Back to Journals » Infection and Drug Resistance » Volume 14
Occurrence, Risk Factors, and Antimicrobial Susceptibility Test of Thermophilic Campylobacter Species of Bovine Carcass at Municipal Abattoir and Butcher Shops of Jimma Town, Southwest Ethiopia
Authors Berhanu L , Bedru H , Gume B, Tolosa T , Kassa T , Getaneh A, Mereta ST
Received 30 July 2021
Accepted for publication 2 September 2021
Published 15 September 2021 Volume 2021:14 Pages 3753—3762
DOI https://doi.org/10.2147/IDR.S331040
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Leykun Berhanu,1,* Habib Bedru,2,* Beje Gume,3 Tadele Tolosa,4 Tesfaye Kassa,5 Assegid Getaneh,4 Seid Tiku Mereta3
1Department of Environmental Health, Wollo University, Dessie, Ethiopia; 2Department of Livestock and Fishery Development, Jimma, Ethiopia; 3Department of Environmental Health Science and Technology, Jimma University, Jimma, Ethiopia; 4School of Veterinary Medicine, Jimma University, Jimma, Ethiopia; 5School of Medical Laboratory Science, Jimma University, Jimma, Ethiopia
*These authors contributed equally to this work
Correspondence: Leykun Berhanu Email [email protected]
Background: Although Campylobacter jejuni (C. jejuni) and Campylobacter coli (C. coli) cause acute diarrheal diseases in people all over the world, they are most commonly seen in other mammalian species and are a seemingly healthy carrier condition. Humans in Ethiopia, on the other hand, are largely unaware of the existence of Campylobacter in food animals as potential sources of infection.
Objective: To determine the occurrence, risk factors, and antimicrobial susceptibility pattern of thermophilic Campylobacter species in bovine raw meat from the abattoir and butcher shops.
Methods: Swab samples were collected from 177 cattle carcasses (from abattoir 93 and butcher shops 84) and cultured using standard methods. An antimicrobial susceptibility test was performed using the disk diffusion method towards eleven antimicrobial agents.
Results: The overall prevalence of thermophilic Campylobacter species was 14 (7.9%). From these, 11 (78.6%) were identified to be C. jejuni and 3 (21.4%) were C. coli. Lack of handwashing before meat processing and after visiting the toilet, meat contact with floors, walls, or soiling during preparation, and lack of training were the most important factors independently associated with (p< 0.05) the prevalence of Campylobacter species contamination. The highest level of antimicrobial resistance of the Campylobacter isolates was recorded to ampicillin (10μg) (100%), followed by amoxicillin (30μg) (78.6%) and sulphamethoxazole-trimethoprim (57.1%) while the least resisted antimicrobials were streptomycin (25μg), erythromycin (15μg), oxytetracycline (30μg) (each 28.6%), kanamycin (30μg) 14.3%, chloramphenicol (30μg) and gentamycin (10μg) (each 7.1%).
Conclusion: Despite the low prevalence of thermophilic Campylobacter in the current investigation, it may pose a significant public health threat. As a result, it is vital to give retailers and customers extensive education, training, and knowledge about the correct handling and cooking of animal-derived goods. Furthermore, antimicrobials should be used with caution in both veterinary and human treatment regimens as well as a wider examination of antimicrobial resistance patterns for the use of well-targeted antimicrobials.
