Back to Journals » Clinical Optometry » Volume 14
Ocular Dimensions, Refractive Error, and Body Stature in Young Chinese Children with Myopia in Kuala Lumpur, Malaysia
Authors Mohd-Ali B , Low YC , Shahimin MM , Arif N, Abdul Hamid H, Wan Abdul Halim WH , Mokri SS, Baseri Huddin A, Mohidin N
Received 30 March 2022
Accepted for publication 4 July 2022
Published 22 July 2022 Volume 2022:14 Pages 101—110
DOI https://doi.org/10.2147/OPTO.S368672
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Mr Simon Berry
Bariah Mohd-Ali,1 Yu Chen Low,1 Mizhanim Mohamad Shahimin,1 Norlaili Arif,1 Hamzaini Abdul Hamid,2 Wan Haslina Wan Abdul Halim,3 Siti Salasiah Mokri,4 Aqilah Baseri Huddin,4 Norhani Mohidin5
1Optometry and Vision Science Program, Research Centre for Community Health, Faculty of Health Science, Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia; 2Department of Radiology, Faculty of Medicine, Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia; 3Department of Ophthalmology, Faculty of Medicine, Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia; 4Department of Electrical, Electronics and Systems Engineering, Faculty of Engineering and Built Environment, Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia; 5Optometry Centre, Faculty of Health Science, Universiti Teknologi Mara, Kuala Lumpur, Malaysia
Correspondence: Bariah Mohd-Ali, Optometry and Vision Science Program & Research Centre for Community Health (REACH), Faculty of Health Science, Universiti Kebangsaan Malaysia, Kuala Lumpur, 50300, Malaysia, Tel +6019-3296835, Email [email protected]
Purpose: Eyeball shape varies with refraction and body stature. Nevertheless, there are few reports on three-dimensional measurements of eyeball shape in children. The aim of this cross-sectional observational study was to investigate the associations between three-dimensional measurements of ocular dimensions, refractive error, and body stature in young Chinese children with myopia in Kuala Lumpur.
Materials and Methods: Thirty-five female and 35 male school children aged 8– 9 years old were recruited in this study. Cycloplegic spherical equivalent (SE) and visual acuity (VA) were determined using a logarithm of the minimum angle of resolution (logMAR) chart. Body mass index (BMI), body height, and head circumference were ascertained. Three ocular dimensions, that is, longitudinal axial length (LAL), horizontal width (HW), and vertical height (VH), were determined using magnetic resonance imaging (MRI).
Results: There were significant differences among the ocular dimensions in the myopic children. Bonferroni-corrected pairwise t-tests showed that LAL was significantly longer (mean difference, 0.318 mm) than VH, which was in turn significantly longer (mean difference, 0.245 mm) than HW. Body height was significantly correlated with LAL (p < 0.001) and SE (p < 0.001), and multivariate linear regression confirmed that longer LAL and more myopic SE were associated with increased body height (p < 0.001 for both) but not BMI (p = 0.894 and p = 0.413) or head circumference (p = 0.305 and p = 0.226).
Conclusion: This study confirms previous reports that changes in ocular dimensions are associated with body height in young children. Axial elongation (forming a prolate profile) occurs in myopic children of both genders at a young age.
