaccine outweigh the potential risks.
Risk of Generalization of OMG
The reported range of conversion to GMG varies between 20–85% across different studies.1,3 The wide variation in the reported conversion rates of OMG to GMG is inherent in the retrospective study design of most of these studies, with the variable inclusion criteria for the duration of isolated ocular symptoms to be considered as OMG rather than GMG with ocular symptoms at onset, and the lack of well defined, clinically relevant criteria for diagnosis. Some studies required that patients should have only ocular symptoms for at least three months to be considered true OMG and reported a conversion rate of 21.1% among all patients at an average of four years after diagnosis and a lower rate of conversion (13.7%) if seronegative for AchR and MuSK antibodies.76
Predictors for generalization of OMG include AchR seropositivity and high AchR titer, thymoma or thymic hyperplasia, age over 50, abnormal RNS, and severity of symptoms.3,34,76–79 Evidence of reducing the risk of generalization of OMG by treatment with immunosuppression (steroids) is derived only from retrospective studies, and no randomized control trials have addressed this so far.38,80 A multi-center retrospective study to create a prognostic score to predict the risk of conversion of OMG into GMG found that AchR seropositivity, presence of comorbidities, and thymic hyperplasia were independently associated with increased generalization rates of OMG.81 Prognostic models can be helpful in the clinical decision in management and for selecting suitable patients for early interventional treatment in future randomized clinical trials to study the effects of immunosuppression on the generalization of OMG.81,82 Asian ethnic background has been reported to have a lower conversion rate to GMG (11.4–29%) than a caucasian background.83,84 A recent study in China of 228 patients showed a generalization rate of 17% over four years. The prognostic factors for generalization were higher age of onset and positive facial nerve RNS.38
Despite the growing evidence from animal models of auto-antibodies pathogenicity in myasthenia, their titer levels do not correlate with disease severity.85 This calls for a reliable biomarker to determine disease severity and possibly to predict the progression and risk of generalization in OMG consistently. A recent systemic review found a correlation between autoantibody levels, such as AchR, titin receptor antibodies, and ryanodine receptor antibodies, and disease activity in patients with myasthenia. However, little evidence to recommend routine clinical use of autoantibody-level testing for this purpose. Instead, clinical disease characteristics and laboratory data (eg, autoantibody status, thymus histology) should guide management alongside the clinical outcomes.86 Recently, microRNAs (miRNAs), non-coding RNA secreted by many cell types, emerged as relevant molecules in the pathogenesis of several human disorders, including neuromuscular disorders such as myasthenia. Of these miRNAs, miR-150-5p, miR-21-5p, and miR-30e-5p levels have been reported to correlate with the clinical response after immunosuppression and to predict the generalization of OMG in some studies.87,88 More studies are needed to assess the utility of miRNAs in determining disease severity, response to treatment, and as a predictor for generalization of OMG.
Current Treatment for OMG
Medical Treatment
There is a general lack of evidence-based guidelines and consensus on the treatment for OMG, as most randomized-controlled trials recruited GMG patients. Therefore, the results and findings do not apply directly to OMG. The most commonly used medical treatment agents are cholinesterase inhibitors (pyridostigmine), oral steroids, second-line immunosuppressive agents (azathioprine, mycophenolate mofetil, cyclophosphamide, cyclosporine, tacrolimus), intravenous immunoglobulins, plasma exchange; and biologic monoclonal antibody therapy: eculizumab and rituximab. Surgical treatment includes thymectomy, ptosis repair, or strabismus surgery in stable ptosis and diplopia cases.89 Pyridostigmine is helpful in patients with mild ocular symptoms and is more effective in alleviating ptosis than diplopia. It can be prescribed from 60 mg up to 300 mg/day depending on the clinical response, the duration of the response to a single dose, and how effectively it eliminates symptoms.89 Cholinesterase inhibitors are symptomatic treatments and do not affect the natural history of the disease. Side effects include diarrhea and increased weakness from a cholinergic crisis. The drug should be given carefully to patients with asthma, cardiac arrhythmia, or urinary obstruction.