Keywords: antimicrobial resistance, cattle carcass, thermophilic Campylobacter, Jimma town
Introduction
Foodborne illnesses are caused by eating contaminated foods, particularly animal products such as diseased animal flesh or food contaminated with pathogenic microbes.1 With an estimated 400 million cases each year, Campylobacter is one of the most common pathogens involved in foodborne diseases.2,3 The bacteria that cause campylobacteriosis in humans cause watery or bloody diarrhea, abdominal pain, and nausea in many countries. Peripheral neuropathies, such as Guillain–Barre syndrome (GBS) and Miller Fisher syndrome (MFS), and functional bowel illnesses, such as irritable bowel syndrome, can all be major long-term repercussions of an acute infection.4
The use of unpasteurized milk and meat has been connected to outbreaks and in rare cases, involving cattle and cow products.5 Beef is not regarded as a key vehicle of transmission in human infections since Campylobacter is not commonly detected on carcasses or in beef. Prevalence studies of Campylobacter species as human gastrointestinal pathogens in Tanzania reported isolation rates ranging from 9.3% to 18.8%.6,7 The epidemiology of various Campylobacter species has been recorded in several countries in cattle.8–10
Several species of public and animal health importance are found in the Campylobacter bacterial genera. Campylobacter jejuni (C. jejuni) and Campylobacter coli (C. coli) are the most common causes of gastroenteritis, accounting 400 million cases of diarrhea each year. The bacteria is found 3–4 times more common in persons with gastrointestinal problems than other bacterial enteropathogens such as salmonella or Escherichia coli.4,11
Over the last 10 years, the incidence and prevalence of campylobacteriosis have increased in both developed and developing countries. In Australia, Europe, and North America, there has been a significant increase in the number of reported cases.12 In the United States, an incidence of 14.3 campylobacteriosis per 100,000 inhabitants was reported for the period between 1996 and 2012. An annual incidence of 35.2 incidents per 100,000 people was observed in Quebec, Canada. Since 2005, Campylobacter has surpassed E. coli as the most often reported bacterial pathogen causing human gastrointestinal illnesses in Europe. In 2013, the European Union (EU) member states reported 214,779 confirmed cases, corresponding to a notification rate of 64.8 per 100,000 people.13
Despite the fact that data from African countries is scarce, it appears that Campylobacter infections are most common among children. In Malawi, 14% of the non-diarrheic children and 28% of the diarrheal children tested positive for C. jejuni and C. coli by PCR. In Madagascar and Kenya, C. jejuni and C. coli were also shown to be endemic in children.14,15 In Ethiopia, it is difficult to give an accurate picture of the disease’s burden mainly due to lack of a national surveillance program, limited routine culture availability for Campylobacter species isolation in clinical and research settings, and the requirement for selective media.16
Children, the elderly, and those with weakened immune systems (such as cancer patients, HIV/AIDS patients, and transplant recipients) are particularly vulnerable. Because of the severity of diarrhea and the possibility of squeals, campylobacteriosis is a major public health threat having a significant socioeconomic implications.17 Campylobacter species is a bacteria that causes food poisoning. Much domestic livestock has parasites in their intestinal tracts.3,18 Campylobacter has been linked to antimicrobial resistance across the world.7,8,19,20
There is an escalating number of Campylobacter isolates resistant to many of the antibiotics.7 Antimicrobials’ use in livestock has resulted in the emergence and spread of antimicrobial-resistant bacteria such as Campylobacter. In developing nations, where antimicrobial use is widespread and uncontrolled, the situation appears to be deteriorating faster.21 Despite the lack of statistics, evidence indicated that the burden of the disease caused by Campylobacter infection is significant.22 A few Ethiopian studies have reported on the presence of Campylobacter strains on humans as well as antimicrobial susceptibility tests.8,23 However, there are just a few investigations on the state of Campylobacter species contamination in abattoir environments. The current study, therefore, aimed to assess the occurrence, risk factors, and antimicrobial susceptibility pattern of thermophilic Campylobacter species in bovine raw meat from the butcher shops and abattoir of Jimma Municipality.
Methods
Study Area Description
The research was carried out in Jimma town, 350 km southwest of Addis Ababa. The town is located at 7°41ʹ N latitude and 36°50ʹ E longitude. The town receives an abundance of mean annual rainfall ranging from 1800 to 2300mm, making it one of Ethiopia’s best-watered highland areas, ideal for agricultural output.24 Ethiopia’s federal statistical agency estimates that there are 177,943 people in the town, with 89,233 (50.1%) of them being women. The town serves as a commercial hub for the surrounding area, increasing food service establishments. The lack of regular monitoring of these establishments’ sanitary conditions is a major issue in foodservice investment.25,26 The Jimma Municipality Abattoir was established in 1942 as a Governmental organization for the slaughtering and distribution of meat around the town. The abattoir has a daily slaughter capacity of 50 cattle and 20 sheep. In this abattoir there is one Christian slaughtering room and one Muslim slaughtering room, as wells as two vehicles for transporting meat to the customers.
Study Design and Period
A laboratory-based cross-sectional investigation was conducted between January 2018 and January 2019.
Study Population
Healthy adult male cattle slaughtered in the Jimma Municipal abattoir throughout the study period, as well as meat handlers working in the abattoir and butcher shops, were the study population.
