Back to Journals » Journal of Pain Research » Volume 16
Nurse-Led Telephone Follow-Up Intervention for Titrating and Tapering Opioids in Chronic Pain Patients – A Feasibility Study
Authors Halvorsen U, Bjørnnes AK , Ljosaa TM
Received 28 October 2022
Accepted for publication 8 March 2023
Published 17 April 2023 Volume 2023:16 Pages 1285—1300
DOI https://doi.org/10.2147/JPR.S394878
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Timothy Atkinson
Unni Halvorsen,1 Ann Kristin Bjørnnes,2 Tone Marte Ljosaa1
1Department of Pain Management and Research, Oslo University Hospital, Oslo, Norway; 2Institute of Nursing and Health Promotion, Oslo Metropolitan University, Oslo, Norway
Correspondence: Unni Halvorsen, Department of Pain Management and Research, Oslo University Hospital, Pb 4950 Nydalen, Oslo, 0424, Norway, Tel + 47 23 02 61 61, Fax + 47 23 02 74 02, Email [email protected]
Background and Purpose: Opioids in chronic non-cancer pain are debated, but remain a part of the pain treatment for selected patients. Research is scarce on the relieving and adverse effects of opioids, and how to deliver opioid treatment in this patient group. This study’s purpose was to assess the feasibility of a nurse-led telephone follow-up intervention for titrating or tapering opioids, including a pilot study of the intervention outcomes.
Patients and Methods: The feasibility assessment and process evaluation were performed according to the UK Medical Research Council (MRC) framework for evaluating complex interventions. A pilot study of the intervention outcomes was also performed. With a retrospective, descriptive, and longitudinal approach, we analyzed reports from 32 patients who titrated or tapered opioids. Information on demography, sleep satisfaction, health status, pain intensity/bothersomeness, opioid doses, and side effects was derived from the Oslo Pain Registry. Descriptive statistics, t-tests, and chi-square tests were used to analyze registry data.
Results: The study findings indicate that the intervention is feasible. Areas of impact were lack of a sound theory basis, unclear allocation criteria, and inconsistent and non-validated measurement tools. Mechanisms of change were interprofessional collaboration, nurses’ knowledge and competencies, predictability, and availability. Statistical analyses showed no between-groups differences in demographical-, clinical-, and pain characteristics, except those who titrated opioids were significantly older than patients tapering opioids (P=0.010). All patients reported poor health and side effects at baseline. Those who tapered opioids had a significant reduction in opioid use (P=0.004). Titrating opioids was associated with a significant increase in side effects (P=0.038).
Conclusion: Considering the limitations and the strengths of the intervention, the present study indicates that the nurse-led telephone follow-up program is a feasible intervention. With the right adjustments and improvements, the intervention is eligible for a larger efficacy study.
Keywords: opioids, feasibility study, register study, pilot study, process evaluation, health care personnel, chronic pain, nurse intervention
Introduction
Patients with chronic non-cancer pain (CNCP) often have complex pain conditions,1 and some patients spend years searching for effective treatment. Over the past 20 years, the use of opioids for CNCP has increased worldwide.2 With the opioid epidemic, governments and health-care personnel call for safer and better ways of treating pain with opioids.3 Although opioids may be effective in reducing acute pain and pain intensity,4 this type of drug is debated in chronic pain management due to long-term side effects, as well as the risk of misuse, addiction,3,5,6 and overdose death.3 Therefore, treatment with opioids for chronic pain must have a holistic and longitudinal approach considering treatment effects, side effects, and biopsychosocial aspects affecting the pain condition.3,7
Treatment with opioids requires a comprehensive treatment plan including functional goals,8 titration or tapering schedules, regularly scheduled follow-ups (according to the patients’ needs), and interventions for expected side effects or abstinences.3,9 An interdisciplinary approach can ensure safety, firm frames, and control over opioid consumption and side effects.10 The Norwegian Directorate of Health defines the nurse’s role as a part of the interdisciplinary team in outpatient pain clinics, and specifies that nurses have knowledge and skills to contribute to the treatment of patients with CNCP.11 However, following up the treatment plan for titrating or tapering opioids requires that nurses have comprehensive medical knowledge and a biopsychosocial understanding of the effects and problems related to opioids. Communication skills are essential,12 as nurses need to address the patients’ expectations, goals of treatment, possible risk factors, and alternative medication, as well as individually assess and discuss if the positive outcomes overweigh negative side effects.13
Nurse-led telephone follow-up programs are frequently utilized interventions in health care in recent years due to technological development.14 Telephone follow-ups have several advantages as they are individually arranged and save time and resources for both the clinician and the patients who do not have to travel to the clinic for consultations.15 However, lack of information from non-verbal communication may affect the dialogue in telephone consultations,16 and it is difficult to control whether the patients follow recommendations.17 Although communication by telephone may be challenging, this type of intervention still has the potential to support behavior change and treatment adherence.18,19 Treatment with opioids, whether the patient titrates or tapers doses, requires individually tailored monitoring and availability,20 which the nurse can provide through telephone follow-ups. Previous research indicates that a designated nurse in the clinic who supports and is available for the patients during the follow-up is associated with a more successful outcome.12,16,21,22 The use of guidelines and templates for opioid treatment makes follow-ups more standardized, thus making it possible to systematically evaluate the intervention.15
Nurse-led telephone follow-up programs are frequently used in health care for behavioral change, such as smoking cessation and self-management of diabetes.23 Only a few studies describe and evaluate nurse-led telephone follow-up programs for titrating opioids.24,25 These studies show that close follow-ups are important in order to prevent and handle side effects and adverse events associated with changes in opioid treatment.24,26 To assess feasibility and identify the factors of follow-up programs that promote change, research should follow procedures for development and evaluation of complex interventions, such as the UK Medical Research (MRC) framework.27 Complex interventions are defined as interventions comprising several interacting components, their implementation is complex, and they target several organizational levels.28 Follow-up programs meet these criteria, and are therefore described as complex interventions. A process evaluation must aim to see if the intervention is effective in daily practice, and assesses key components such as theoretical basis, development and implementation, context, uncertainties, mechanisms of change, and feasibility (eg, recruitment, retention, sample size, cost-effectiveness).27–29 The MRC framework emphasizes that complex interventions work best if tailored to local context rather than being standardized.29
The present study aimed to assess the feasibility and describe patients’ outcomes from a nurse-led telephone follow-up program for patients with CNCP who titrated or tapered opioids. The specific objectives were to 1) perform a process evaluation to assess the feasibility of the complex intervention, 2) describe patients’ demographics, clinical and pain characteristics at baseline, and 3) assess change in pain intensity, pain bothersomeness, sleep satisfaction, total symptom burden including opioid side effects, and change in morphine equivalents (MME) consumption from baseline to last follow-up.