Oral steroids are frequently used in diplopia or ptosis with diplopia, where cholinesterase inhibitors become largely ineffective. In a non-randomized retrospective series, oral prednisone showed resolution of primary gaze diplopia in 73% of patients. Pyridostigmine achieved resolution in only 6.9%, and the prism cover orthoptic measurements improved in the prednisone group only (p=0.003).90 Steroids can be started at a high dose (60 mg) and tapered down slowly or at an initial low dose (10 mg) and increased over several weeks. Maintenance doses continued for 6 to 12 weeks and are followed by a slow taper over several months while monitoring the clinical improvement and then either discontinued or maintained on a daily or alternate day low dose of 2.5–10 mg.91,92 The EPITOME study was designed to be a randomized controlled trial to investigate the clinical utility of oral prednisone in OMG. Subjects were treated with pyridostigmine, and those who did not respond were randomized to receive an escalating dose of oral steroids or a placebo for a 16-week double-blind therapy. The study was terminated early due to slow recruitment and low sample size (n=11). However, a clinical and statistically significant benefit of prednisone was found compared with placebo as five of 6 participants (83%) in the prednisone group achieved the primary endpoint of remission at a median of 14 weeks on a median prednisone dose of 15 mg/day, compared with 0 of 5 participants were in the placebo group.92 A recent randomized trial comparing two dosing regimens for GMG with concurrent use of azathioprine; a low-tapering regimen with a gradual increase of the prednisone dose to 1.5 mg/kg every other day and a slow-tapering and rapid-tapering regimen of immediate high-dose daily of 0.75 mg/kg, followed by an earlier and rapid decrease once improved myasthenia status was attained.93 The rapid-tapering regimen facilitated quicker prednisone discontinuation (ie, before 12 months), with a 4-fold increase in the proportion of patients reaching minimal manifestation status of myasthenia (no symptoms or functional limitations from myasthenia) at 12 months. A recent retrospective study found no difference between a low initial prednisone dosing of 10 mg/day (dosed 20 mg every other day) and a higher dose of 30 mg/day in controlling the symptoms of OMG at five weeks and six-month intervals from starting the treatment.94 This suggests that a low-dose steroids regimen may be appropriate for the initial dosing of OMG with fewer adverse effects. Intravenous immunoglobulins and plasma exchange are commonly used for severe GMG but are usually not required for pure OMG with no systemic symptoms.
OMG may not respond to steroids, and about 20% of patients may develop treatment-resistant ophthalmoplegia.95 Since the pathophysiologic mechanism of myasthenia can vary depending on the autoantibody driving the immunopathologic response, specific therapy may vary. Most cases of OMG involve AchR-positive antibodies, which are IgG1 and IgG3, and therefore can activate complement-mediated effector mechanisms causing damage to the muscle endplate. MuSK antibodies are predominantly IgG4 and less effective in activating the complement pathway. Therefore, complement inhibitors can be effective in AchR myasthenia but are expected to be less effective in MuSK myasthenia.95
Patients who fail steroid treatment or develop significant adverse effects should be offered second-line immunosuppressive agents, such as mycophenolate mofetil, azathioprine, cyclosporine, cyclophosphamide, or tacrolimus. These drugs have been used in GMG, but they have also succeeded in OMG.89,96
Rituximab (anti-CD20) has been used in refractory myasthenia, particularly in MuSK myasthenia.97 Furthermore, anti-CD19 therapy is a potential option for both MuSK and AchR-positive myasthenia as it could have an increased effect on the AchR autoantibody-producing B cell subsets.98 Proteasome inhibitors such as Bortezomib act by deleting plasma cells and have been proposed to treat this AchR-positive myasthenia.99 Complement Inhibitors are available as two different therapies; eculizumab is a monoclonal antibody, and Zilucoplan is a synthetic macrocyclic peptide. Both act by binding to C5 and thus inhibit the terminal complement pathway and have been tested in refractory AchR-positive GMG.100
Surgery for Ptosis and Strabismus
It is generally accepted that treatment of ptosis, ophthalmoplegia, and strabismus in OMG should be through optimal medical treatment initially with either pyridostigmine or steroids/immunosuppressive treatment. However, if the ptosis of strabismus is stable for an extended period (at least two years), then ptosis surgery or strabismus may be appropriate. Many older OMG patients may also have an involutional element of ptosis through levator dehiscence and dermatochalasis and, therefore, may benefit from ptosis surgery. The technique of ptosis repair, just like in other types of ptosis, is chosen based on the levator function, the risk of corneal exposure, and whether Bell’s phenomenon is intact.101 Similarly, strabismus surgery can be performed in a highly selected group of patients if their orthoptic measurements are stable, especially patients with a large angle of