Keywords: height, BMI, ocular shape, MRI analysis, axial length
Introduction
The refractive status of the eye is determined by the balance of the corneal and crystalline lens refractive power and the longitudinal axial length (LAL) of the eye. Elongation of LAL and change in refraction happen at the same time as the increase in body height during puberty, and an emmetropization mechanism takes place to prevent the development of ametropia.1,2 Despite this, LAL continues to elongate in some children, producing myopia. Myopia is the most common ocular anomaly worldwide. It has the highest prevalence in East Asian countries, including China, Japan, and Singapore.3,4 Estimates show that half of the world’s population will have myopia by 2050, and 10% of these cases are expected to involve high myopia.5 High myopia is associated with serious and potentially blinding ocular diseases such as cataract, retinal detachment, glaucoma, and maculopathy.6 The associations of LAL and refraction with anthropometric measurements have been investigated by several research groups. Dirani et al investigated the relationship between body stature and myopia using data from an Australian twin cohort study and found that females in the heaviest weight quartile were at greater risk of myopia than those in the lightest quartile.7 Kearney et al investigated the change in body height, LAL, and refractive status over a 4-year period in 140 Caucasian children and young adults.8 Their results showed that body growth and LAL elongation were correlated in emmetropes. However, in subjects with myopia, body growth appeared to stabilize whilst LAL elongation accelerated, indicating dysregulation of normal ocular growth. Nevertheless, in a cross-sectional study of 4681 male Danish school students ages 8–13 years, no relationship was found between myopia and BMI or height, but number of years in education and IQ test score were related to myopia.9 Similarly, Huang et al investigated the associations of lifestyle and body growth with LAL elongation among Taiwanese elementary school children aged 7–9 years and they showed that although LAL change was positively correlated with the body height change (p<0.001) the refractive changes were not correlated to body height changed (p=0.640). In their study, refractive changes were only significant in children who were engaged in a lot of near work (p<0.01). The study further concluded that while genetic factors such as parental myopia and body height were associated to myopia development, environment factor such as near work intensity was related to myopia progression.10 Using computerized tomography data, the relationships between eye shape, weight, age, and refraction in Koreans aged <20 years were analyzed.11 The results showed a strong correlation between the LAL and horizontal width (HW) of the eye, and both parameters increased with age and body weight.
The ocular shape has been reported to be associated with refractive development and has become an important area in refractive research.12 Magnetic resonance imaging (MRI) allows us to directly measure the entire ocular globe in three dimensions. Atchison et al13 studied ocular dimensions in 88 young adults aged 18–36 years, based on MRI, and reported that myopic eyes were elongated more in the LAL dimension, and that myopic eyes fitted the global expansion and axial elongation model. However, there is a limited number of MRI studies on ocular dimensions in children. Lim et al14 studied the variations in eye volume and shape with refractive error in young Singaporean children using MRI and showed that increased myopic spherical equivalent (SE) was associated with greater LAL and HW, but not VH.
No studies have investigated ocular dimensions in Malaysian children using MRI. As myopia onset typically occurs in school-aged children, it is particularly informative to find out if eye growth is correlated with body growth in early childhood. This study aimed to determine the associations among ocular dimensions (longitudinal axial length [LAL], vertical height [VH], and horizontal width [HW], measured using MRI), spherical equivalent [SE], corneal curvature, and body stature (body height, body mass index [BMI], and head circumference) in Chinese children with myopia in Kuala Lumpur, Malaysia.
Materials and Methods
Subjects and Ethics Approval
Primary school-aged Chinese children (age 8 to 9 years old) from around the Kuala Lumpur area, who attended a primary eye care clinic for regular eye checkups, were recruited for this cross-sectional study. Eligible subjects were selected by using simple random sampling. This research was approved by the Universiti Kebangsaan Malaysia research ethics committee (UKM PPI-800-1/1/5 JEP-2017-422) and followed the tenets of the Declaration of Helsinki. The nature of the research procedures was explained to the parents and subjects, and written consent was obtained from the parents prior to data collection.
The inclusion criteria were as follows: spherical equivalent (SE) between −0.50 D15 and −4.00 D, astigmatism of no more than 1.50 D, a best corrected visual acuity (BCVA) of 0.0 logarithm of the minimum angle of resolution (logMAR) or better in both eyes, no anisometropia, no history of ocular or systemic diseases, no binocular vision anomalies, and not undergoing any myopia treatment. Children with contraindications for MRI (for example, metallic implants, braces, pacemakers, or claustrophobia) were excluded.
Eye Examination
Cycloplegic refraction was determined using subjective refraction. Two drops of cyclopentolate (1%) were instilled within a 5-min interval and refraction was measured when the pupil size was >5 mm. Visual acuity (VA) was determined using a logMAR chart and the anterior segment of the eye was examined using a slit-lamp biomicroscope (Righton MW50D LED; Tokyo, Japan).