Sample Size Determination and Sampling Procedure
The sample size was determined using the following formula, which took into account the expected prevalence of Campylobacter in abattoirs and butcher shops to be 6.5% and 5.8%, respectively.27,5% of desired absolute precision, and 95% confidence interval.
n=1.962 Pexp (1-Pexp) /d2
where n=required sample size, Pexp=expected prevalence, d=desired absolute precision. Therefore, the required sample size for this study was 93 and 84 cattle carcasses swabs, from abattoir and butcher shops, respectively. Totally, 177 swab samples were collected. In addition, 177 meat handlers (84 from Butcher shops and 93 from abattoir) were selected for a face-to-face interview.
Meat Samples Collection
Randomly selected carcasses were swabbed using a sterile cotton-tipped swab (2×3cm) fitted with a shaft on specific sites of a carcass, the abdomen (flank), thorax (lateral), crutch, breast (lateral), which are sites with the highest rate of contamination.28 To take a sample from each site, a sterile cotton was first soaked in peptone water (Oxoid Ltd., Hampshire, England), then rubbed horizontally and vertically on the carcasses multiple times. The shaft was broken by pressing it against the inner wall of the test tube once the rubbing operation was completed, and the cotton swab was left in the test tube with the screw sealed. Over the entire sampled region, a second dry sterile cotton swab of the same type was applied as before. Swab samples were taken with commercially available transport tubes that contained peptone water, which protects Campylobacter species from drying out and the damaging effects of oxygen, as advised.10 Because Campylobacter is highly sensitive to environmental factors such as dehydration, atmospheric oxygen, sunlight, and high temperatures, all carcass swab samples from abattoir and butcher shops were transported to Jimma University’s laboratory in an icebox with ice packs and processed within 4 hours of collection.
Data Collection
A semi-structured interview questionnaire was presented with the intent of determining meat contamination. The information collected included scodio-demoghraphic charactestics of meat handlers, status of washing hands before meat processing, washing knife before and after use, hygienic condition of apron/white coat and head cover, daily cleanliness of abattoir/butcher shop, hygienic condition of wood chopping block for cutting meat, use of detergent/disinfectant for cleaning the abattoir/butcher shop, sterilization of equipment, routine control of flies and another insect in the abattoir/butcher shops, meat contact with the floor, walls or soiling in preparation, attending of any courses related to their work, frequency of healthy checkup for workers and accessibility to clean and safe water.
Isolation and Identification of Campylobacter Species
For the isolation of Campylobacter, two enrichment procedures were followed because of cellular susceptibility outside the gastrointestinal environment resulting in possible sub-lethal effects on the cells. In the first enrichment, the buffered peptone water culture was inoculated into Mueller–Hinton broth containing the mExeter antimicrobials mix (10mg sulphamethoxazole–trimethoprim, 15mg cefoperazone, and 2mg amphotericin B (TCA) and incubated for 8 hours at 37°C. Then, 1 mL of the culture was transferred to the full mExeter mix (MH broth containing TCA and 5mg rifampicin and 2500 IU polymyxin B) and incubated as before. One loopful of the second enrichment was streaked on MH-full mExeter agar, incubated for 24–48 hours at 37°C under microaerophilic conditions. The traditional diagnostic process requires that suspicious specimens be cultured on selective agar at 42°C under microaerophilic conditions for up to 72 hours before a negative report is issued, with favorable transportation and storage environments including the use of transport media in the pre-analytical phase.29
Preliminary Campylobacter species identification was done using microscopy to show characteristic darting movement with the iris diaphragm effectively closed to contrast the field. Gram-stained morphology revealed a gram-negative bacterium in an ‘S’ shape. The thermotolerant Campylobacter genera were identified by positive oxidase and catalase tests. Campylobacter colonies were selected out with a sterile cotton swab from blood agar medium and placed in micro tubes containing storage medium (brain-heart-infusion broth medium) for detection. Hippocrates hydrolysis and susceptibility to nalidixic acid (30μg) disk were analyzed and reported; these variables constituted the basis for the identification of C. jejuni, and C. coli.
Antimicrobial Susceptibility Test
The usual agar disk diffusion method was used to determine antimicrobial susceptibility for Campylobacter species as suggested by Clinical and Laboratory Standards Institutions.30 Isolates were screened for the following antimicrobial agents (Oxoid Ltd. UK) ampicillin (10μg) (AMP) 10μg, amoxicillin (30μg) (AMC) 30μg, oxytetracycline (OT) 30μg, chloramphenicol (30µg), gentamycin (G) 10μg, erythromycin (E) 15μg, sulphamethoxazole-trimethoprim (SXT) 25μg, ciprofloxacin (Cip) 5μg, kanamycin (K) 30μg, streptomycin (S) 25μg, and nalidixic acid (NA) 30μg.