deviation that respond poorly to medical treatment.102 Despite maximal immunosuppression, some patients may continue to have resistant ophthalmoplegia. There are anatomical and ultrastructural changes secondary to muscle denervation that may explain the lack of response of extraocular muscles to steroids, which include reduced increased endomysial collagen deposition and the presence of adipocytes replacing atrophic muscle fibers, increased myofiber size variation, myofiber whorling and splitting myofibrillar disarray with Z-band streaming, and subsarcolemmal aggregates of swollen mitochondria.103 This will ultimately contribute to the tipping point in developing treatment-resistant ophthalmoplegia in myasthenia; therefore, strabismus surgery here would probably be a more appropriate treatment.104 Botulinum toxin injection has also been performed in small case series for patients with strabismus for both cosmetic and visual rehabilitative purposes with some success, and the interval between injections varied between 6 and 12 months.105
Thymectomy for OMG
Although the thymus has a crucial role in inducing acetylcholine receptor antibody production, and its removal has shown a clear benefit in GMG, its therapeutic value in patients with OMG is still controversial due to the lack of randomized controlled trials proving its benefit.106 A recent meta-analysis of studies examining the outcome of thymectomy in patients with non-thymomatous OMG concluded that thymectomy might benefit patients’ remission rate of 50%.107 However, studies supporting the clinical benefit of thymectomy for OMG lacked randomization, methodological differences, and definitions of remission and relapse.108–110 A recent non-randomized series found that age below 40 years was the only independent prognostic factor significantly associated with complete remission of OMG in patients who underwent thymectomy.111 In A recent retrospective study to assess the benefit of transcervical thymectomy in OMG, there was no difference in the daily prednisone dose, symptom severity, and risk of conversion to GMG between thymectomy and the non-thymectomy group.112 Therefore, at this point, there is not enough evidence for advocating thymectomy in patients with pure OMG, and it should probably be reserved for patients with GMG or, in some cases, refractory to immunosuppressive therapy.113
In summary, although OMG is a focal form of myasthenia with peculiar clinical, serological, and management aspects, it represents diverse subtypes with distinct underlying pathophysiologic mechanisms and clinical features. Most cases of OMG are treated successfully with pyridostigmine and steroids; however, there are still challenges in the management due to the paucity of evidence-based guidelines. The risks for conversion to GMG include AchR seropositivity and high AchR titer, thymoma or thymic hyperplasia, age over 50, abnormal RNS, and severity of symptoms. More randmozed clinical trials are needed to determine the effect of immunosuppressive treatment and thymectomy on the natural history of the disease and its progression toward GMG. The growing use of ICI in cancer management will increase the prevalence of ICI-related autoimmune disorders, including ICI-related myasthenia. Clinicians need to be aware of this entity since it carries higher morbidity than the predominant form of myasthenia. Finally, reliable biomarkers to assess disease activity and severity are needed to guide treatment.
Acknowledgments
Dr. Ali Behbehani for providing the medical illustration for Figure 1. Patient in Figures 2 and 3 have provided consent for use of the images for educational purposes including publication.
Disclosure
The author reports no financial conflict to disclose.
References
1. Hendricks TM, Bhatti MT, Hodge DO, Chen JJ. Incidence, epidemiology, and transformation of ocular myasthenia gravis: a population-based study. Am J Ophthalmol. 2019;205:99–105. doi:10.1016/j.ajo.2019.04.017
2. Robertson NP, Deans J, Compston DA. Myasthenia gravis: a population based epidemiological study in Cambridgeshire, England. J Neurol Neurosurg Psychiatry. 1998;65(4):492–496. doi:10.1136/jnnp.65.4.492
3. Bever CT
4. Beekman R, Kuks JB, Oosterhuis HJ. Myasthenia gravis: diagnosis and follow-up of 100 consecutive patients. J Neurol. 1997;244(2):112–118. doi:10.1007/s004150050059
5. Grob D, Brunner N, Namba T, Pagala M. Lifetime course of myasthenia gravis. Muscle Nerve. 2008;37(2):141–149. doi:10.1002/mus.20950
6. Zong Y, Jin R. Structural mechanisms of the agrin-LRP4-MuSK signaling pathway in neuromuscular junction differentiation. Cell Mol Life Sci. 2013;70(17):3077–3088. doi:10.1007/s00018-012-1209-9
7. Berrih-Aknin S, Le Panse R. Myasthenia gravis and autoantibodies: pathophysiology of the different subtypes [Myasthenie et auto-anticorps: physiopathologie des differentes entites]. Rev Med Interne. 2014;35(7):413–420. French. doi:10.1016/j.revmed.2013.09.012
8. Ciafaloni E. Myasthenia gravis and congenital myasthenic syndromes. Continuum. 2019;25(6):1767–1784. doi:10.1212/CON.0000000000000800
9. Leprince C, Cohen-Kaminsky S, Berrih-Aknin S, et al. Thymic B cells from myasthenia gravis patients are activated B cells. Phenotypic and functional analysis. J Immunol. 1990;145(7):2115–2122.