MRI Acquisition
Measurements were taken using MRI, by an experienced pediatric radiologist and radiographer at the Radiology Department of Hospital Canselor Tuanku Mukhriz (Kuala Lumpur). Each subject was scanned with a whole-body MRI scanner (3-Tesla Trio; Siemens, Erlangen, Germany). T2-weighted scans were performed with the following parameters: (1) 176 sagittal slices; 516 pixels; 512 matrices of 1-mm thickness with no gap (field of view [FOV], 250×250 mm; repetition time [TR], 3200 ms; echo time [TE], 409 ms; flip angle, 120) and (2) 60 axial slices; 381 pixels×384 matrices of 0.8-mm thickness with no gap (FOV, 199×199 mm; TR, 1270 ms; TE, 132 ms; flip angle, 120). To provide a clear image and high-contrast delineation of the edges of the eye, subjects were asked to lie still in a supine position and keep their eyes closed so that less motion would occur throughout the fast image acquisition process. The subjects were provided with earplugs and headphones to minimize the noise during the procedure and the whole duration of the procedure was around 10 min per eyeball.
MRI Segmentation
The MRI segmentation method was based on the Chan-Vese model.16 The advantage of this method is its efficiency to segment regions of complex curves through numerical calculations and solve problems related to angle production and curvature.17,18 Initially, the sclera of the eyes, as seen from the axial view, was segmented. By browsing through the three-dimensional MRI ocular images, an axial slice was selected, and a rectangle was defined as the initial curve (Figure 1).
 |
Figure 1 Yellow rectangle- Initial selection of both eyeballs on axial view; Red line- Defining the outline of the selected eyeball shape. |
A commercially available graphics program (OsiriX DICOM viewer; www.osirix-viewer.com) was used to display and magnify the images. The axial image of the eye was displayed at 50X magnification on a computer screen, and the contrast was adjusted until the edges of interest were clearly defined. At this point, the left and right eyes were segmented by manually defining the initial curves for each eye as an initialization of the Chan-Vese level set segmentation method. Thereafter, a sagittal view of each eyeball was displayed for segmentation. Again, a rectangular curve was defined that covered the eyeball before it was guided to track the edge using the Chan-Vese level set method (Figure 2).
 |
Figure 2 Yellow rectangle- Initial segmentation of the Right eyeball on sagittal view; Red line- Defining the outline of the selected eyeball shape. |
Following these results, the pertinent lines to be used to perform the assessment of the eyeball shape were automatically measured and analyzed. The details of the MRI segmentation method were reported previously.17 A summary of the algorithm for the segmentation and measurement of LAL, VH, and HW based on the MRI images is shown in Figure 3.
 |
Figure 3 Summary of algorithm for segmentation and measurement of the MRI images. |
Validation of MRI Measurements
To validate the MRI measurements, the LAL of all subjects was also measured using an ultrasound A-Scan (PacScan Plus; Sonomed Escalon, New Hyde Park, NY, USA), and the results were compared using Pearson’s correlation analysis. The LAL measured using the A-Scan was highly correlated (r2=0.950, p<0.001) with the LAL measured using MRI. The mean LAL based on MRI was 23.72±0.82 mm, compared to 23.54±0.55 mm based on ultrasound A-Scan, which is very similar. The corresponding scatter plot with a fitted regression line is shown in Figure 4.
 |
Figure 4 Correlation between LAL measured using ultrasound A-Scan and MRI. Abbreviation: LAL, longitudinal axial length. |
Body Measurements
Body height, body weight, and head circumference were measured for all subjects. Body height was determined to the nearest 0.5 cm in a standardized manner without shoes and with a wall-mounted measuring tape. Body weight was measured to the nearest 0.1 kg using a digital step-on scale (Novoscale CB501; Novoplus, Penang, Malaysia). Body mass index (BMI; kg/m2) was calculated based on weight (kg) and height (m). Head circumference was measured to the nearest 0.1 cm by passing the measuring tape around the subject’s head and placing it on the most anterior protuberance of the forehead and the most posterior protuberance of the back of the head to measure the maximum head circumference.
