Back to Journals » Clinical Ophthalmology » Volume 16
Ocular Graft-versus-Host Disease Underdiagnosis: A Survey Study
Authors Colarusso BA, Bligdon SM, Ganjei AY , Kwok A, Brocks D , Luo ZK
Received 22 January 2022
Accepted for publication 21 April 2022
Published 3 May 2022 Volume 2022:16 Pages 1419—1426
DOI https://doi.org/10.2147/OPTH.S359539
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Bradley A Colarusso,1 Shannon M Bligdon,2 Allen Y Ganjei,3 Alan Kwok,4 Daniel Brocks,4 Zhonghui K Luo1
1Massachusetts Eye and Ear, Boston, MA, USA; 2Ajax Eye Care, Ajax, ON, Canada; 3Drexel University College of Medicine, Philadelphia, PA, USA; 4BostonSight, Needham, MA, USA
Correspondence: Zhonghui K Luo, Massachusetts Eye and Ear, 800 Huntington Avenue, Boston, MA, 02115, USA, Tel +1-617-936-6092, Fax +1-617-936-6186, Email [email protected]
Purpose: To understand the degree and explore the possible causes of ocular graft-versus-host disease (oGVHD) underdiagnosis in patients following allogeneic hematopoietic stem cell transplantation (allo-HSCT).
Patients and Methods: A 15-question survey was emailed to 6032 subscribers to the Blood and Marrow Transplant Information Network. A total of 371 respondents confirmed the history of allo-HSCT, of which 335 were symptomatic. Their self-reported symptoms, onset, treatments tried, degree of symptom control and established diagnoses of systemic chronic graft-versus-host disease (cGVHD) and oGVHD were analyzed.
Results: Among the 335 symptomatic survey respondents, 306 reported their ocular symptom onset was after allo-HSCT, with only 170 [55.6% (170/306)] ever receiving a diagnosis of oGVHD; 23 reported worsening pre-existing ocular symptoms after allo-HSCT, with only 5 [21.7% (5/23)] ever receiving a diagnosis of oGVHD; 6 reported stable symptoms before and after allo-HSCT, with 1 ever receiving a diagnosis of oGVHD. Of the 176 respondents carrying the diagnosis of oGVHD, 167 [94.9% (167/176)] also had the diagnosis of cGVHD. Logistic regression analysis showed that the diagnosis of oGVHD was highly correlated with the number of symptoms and treatments one reported. Furthermore, 35% of the respondents with new onset ocular symptoms reported onset within the first 6 months after allo-HSCT (previously reported), as well as 39% of the respondents with worsened existing symptoms.
Conclusion: oGVHD underdiagnosis is likely associated with the previous diagnostic criteria, in which cGVHD of another organ system was required. The correct notion that oGVHD commonly causes severe dry eye disease has likely led to its underdiagnosis in patients with fewer number of symptoms and/or who tried fewer treatments.
Keywords: oGVHD, cGVHD, dry eye, keratoconjunctivitis sicca, hematopoietic stem cell transplantation
Introduction
Ocular graft-versus-host disease (oGVHD) is an immune-mediated inflammatory process that damages the lacrimal glands, meibomian glands and other accessory glands, leading to severe ocular surface homeostasis disruption.1 It is believed to occur in more than 50% of individuals following allogeneic hematopoietic stem cell transplantation (allo-HSCT) as a curative measure for hematologic malignancies.2,3 All three forms of graft-versus-host disease (GVHD), the acute, chronic and overlap syndrome, can affect the eye. To simplify the subject, we will focus on chronic oGVHD, which is the most common form among ambulatory patients that can occur a few months to decades after allo-HSCT.4–6 oGVHD leaves patients in a state of constant discomfort, therefore exerting a profound effect on quality of life.7,8 Furthermore, it predisposes patients to vision-threatening complications such as corneal ulceration, melt and perforation.9–11
Despite the eyes being the third most involved organ at the time of chronic GVHD (cGVHD) diagnosis,12 oGVHD is often underdiagnosed due to the relatively small population of people who have undergone allo-HSCT. Another cause is the evolving diagnostic criteria over the years. Historically, ocular symptoms and findings were not considered sufficient for the diagnosis of ocular cGVHD unless accompanied by at least one other organ involvement.13 In 2013 the International Chronic Ocular GVHD Consensus Group (ICCGVHD) proposed a set of new scoring schema,14 which was validated in a 2017 report for the diagnosis and severity determination of oGVHD.15 However, the sophisticated 4-part scoring system has not been widely adopted in routine clinical practices. The ICCGVHD also proposed to include dry eye as a concrete diagnostic sign of chronic Graft-versus-Host Disease (cGVHD) without the requirement of involvement in other organ systems, which was adopted by the 2014 NIH consensus criteria and was supported by other studies.16,17 Thus, a combination of new onset ocular symptoms and keratoconjunctivitis sicca confirmed by an ophthalmologist (with Schirmer test) became sufficient to establish the diagnosis of ocular cGVHD. The new criteria allows oGVHD diagnosis to be given to patients who have and only have ocular manifestations. Therefore, the underdiagnosis of oGVHD would lead to the underdiagnosis of cGVHD.