10. Hara H, Hayashi K, Ohta K, Itoh N, Nishitani H, Ohta M. Detection and characterization of blocking-type anti-acetylcholine receptor antibodies in sera from patients with myasthenia gravis. Clin Chem. 1993;39(10):2053–2057. doi:10.1093/clinchem/39.10.2053
11. Sahashi K, Engel AG, Linstrom JM, Lambert EH, Lennon VA. Ultrastructural localization of immune complexes (IgG and C3) at the end-plate in experimental autoimmune myasthenia gravis. J Neuropathol Exp Neurol. 1978;37(2):212–223. doi:10.1097/00005072-197803000-00008
12. Niks EH, van Leeuwen Y, Leite MI, et al. Clinical fluctuations in MuSK myasthenia gravis are related to antigen-specific IgG4 instead of IgG1. J Neuroimmunol. 2008;195(1–2):151–156. doi:10.1016/j.jneuroim.2008.01.013
13. Koneczny I, Cossins J, Waters P, Beeson D, Vincent A. MuSK myasthenia gravis IgG4 disrupts the interaction of LRP4 with MuSK but both IgG4 and IgG1-3 can disperse preformed agrin-independent AChR clusters. PLoS One. 2013;8(11):e80695. doi:10.1371/journal.pone.0080695
14. Koneczny I, Stevens JA, De Rosa A, et al. IgG4 autoantibodies against muscle-specific kinase undergo Fab-arm exchange in myasthenia gravis patients. J Autoimmun. 2017;77:104–115. doi:10.1016/j.jaut.2016.11.005
15. Vergoossen DLE, Plomp JJ, Gstottner C, et al. Functional monovalency amplifies the pathogenicity of anti-MuSK IgG4 in myasthenia gravis. Proc Natl Acad Sci U S A. 2021;118(13). doi:10.1073/pnas.2020635118
16. Higuchi O, Hamuro J, Motomura M, Yamanashi Y. Autoantibodies to low-density lipoprotein receptor-related protein 4 in myasthenia gravis. Ann Neurol. 2011;69(2):418–422. doi:10.1002/ana.22312
17. Romi F, Suzuki S, Suzuki N, Petzold A, Plant GT, Gilhus NE. Anti-voltage-gated potassium channel Kv1.4 antibodies in myasthenia gravis. J Neurol. 2012;259(7):1312–1316. doi:10.1007/s00415-011-6344-y
18. Berrih-Aknin S. Cortactin: a new target in autoimmune myositis and myasthenia gravis. Autoimmun Rev. 2014;13(10):1001–1002. doi:10.1016/j.autrev.2014.08.037
19. Zoltowska Katarzyna M, Belaya K, Leite M, Patrick W, Vincent A, Beeson D. Collagen Q--a potential target for autoantibodies in myasthenia gravis. J Neurol Sci. 2015;348(1–2):241–244. doi:10.1016/j.jns.2014.12.015
20. Lisak RP. Antibodies to LRP4 and agrin are pathogenic in myasthenia gravis: at the junction where it happens. Neurology. 2021;97(10):463–464. doi:10.1212/WNL.0000000000012471
21. Soltys J, Gong B, Kaminski HJ, Zhou Y, Kusner LL. Extraocular muscle susceptibility to myasthenia gravis: unique immunological environment? Ann N Y Acad Sci. 2008;1132:220–224. doi:10.1196/annals.1405.037
22. Singman EL, Matta NS, Silbert DI. Use of the Cogan lid twitch to identify myasthenia gravis. J Neuroophthalmol. 2011;31(3):239–240. doi:10.1097/WNO.0b013e3182224b92
23. Gerling J, Meyer JH, Kommerell G. Visual field defects in optic neuritis and anterior ischemic optic neuropathy: distinctive features. Graefes Arch Clin Exp Ophthalmol. 1998;236(3):188–192. doi:10.1007/s004170050062
24. Osher RH, Griggs RC. Orbicularis fatigue: the ‘peek’ sign of myasthenia gravis. Arch Ophthalmol. 1979;97(4):677–679. doi:10.1001/archopht.1979.01020010333009
25. Colavito J, Cooper J, Ciuffreda KJ. Non-ptotic ocular myasthenia gravis: a common presentation of an uncommon disease. Optometry. 2005;76(7):363–375. doi:10.1016/j.optm.2005.05.004
26. Pike-Lee T, Hill J, Li J, Kosmorsky GS, Li Y. Comitant ocular deviation in myasthenia gravis. J Neuroophthalmol. 2021;41(4):e619–e621. doi:10.1097/WNO.0000000000001056
27. Bandini F, Faga D, Simonetti S. Ocular myasthenia mimicking a one-and-a-half syndrome. J Neuroophthalmol. 2001;21(3):210–211. doi:10.1097/00041327-200109000-00010
28. McClard CK, Lyons LJ, Yalamanchili S. Bilateral pseudo-internuclear ophthalmoplegia in a patient with myasthenia gravis. Am J Ophthalmol Case Rep. 2018;12:76–78. doi:10.1016/j.ajoc.2018.09.008
29. Kubis KC, Danesh-Meyer HV, Savino PJ, Sergott RC. The ice test versus the rest test in myasthenia gravis. Ophthalmology. 2000;107(11):1995–1998. doi:10.1016/S0161-6420(00)00458-9
30. Odel JG, Winterkorn JM, Behrens MM. The sleep test for myasthenia gravis. A safe alternative to tensilon. J Clin Neuroophthalmol. 1991;11(4):288–292.
31. Diamond S, Schear HE, Leeds MF. Pseudo-internuclear oculomotor ophthalmoplegia secondary to Guillain-Barre polyneuronitis simulating myasthenia gravis in a air transport pilot. Aviat Space Environ Med. 1975;46(2):204–207.