From a fresh culture, three to four morphologically indistinguishable bacteria colonies were selected and suspended in sterile normal saline. The broth culture’s turbidity was measured using a turbidity meter with an absorbance range of 0.08 to 0.1, which is equivalent to 0.5 McFarland turbidity standards. A loop of bacterial suspension was placed in the center of Muller–Hinton agar media (Oxoid, Ltd) supplemented with 5% sheep blood and spread evenly with a sterile cotton-tipped applicator. Following drying, the 11 antimicrobial disks were placed on 120mm Petri dishes and incubated in anaerobic jars at 42°C for 48 hours using CO2-producing kits (CampyGenTMOxoid Ltd). Finally, using a metal caliper, the diameter of the zone of inhibition around the disks was measured to the nearest millimeter, and the isolates were classed as Sensitive (S), Intermediate (I), or Resistant (R) according to the manufacturer’s standardized table.31 C. jejuni and C. coli were identified as Campylobacter species that were sensitive to nalidixic acid (30μg).32
Data Processing and Analysis
Data obtained from both laboratory results and questionnaire surveys were entered and stored in Microsoft (MS) Excel spreadsheet program and analyzed using Statistical Package for Social Science (SPSS) software version 20. Descriptive statistics were performed for frequencies and percentages. The occurrence of Campylobacter species was calculated by dividing the number of positive samples to the total number of samples tested for both abattoir and butcher shops. Bivariable logistic regression analysis was used to select candidate variables for multivariable analysis and the variables with p<0.25 in the bivariable analysis were further subjected to multivariable logistic regression analysis. The strength of association between the risk factors and the occurrence of the thermophilic Campylobacter was assessed using adjusted odds ratio (AOR) at a 95% confidence level. A p-value of <0.05 was used as a cut of point.
Results
Socio-Demographic Characteristics of the Respondents
Of 177 respondents, about one-third 55(31.1%) were female while the remaining 122(68.9%) were male. About half 83(46.9%) of the respondents could not read or write. About two-fifths 70(39.5%) of them had meat handling experience of 1to 2 years (Table 1).
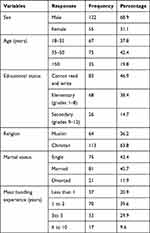 |
Table 1 Socio-Demographic Characteristics of the Meat Handlers Working in Municipality Abattoir and Butcher Shops in Jimma Town, Southwest, Ethiopia |
Meat Handling Practices of the Respondents
Among 177 respondents, about three-fourth 134 (75.7%) of them wash their hands before meat processing, and about one-third 55 (31.1%) wash knives before and after using. In addition, about two-thirds 114 (64.4%) wash their hands before visiting the toilet (Table 2).
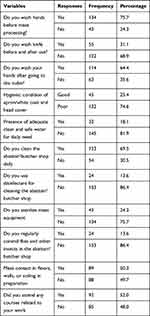 |
Table 2 Meat Handling Practices of the Respondents Working in a Municipal Abattoir and Butcher Shops in Jimma Town, Southwest, Ethiopia |
Prevalence of Thermophilic Campylobacter
Among 177 meat samples analyzed, 14(7.9%) of them were positive for thermotolerant Campylobacter species. From the abattoir samples, 8 (8.6%) of them were positive for campylobacter (Table 3).
 |
Table 3 Prevalence of Thermophilic Campylobacter from Cattle Carcass in the Municipal Abattoir and Butcher Shops in Jimma town, Southwest, Ethiopia |
Prevalence of Campylobacter Species
Out of the 14 Campylobacter species isolated from cattle carcasses, 11(78.6) of them were found to be positive for C. jejuni, whereas the remaining 3 of them were C. coli (Table 4).
