Back to Journals » Journal of Inflammation Research » Volume 15
Novel Th17 Lymphocyte Populations, Th17.1 and PD1+Th17, are Increased in Takayasu Arteritis, and Both Th17 and Th17.1 Sub-Populations Associate with Active Disease
Authors Singh K, Rathore U , Rai MK, Behera MR , Jain N, Ora M , Bhadauria D , Sharma S, Pande G, Gambhir S, Nath A, Kumar S, Sharma A, Agarwal V , Misra DP
Received 26 December 2021
Accepted for publication 16 February 2022
Published 1 March 2022 Volume 2022:15 Pages 1521—1541
DOI https://doi.org/10.2147/JIR.S355881
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Video abstract presented by Durga Prasanna Misra.
Views: 375
Kritika Singh,1,* Upendra Rathore,1,* Mohit Kumar Rai,1 Manas R Behera,2 Neeraj Jain,3 Manish Ora,4 Dharmendra Bhadauria,2 Supriya Sharma,5 Gaurav Pande,6 Sanjay Gambhir,4 Alok Nath,7 Sudeep Kumar,8 Aman Sharma,9 Vikas Agarwal,1 Durga Prasanna Misra1
1Department of Clinical Immunology and Rheumatology, Sanjay Gandhi Postgraduate Institute of Medical Sciences (SGPGIMS), Lucknow, Uttar Pradesh, 226014, India; 2Department of Nephrology, Sanjay Gandhi Postgraduate Institute of Medical Sciences (SGPGIMS), Lucknow, Uttar Pradesh, 226014, India; 3Department of Radiodiagnosis, Sanjay Gandhi Postgraduate Institute of Medical Sciences (SGPGIMS), Lucknow, Uttar Pradesh, 226014, India; 4Department of Nuclear Medicine, Sanjay Gandhi Postgraduate Institute of Medical Sciences (SGPGIMS), Lucknow, Uttar Pradesh, 226014, India; 5Department of Surgical Gastroenterology, Sanjay Gandhi Postgraduate Institute of Medical Sciences (SGPGIMS), Lucknow, Uttar Pradesh, 226014, India; 6Department of Medical Gastroenterology, Sanjay Gandhi Postgraduate Institute of Medical Sciences (SGPGIMS), Lucknow, Uttar Pradesh, 226014, India; 7Department of Pulmonary Medicine, Sanjay Gandhi Postgraduate Institute of Medical Sciences (SGPGIMS), Lucknow, Uttar Pradesh, 226014, India; 8Department of Cardiology, Sanjay Gandhi Postgraduate Institute of Medical Sciences (SGPGIMS), Lucknow, Uttar Pradesh, 226014, India; 9Clinical Immunology and Rheumatology Services, Department of Internal Medicine, Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh, 160012, India
*These authors contributed equally to this work
Correspondence: Durga Prasanna Misra, Department of Clinical Immunology and Rheumatology, Sanjay Gandhi Postgraduate Institute of Medical Sciences (SGPGIMS), Lucknow, 226014, India, Tel +91 5222495273, Fax + 91 522-2668812, Email [email protected]
Purpose: We evaluated T helper lymphocyte profile, including novel Th17 subsets Th17.1 (secrete IFN-γ, associate with corticosteroid resistance) and PD1+Th17 (secrete TGF-β 1, implicated in fibrosis), and related cytokines in peripheral blood of Takayasu arteritis (TAK).
Materials and Methods: We evaluated circulating Th1, Th2, Th17, Th17.1, PD1+CD4+ T lymphocytes, PD1+Th17, and Treg lymphocytes, inflammatory (IFN-γ, IL-4, IL-6, IL-17A, IL-23, IL-1β, TNF-α) and regulatory (IL-10, TGF-β 1) cytokines in peripheral blood of TAK (n = 57; median age 35 (interquartile range 26– 45) years; 40 females) in a cross-sectional design. We studied inflammatory and regulatory cytokines in culture supernatant of peripheral blood mononuclear cells (PBMCs) from TAK following stimulation with anti-CD3/anti-CD28 and their modulation by tacrolimus (immunosuppressive) with/without tadalafil (anti-fibrotic). Furthermore, we followed up immunosuppressive-naïve active TAK (n = 16) and compared T helper lymphocyte populations and cytokines before and after immunosuppressive therapy. Healthy controls (HC, n = 21) and sarcoidosis (disease control, n = 11) were compared against TAK.