32. Dirr LY, Donofrio PD, Patton JF, Troost BT. A false-positive edrophonium test in a patient with a brainstem glioma. Neurology. 1989;39(6):865. doi:10.1212/wnl.39.6.865
33. Vincent A, Newsom Davis J. Anti-acetylcholine receptor antibodies. J Neurol Neurosurg Psychiatry. 1980;43(7):590–600. doi:10.1136/jnnp.43.7.590
34. Peeler CE, Lott LB, Nagia L, Lemos J, Eggenberger ER, Cornblath WT. Clinical utility of acetylcholine receptor antibody testing in ocular myasthenia gravis. JAMA Neurol. 2015;72:1170–1174. doi:10.1001/jamaneurol.2015.1444
35. Chung IY, Sheth SJ, Wells KK, Campbell TG. The usefulness of anti-acetylcholine receptor binding antibody testing in diagnosing ocular myasthenia gravis. J Neuroophthalmol. 2020. doi:10.1097/WNO.0000000000001061
36. Rodriguez Cruz PM, Al-Hajjar M, Huda S, et al. Clinical features and diagnostic usefulness of antibodies to clustered acetylcholine receptors in the diagnosis of seronegative myasthenia gravis. JAMA Neurol. 2015;72(6):642–649. doi:10.1001/jamaneurol.2015.0203
37. Monte G, Spagni G, Damato V, Iorio R, Marino M, Evoli A. Acetylcholine receptor antibody positivity rate in ocular myasthenia gravis: a matter of age? J Neurol. 2021;268(5):1803–1807. doi:10.1007/s00415-020-10342-3
38. Ding J, Zhao S, Ren K, et al. Prediction of generalization of ocular myasthenia gravis under immunosuppressive therapy in Northwest China. BMC Neurol. 2020;20(1):238. doi:10.1186/s12883-020-01805-1
39. Behbehani R, Ali A, Al-Moosa A. Ocular myasthenia: clinical course and the diagnostic utility of assaying acetylcholine receptor antibodies. Neuroophthalmology. 2022;46(4):220–226. doi:10.1080/01658107.2022.2037662
40. Soliven BC, Lange DJ, Penn AS, et al. Seronegative myasthenia gravis. Neurology. 1988;38(4):514–517. doi:10.1212/wnl.38.4.514
41. Hoch W, McConville J, Helms S, Newsom-Davis J, Melms A, Vincent A. Auto-antibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. Nat Med. 2001;7(3):365–368. doi:10.1038/85520
42. Bau V, Hanisch F, Hain B, Zierz S. Ocular involvement in MuSK antibody-positive myasthenia gravis [Okulare Beteiligung bei MuSK-Antikorper-positiver Myasthenia gravis]. Klin Monbl Augenheilkd. 2006;223(1):81–83. German. doi:10.1055/s-2005-858629
43. McConville J, Farrugia ME, Beeson D, et al. Detection and characterization of MuSK antibodies in seronegative myasthenia gravis. Ann Neurol. 2004;55(4):580–584. doi:10.1002/ana.20061
44. Bacchi S, Kramer P, Chalk C. Autoantibodies to low-density lipoprotein receptor-related protein 4 in double seronegative myasthenia gravis: a systematic review. Can J Neurol Sci. 2018;45(1):62–67. doi:10.1017/cjn.2017.253
45. Zouvelou V, Zisimopoulou P, Rentzos M, et al. Double seronegative myasthenia gravis with anti-LRP 4 antibodies. Neuromuscul Disord. 2013;23(7):568–570. doi:10.1016/j.nmd.2013.03.013
46. Tsivgoulis G, Dervenoulas G, Kokotis P, et al. Double seronegative myasthenia gravis with low density lipoprotein-4 (LRP4) antibodies presenting with isolated ocular symptoms. J Neurol Sci. 2014;346(1–2):328–330. doi:10.1016/j.jns.2014.09.013
47. Madhavan R, Gong ZL, Ma JJ, Chan AW, Peng HB. The function of cortactin in the clustering of acetylcholine receptors at the vertebrate neuromuscular junction. PLoS One. 2009;4(12):e8478. doi:10.1371/journal.pone.0008478
48. Pinal-Fernandez I, Pak K, Gil-Vila A, et al. Anti-cortactin autoantibodies are associated with key clinical features in adult myositis but are rarely present in juvenile myositis. Arthritis Rheumatol. 2022;74(2):358–364. doi:10.1002/art.41931
49. Cortes-Vicente E, Gallardo E, Martinez MA, et al. Clinical characteristics of patients with double-seronegative myasthenia gravis and antibodies to cortactin. JAMA Neurol. 2016;73(9):1099–1104. doi:10.1001/jamaneurol.2016.2032
50. Stathopoulos P, Kumar A, Nowak RJ, O’Connor KC. Autoantibody-producing plasmablasts after B cell depletion identified in muscle-specific kinase myasthenia gravis. JCI Insight. 2017;2(17). doi:10.1172/jci.insight.94263
51. Tandan R, Hehir MK 2nd, Waheed W, Howard DB. Rituximab treatment of myasthenia gravis: a systematic review. Muscle Nerve. 2017;56(2):185–196. doi:10.1002/mus.25597