Statistical Analysis
The data were analyzed using SPSS version 21.0 (IBM Corp., Armonk, NY, USA) and only data from the right eye were used for all analyses. Data normality was assessed using the Shapiro–Wilk test and all data were normally distributed. Age, height, body weight, head circumference, BMI, SE, visual acuity, corneal curvature, LAL, VH, and HW were described using descriptive statistics.
Repeated-measures analysis of variance (ANOVA) was used to assess the differences among the ocular dimensions (LAL, VH, and HW). This was followed by Bonferroni-corrected t-tests, to compare the pairwise differences in LAL, VH, and HW.
Pearson’s correlation analysis was used to determine the correlations among ocular dimensions, SE, and body stature (body height, BMI, and head circumference).
Multivariate linear regression was used to assess the increase in ocular dimensions, SE (refractive error), and corneal curvature with increasing body height, BMI, and head circumference (all three were included in each model). The differences were considered statistically significant when p<0.05.
Results
Of the 80 children that underwent MRI scans, 70 (87.5%) children (35 females and 35 males; mean age, 8 years; range, 8–9 years) were included in the analyses. Ten (12.5%) children were excluded due to having blurred MRI images. Mean SE was −2.77±1.10 D (range, −0.75 to −4.59 DS), mean BCVA was −0.01±0.07, and mean corneal curvature was 43.43±1.21 D. Mean body height was 125.24±7.54 cm, mean body weight was 26.67±5.74 kg, and mean head circumference was 50.46 ±0.94 cm. Mean BMI was 16.95±2.89 kg/m2. No significant differences were observed in age (p=0.393), body height (p=0.167), body weight (p=0.295), head circumference (p=0.830), BMI (p=0.132), SE (0.241), visual acuity (0.181), or corneal curvature (0.362) between genders. Regarding the mean ocular dimensions, LAL was 23.72±0.82 mm, VH was 23.40±0.82 mm, and HW was 23.16±0.80 mm. No significant differences were noted in ocular dimensions between genders (p>0.05). The demographic characteristics of the subjects (by gender) are shown in Table 1.
 |
Table 1 Demographic Characteristics of Subjects |
Repeated-measures ANOVA showed that there was a significant difference in the ocular dimensions in the myopic children. LAL was the largest ocular dimension (23.72±0.82 mm), followed by VH (23.4±0.73 mm), and HW (23.16±0.8 mm) (p<0.001 for all). Bonferroni-corrected pairwise t-tests confirmed that LAL was significantly longer (mean difference, 0.318 mm) than VH, which was in turn significantly longer (mean difference, 0.245 mm) than HW (Table 2).
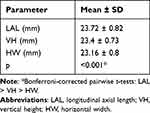 |
Table 2 Summary of Ocular Dimensions in Myopic Children |
Pearson’s correlation analysis was used to determine the associations of ocular dimensions, SE, and corneal curvature with body stature. LAL (r2=0.497, p<0.001) and SE (r2=−0.597, p<0.001) were significantly positively and negatively correlated, respectively, with body height, but they were not correlated with BMI or head circumference (Figures 5 and 6). Multivariate linear regression confirmed that increased body height was associated with longer LAL (R2=0.260, p<0.001) and a more negative SE (R2=0.349, p<0.001). For every 1-cm increase in body height, there was a 0.056 mm increase in LAL (p<0.001) and a −0.081 D increase in SE (leading to a more negative SE) (p<0.001) (Table 3).