 |
Table 4 The Percentages of Thermophilic Campylobacter Species Isolated from Cattle Carcasses at municipal Abattoir and Butcher Shops in Jimma town, Southwest, Ethiopia |
Antimicrobial Susceptibility Pattern of Campylobacter Isolates
Fourteen Campylobacter species isolated from cattle carcass were subjected to antimicrobial susceptibility test using disk diffusion method towards eleven antimicrobial agents. All Campylobacter species were tested for antimicrobial susceptibility. Both Campylobacter species were tested independently for antimicrobial susceptibility in this investigation. Nalidixic acid (30μg) susceptibility was found in all (100%) C. jejuni species. The antimicrobial susceptibility test for C. coli isolates showed that 100% sensitive to ciprofloxacin (5μg) and nalidixic acid (30μg), and 66.7% sensitive to chloramphenicol (30μg), sulphamethoxazole–trimethoprim, kanamycin (30μg), streptomycin (25μg), gentamycin (10μg), and erythromycin (15μg). Higher resistance rates, 100%, and 66.7% were also observed for ampicillin (10μg), and amoxicillin (30μg), respectively, in C. coli isolates (Table 5).
 |
Table 5 The Antimicrobial Susceptibility Pattern of C. jejuni and C. coli Isolated from Carcasses of Cattle at Municipal Abattoir and Butcher Shops in Jimma town, Southwest, Ethiopia |
Factors Affecting the Presence of Thermotolerant Campylobacter Species
From the bivariable analysis, five variables with p<0.25 were selected for multivariable analysis. These are “lack of handwashing before meat processing” (p=0.001), lack of handwashing after visiting toile (p=0.049), “hygienic condition of apron/white coat and headcover” (p=0.136), “meat contact with floors, walls or soiling in preparation” (p=0.015), and “lack of attending any courses related to their work” (p=0.079).
From the multivariable analysis, four variables, namely lack of handwashing before meat processing; lack of washing hands after visiting the toilet; meat contact with floors, walls, or soiling in preparation; and lack of attending any courses related to meat handling were all factors that increase the odds of thermophiles Campylobacter presence. Those meat handlers who do not wash their hands before meat processing had 11.6 times more likely to increase the thermotolerant Campylobacter species to the meat they handle than those who wash their hands. Those meat handlers who did not wash their hands after visiting a toilet had 4.5 times more likely to transfer thermotolerant Campylobacter species than those who wash their hands after visiting a toilet. In addition, meat contact in floors, walls, or soiling in preparation had 70% more likely to increase the presence of campylobacter in meat samples. Moreover, those meat handlers who did not take any course related to meat handling had 15.9 times more likely to increase the thermotolerant species than those who took the course related to meat handling (Table 6).
 |
Table 6 Factors Affecting the Presence of Thermotolerant Campylobacter Species in Meat Samples Collected from Abattoir and Butcher Shops in Jimma Town, Southwest, Ethiopia |
Discussion
Thermophilic Campylobacter bacterial genera contain several species of both public and animal health importance. C. jejuni and C. coli are the most common cause of gastroenteritis in humans. Campylobacteriosis is predominantly acquired through the consumption of contaminated foods.4 In the present study, both carcasses from abattoir and butcher shops were contaminated with Campylobacter species, where 8.6% of the cattle carcasses in the abattoir and 7.1% of the carcasses in the butcher shops were found to be contaminated by thermophilic Campylobacter species. This finding is higher than the previous prevalence reported by Dadi and Asrat.27 where 6.5% and 5.8% of the analyzed carcasses from the abattoir and butcher shops were positive for thermophilic Campylobacter species, respectively. Also, higher result was reported by Rahimi.21, where the prevalence of Campylobacter species in sheep and goat meat samples was found to be 13.2% and 6.4%, respectively. The higher prevalence of Campylobacter species in the present study may be due to cross contamination during manual skinning, evisceration, and processing in the slaughterhouse or insufficient hygiene during storage transport and boning in the butcheries, particularly in small butcher shops where there may have been closer proximity to meat from other food animal species.7,21
Foods of animal origin have been incriminated for being the main source of Campylobacter infection in humans. Since raw meat from beef is widely consumed in Ethiopia, the occurrence of Campylobacter in meat increases the likelihood of the pathogen’s transmission to humans. In this study, among thermophilic Campylobacter species isolated from cattle carcass, C. jejuni accounted for 78.6% and C. coli for 21.4%. Consistent finding has been reported in Ethiopia,27 where the prevalence of C. jejuni and C. coli was reported to be 78% and 25%, respectively. The presence of campylobacter species in meat samples may indicates poor sanitation of the abattoir and bucther shops as well as unhygienic condition of the meat handlers and meat preparion area.