Although there is no Food and Drug Administration (FDA) pharmacologic treatment specifically approved for oGVHD, scleral lenses and prosthetic replacement of the ocular surface ecosystem devices (SL/PD), (PROSE, BostonSight, Needham, MA) are FDA approved for a number of ocular surface diseases including oGVHD.18 However, SL/PDs have been utilized only for severe oGVHD by many referring providers historically. Our recent publication based on the first portion of the survey showed that despite the known benefits, SL/PDs have been greatly underutilized in oGVHD care.19 Through the survey-collected data, we also had the opportunity to look into the potential biases that might have contributed to the underdiagnosis of oGVHD.
Methods
Study Design
The authors created a 15-question survey (Appendix 1: Survey) to characterize the participants’ GVHD history, ocular symptoms, oGVHD diagnosis, prior treatments and history of SL/PD use as previously reported.19 The survey questions asked respondents if they have ever experienced one or more of the nine ocular symptoms including vision changes throughout the day, burning and/or stinging, gritty and/or dry eyes, feeling as if something is stuck in the eyes, eye pain or discomfort, light sensitivity, sensitivity to wind or airflow, eye and/or eyelid redness, or excessive tearing. The respondents were asked to check all symptoms that applied.19 The next set of questions revolved around treatment use to mitigate symptom presentation and severity. Respondents were asked to check all the treatments they have ever tried from a comprehensive list including artificial tears or lubricant drops, artificial tear ointment or lubricant ointment or gel, Restasis or Cequa eye drops, Xiidra eye drops, steroid eye drops, serum tears eye drops, punctal plugs, bandage contact lenses, scleral lenses, Vitamin A ointment, lacriserts, amniotic membrane graft, lid gland exfoliation, oral doxycycline antibiotic pill, or tarsorrhaphy.19 The subjects were guided through the survey by question logic to stay on respective pathways given their personal experience with ocular symptoms and treatments.
Data Collection
As previously reported,19 a link to the study survey (SurveyMonkey, San Mateo, CA) was emailed to 6032 blood and bone marrow transplant patients who were members of the Blood and Marrow Transplant Information Network (BMTinfonet https://www.bmtinfonet.org), a not-for-profit 501(c)3 transplant advocacy organization. The email was sent to the subjects on January 17th, 2021. Those who did not respond received the same email again on January 24th, 2021. Subjects who completed the survey were entered into a drawing, in which the winner received a $200 gift card prize.
Statistical Analysis
Logistic regression analysis was performed using Microsoft Excel and the Statistical Package for the Social Sciences (SPSS) program version 21.0 (IBM Corp. Armonk, New York, NY, USA). The dependent variable is the diagnosis of oGVHD, with YES =1 and No = 0. The independent variables are the number of symptoms one has reported, and the number of treatments tried. Significance was set to a P-value of ≤0.05.
Ethical Approval
The study was exempted from IRB review under category #2(ii) as detailed in 45 CFR 46.104(d) and BRANY’s standard operating procedure, by the BRANY IRB (an independent Institutional Review Board) on 1/19/2021, BRANY IRB File # 21–12-019-784. HIPPA requirements do not apply because the data collected did not include PHI. All procedures and activities were performed in accordance with relevant state and local law.