 |
Table 3 Multivariate Linear Regression Analyses of the Associations of Body Stature Variables with Ocular Dimensions, Corneal Curvature, and SE |
 |
Figure 5 Correlation between body height and LAL. Abbreviation: LAL, longitudinal axial length. |
 |
Figure 6 Correlation between body height and refractive error. Abbreviation: SE, spherical equivalent. |
Discussion
There were significant differences among the ocular dimensions in the myopic children in this study. The longest ocular dimension was LAL, followed by VH and HW. This indicates that myopia involves a prolate shape, which is consistent with previous studies.13,14
Atchison et al compared the shape of the retinal surface in 21 emmetropic and 66 myopic eyes in subjects aged 18–36 years.13 They found that almost all emmetropic eyes are oblate in shape, with the axial dimensions being smaller than the vertical and horizontal dimensions. As myopia increased, all ocular dimensions increased, with the axial dimension increasing more than the vertical or horizontal dimensions, indicating a decrease in oblate profile. Our results agree with this observation. In our myopic children, the growth of the ocular dimensions had occurred to different degrees; LAL was the largest dimension, followed by VH and then HW, leading to a prolate shape. Lim et al evaluated three-dimensional variations in the eye dimensions and shapes of 134 eyes from 67 young Singaporean Chinese boys (mean age, 77.9±3.9 months) enrolled in the population-based Strabismus, Amblyopia, and Refractive error study.14 Their results showed that refractive error was significantly associated with eye surface area, SE was correlated with LAL (p<0.001) and HW (p<0.001) but not VH (p=0.70).
Previous reports have shown an association between LAL and body height in children.19–21 Similarly, we found that each centimeter increase in body height was associated with a longer LAL (0.056 mm) and a more negative SE (−0.081 D), with an adjusted R2 of 0.226 and an R2 of 0.319. In a longitudinal study in China, Wang et al found a positive correlation between body height and LAL in school children aged 7–15 years.19 However, detailed information about the association was unavailable. In a school-based study of 1449 Chinese children aged 7–9 years in Singapore, Saw et al20 showed that taller children were more likely to have eyes with longer LAL (+0.46 mm, p<0.01) and refraction that trended towards myopia (−0.47 D, p<0.01).
Tideman et al determined the correlations of MRI-derived ocular height, width, and posterior segment length with refractive error, birth weight, and body height in European children in the population-based birth cohort study Generation R.21 Their results showed that refractive error had the highest correlation with posterior segment length (p<0.001), whereas the correlation with body height was highest for ocular height (p<0.01) and the correlation with birth weight was highest for ocular width (p<0.01). Similarly, in the present study of children with myopia, LAL was longer than VH and HW (p<0.001). However, when comparing the associations between ocular dimensions and body stature, VH and HW showed no association with body height, BMI, or head circumference in our study. The discrepancies in the results may be due to differences in ethnicity and the SE in their study being lower than in our study.
Positive associations of anthropometric indicators (height, weight, and BMI) with both refraction and ocular biometrics in Chinese school children have been reported in Tianjin, China. Ye et al22 showed that higher body heights and heavier weights were associated with longer LAL and more negative refraction. The authors concluded that a shared mechanism may regulate the coordinated growth of body and eye size in children, and our results concurred with this hypothesis. Our results showed a significant association of LAL with body height but not BMI. This was probably due to the small sample size and narrow age range of the subjects. Although the exact underlying biological mechanism remains unknown, studies have reported that some systemic hormones can regulate longitudinal bone growth factors during childhood which are also involved in the development of myopia.22,23 Earlier epidemiological studies have also reported that children with growth hormone deficiency have shorter body stature and LAL than usual.24 Therefore, physical growth and the development of refractive error may be regulated by similar mechanisms.
It is evident that LAL plays a role in myopia development, with several studies reporting that the prolate ocular shape is associated with myopia progression.12–15,25 Due to the prolate shape, peripheral light rays are progressively focused behind the central retina. The prolate shape induces a relative hyperopic defocus in the peripheral refraction compared to the central fovea, making it a potential trigger for myopia progression. Nevertheless, although AL increases at the same time as body height in children, studies have proven that those with a high genetic risk in addition to a high level of education and high near work intensity have a greater risk of myopia progression.9,10 A large European study, has revealed a significant relationship between higher education level and greater prevalence of myopia.26 Other studies have reported that recent indoor confinement and prolonged home- based online classes due to coronavirus disease 2019 appeared to be associated with a substantial myopic progression in children.27 When comparing the prevalence of myopia in children to the previous years, the prevalence of myopia in 2020 is higher than in between 2015 to 2019 for children between the age of 5 to 8 years old.28 Increased in myopia progression and axial length elongation was associated with significant decrease in outdoor time and increase in screen time among schoolchildren in Hong Kong during the COVID-19 pandemic.29
A potential limitation of this study should be mentioned. The most significant limitation of this study is the small number of participants. A larger sample size followed for a long period would elicit more variability and allow factors such as age and sex to be explored in more detail with respect to the observed association between the changes in ocular dimensions and body growth during myopia progression. Further studies with a larger sample size and longer follow-up period are necessary to confirm the results.