Materials and Methods
Study Design and Setting
This is a feasibility study including a process evaluation of an already developed and implemented complex intervention that is the nurse-led telephone follow-up program. The study also involves a pilot study of intervention outcomes using a retrospective, descriptive, and longitudinal design. In line with the MRC framework28 (Figure 1) the intervention is presently at the feasibility and piloting stage which focuses on identifying areas of impact from the development, implementation, and content of the intervention.27,29
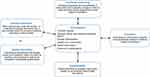 |
Figure 1 MRC framework for the process evaluation of complex intervention.27. |
The study took place in the largest interprofessional outpatient pain clinic in Norway (the Department of Pain Management and Research at Oslo University Hospital (OUS)).
Development and Implementation of the Intervention
The nurse-led telephone follow-up program was collaboratively developed by specialized pain management nurses and physicians working at the OUS pain clinic from 2014 to 2015. The program was developed and implemented to meet the need for comprehensive follow-up of patients on analgesics. In the development phase, key clinicians and leaders of the department held meetings to design and implement the program in the outpatient clinic. Recommendations and instructions were prepared, such as: standardized procedures for titration/tapering of analgesics, template for electronic journal documentation, standardized interventions for side effects, and standardized outcome measures for telephone consultations (ie, simple NRS and Likert scale questions regarding symptoms and side effects). Interprofessional and monoprofessional meetings were held with all clinicians at the outpatient clinic to inform, discuss, and shape the program before implementation.
The program was implemented as a quality improvement project in 2015. One to three nurses were involved in the program at any time. Some pain physicians chose to abstain from the program due to various reasons (eg, did not include analgesics in the treatment they offered, needed to do the follow-up of patients themselves, doubted the nurses’ medical competence). In 2020, the program changed status from a quality improvement project to an integrated interprofessional intervention in the outpatient clinic.
A logic model of the nurse-led telephone follow-up intervention was made to clarify features of the program and guide the process evaluation and feasibility assessment.28 Figure 2 is based on the template of Moore et al28 of a logic model for a complex intervention according to the MRC framework.
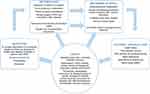 |
Figure 2 Logic model of process evaluation of the nurse-led telephone follow-up intervention for titrating and tapering opioids for CNCP. |
Description of the Intervention
The nurse-led telephone follow-up program is a complex intervention offered to patients with CNCP who attended the pain clinic and needed support in the titration or tapering of their opioid treatment. Nurses at the pain clinic organized and ran the program. The pain physicians allocated the patients and designed individual treatment plans, including treatment goals, type and doses of opioids, titration or tapering intervals, and advice regarding side effects. Overall treatment goals involved improved function and increased quality of life. Specific treatment goals were titration to the lowest possible dose that provided pain relief with a minimum of side effects, tapering to the lowest possible dose with adequate pain relief and tolerable side effects, or tapering to discontinuation. Nurses, pain physicians, and often primary care physicians collaborated interprofessionally on the opioid treatment, solved treatment challenges, and adjusted treatment plans when needed. All enrolled patients received follow-up consultations by phone, conducted by a designated nurse. A phone follow-up lasted from 30 to 60 minutes, and patients received on average 5 follow-ups (ranging from 1 to 20) during 14 weeks on average (ranging from 2 to 52 weeks). The phone follow-up included standardized questions regarding symptoms and side effects, individual advice based on the patients’ experience and measures, as well as adjustment in opioid-dose, based on standardized procedures and the individual treatment plan. If needed, the patients also received psychoeducation and cognitive therapy strategies (CBT) to better cope with psychosocial aspects of pain and opioid treatment. The experienced nurses had education in CBT, behavioral therapy, teaching and supervision and/or palliative care.
Patient Sample
In this study’s pilot, registry data was retrieved from 32 patients with CNCP who titrated or tapered opioids. Physicians at the pain clinic allocated patients to the nurse-led telephone program based on their discretionary evaluation of the patients’ need for support in analgesic treatment. The inclusion criteria were age 18 years or older, and the ability to understand and speak Norwegian. Patients were excluded if they had cognitive impairment prohibiting them from answering questionnaires or using a numeric rating scale. Data on the patients were retrieved from the Oslo University Hospital Pain Registry (OPR) from January 2017 until March 2020 and was used in the outcome analyses. OPR is a local registry at the largest university and interdisciplinary outpatient pain clinic in Norway.30 Data is included in the OPR if patients sign a written informed consent form before their first consultation at the clinic.31
Measures
The patients entered information on demography and health status by self-report questionnaires in the OPR before their first scheduled appointment at the pain clinic (baseline 1). In the phone follow-up, the nurse asked the patients about the total symptom burden, pain, sleep, opioid doses, and side effects related to titration or tapering of opioids. This information was registered by the nurse in the OPR and the electronic patient journal. In the pilot study, information collected by the nurse at the entry (baseline 2) and the last follow-up consultation was included in the analyses.