Results
Of the 6032 emails sent with the attached survey, 391 surveys were returned. Out of this cohort, 20 survey responses were excluded due to incompletion. The remaining 371 responders confirmed they had indeed undergone an allo-HSCT. Among the 371 respondents, 335 checked one or more ocular symptoms on the questionnaire. Thirty-six respondents chose none of the symptoms and were therefore excluded from the analyses. Gritty and/or dry eyes, light sensitivity and burning and/or stinging are the three most reported symptoms as previously reported.19
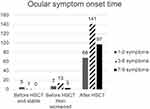
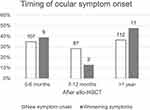
Six of the symptomatic subjects [1.8% (6/335)] reported their ocular symptoms started before the HSCT and remained mild and stable; 23 [6.9% (23/335)] reported symptoms started before the HSCT but worsened after the HSCT, while 306 [91.3% (306/335)] reported symptoms onset was after the HSCT with most of them experiencing at least three symptoms (Figure 1). As previously reported, among the 306 subjects with newly onset ocular symptoms,107 [35.0% (107/306)] reporting onset within 6 months after HSCT; 87 [28.4% (87/306)] within 7 to 12 months, and 112 [36.6% (112/306)] after a year.19 Here we looked further into the 23 individuals who reported worsening of existing ocular symptoms after the allo-HSCT. Nine [(39.1% (9/23)] indicated their symptoms worsened within 0 to 6 months after their transplantation, 3 [13.0% (3/23)] had symptoms that worsened within 7 to 12 months, and 11 [47.8% (11/23)] had symptoms that worsened after a year or more (Figure 2). Thus, consistently over a third of their ocular symptoms started or worsened within 6 months of the allo-HSCT, only slightly less than the number of individuals reporting so more than a year after.
We then looked at the diagnoses of systemic cGVHD and oGVHD in the 335 symptomatic participants, 286 [85.4% (286/335)] of whom had a diagnosis of systemic cGVHD at the time of the survey. One hundred sixty-seven individuals reported having both oGVHD and systemic cGVHD diagnoses; 119 had systemic cGVHD diagnosis only; 9 had oGVHD diagnosis only; 40 had neither (Figure 3). Therefore, a total of 154 [46.0% (154/335)] subjects including 136 with new onset symptoms and 18 with worse ocular symptoms after allo-HSCT never received a diagnosis of oGVHD, despite a high likelihood of meeting the 2014 NIH diagnostic criteria.16
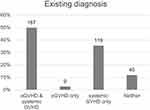 |
Figure 3 The diagnoses of oGVHD and systemic cGVHD reported by the 335 subjects with ocular symptoms. The actual number of subjects is labeled above each bar. |
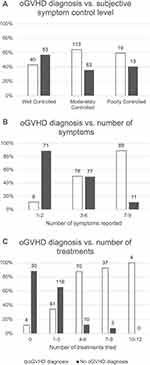
We wanted to understand who among all the symptomatic subjects were more likely diagnosed with oGVHD. At this step of the survey, 301 out of the 335 symptomatic participants answered the question if their symptoms were well, moderately, or poorly controlled (Appendix 1, question 9). Among the 93 [30.9% (93/301)] subjects who reported well controlled symptoms, 40 [43.0% (40/93)] had oGVHD diagnosis and 53 [57.0% (53/93)] did not. One hundred seventy-six subjects reported moderately controlled symptoms, 113 [64.2% (113/176)] of whom had oGVHD diagnosis and 63 [35.8% (63/176)] did not. Among the 32 subjects who reported poorly controlled symptoms, 19 [59.4% (19/32)] had oGVHD diagnosis and 13 [40.6% (13/32)] did not (Figure 4A). Therefore, the oGVHD diagnosis did not correlate with self-reported symptom control level. However, we found that the percentage of subjects with the diagnosis of oGVHD increases steadily with the number of symptoms they have reported (Figure 4B). 88.8% of subjects with 1–2 symptoms did not get a diagnosis of oGVHD while 89.0% of them with 7–9 symptoms did. The median number of symptoms of the 176 subjects diagnosed with oGVHD was 7, whereas that of the 159 subjects without oGVHD diagnosis was 3. The same pattern was observed with the number of treatments (Figure 4C). One hundred and eleven out of the 124 subjects (89.5%) who had tried 4 or more treatments were diagnosed with oGVHD, while only 65 out of the 213 subjects (30.5%) who had tried less than 4 treatments got the diagnosis. Logistic regression analysis showed that the diagnosis of oGVHD was statistically significantly dependent on the number of symptoms (p < 0.001) the individual had reported and/or the treatments they had received (p < 0.001).