In this study, the highest level of resistance of the Campylobacter isolates was recorded to ampicillin (10μg) (100%), amoxicillin (30μg) (78.57%), and sulphamethazole–trimethoprim (25μg) (57.1%) while the least resisted antimicrobials for this specific test were streptomycin (25μg), erythromycin (15μg), oxytetracycline (30μg) (each 28.6%), kanamycin (30μg) 14.3%, chloramphenicol (30μg) and gentamycin (10μg) (each 7.1%). Similar antimicrobial susceptibility patterns have been observed in a previous study conducted in Ethiopia for these antimicrobial agents.8,27 Thus, there is convincing evidence today that quinolone resistance emerged and increased among food animals because of the use of antimicrobials in animal production and then spread to via food chain and caused infection in man.27 The increase in resistance to antimicrobial agents could be associated with extensive use of antimicrobials not only as therapeutic agents for human infections but also for prophylaxis and growth promotion in animal husbandry.7
Relative resistance of thermophilic Campylobacter strains showed the higher resistance frequencies of C. jejuni were observed against ampicillin (10μg) (100%), amoxicillin (30μg) (81.8%), and sulphamethoxazole-trimethoprim (25μg) (63.6%). Higher resistance of the C. coli isolates was shown to ampicillin (10μg) (100%), amoxicillin (30μg) (66.7%) followed by sulphamethazole–trimethoprim (25μg), oxytetracycline (30μg), streptomycin (25μg), chloramphenicol (30μg), gentamycin (10μg), and erythromycin (15μg) (each 33.3%). The resistance to erythromycin is of public health concern, as there are currently limited options in the choice of treatment of Campylobacter infections.7
In the present study, some workers had no habits of washing their hands with water and soap before and after processing meat that contributes to contamination of meat. Meat handlers are the potential sources of contamination of beef with microorganisms. It is important to maintain hygiene in the floors and walls since such structures can act as a source of contamination of carcasses especially during skinning the meat might be contaminated with cattle feces. Meat contact in floors, walls, or soiling during preparation was found to be 70% more likely to enhance the prevalence of Campylobacter species at abattoirs and butcher shops in the current study. This could be due to the fact that all of the slaughtering, skinning, evisceration, and quartering of the carcasses took place don a dirty floor, exposing the meat to contamination with microbes. After being skinned and eviscerated, the carcasses were hung on the slaughter hall before being inspected.
However, the heads were left on the floor and inspected onsite, practices that may contribute to contamination of meat from the head as the floor was in poor hygienic condition. This finding is similar to those reported by Adzitey et al.33 where 65% of abattoir workers dressed carcasses on a bare floor in the abattoir, 16% dressed carcasses on unclean slaughter slabs, and 19% on both the slaughter slabs and bare floor in which the slaughter floor and slabs were smeared with blood, rumen contents and other wastes from previously dressed animals, which increased the risk of contamination of subsequent carcasses. Animals are often slaughtered and eviscerated on the floor because of the absence of mechanical or manual hoists a factor, which contributed to a major source of contamination. Efforts being made to maintain some level of cleanliness before and after the close of work appeared to be insufficient due to fewer cleaners who also lacked cleaning facilities, poor drainage systems, and insufficient water. In this study, lack of attending of any courses related to their work (p=0.001), increased the odds of thermophilic Campylobacter presence at the abattoir and butcher shops by 16.0 (95% CI: 3.1–82.2).
Conclusion
Despite the low prevalence of thermophilic Campylobacter in the current investigation, the disease poses a significant public health threat. There was a higher occurrence of thermophilic Campylobacter in abattoirs compared to butcher shops. Lack of handwashing before meat processing and after visiting the toilet, meat contact in floors, walls, or soiling in preparation, and attending training related to meat handling were the most important factors independently associated with the prevalence of Campylobacter species contamination of carcasses at abattoir and butcher shops. The study also revealed that the highest level of resistance Campylobacter species was recorded to ampicillin (10μg), amoxicillin (30μg), and sulphamethoxazole-trimethoprim (25μg).
As a result, it is critical to provide intensive education, training, and awareness to retailers and customers on the correct handling and cooking of animal-derived foods. Furthermore, public education is fundamental in preventing the consumption of raw meat and other undercooked animal-origin items. Furthermore, antimicrobials should be used with caution in both veterinary and human treatment regimens, as well as a wider examination of antimicrobial resistance patterns for well-targeted antimicrobial use. To determine the prevalence of zoonotic enteric campylobacteriosis in humans and the epidemiological involvement of cattle and other animals in the study region, more researches are needed. In the same way, a further epidemiological study is needed to determine the role of cattle as a disease reservoir source.