52. Costa J, Evangelista T, Conceicao I, de Carvalho M. Repetitive nerve stimulation in myasthenia gravis--relative sensitivity of different muscles. Clin Neurophysiol. 2004;115(12):2776–2782. doi:10.1016/j.clinph.2004.05.024
53. Giannoccaro MP, Di Stasi V, Zanesini C, Donadio V, Avoni P, Liguori R. Sensitivity and specificity of single-fibre EMG in the diagnosis of ocular myasthenia varies accordingly to clinical presentation. J Neurol. 2020;267(3):739–745. doi:10.1007/s00415-019-09631-3
54. Witoonpanich R, Dejthevaporn C, Sriphrapradang A, Pulkes T. Electrophysiological and immunological study in myasthenia gravis: diagnostic sensitivity and correlation. Clin Neurophysiol. 2011;122(9):1873–1877. doi:10.1016/j.clinph.2011.02.026
55. Baruca M, Leonardis L, Podnar S, et al. Single fiber EMG as a prognostic tool in myasthenia gravis. Muscle Nerve. 2016;54(6):1034–1040. doi:10.1002/mus.25174
56. Oh SJ, Ohira M. Single-fiber EMG and clinical correlation in Lambert-Eaton myasthenic syndrome. Muscle Nerve. 2013;47(5):664–667. doi:10.1002/mus.23638
57. Giannoccaro MP, Paolucci M, Zenesini C, et al. Comparison of ice pack test and single-fiber EMG diagnostic accuracy in patients referred for myasthenic ptosis. Neurology. 2020;95(13):e1800–e1806. doi:10.1212/WNL.0000000000010619
58. Qin S, Xu L, Yi M, Yu S, Wu K, Luo S. Novel immune checkpoint targets: moving beyond PD-1 and CTLA-4. Mol Cancer. 2019;18(1):155. doi:10.1186/s12943-019-1091-2
59. Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8(9):1069–1086. doi:10.1158/2159-8290.CD-18-0367
60. Safa H, Johnson DH, Trinh VA, et al. Immune checkpoint inhibitor related myasthenia gravis: single center experience and systematic review of the literature. J Immunother Cancer. 2019;7(1):319. doi:10.1186/s40425-019-0774-y
61. Johansen A, Christensen SJ, Scheie D, Hojgaard JLS, Kondziella D. Neuromuscular adverse events associated with anti-PD-1 monoclonal antibodies: systematic review. Neurology. 2019;92(14):663–674. doi:10.1212/WNL.0000000000007235
62. Becquart O, Lacotte J, Malissart P, et al. Myasthenia gravis induced by immune checkpoint inhibitors. J Immunother. 2019;42(8):309–312. doi:10.1097/CJI.0000000000000278
63. Al-Hashel J, Rashad HM, Rousseff RT. An adult patient with ocular myasthenia and unusually long spontaneous remission. Case Rep Neurol Med. 2014;2014:372769. doi:10.1155/2014/372769
64. So H, Ikeguchi R, Kobayashi M, Suzuki M, Shimizu Y, Kitagawa K. PD-1 inhibitor-associated severe myasthenia gravis with necrotizing myopathy and myocarditis. J Neurol Sci. 2019;399:97–100. doi:10.1016/j.jns.2019.02.023
65. Rubio-Infante N, Ramirez-Flores YA, Castillo EC, Lozano O, Garcia-Rivas G, Torre-Amione G. Cardiotoxicity associated with immune checkpoint inhibitor therapy: a meta-analysis. Eur J Heart Fail. 2021;23(10):1739–1747. doi:10.1002/ejhf.2289
66. Rubio-Infante N, Ramirez-Flores YA, Castillo EC, et al. Review of the mechanisms involved in immune checkpoint inhibitors cardiotoxicity and challenges to improve clinical safety. Front Cell Dev Biol. 2022;10:851032. doi:10.3389/fcell.2022.851032
67. Shi J, Tan Y, Huang Y, et al. Association between clinical factors and result of immune checkpoint inhibitor related myasthenia gravis: a single center experience and systematic review. Front Neurol. 2022;13:858628. doi:10.3389/fneur.2022.858628
68. Sriwastava S, Tandon M, Kataria S, Daimee M, Sultan S. New onset of ocular myasthenia gravis in a patient with COVID-19: a novel case report and literature review. J Neurol. 2020. doi:10.1007/s00415-020-10263-1
69. Restivo DA, Centonze D, Alesina A, Marchese-Ragona R. Myasthenia gravis associated with SARS-CoV-2 infection. Ann Intern Med. 2020;173(12):1027–1028. doi:10.7326/L20-0845
70. Muhammed L, Baheerathan A, Cao M, Leite MI, Viegas S. MuSK antibody-associated myasthenia gravis with SARS-CoV-2 infection: a case report. Ann Intern Med. 2021;174(6):872–873. doi:10.7326/L20-1298