Discussion
oGVHD has become a more prominent complication as the number of allo-HSCT increases rapidly world-wide along with the survival rate. However, oGVHD remains underdiagnosed14–16 despite the recognition that early diagnosis of oGVHD is imperative to patient quality of life. Our survey showed that of the 306 subjects whose ocular symptoms occurred after HSCT, 136 [44.4% (136/306)] of them never got a diagnosis of oGVHD. Out of the 23 individuals whose symptom worsened after allo-HSCT, only 5 [21.7% (5/23)] of them got a diagnosis of oGVHD. Although the survey did not have access to their health record, and the self-reported onset time was based on the individual’s memory, it is likely that a number of the subjects without oGVHD diagnosis would have met the 2014 NIH diagnostic criteria.16 It highlights the need to integrate the access to experienced eyecare providers as part of the allo-HSCT planning. Furthermore, based on our clinical experience, there is a real need to increase the awareness of oGVHD among eyecare as well as transplant providers to ensure timely and accurate diagnosis.
We showed via logistic regression analysis that the likelihood of a post-HSCT patient with ocular symptoms to be diagnosed with oGVHD is highly dependent on the number of symptoms and treatments they reported. The median number of symptoms within the no diagnosis cohort was found to be 3 while the diagnosed cohort was 7; the median number of treatments tried were 1 and 4, respectively. It is possible that patients who suffer from more symptoms and/or report more treatments tried are more likely to be seen by a cornea specialist who is more familiar with the oGVHD diagnosis. It is also possible that due to the severe ocular surface impact oGVHD can cause, providers less familiar with oGVHD and its diagnostic criteria would be more comfortable giving the diagnosis to more symptomatic patients. The onset of the symptoms may or may not always be factored in consistently. It is also possible that patients with few symptoms were being managed with over-the-counter self-treatments and not being referred for ophthalmologic care. It is worth repeating that new onset ocular symptoms following allo-HSCT and keratoconjunctivitis sicca confirmed by an ophthalmologist are sufficient to establish the diagnosis of ocular cGVHD per the 2014 NIH consensus criteria.16 Therefore, new onset ocular symptoms alone should raise the clinical suspicion in general providers (including but not limited to transplant center providers, primary care providers, optometrists and general ophthalmologists) to expedite referral to a cornea specialist. In our first survey paper we discussed the need to increase the awareness of SL/PD as an effective treatment option for oGVHD. It could not be achieved without raising the awareness of oGVHD first. Furthermore, the diagnosis of oGVHD is no longer dependent on systemic cGVHD.16 Practitioners should be empowered to make the diagnosis in the absence of another organ involvement.4
Our survey respondents revealed over 35% of them had new onset ocular symptoms or worsening existing symptoms in the first 6 months following allo-HSCT (Figure 2). This is a great divergence from previous notions that chronic oGVHD began at 6 months or later following allo-HSCT.17,20 In fact, it has been increasingly recognized that chronic oGVHD can be seen much less than 6 months after allo-HSCT.21 Our own clinical observations have suggested so as well. This calls for reexamination of the assumed pattern of oGVHD onset and progression, in order for earlier recognition and intervention.
Six subjects reported their ocular symptoms started before allo-HSCT and stayed stable after. It has been strongly advocated that a pre-HSCT comprehensive eye exam should be performed on patients scheduled to undergo allo-HSCT14,21–23 because such practice is necessary to separate preexisting ocular surface diseases from new onset or change in severity following HSCT. In fact, 1 of those 6 subjects did get a diagnosis of oGVHD, which is possibly prompted by the systemic cGVHD he/she had.