Demography
Patients provided information on age, gender, marital status, cohabitation, number of children, level of education, employment, social benefits, application for disability pension and litigation due to pain condition.
Health Status
The EQ-5D VAS is a quantitative measure of patients’ perceived health status.32 Patients self-rated their health on a vertical visual analogue scale, with the endpoints 100 (the best health you can imagine) and 0 (the worst health you can imagine). EQ-5D VAS, as a part of the EQ-5D questionnaire, has a high test-retest reliability of 0.8 (ie, intraclass correlation). A total score of 87.0–68.8 on the EQ-5D VAS is regarded as a normal range of self-perceived health status according to the Norwegian population norm.33
Opioids
The type and daily doses (in mg) of opioids were registered based on the patients’ medical journals, treatment plans, and self-reports.
Pain Intensity and Bothersomeness
The patients indicated their pain intensity since the last consultation on a numeric rating scale (NRS) with anchors of 0 (no pain) and 10 (worst imaginable pain), both when active and at rest. Patients also indicated how bothersome their pain was, using a NRS with anchors 0 (no bothersomeness) and 10 (worst imaginable bothersomeness). The reliability and validity of the 11-point NRS for pain intensity are well documented.34 The NRS allows comparison over time, is easy for the patient to understand and use, and is also commonly used to assess other aspects of pain than intensity.35
Sleep Satisfaction
Patients indicated how satisfied they were with their sleep since the last consultation on a reversed NRS with anchors of 10 (very satisfied) and 0 (not satisfied at all). NRS is commonly used to grade pain interference with function, such as sleep.35 The inverse use of the NRS to assess sleep satisfaction is a tailor-made measure for the OPR and is based on clinical experience.
Total Symptom Burden
Patients reported their experience of total symptom burden, comprising pain, side effects, and abstinences of opioid treatment. Patients ranged their answers on a 7-point Likert scale36 from much better to much worse. This Likert scale presents logically ranked answers based on clinical experience with medication follow-up of patients with CNCP.
Opioid Side Effects and Abstinences
The presence of side effects and abstinences with titration or tapering of opioids was identified from a list of 20 symptoms retrieved from a literature review,37–39 and were recorded as “present” or “not present” in this study. Additional symptoms not covered by the list were also registered.
Analyses
Data Analyses
Statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) (version 25). P<0.05 was considered statistically significant for all analyses. Descriptive statistics were used to describe the characteristics of the sample. Categorical variables were presented as frequencies and percentages. Continuous variables were presented with means and standard deviation (SD) for variables with normal distribution, and with median and interquartile range (IQR) for variables with skewed distribution.
To allow for comparison across different types of opioids, the doses were converted into Oral Morphine Milligram Equivalent (MME) using the morphine equivalent calculator of The Norwegian Health Economic Administration (HELFO).40
Independent students t-tests, Mann–Whitney U-tests, chi-square tests, and Fishers exact tests were used to compare the two patient groups (ie, those who titrated versus those who tapered opioids), on demographic and clinical characteristics. Paired sample t-tests were used to compare scores at baseline and the last follow-up for the three variables MME, number of side effects, and pain intensity.
The variable “age” was recoded from a continuous to a categorical variable and presented as frequencies. Opioids by “MME” were also recoded into a categorical variable and presented as frequencies with categories of 25–50 MME. The categorical variables marital status and education were collapsed and recoded into dichotomous variables, and Fischer’s exact tests were used to compare the groups.
Sample Size Calculation
An a-priory sample size calculation was based on information from the literature and results from the pilot study (ie, changes in pain intensity and MME). We used the MedCalc Software Ltd sample size calculator for paired t-test and proportions.41
For pain intensity, previous research suggests that a minimum clinically significant reduction in patient with chronic pain is 1–2 units or 30% on a 0–10 NRS.42 Being conservative but within clinical relevance, we set the minimum detected difference to 1.25 units. In the present feasibility study, an SD of 2.5 was found for pain intensity reduction at rest, thus chosen as basis for our sample size calculation. A difference of 1.25 and a SD of 2.5 gives a Cohen’s D of 0.5 which corresponds to a moderate effect size. With a power of 0.80, an alpha value of 0.05, we need a sample size of 34 patients. To compensate for potential dropouts and incomplete registrations, we add 10% to the estimated sample size. Thus, a total of 38 patients titrating opioids are needed.
There is no previous research estimating a minimal important clinical reduction of MME or other measures in patients with chronic pain.42 However, we consider a 30% reduction of MME as clinically relevant. In the present pilot study, 61% of patients achieved at least a 30% reduction of MME. For a one-sample proportion test assuming that the intervention reduces the proportion of patients achieving 30% MME reduction by an absolute difference of 30%, with a power of 0.08 and an alpha value of 0.05, we need a sample size of 14 patients. To compensate for potential dropouts and incomplete registrations, we add 10% to the estimated sample size. Thus, a total of 16 patients tapering opioids are needed.
Ethical Considerations
The study was conducted in accordance with the Declaration of Helsinki and inclusion of patient data in the OPR was approved by the Data Protection Officer at OUS (file number 20/08986).43 All participants provided electronically informed consent. Data were available for the pilot study after further approval by the Data Protection Officer at OUS (file number 19/15083), and access to the data was granted by the Head of Department of Pain Management and Research at OUS.