Ethical Consideration
The study’s techniques were all carried out in conformity with the Helsinki Declaration. As a result, Jimma University’s ethical review board granted permission with a reference number of JU/245/17 dated August 17, 2017. A formal letter of cooperation was also written to the Jimma town livestock and fishery development office. Written consent was obtained from study participants, butcher shops, and town municipality offices before the beginning of the sample and data collection. Before starting the interview, the data collector explained the purpose of the study to all the participants. All the information obtained from each study participant was kept confidential.
Acknowledgments
Jimma University is praised for paying for the study. The authors would like to thank the research assistants who helped with data collection, supervision, and sample analysis. Jimma University’s Environmental Health Sciences and Technology, as well as its Medical Microbiology Laboratories, are also credited with providing laboratory space. The authors are appreciative of the livestock and fishery development offices in Jimma town, as well as the proprietors of butcher shops and study participants.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Rosef O, Johnsen G, Stølan A, Klæboe H. Similarity of campylobacter lari among human, animal, and water isolates in Norway. Foodborne Pathog Dis. 2008;5(1):33–39. doi:10.1089/fpd.2007.0027
2. Hassanain A. Antimicrobial-resistant Campylobacter jejuni isolated from humans and animals in Egypt. Glob Vet. 2011;6(2):195–200.
3. Mpalang R, Boreux R, Melin P, Akir Ni Bitiang K, Daube G, De Mol P. Prevalence of Campylobacter among goats and retail goat meat in Congo. J Infect Dev Ctries. 2014;8(2):168–175. doi:10.3855/jidc.3199
4. Center for diseases control and prevention. Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food — 10 states, 2009. MMWR Morb Mortal Wkly Rep. 2010;59:418–422.
5. Nielsen E. Occurrence and strain diversity of thermophilic Campylobacters in cattle of different age groups in dairy herds. Lett Appl Microbiol. 2002;35(1):85–89. doi:10.1046/j.1472-765X.2002.01143.x
6. Mdegela R, Nonga H, Ngowi H, Kazwala R. Prevalence of thermophilic Campylobacter infections in humans, chickens, and crows in Morogoro, Tanzania. J Vet Med Series B. 2006;53(3):116–121. doi:10.1111/j.1439-0450.2006.00926.x
7. Gahamanyi N, Mboera L, Matee M, Mutangana D, Komba E. Prevalence, risk factors, and antimicrobial resistance profiles of thermophilic campylobacter species in humans and animals in Sub-Saharan Africa: a Systematic Review. Int J Microbiol. 2020;2020:1–12. doi:10.1155/2020/2092478
8. Kassa T, Gebre-Selassie S, Asrat D. The prevalence of thermotolerant Campylobacter species in food animals in Jimma Zone, Southwest Ethiopia. Ethiop J Health Dev. 2006;19(3). doi:10.4314/ejhd.v19i3.10002
9. Stanley K, Jones K. Cattle and sheep farms as reservoirs of Campylobacter. J Appl Microbiol Symp Suppl. 2003;94(32):104–113. doi:10.1046/j.1365-2672.94.s1.12.x
10. Aquino M, Regua M, Filgueiras A, Teixeira L, Ferreira M, Tibana A. Use of a multiplex PCR-based assay to differentiate Campylobacter jejuni and Campylobacter coli strains isolated from human and animal sources. Vet J. 2002;163(1):102–104. doi:10.1053/tvjl.2001.0632
11. Nonga HE, Sells P, Karimuribo ED. Occurrences of thermophilic Campylobacter in cattle Slaughtered at Morogoro municipal abattoir, Tanzania. Trop Anim Health Prod. 2010;42:73–78. doi:10.1007/s11250-009-9387-7
12. Nguyen NH, Nguyen TNM, Hotzel H, et al. Thermophilic campylobacter-neglected foodborne pathogens in Cambodia, Laos and Vietnam. Gastroenterol Hepatol Open Access. 2017;8(3):
13. European Food Safety Authority. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2013. EFSA J. 2015;13(1):1–165. doi:10.2903/j.efsa.2015.3991
14. Randremanana RV, Randrianirina F, Sabatier P, et al. Campylobacter infection in a cohort of rural children in Moramanga, Madagascar. BMC Infect Dis. 2014;14(372):1471–2334. doi:10.1186/1471-2334-14-372
15. Swierczewski BE, Odundo EA, Koech MC, et al. Enteric pathogen surveillance in a case-control study of acute diarrhoea in the town of Kisii, Kenya. J Med Microbiol. 2013;62(11):1774–1776. doi:10.1099/jmm.0.059139-0
16. Hagos Y, Gugsa G, Awol N, et al. Isolation, identification, and antimicrobial susceptibility pattern of Campylobacter jejuni and Campylobacter coli from cattle, goat, and chicken meats in Mekelle, Ethiopia. PLoS One. 2021;16(2):1–13.