We clearly recognize the bias in this study, which was previously discussed.19 The method for conducting this survey involved an email, which prompted individuals to decide if they wished to respond. This establishes an inherent bias as those who respond are more likely to have ocular issues. We recognized the data depended heavily on the participants’ recollection, therefore was less accurate compared to a retrospective review of health records. However, it served as a channel for the participants to report their subjective symptoms onset and development, which created a unique perspective. In the survey, when we inquired patient’s GVHD history (ocular and systemic), we did not provide a choice between acute vs chronic forms to minimize confusion. Furthermore, as the survey was only conducted in a virtual format, only individuals with access to computers and the ability to navigate themselves through the survey were able to complete it. Therefore, individuals with less severe symptoms, no symptoms at all, lacking computer access, or lacking the knowledge to navigate themselves through the survey were less likely to fill out the survey. On the other hand, socioeconomic status often correlates with race and non-Caucasian racial status is a known risk factor associated with the onset of oGVHD which could be a missing population from the presented data which could have increased the presence of underdiagnosed oGVHD.24
Conclusion
Despite its limitations, our survey analysis provided a channel for symptomatic patients to describe their experience, albeit in simplified forms. It peeked into the possible factors leading to oGVHD underdiagnosis and suggested a significant fraction of patients may have disease onset less than 6 months after allo-HSCT. oGVHD is a formidable complication that diminishes the quality of life of allo-HSCT survivors. The search for curative or highly effective symptomatic treatment is on. We hope to raise the awareness of oGVHD in patients as well as providers. We hope to point direction for future studies to establish better and earlier diagnosis and treatment.
Data Sharing Statement
The authors confirm that the data supporting the findings of the study are available with the article. Any request of the data should be sent to the corresponding author.
Acknowledgments
The authors acknowledge the support of BMTInfonet (bmtinfonet.org) in disseminating the survey.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. It was supported by the authors’ sundry funds and other institutional resources.
Disclosure
A. Kwok and D. Brocks are salaried employees of BostonSight, Needham, MA, where BostonSight PROSE treatment and BostonSight SCLERAL lenses were developed. None of the authors have a financial or any other conflict of interest in this work.
References
1. Ogawa Y, Kawakami Y, Tsubota K. Cascade of inflammatory, fibrotic processes, and stress-induced senescence in chronic GVHD-related dry eye disease. Int J Mol Sci. 2021;22:11. doi:10.3390/ijms22116114
2. Munir SZ, Aylward J. A review of ocular Graft-versus-Host Disease. Optom Vis Sci. 2017;94(5):11. doi:10.1097/OPX.0000000000001071
3. Nassiri N, Eslani M, Panahi N, Mehravaran S, Ziaei A, Djalilian AR. Ocular graft versus host disease following allogeneic stem cell transplantation: a review of current knowledge and recommendations. J Ophthalmic Vis Res. 2013;8(4):8.
4. Shikari H, Antin JH, Dana R. Ocular Graft-versus-Host Disease: a review. Surv Ophthalmol. 2013;58(3):233–251. doi:10.1016/j.survophthal.2012.08.004
5. Hirst LW, Jabs DA, Tutschka PJ, Green WR, Santos GW. The eye in bone marrow transplantation I. Clin Study. 1983;101(4):5.
6. Carreno-Galeano JT, Dohlman TH, Kim S, Yin J, Dana R. A review of ocular Graft-versus-Host Disease: pathophysiology, clinical presentation and management. Ocul Immunol Inflamm. 2021:29;1–10.
7. Inamoto Y, Valdes-Sanz N, Ogawa Y, et al. Ocular Graft-versus-Host Disease after hematopoietic cell transplantation: expert review from the late effects and quality of life working committee of the CIBMTR and transplant complications working party of the EBMT. Bone Marrow Transplant. 2019;54(5):662–673. doi:10.1038/s41409-018-0340-0
8. Sun YC, Chai X, Inamoto Y, et al. Impact of ocular chronic Graft-versus-Host Disease on quality of life. Biol Blood Marrow Transplant. 2015;21(9):1687–1691. doi:10.1016/j.bbmt.2015.05.020
9. Groth JB, Conto J, Pasquini M. Chronic ocular graft versus host disease: an update and review. J Dry Eye Ocul Surface Dis. 2020;3(1):21.