71. Murthy JMK, Gutta AK, Yerasu MR, et al. COVID-19 in patients with myasthenia gravis: mechanisms of respiratory failure. Neurol India. 2021;69(6):1772–1776. doi:10.4103/0028-3886.333460
72. Fanella G, Baiata C, Candeloro E, et al. New-onset myasthenia gravis after mRNA SARS-CoV-2 vaccination: a case series. Neurol Sci. 2022;43(10):5799–5802. doi:10.1007/s10072-022-06284-5
73. Kaulen LD, Doubrovinskaia S, Mooshage C, et al. Neurological autoimmune diseases following vaccinations against SARS-CoV-2: a case series. Eur J Neurol. 2022;29(2):555–563. doi:10.1111/ene.15147
74. Tereshko Y, Gigli GL, Pez S, De Pellegrin A, Valente M. New-onset myasthenia gravis after SARS-CoV-2 infection: case report and literature review. J Neurol. 2022;1–9. doi:10.1007/s00415-022-11472-6
75. Mirmosayyeb O, Moases Ghaffary E, Mazdak M, Bagheri Z, Bagherieh S, Shaygannejad V. Is myasthenia gravis a real complication of the COVID-19 vaccine? A case report-based systematic review. Can J Infect Dis Med Microbiol. 2022;2022:5009450. doi:10.1155/2022/5009450
76. Galassi G, Mazzoli M, Ariatti A, Kaleci S, Valzania F, Nichelli PF. Antibody profile may predict outcome in ocular myasthenia gravis. Acta Neurol Belg. 2018;118(3):435–443. doi:10.1007/s13760-018-0943-7
77. Grob D, Arsura EL, Brunner NG, Namba T. The course of myasthenia gravis and therapies affecting outcome. Ann N Y Acad Sci. 1987;505:472–499. doi:10.1111/j.1749-6632.1987.tb51317.x
78. Nagia L, Lemos J, Abusamra K, Cornblath WT, Eggenberger ER. Prognosis of ocular myasthenia gravis: retrospective multicenter analysis. Ophthalmology. 2015;122(7):1517–1521. doi:10.1016/j.ophtha.2015.03.010
79. Li F, Hotter B, Swierzy M, Ismail M, Meisel A, Ruckert JC. Generalization after ocular onset in myasthenia gravis: a case series in Germany. J Neurol. 2018;265(12):2773–2782. doi:10.1007/s00415-018-9056-8
80. Kupersmith MJ, Latkany R, Homel P. Development of generalized disease at 2 years in patients with ocular myasthenia gravis. Arch Neurol. 2003;60(2):243–248. doi:10.1001/archneur.60.2.243
81. Wong SH, Petrie A, Plant GT. Ocular myasthenia gravis: toward a risk of generalization score and sample size calculation for a randomized controlled trial of disease modification. J Neuroophthalmol. 2016;36(3):252–258. doi:10.1097/WNO.0000000000000350
82. Ruan Z, Sun C, Lang Y, et al. Development and validation of a nomogram for predicting generalization in patients with ocular myasthenia gravis. Front Immunol. 2022;13:895007. doi:10.3389/fimmu.2022.895007
83. Hong YH, Kwon SB, Kim BJ, et al. Prognosis of ocular myasthenia in Korea: a retrospective multicenter analysis of 202 patients. J Neurol Sci. 2008;273(1–2):10–14. doi:10.1016/j.jns.2008.05.023
84. Teo KY, Tow SL, Haaland B, et al. Low conversion rate of ocular to generalized myasthenia gravis in Singapore. Muscle Nerve. 2018;57(5):756–760. doi:10.1002/mus.25983
85. Yu Z, Zhang M, Jing H, et al. Characterization of LRP4/agrin antibodies from a patient with myasthenia gravis. Neurology. 2021;97(10):e975–e987. doi:10.1212/WNL.0000000000012463
86. Meisel A, Baggi F, Behin A, et al. The role of autoantibody levels as biomarkers in the management of patients with myasthenia gravis: a systematic review and expert appraisal. Eur J Neurol. 2022. doi:10.1111/ene.15565
87. Sabre L, Maddison P, Wong SH, et al. miR-30e-5p as predictor of generalization in ocular myasthenia gravis. Ann Clin Transl Neurol. 2019;6(2):243–251. doi:10.1002/acn3.692
88. Sabre L, Punga T, Punga AR. Circulating miRNAs as potential biomarkers in myasthenia gravis: tools for personalized medicine. Front Immunol. 2020;11:213. doi:10.3389/fimmu.2020.00213
89. Cornblath WT. Treatment of ocular myasthenia gravis. Asia Pac J Ophthalmol. 2018;7(4):257–259. doi:10.22608/APO.2018301
90. Kupersmith MJ, Ying G. Ocular motor dysfunction and ptosis in ocular myasthenia gravis: effects of treatment. Br J Ophthalmol. 2005;89(10):1330–1334. doi:10.1136/bjo.2004.063404
91. Kupersmith MJ. Ocular myasthenia gravis: treatment successes and failures in patients with long-term follow-up. J Neurol. 2009;256(8):1314–1320. doi:10.1007/s00415-009-5120-8