Results
According to the MRC framework, a feasibility assessment involves a process evaluation of areas of impact such as uncertainties, mechanisms of change, recruitment and retention, sample size, cost-effectiveness, and outcomes of the intervention.27,29
Feasibility of the Intervention
Uncertainties
We identified a lack of a sound theoretical basis as an uncertainty in the development and implementation phases of the intervention. Further, the physicians’ allocation of patients to the program was based on discretional evaluation of the patients’ individual needs rather than a fixed set of inclusion criteria. Interviews with the physicians revealed several rationales for the need for close follow-ups, such as high opioid doses and excess consumption, unstructured or lack of control of opioid consumption, and/or a need of support and help beyond the administration of opioids. Another uncertainty was variation in commencement of opioid dose titration or tapering (ie, before or after the first nurse consultation). This procedure led in some cases to an improvement or exacerbation of outcomes before baseline assessment, and possibly influenced the results from analyses of change between baseline and last follow-up. We also identified uncertainties concerning the assessment of outcomes. Some of the outcomes were tailor-made to the intervention and non-validated measures (ie, total symptom burden, sleep satisfaction, pain bothersomeness). In addition, the questionnaire of health status (EQ-5D) was filled out for the OPR at the first visit to the pain clinic and one year later, and not at the allocation to and completion of the nurse-led telephone program. These factors make it uncertain whether we have measured what we intended to, and other factors than the intervention may have an impact on the patients’ health status.
Mechanisms of Change
A major mechanism of change was the close interprofessional collaboration between nurses and physicians in adjusting the intervention and treatment plan to the patients’ needs according to effects and abstinences. In addition, the nurses’ knowledge and competencies in both medical and non-medical approaches (eg, opioid treatment, pain psychoeducation, CBT) enhanced the complex intervention as a more holistic and bio-psycho-social approach. The nurses’ availability for the patients’ questions and needs, and the application of a primary nursing model for pain management were also considered important factors for making changes.
Likely Rates of Recruitment and Retention
The number of patients who were asked and allocated to attend the program by the pain physicians (and those who declined) was not systematically recorded. However, from clinical experience, few patients declined participation or dropped out of the program. The recruitment of participants from the registry to the pilot study was satisfactory. A total of 78% (32/41) of the eligible participants titrating or tapering opioids were included in the analysis. The retention of participants in the pilot study was also satisfactory with all 32 patients completing the program from baseline to follow-up. Missing outcome data was neither a major issue, with 1–9 missing single items among the seven outcome variables at both baseline and follow-up.
Sample Size
The sample size calculations showed that 38 patients titrating opioids is sufficient to explore the change in pain intensity after a nurse-led telephone follow-up intervention. A small sample size of 16 patients tapering opioids is sufficient to explore the change in MME. The nurse-led intervention was highly effective with a proportion of 61% of the patients receiving at least 30% MME reduction, and therefore the sample size needed for further research is low.
Cost-Effectiveness
The nurse-led intervention is cost-effective in terms of lower salary expenses for nurses compared to physicians in the follow-up of patients. There are no travel costs to the outpatient clinic for the patients, and they need take less time off work for attending a consultation. There might also be long-term socio-economic benefits of successful treatment of chronic pain, in terms of less health-care use and expenses, as well as possible return to normal life and work.
Pilot Study Outcomes
Sociodemographic Characteristics
A total of 32 patients were included in the nurse-led follow-up program (Figure 3). Twelve (38%) titrated, and 20 (63%) tapered their opioids during the follow-up period.
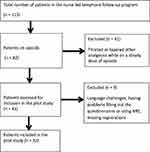 |
Figure 3 Patients included in the pilot study. |
A majority of patients were female (n = 22, 69%). Patients had a mean age of 60.0 years (SD = 19.9), with a range of 19–98 years. Results showed that 34% (n = 11) were between the age of 39–58. Those who tapered opioids were statistically younger than those who titrated opioids during follow-up (P=0.010). As many as 84% of the patients had children. The highest degree of education was high school in 52% (n = 16), 81% (n = 26) of the patients were unemployed, 34% (n = 11) received a full disability pension, and 17% (n = 5) had ongoing litigation due to their pain condition (Table 1).
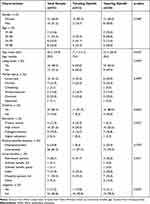 |
Table 1 Demographic and Socioeconomic Characteristics at Baseline |
Health Status
At baseline, patients rated their health status (EQ-5D VAS) to a mean of 39.6 (SD = 22.7).
Opioid Consumptions
In the total sample at baseline 46.9% (n = 15) used oxycodone, and 25% (n = 7) used tramadol (Figure 4). Patients who titrated their opioid doses had a mean increase of only 3.5 MME. Patients who tapered opioids had a statistically significant reduction of MME during the follow-up period (P=0.004), with a mean reduction of 30.8 MME (Table 2).
 |
Table 2 Opioid Consumption from Baseline to Last Follow-Up |
 |
Figure 4 Percentages of patients using different types of opioids at baseline. |
Change in Pain and Sleep
Reduction of mean pain intensity in activity and at rest was found in both groups, but the differences were not statistically significant. The reduction of NRS at rest for patients increasing their opioid doses was 1.5 (P=0.081). No change in pain bothersomeness and sleep satisfaction was found in any group during the follow-up (Table 3).
 |
Table 3 Change in Pain Intensity, Pain Bothersomeness, and Sleep Satisfaction from Baseline to Follow-Up |
Total Symptom Burden
At baseline, 44.4% of all patients reported “no change”, 22% reported “slightly better”, and 22% reported “slightly worse” total symptom burden. At follow-up, 33% of patients titrating opioids experienced no change in total symptom burden. As many as 22% felt slightly worse, while 33% felt slightly better. Of those tapering opioids, 31% felt no change in total symptom burden, 31% felt slightly worse and 31% felt slightly better. Only two patients felt moderately better or worse (Table 4).
 |
Table 4 Total Symptom Burden During Follow-Up |
Side Effects and Abstinences
For patients titrating opioids, nausea/vomiting, sleepiness, constipation, and dizziness were the most common side effects at baseline. Most side effects were less frequent at the last follow-up, except constipation and dry mouth. A significant increase in the number of side effects was observed for this group (P=0.038). For patients tapering opioids, constipation, sleepiness, nausea/vomiting and change in cognitive function – memory, were the most common side effects at baseline. Most side effects and abstinences were less frequent at the last follow-up, while constipation was equally frequent (Table 5). Eight symptoms from the literature list were not reported by the patients (ie, diarrhea, flatulence, cramps, change in cognitive function – confusion, impotence, libido, paresthesia, skin rash). A few patients reported “other” symptoms (ie, low energy, increased pain, anxiety).