10. Adrean SD, Puklin JE. Perforated corneal ulcer with subsequent endophthalmitis in a patient with Graft-versus-Host Disease. Cornea. 2006;26(1):2.
11. Inagaki E, Ogawa Y, Matsumoto Y, Kawakita T, Shimmura S, Tsubota K. Four cases of corneal perforation in patients with chronic Graft-versus-Host Disease. Mol Vis. 2011;17:9.
12. Arai S, Jagasia M, Storer B, et al. Global and organ-specific chronic Graft-versus-Host Disease severity according to the 2005 NIH Consensus Criteria. Blood. 2011;118(15):4242–4249. doi:10.1182/blood-2011-03-344390
13. Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic Graft-versus-Host Disease: I. Diagnosis and staging working group report Biol Blood Marrow Transpl. 2005;11(12):945–956. doi:10.1016/j.bbmt.2005.09.004
14. Ogawa Y, Kim SK, Dana R, et al. International Chronic Ocular Graft-vs-Host-Disease (GVHD) Consensus Group: proposed diagnostic criteria for chronic GVHD (Part I). Sci Rep. 2013;3:3419. doi:10.1038/srep03419
15. Rapoport Y, Freeman T, Koyama T, et al. Validation of International Chronic Ocular Graft-Versus-Host Disease (GVHD) group diagnostic criteria as a chronic ocular GVHD-specific metric. Cornea. 2017;36(2):258–263. doi:10.1097/ICO.0000000000001109
16. Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic Graft-versus-Host Disease: I. the 2014 diagnosis and staging working group report. Biol Blood Marrow Transplant. 2015;21(3):389–401.e1. doi:10.1016/j.bbmt.2014.12.001
17. Shikari H, Amparo F, Saboo U, Dana R. Onset of ocular Graft-versus-Host Disease symptoms after allogeneic hematopoietic stem cell transplantation. Cornea. 2015;34(3):243–247. doi:10.1097/ICO.0000000000000340
18. Takahide K, Parker PM, Wu M, et al. Use of fluid-ventilated, gas-permeable scleral lens for management of severe keratoconjunctivitis sicca secondary to chronic Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2007;13:6. doi:10.1016/j.bbmt.2007.05.006
19. Bligdon SM, Colarusso BA, Ganjei AY, Kwok A, Luo ZK, Brocks D. Scleral lens and prosthetic replacement of the ocular surface ecosystem utilization in ocular Graft-versus-Host Disease: a survey study. Clin Ophthalmol. 2021;15:4829–4838. doi:10.2147/OPTH.S337824
20. Ogawa Y, Okamoto S, Wakui M, et al. Dry eye after haematopoietic stem cell transplantation. Br J Ophthalmol. 1999;83(10):1125–1130. doi:10.1136/bjo.83.10.1125
21. Giannaccare G, Bonifazi F, Sessa M, et al. Ocular surface analysis in hematological patients before and after allogeneic hematopoietic stem cell transplantation: implication for daily clinical practice. Eye. 2017;31(10):1417–1426. doi:10.1038/eye.2017.78
22. Kitko CL, Pidala J, Schoemans HM, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: Iia. The 2020 clinical implementation and early diagnosis working group report. Transplant Cell Ther. 2021;27(7):545–557. doi:10.1016/j.jtct.2021.03.033
23. Wolff D, Radojcic V, Lafyatis R, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IV The 2020 Highly morbid forms report. Transpl Cell Ther. 2021;27(10):817–835.
24. Wang JC, Teichman JC, Mustafa M, O’Donnell H, Broady R, Yeung SN. Risk factors for the development of ocular Graft-versus-Host Disease (GVHD) dry eye syndrome in patients with chronic GVHD. Br J Ophthalmol. 2015;99(11):1514–1518. doi:10.1136/bjophthalmol-2014-306438
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.