Conclusion
The results of this study showed that one of the ocular dimensions (LAL) and refractive error are associated with body height in myopic children. Myopic eyes tend to elongate more in the axial dimension, and the correlation between LAL and body height was positive. This indicates that LAL elongation needs to be monitored in children undergoing rapid body height growth spurts. To the best of our knowledge, this is the first study that investigated the relationship between body growth and ocular dimensions using MRI measurements.
Acknowledgments
This study was funded by Menicon Pty Ltd (NN-2017-180).
Disclosure
Prof. Dr. Bariah Mohd-Ali reports grants from Menicon Ltd, Japan, during the conduct of the study. Dr Mizhanim Mohamad Shahimin reports grants from Menicon Co., Ltd., during the conduct of the study. In addition, Dr Mizhanim Mohamad Shahimin has a patent ORTHOKERATOLOGY (ORTHO-K) Instruction For Wearers licensed to Bariah Mohd Ali. The authors report no other conflicts of interest in this work.
References
1. Yip VC, Pan CW, Lin XY, et al. The relationship between growth spurts and myopia in Singapore children. Invest Ophthalmol Vis Sci. 2012;53(13):7961–7966. doi:10.1167/iovs.12-10402
2. Goss DA, Cox VD, Herrin-Lawson GA, Nielsen ED, Dolton WA. Refractive error, axial length, and height as a function of age in young myopes. Optom Vis Sci. 1990;67(5):332–338. doi:10.1097/00006324-199005000-00006
3. Morgan IG, French AN, Ashby RS, et al. The epidemics of myopia: aetiology and prevention. Prog Retin Eye Res. 2018;62:134–149. doi:10.1016/j.preteyeres.2017.09.004
4. Pan CW, Dirani M, Cheng CY, Wong TY, Saw SM. The age-specific prevalence of myopia in Asia: a meta-analysis. Optom Vis Sci. 2015;92(3):258–266. doi:10.1097/OPX.0000000000000516
5. Holden BA, Fricke TR, Wilson DA, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123(5):1036–1042. doi:10.1016/j.ophtha.2016.01.006
6. Chen SJ, Lu P, Zhang WF, Lu JH. High myopia as a risk factor in primary open angle glaucoma. Int J Ophthalmol. 2012;5(6):750–753. doi:10.3980/j.issn.2222-3959.2012.06.18
7. Dirani M, Islam A, Baird PN. Body stature and myopia-The Genes in Myopia (GEM) twin study. Ophthalmic Epidemiol. 2008;15(3):135–139. doi:10.1080/09286580801957751
8. Kearney S, Strang NC, Cagnolati B, Gray LS. Change in body height, axial length and refractive status over a four-year period in Caucasian children and young adults. J Optom. 2020;13(2):128–136. doi:10.1016/j.optom.2019.12.008
9. Jacobsen N, Jensen H, Goldschmidt E. Prevalence of myopia in Danish conscripts. Acta Ophthalmol Scand. 2007;85(2):165–170. doi:10.1111/j.1600-0420.2006.00789.x
10. Huang CY, Hou CH, Lin KK, Lee JS, Yang ML. Relationship of lifestyle and body stature growth with the development of myopia and axial length elongation in Taiwanese elementary school children. Indian J Ophthalmol. 2014;62(8):865–869. doi:10.4103/0301-4738.141047
11. Song HT, Kim YJ, Lee SJ, Moon YS. Relations between age, weight, refractive error and eye shape by computerized tomography in children. Korean J Ophthalmol. 2007;21(3):163–168. doi:10.3341/kjo.2007.21.3.163