 |
Table 5 Symptoms Associated with Adjustment of Opioid Doses |
Discussion
Discussion of Outcomes
The present study describes and evaluates the feasibility and outcomes of a nurse-led follow-up intervention for titrating or tapering opioids in patients with CNCP in an interprofessional outpatient clinic setting. Patients tapering opioids had a significant reduction in MME (30.8 mg) from baseline to follow-up, but no significant exacerbation of pain and sleep problems or more adverse effects. We believe these successful outcomes imply that the tapering of opioids was well controlled under nurse guidance through telephone follow-ups. The opioid doses, intervals tapering, as well as side effects and abstinences were assessed and dealt with considering the patients’ individual needs. Interestingly, patients tapering their opioid doses had insignificant, but some reduction in pain intensity and bothersomeness. These results may be due to coincidence and small sample size, or reasons not investigated in this pilot study, such as cessation of bothersome side effects or opioid-induced hyperalgesia,44,45 that high doses of opioids over a long period is ineffective in relieving CNCP intensity, and concomitant relieving effects of other analgesics in a multimodal treatment strategy as recommended for CNCP.3,46 The reduction in pain intensity may also come from additional pain-relieving effects of psychoeducation and cognitive strategies for better coping with pain, which is a central part in the nurse-led phone follow-up program. Future studies should look more into the suggested multimodal mechanisms of pain relief with opioid tapering as well as the nurses’ role and impact on opioid management in patients with CNCP.
Patients titrating opioids had only a small increase in MME from baseline to follow-up with no significant pain intensity relief when active or at rest. These findings may indicate that the nurses did not titrate the opioids sufficiently in order to relieve pain with activity. On the other hand, the minimal titration may be a result of well-controlled nursing follow-up with assessments, as well as handling of side effects and misuse behaviors. Note that opioids are often ineffective in relieving CNCP,46 and high doses of opioids are not necessarily more effective but a consequence of opioid tolerance development.7 Therefore, the nurse guidance and close follow-up may have prevented opioid titration to undesirably high doses, and opioid misuse problems among the patients in the study.
Neither pain intensity, pain bothersomeness, sleep satisfaction, nor total symptom burden changed considerably from baseline to follow-up while adjusting opioids. However, the number of side effects did increase significantly with titrating opioids, which is also found in previous research.46 One of the benefits of close follow-up is that the experienced nurse can assess the occurrence and severity of side effects and abstinences, adjust further titration or tapering, and initiate treatment to relieve bothersome symptoms. Note that in the present study the intensity and bothersomeness of the side effects were assessed by the nurses, but data were not retrievable from the OPR registry. Speculatively, the stable total symptom burden found in the present study may indicate that the bothersomeness of the side-effects of titrating opioids was minor and successfully treated. However, further data analyses are needed to fully understand how the various symptoms of opioid treatment affect the patients’ perceived total symptom burden.
Patients allocated to the nurse-led follow-up were mostly women (69%), all received social benefits, and 2/3 had lower levels of education. Patients also perceived their health to be very poor.47 The fact that the patients on opioids in the present study had poor socioeconomic status and health, maybe due to bias in small sample size, but is also supported by national and international research on opioid use in the general population48 and in patients with CNCP.7,47
In the current study, patients who titrated opioids were significantly older than those who tapered opioids. This finding may be explained by a treatment strategy to increase the quality of life and improve function for older patients when other analgesics (ie, NSAIDs, antiepileptics, antidepressants) are contraindicated. Interestingly, younger patients were more likely to be recommended tapering opioids. These findings may be due to younger people having the prospect of years of a normal work- and family life, which is often not comprehendible with effects and consequences of long-term opioid treatment (eg, fatigue, changes in cognitive function, endocrine dysfunction).49–51
Discussion of the Feasibility Assessment
To the best of our knowledge, this study is the first to assess the feasibility of a nurse-led follow-up program for titrating or tapering opioids in patients with CNCP in an interprofessional outpatient pain clinic setting, and doing so according to MRC framework for evaluating such interventions. Previous studies of nurse-led follow-up programs for opioid treatment have analyzed the outcomes and effects but not the feasibility of the intervention.24,25
The feasibility assessment in the present study aimed to illuminate the context, clarify areas of impact, and describe the outcomes of the intervention in order to evaluate whether the intervention was and can be effective in clinical practice.28 The process evaluation focused on the development, implementation and content of the intervention.29 One of the identified areas of impact was the lack of a sound theoretical basis as an uncertainty of the nurse-led telephone follow-up program. According to the MRC framework, best practice is to develop interventions based on evidence and theory. However, refining the theory basis along the development phases of the intervention is also considered acceptable and good practice.29 Therefore, in this feasibility study, we made an updated literature review to draw out an evidence-based theory basis. We also prepared a logic model (Figure 2) to visually describe the intervention and clarify important aspects of the program. The logic model will be a valuable tool in further work on developing and evaluating the nurse-led telephone follow-up program for medical pain treatment.28
This study also identified uncertainties regarding how the intervention was delivered, such as unclear allocation criteria, discretional evaluation of patients’ needs, and inconsistent commencement of titrating or tapering opioids (ie, before or after baseline measurements).28 Our evaluation revealed, however, that these practices work well in a clinical setting because they are tailored to a local context. The physicians’ immediate evaluation of the situation and the patients’ individual needs may be important factors for the successful outcome of the intervention. Today, the program functions in loose frames as opposed to the strict frames needed for efficacy studies. Therefore, this complex intervention may be suitable “as it is” for registry studies but not for a more controlled research design (eg, RCT). However, we do believe that following stricter criteria for allocation of patients and commencement of opioid titration/tapering is feasible in a period of data collection of a larger efficacy study.