92. Benatar M, McDermott MP, Sanders DB, et al. Efficacy of prednisone for the treatment of ocular myasthenia (EPITOME): a randomized, controlled trial. Muscle Nerve. 2016;53(3):363–369. doi:10.1002/mus.24769
93. Sharshar T, Porcher R, Demeret S, et al. Comparison of corticosteroid tapering regimens in myasthenia gravis: a randomized clinical trial. JAMA Neurol. 2021;78(4):426–433. doi:10.1001/jamaneurol.2020.5407
94. Shah YS, Henderson AD, Carey AR. Effect of initial prednisone dosing on ocular myasthenia gravis control. J Neuroophthalmol. 2021;41(4):e622–e626. doi:10.1097/WNO.0000000000001058
95. Heckmann JM, Nel M. A unique subphenotype of myasthenia gravis. Ann N Y Acad Sci. 2018;1412(1):14–20. doi:10.1111/nyas.13471
96. Chan JW. Mycophenolate mofetil for ocular myasthenia. J Neurol. 2008;255(4):510–513. doi:10.1007/s00415-008-0718-9
97. Di Stefano V, Lupica A, Rispoli MG, Di Muzio A, Brighina F, Rodolico C. Rituximab in AChR subtype of myasthenia gravis: systematic review. J Neurol Neurosurg Psychiatry. 2020;91(4):392–395. doi:10.1136/jnnp-2019-322606
98. Menon D, Barnett C, Bril V. Novel treatments in myasthenia gravis. Front Neurol. 2020;11:538. doi:10.3389/fneur.2020.00538
99. Gomez AM, Willcox N, Vrolix K, et al. Proteasome inhibition with bortezomib depletes plasma cells and specific autoantibody production in primary thymic cell cultures from early-onset myasthenia gravis patients. J Immunol. 2014;193(3):1055–1063. doi:10.4049/jimmunol.1301555
100. Howard JF
101. Brogan K, Farrugia ME, Crofts K. Ptosis surgery in patients with myasthenia gravis: a useful adjunct to medical therapy. Semin Ophthalmol. 2018;33(3):429–434. doi:10.1080/08820538.2017.1284871
102. Park KA, Oh SY. Treatment for diplopia in patients with myasthenia gravis. Graefes Arch Clin Exp Ophthalmol. 2013;251(3):895–901. doi:10.1007/s00417-012-2227-x
103. Rautenbach RM, Pillay K, Murray ADN, Heckmann JM. Extraocular muscle findings in myasthenia gravis associated treatment-resistant ophthalmoplegia. J Neuroophthalmol. 2017;37(4):414–417. doi:10.1097/WNO.0000000000000534
104. Europa TA, Nel M, Heckmann JM. A review of the histopathological findings in myasthenia gravis: clues to the pathogenesis of treatment-resistance in extraocular muscles. Neuromuscul Disord. 2019;29(5):381–387. doi:10.1016/j.nmd.2019.03.009
105. Bentley CR, Dawson E, Lee JP. Active management in patients with ocular manifestations of myasthenia gravis. Eye. 2001;15(Pt 1):18–22. doi:10.1038/eye.2001.6
106. Wolfe GI, Kaminski HJ, Aban IB, et al. Randomized trial of thymectomy in myasthenia gravis. N Engl J Med. 2016;375(6):511–522. doi:10.1056/NEJMoa1602489
107. Zhu K, Li J, Huang X, et al. Thymectomy is a beneficial therapy for patients with non-thymomatous ocular myasthenia gravis: a systematic review and meta-analysis. Neurol Sci. 2017;38(10):1753–1760. doi:10.1007/s10072-017-3058-7
108. Schumm F, Wietholter H, Fateh-Moghadam A, Dichgans J. Thymectomy in myasthenia with pure ocular symptoms. J Neurol Neurosurg Psychiatry. 1985;48(4):332–337. doi:10.1136/jnnp.48.4.332
109. Nakamura H, Taniguchi Y, Suzuki Y, et al. Delayed remission after thymectomy for myasthenia gravis of the purely ocular type. J Thorac Cardiovasc Surg. 1996;112(2):371–375. doi:10.1016/S0022-5223(96)70264-7
110. Liu Z, Feng H, Yeung SC, et al. Extended transsternal thymectomy for the treatment of ocular myasthenia gravis. Ann Thorac Surg. 2011;92(6):1993–1999. doi:10.1016/j.athoracsur.2011.08.001
111. Liu X, Zhou W, Hu J, et al. Prognostic predictors of remission in ocular myasthenia after thymectomy. J Thorac Dis. 2020;12(3):422–430. doi:10.21037/jtd.2020.01.17
112. Hamedani AG, Pistilli M, Singhal S, et al. Outcomes after transcervical thymectomy for ocular myasthenia gravis: a retrospective cohort study with inverse probability weighting. J Neuroophthalmol. 2020;40(1):8–14. doi:10.1097/WNO.0000000000000814
113. Wolfe GI, Kaminski HJ, Cutter GR. Randomized trial of thymectomy in myasthenia gravis. N Engl J Med. 2016;375(20):2006–2007. doi:10.1056/NEJMc1611704
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.