17. European Food Safety Authority. Scientific opinion on quantification of the risk posed by broiler meat to human campylobacteriosis in the EU. EFSA J. 2010;8(1):1–89.
18. Pezzotti G, Serafin A, Luzzi I, Mioni R, Milan M, Perin R. Occurrence and resistance to antibiotics of Campylobacter jejuni and Campylobacter coli in animals and meat in northeastern Italy. Int J Food Microbiol. 2003;82(3):281–287. doi:10.1016/S0168-1605(02)00314-8
19. Ekin IH, Gürtürk K, Arslan A, Boynukara B. Prevalence and Characteristics of campylobacter species isolated from gallbladder of slaughtered sheep in van, (Eastern) Turkey. Acta Vet Brno. 2006;75(1):145–149. doi:10.2754/avb200675010145
20. United States Department of Agriculture: Animal and Plant Health Inspection Services. Campylobacter on U. S. Sheep and Lamb Operations; 2014. Available from: www.aphis.usda.gov›animal_health›nahms. Accessed on May, 2020.
21. Rahimi E. Occurrence and resistance to antibiotics of Campylobacter species in retail raw sheep and goat meat in Shahr-e Kord, Iran. Glob Vet. 2010;4(5):504–509.
22. WHO. The Global View of Campylobacteriosis: Report of an Expert Consultation. Vol. 72. Plant Engineering; 2018.
23. Gedlu E, Aseffa A. Campylobacter enteritis among children in northwest Ethiopia: a 1-year prospective study. Ann Trop Paediatr. 1996;16(3):207–212. doi:10.1080/02724936.1996.11747828
24. Weldezgina D, Muleta D. Bacteriological contaminants of some fresh vegetables irrigated with Awetu River in Jimma Town, Southwestern Ethiopia. Adv Biol. 2016;2016:1–11. doi:10.1155/2016/1526764
25. Neme K, Hailu B, Belachew T. Assess sanitary condition and food handling practices of restaurants in Jimma Town, Ethiopia: implication for food borne infection and food intoxication. Food Sci Qual Manag. 2017;60:2224–6088.
26. Berhanu L, Mereta S, Gume B, et al. Effect of microbial quality of washing water on hand hygiene status of food handlers in Jimma town: implication for food hygiene and safety. J Multidiscip Healthc. 2021;14:1129–1134. doi:10.2147/JMDH.S306359
27. Dadi L, Asrat D. Prevalence and antimicrobial susceptibility profiles of thermotolerant Campylobacter strains in retail raw meat products in Ethiopia. Ethiop J Health Dev. 2009;22(2):1–7. doi:10.4314/ejhd.v22i2.10072
28. International Organization for Standardization. Microbiology of the food chain: carcass sampling for microbiological analysis this version remains current; 2020. Available from: committee.iso.org›standard›62769. Accessed on April, 2020.
29. Senok A, Botta G. Campylobacter enteritis in the Arabian Gulf. J Infect Dev Ctries. 2009;3(2):74–82. doi:10.3855/jidc.52
30. CLSI. Performance standards for antimicrobial susceptibility testing; Twenty-Second Informational Supplement. CLSI Document M100-S22, Clinical and Laboratory Standards Institute, Wayne. Am J Ind Bus Manag. 2016;6(8):1–3.
31. Mulla S, Kumar A, Rajdev S. Performance standards for antimicrobial susceptibility testing in biofilm forming clinical bacterial isolates. Adv Microbiol. 2016;06(02):73–78. doi:10.4236/aim.2016.62007
32. Wayne P. M100-S24 performance standards for antimicrobial susceptibility testing: 24th informational supplement. Clinical and Laboratory Standards Institute; 2014: 1–230.
33. Adzitey F, Teye G, Dinko M. Pre and post-slaughter animal handling by butchers in the Bawku Municipality of the Upper East Region of Ghana. Livestock Res Rural Dev. 2011;23(2):1–7.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