In the present study, simple and unidimensional NRS- and Likert scales were used over the phone to assess the patients’ experience of pain intensity, pain bothersomeness, side-effects and abstinences, sleep satisfaction and total symptom burden – as recommended for the assessment of CNCP.52 These measurement tools were easy to explain and understand verbally without visual aids. Their outcomes contributed to a biopsychological understanding of the patients’ pain problems in the clinical setting.35,53 However, in a larger efficacy study of the complex intervention, it is necessary to improve the assessment procedure. To ensure trustworthy outcomes of effect, validated and multidimensional assessment tools for biopsychological aspects related to pain and its management should be implemented35 at appropriate time points for baseline and last follow-up of the opioid treatment.
A number of mechanisms of change were identified in the feasibility assessment, such as the interprofessional collaboration which provided the competency needed for managing CNCP patients on opioids. In addition, the nurses’ knowledge in opioid treatment, cognitive approaches, communication and psychoeducation skills were of uttermost importance for achieving desired changes and reach treatment goals. Note that our evaluation is based on stakeholders’ experiences and patients’ feedback rather than systematic data collection. Therefore, further research is needed to fully understand if and how these assumed mechanisms cause changes.
The purpose of the sample size calculations as part of the feasibility assessment, was to estimate the number of patients needed, as well as the commencement date, for a larger efficacy study. The analyses showed that 38 patients titrating opioids is sufficient to explore the change in pain intensity after a nurse-led telephone follow-up program. Interestingly, a small sample size of 16 patients is sufficient to explore the change in MME in patients tapering opioids. In terms of a larger study, we can estimate from the inclusion rate (20 patients from 2017 to 2020) that data withdrawal on patients tapering opioids can commence in March 2023. Due to the legacy of the opioid epidemic and changes in treatment routines of chronic pain in the pain clinic,54 we see a large reduction of patients titrating opioids from 2020. Therefore, it is not possible to estimate a commencement date for a larger study on patients titrating opioids.
The cost-effectiveness of a nurse-led telephone follow-up program is linked to expenses of salaries, travel, and possibly long-term health and societal benefits of improved pain condition and less opioid consumption. In the Norwegian health-care setting, opioid treatment and any follow-up would traditionally be carried out by specialist physicians and general practitioners whose salaries are higher than specialized nurses’. Involving nurses with competence in opioid treatment can therefore provide a qualitatively sound and less expensive treatment option. Conducting a follow-up over the phone is also cost-effective since these consultations save time, and travel-expenses. Although the opioid epidemic is per today not established in Norway,54 the individual and socioeconomic costs of long-term opioid treatment for CNCP must be considered. Successful alleviation of CNCP or less opioid consumption may lead to long-term benefits in terms of less health-care expenses and possible return to normal life and work. However, due to the complexity CNCP and opioid treatment, as well as numerous influencing factors, it is rather difficult to estimate concrete figures of cost effectiveness for a nurse-led follow-up intervention for titrating or tapering opioids.
Limitations
A major limitation of this pilot study is the generalizability of the results. The study sample was small; however, this limitation is justified as this study’s purpose was to evaluate the intervention nurse-led follow-up program, prepare the ground and improve the quality of a future larger study. In addition, the sample was drawn from one specialized outpatient pain clinic, and may thus involve more complex patients with CNCP than we usually find in a primary and secondary level of health care. The possibility also remains that the changes found in outcome variables may be caused by factors other than the follow-up program. Finally, while individualized care is emphasized in the process evaluation of the intervention, the important aspect of shared decision-making3 is not explored. Future feasibility assessment should also concern this important aspect of the intervention.
Conclusion
The present study evaluates the feasibility and pilots the outcomes of a nurse-led follow-up intervention for titrating or tapering opioids in patients with CNCP. Analyses of the outcomes show that patients tapering opioids had a significant reduction of MME. Patients who titrated opioids had a significant increase in number of side effects and were significantly older. The feasibility assessment revealed a number of areas of impact such as lack of a sound theory basis, unclear allocation criteria, as well as inconsistent use of non-validated measurement tools. Mechanisms of change were identified as interprofessional collaboration, nurses’ knowledge and competencies, as well as predictability and availability of the nurses. Sample size calculations indicated that 38 patients titrating opioids and 16 patients tapering opioids are needed to perform further efficacy studies. The intervention is also assumed cost-effective in terms of saving costs of salaries, travel, and time off work. In conclusion, challenges and strengths identified in the development, implementation and content of the nurse-led telephone follow-up intervention were manageable, and we believe that with the right adjustments and improvements this complex intervention is feasible for a larger efficacy study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The study is non-funded.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Pergolizzi J, Böger RH, Budd K, et al. Opioids and the management of chronic severe pain in the elderly: consensus statement of an international expert panel with focus on the six clinically most often used World Health Organization step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone). Pain Pract. 2008;8(4):287–313. doi:10.1111/j.1533-2500.2008.00204.x
2. Kaye AD, Jones MR, Kaye AM, et al. Prescription opioid abuse in chronic pain: an updated review of opioid abuse predictors and strategies to curb opioid abuse: part 1. Pain Physician. 2017;20(2s):S93–S109. doi:10.36076/ppj.2017.s109
3. Dowell D, Ragan KR, Jones CM, Baldwin GT, Chou R. Prescribing opioids for pain — the new CDC clinical practice guideline. N Engl J Med. 2022;387(22):2011–2013. doi:10.1056/NEJMp2211040
4. Noble M, Treadwell JR, Tregear SJ, et al. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst Rev. 2010;2010(1):Cd006605. doi:10.1002/14651858.CD006605.pub2
5. Brat GA, Agniel D, Beam A, et al. Postsurgical prescriptions for opioid naive patients and association with overdose and misuse: retrospective cohort study. BMJ. 2018;360:j5790. doi:10.1136/bmj.j5790
6. Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health pathways to prevention workshop. Ann Intern Med. 2015;162(4):276–286. doi:10.7326/m14-2559
7. Eriksen J, Sjøgren P, Bruera E, Ekholm O, Rasmussen NK. Critical issues on opioids in chronic non-cancer pain. PAIN. 2006;125(1):172–179. doi:10.1016/j.pain.2006.06.009
8. Berland D, Rodgers P. Rational use of opioids for management of chronic nonterminal pain. Am Fam Physician. 2012;86(3):252–258.
9. Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2):113–130. doi:10.1016/j.jpain.2008.10.008
10. Cordier Scott L, Lewis S. Opioids for chronic pain. JAMA. 2016;315(15):1672. doi:10.1001/jama.2016.3224
11. Helsedirektoratet. Organisering og drift av tverrfaglige smerteklinikker [Organizing and running interprofessional pain clinics]. Available from: https://www.helsedirektoratet.no/produkter/_/attachment/inline/0173dc62-5281-4ced-8da3-f28afe8ecaaa:4c4a5e20c8dac3ee001c5eef87e8d0c8d0ac16a6/Organisering%20og%20drift%20av%20tverrfaglige%20smerteklinikker%20%E2%80%93%20Veileder.pdf.
12. Leahy M, Krishnasamy M, Herschtal A, et al. Satisfaction with nurse-led telephone follow up for low to intermediate risk prostate cancer patients treated with radical radiotherapy. A comparative study. Eur J Oncol Nurs. 2013;17(2):162–169. doi:10.1016/j.ejon.2012.04.003
13. Meske DS, Lawal OD, Elder H, Langberg V, Paillard F, Katz N. Efficacy of opioids versus placebo in chronic pain: a systematic review and meta-analysis of enriched enrollment randomized withdrawal trials. J Pain Res. 2018;11:923–934. doi:10.2147/jpr.S160255
14. Heritage B, Harvey C, Brown J, et al. The use of telephone communication between nurse navigators and their patients. PLoS One. 2020;15(1):e0227925. doi:10.1371/journal.pone.0227925
15. Anderson B. The benefits to nurse-led telephone follow-up for prostate cancer. Br J Nurs. 2010;19(17):1085–1090. doi:10.12968/bjon.2010.19.17.78565
16. Szöts K, Konradsen H, Solgaard S, Bogø S, Østergaard B. Nurse-led telephone follow-up after total knee arthroplasty--content and the patients’ views. J Clin Nurs. 2015;24(19–20):2890–2899. doi:10.1111/jocn.12905
17. Gaines-Dillard N. Nurse led telephone follow-up improves satisfaction in motorcycle trauma patients. J Trauma Nurs. 2015;22(2):71–77. doi:10.1097/jtn.0000000000000110
18. Bobrow K, Farmer A, Cishe N, et al. Using the medical research council framework for development and evaluation of complex interventions in a low resource setting to develop a theory-based treatment support intervention delivered via SMS text message to improve blood pressure control. BMC Health Serv Res. 2018;18(1):33. doi:10.1186/s12913-017-2808-9
19. Heron KE, Smyth JM. Ecological momentary interventions: incorporating mobile technology into psychosocial and health behaviour treatments. Br J Health Psychol. 2010;15(Pt 1):1–39. doi:10.1348/135910709x466063
20. Hadi MA, Alldred DP, Briggs M, Marczewski K, Closs SJ. Effectiveness of a community based nurse-pharmacist managed pain clinic: a mixed-methods study. Int J Nurs Stud. 2016;53:219–227. doi:10.1016/j.ijnurstu.2015.09.003
21. Malmström M, Ivarsson B, Klefsgård R, Persson K, Jakobsson U, Johansson J. The effect of a nurse led telephone supportive care programme on patients’ quality of life, received information and health care contacts after oesophageal cancer surgery-A six month RCT-follow-up study. Int J Nurs Stud. 2016;64:86–95. doi:10.1016/j.ijnurstu.2016.09.009
22. Cox A, Faithfull S. Aiding a reassertion of self: a qualitative study of the views and experiences of women with ovarian cancer receiving long-term nurse-led telephone follow-up. Support Care Cancer. 2015;23(8):2357–2364. doi:10.1007/s00520-014-2578-4
23. Buhi ER, Trudnak TE, Martinasek MP, Oberne AB, Fuhrmann HJ, McDermott RJ. Mobile phone-based behavioural interventions for health: a systematic review. Health Educ J. 2012;72(5):564–583. doi:10.1177/0017896912452071
24. Bushey MA, Slaven JE, Outcalt SD, et al. Effect of medication optimization vs cognitive behavioral therapy among US veterans with chronic low back pain receiving long-term opioid therapy: a randomized clinical trial. JAMA Netw Open. 2022;5(11):e2242533. doi:10.1001/jamanetworkopen.2022.42533
25. Kroenke K, Krebs E, Wu J, et al. Stepped Care to Optimize Pain care Effectiveness (SCOPE) trial study design and sample characteristics. Contemp Clin Trials. 2013;34(2):270–281. doi:10.1016/j.cct.2012.11.008
26. Avery N, McNeilage AG, Stanaway F, et al. Efficacy of interventions to reduce long term opioid treatment for chronic non-cancer pain: systematic review and meta-analysis. BMJ. 2022;377:e066375. doi:10.1136/bmj-2021-066375
27. Craig P, Dieppe PM, Michie S. Developing and evaluating complex interventions: following considerable development in the field since 2006, MRC and NIHR have jointly commissioned an update of this guidance to be published in 2019. MRC Medical Research Council. Available from: https://mrc.ukri.org/documents/pdf/complex-interventions-guidance/.