12. Stone RA, Flitcroft DI. Ocular shape and myopia. Ann Acad Med Singap. 2004;33(1):7–15.
13. Atchison DA, Jones CE, Schmid KL, et al. Eye shape in emmetropia and myopia. Invest Ophthalmol Vis Sci. 2004;45(10):3380–3386. doi:10.1167/iovs.04-0292
14. Lim LS, Yang X, Gazzard G, et al. Variations in eye volume, surface area, and shape with refractive error in young children by magnetic resonance imaging analysis. Invest Ophthalmol Vis Sci. 2011;52(12):8878–8883. doi:10.1167/iovs.11-7269
15. Flitcroft DI, He M, Jonas JB, et al. IMI - defining and classifying myopia: a proposed set of standards for clinical and epidemiologic studies. Invest Ophthalmol Vis Sci. 2019;60(3):M20–M30. doi:10.1167/iovs.18-25957
16. Chan TF, Vese LA. Active contours without edges. IEEE Trans Image Process. 2001;10(2):266–277. doi:10.1109/83.902291
17. Wang XF, Huang DS, Xu H. An efficient local Chan–Vese model for image segmentation. Pattern Recognit. 2010;43(3):603–618. doi:10.1016/j.patcog.2009.08.002
18. Jiang X, Zhang R, Nie S. Image segmentation based on level set method. Phys Procedia. 2012;33:840–845. doi:10.1016/j.phpro.2012.05.143
19. Wang D, Ding X, Liu B, Zhang J, He M. Longitudinal changes of axial length and height are associated and concomitant in children. Invest Ophthalmol Vis Sci. 2011;52(11):7949–7953. doi:10.1167/iovs.11-7684
20. Saw SM, Chua WH, Hong CY, et al. Height and its relationship to refraction and biometry parameters in Singapore Chinese children. Invest Ophthalmol Vis Sci. 2002;43(5):1408–1413.
21. Tideman W, Marstal K, Polling JR, et al. Do volume, height and width of the eye determine refractive error? Results from 10-year-old European children from the Generation R study. Investig Ophthalmol Vis Sci. 2018;59:2136.
22. Ye S, Liu S, Li W, Wang Q, Xi W, Zhang X. Associations between anthropometric indicators and both refraction and ocular biometrics in a cross-sectional study of Chinese schoolchildren. BMJ Open. 2019;9(5):e027212. doi:10.1136/bmjopen-2018-027212
23. Rada JA, Wiechmann AF. Ocular expression of avian thymic hormone: changes during the recovery from induced myopia. Mol Vis. 2009;15:778–792.
24. Kusakari T, Sato T, Tokoro T. Visual deprivation stimulates the exchange of the fibrous sclera into the cartilaginous sclera in chicks. Exp Eye Res. 2001;73(4):533–546. doi:10.1006/exer.2001.1064
25. Schmid GF. Association between retinal steepness and central myopic shift in children. Optom Vis Sci. 2011;88(6):684–690. doi:10.1097/OPX.0b013e3182152646
26. Williams KM, Bertelsen G, Cumberland P, et al. Increasing prevalence of Myopia in Europe and the impact of education. Ophthalmology. 2015;122(7):1489–1497. doi:10.1016/j.ophtha.2015.03.018
27. Xie Z, Long Y, Wang J, et al. Prevalence of myopia and associated risk factors among primary students in Chongqing: multilevel modeling. BMC Ophthalmol. 2020;20:146. doi:10.1186/s12886-020-01410-3
28. Wang J, Li Y, Musch DC, et al. Progression of Myopia in school-aged children after COVID-19 home confinement. JAMA Ophthalmol. 2021;139(3):293–300. doi:10.1001/jamaophthalmol.2020.6239
29. Zhang X, Cheung S, Chan HN, et al. Myopia incidence and lifestyle changes among school children during the COVID-19 pandemic: a population-based prospective study. Br J Ophthalmol. 2021;2021:1–7.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