28. Moore GF, Audrey S, Barker M, et al. Process evaluation of complex interventions: medical research council guidance. BMJ. 2015;350(h1258). doi:10.1136/bmj.h1258
29. Skivington K, Matthews L, Simpson SA, et al. A new framework for developing and evaluating complex interventions: update of medical research council guidance. BMJ. 2021;374:n2061. doi:10.1136/bmj.n2061
30. Regional kompetansetjenste for smerte. [Regional Advisory Unit on Pain Management]. Elektronisk kartlegging - OUS Smerteregister [Electronic assessment - OUS Pain Registry]; 2021. Available from: https://oslo-universitetssykehus.no/fag-og-forskning/nasjonale-og-regionale-tjenester/regional-kompetansetjeneste-for-smerte-reks/elektronisk-kartlegging-ous-smerteregister.
31. Granan L-P, Reme SE, Jacobsen HB, Stubhaug A, Ljoså TM. The Oslo University Hospital Pain Registry: development of a digital chronic pain registry and baseline data from 1712 patients. Scand J Pain. 2019;19(2):365–373. doi:10.1515/sjpain-2017-0160
32. Foundation E. EQ-5D-5L users guide. Available from: https://euroqol.org/publications/user-guides/.
33. Garratt AM, Hansen TM, Augestad LA, Rand K, Stavem K. Norwegian population norms for the EQ-5D-5L: results from a general population survey. Qual Life Res. 2022;31(2):517–526. doi:10.1007/s11136-021-02938-7
34. Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: a comparison of six methods. Pain. 1986;27(1):117–126. doi:10.1016/0304-3959(86)90228-9
35. Breivik H, Borchgrevink PC, Allen SM, et al. Assessment of pain. Br J Anaesth. 2008;101(1):17–24. doi:10.1093/bja/aen103
36. Nagler A, Schlueter J, Johnson C, et al. Calling for collaboration: piloting smartphones to discover differences between users and devices. Teach Learn Med. 2014;26(3):258–265. doi:10.1080/10401334.2014.910461
37. Relis. Produsentuavhengig legemiddelinformasjon for helsepersonell [Industry-independent drug information for health-care personel]; 2020. Available from: https://relis.no.
38. UpToDate.Lexicomp; 2021. Available from: https://www.wolterskluwer.com/en/solutions/lexicomp?gclid=EAIaIQobChMIk_ChkZSm_QIVA4fVCh3wnAiZEAAYASAAEgJdMvD_BwE.
39. Felleskatalogen. Felleskatalogen [The Norwegian Pharmaceutical Product Compendium]; 2021. Available from: https://www.felleskatalogen.no/medisin/.
40. Helfo. Morfinekvivalenter [Morphine Milligram Equivalent]; 2021. Available from: https://www.helfoweb.com/morfinekvivalenter/.
41. MedCalc easy-to-use statistical software; 2022. Available from: https://www.medcalc.org.
42. Farrar JT, Young JP
43. De nasjonale forskningsetiske komitteene [National Research Ethics Comittees]. Helsinkideklarasjonen [The Declaration of Helsinki]. Available from: https://www.forskningsetikk.no/retningslinjer/med-helse/helsinkideklarasjonen/.
44. Frank JW, Lovejoy TI, Becker WC, et al. Patient outcomes in dose reduction or discontinuation of long-term opioid therapy: a systematic review. Ann Intern Med. 2017;167(3):181–191. doi:10.7326/m17-0598
45. McPherson S, Lederhos Smith C, Dobscha SK, et al. Changes in pain intensity after discontinuation of long-term opioid therapy for chronic noncancer pain. Pain. 2018;159(10):2097–2104. doi:10.1097/j.pain.0000000000001315
46. Busse JW, Wang L, Kamaleldin M, et al. Opioids for chronic noncancer pain: a systematic review and meta-analysis. JAMA. 2018;320(23):2448–2460. doi:10.1001/jama.2018.18472
47. Landmark T, Dale O, Romundstad P, Woodhouse A, Kaasa S, Borchgrevink PC. Development and course of chronic pain over 4 years in the general population: the HUNT pain study. Eur J Pain. 2018;22(9):1606–1616. doi:10.1002/ejp.1243
48. Asfaw A, Alterman T, Quay B. Prevalence and expenses of outpatient opioid prescriptions, with associated sociodemographic, economic, and work characteristics. Int J Health Serv. 2020;50(1):82–94. doi:10.1177/0020731419881336
49. Els C, Jackson TD, Kunyk D, et al. Adverse events associated with medium- and long-term use of opioids for chronic non‐cancer pain: an overview of Cochrane reviews. Cochrane Database Syst Rev. 2017;10. doi:10.1002/14651858.CD012509.pub2
50. Dhingra L, Ahmed E, Shin J, Scharaga E, Magun M. Cognitive effects and sedation. Pain Med. 2015;16(Suppl 1):S37–S43. doi:10.1111/pme.12912
51. O’Rourke TK, Wosnitzer MS. Opioid-Induced Androgen Deficiency (OPIAD): diagnosis, management, and literature review. Curr Urol Rep. 2016;17(10):76. doi:10.1007/s11934-016-0634-y
52. Wenger S, Drott J, Fillipo R, et al. Reducing opioid use for patients with chronic pain: an evidence-based perspective. Phys Ther. 2018;98(5):424–433. doi:10.1093/ptj/pzy025
53. Pergolizzi JV, Varrassi G, Paladini A, LeQuang J. Stopping or decreasing opioid therapy in patients on chronic opioid therapy. Pain Ther. 2019;8(2):163–176. doi:10.1007/s40122-019-00135-6
54. Stubhaug A, Ljoså TM, Granan L-P, Magelssen Vambheim S. Opioidkrisen kan avblåses [The opioid crisis can be called off]. Tidsskr nor Laegeforen. 2021;13. doi:10.4045/tidsskr.21.0621
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