Results: TAK had higher Th17, Th17.1 and PD1+Th17 lymphocytes than HC (p < 0.001), and higher PD1+CD4+ T lymphocytes than sarcoidosis (p < 0.001). Th17 lymphocytes associated with active TAK after multivariable-adjusted logistic regression (p = 0.008). TAK had greater cytokine secretion from PBMCs (IFN-γ, IL-17A, IL-10 versus HC; IL-6, TNF-α, IL-1β versus HC or sarcoidosis) (p < 0.05). In-vitro, PBMCs from TAK showed reduced secretion of all inflammatory cytokines with tacrolimus, with synergistic reduction in IL-17A, IL-6, IL-1β and IL-10 following addition of tadalafil to tacrolimus. Serial follow-up of immunosuppressive-naïve TAK (n = 16) showed reduction in serum IL-6 and TGF-β 1 (p < 0.05) and IL-6 in culture supernatant (p < 0.05) following immunosuppressive therapy.
Conclusion: Novel Th17 sub-populations (Th17.1 and PD1+Th17) are elevated in TAK. Th17 lymphocytes associate with active TAK. In-vitro experiments on cultured PBMCs suggest promise for further evaluation of a combination of immunosuppressive tacrolimus with anti-fibrotic tadalafil (or other anti-fibrotic therapies) in clinical trials of TAK.
Keywords: arteritis, aortic arch syndromes, PD-1 protein, drug resistance, corticosteroid, fibrosis
Introduction
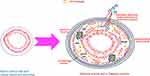
Takayasu arteritis (TAK), a large vessel vasculitis affecting aorta and its major branches, associates with considerable morbidity and increased mortality risk.1–5 Pathology is characterized by granulomatous vascular inflammation resulting in intimal hyperplasia, thickening of the media with inflammatory cell infiltrate and adventitial fibrosis.6–8 Vascular obstruction might result from either inflammatory wall thickening or fibrosis of the hitherto inflamed arterial wall. Less commonly, aneurysms develop in affected vessels.1,9,10 Assessment of disease activity and evaluation of biomarkers is challenging due to difficulty in accessing affected vessels other than during vascular bypass surgeries, therefore, relies on surrogate biomarkers, peripheral blood populations and cytokines.11
Vascular inflammation in TAK results from aberrant immune activation. The adaptive immune system, particularly T lymphocytes, are implicated in the pathogenesis of TAK as well as Giant Cell Arteritis (GCA).6,12,13 Gene signature of T lymphocyte activation associates with active TAK.14 Classically, T helper 1 (Th1) lymphocytes secreting interferon gamma (IFN-γ) have been implicated in vascular inflammation and granuloma formation in TAK.15 An increase in circulating Th17 lymphocytes as well as IL-17A in aortic biopsies from patients with TAK undergoing surgical bypass procedures have been reported recently.15,16 However, these Th17 lymphocytes from TAK were not corticosteroid responsive.15,16
The signature cytokine IL-17A secreted from Th17 lymphocytes attracts neutrophils to the site of inflammation.17,18 Over the past decade, multifarious functions of Th17 lymphocytes have been recognized. Ramesh et al described a sub-population of Th17 lymphocytes which also secreted IFN-γ (signature cytokine of Th1 lymphocytes) along with Th17 cytokines such as IL-17 and expressed the chemokine marker CCR6 (called as Th17.1 or “pathogenic Th17” lymphocytes).19,20 These Th17.1 lymphocytes were resistant to corticosteroids since they expressed the drug efflux protein p-glycoprotein [p-gp, also known as Multidrug Resistant Protein 1 (MRP-1), implicated in resistance to corticosteroid and other immunosuppressive therapy in myriad inflammatory diseases such as rheumatoid arthritis or systemic lupus erythematosus].19–23 Th17 lymphocytes are now also recognized to play a role in granuloma formation.24 Th17.1 lymphocytes are implicated in the pathogenesis of sarcoidosis.25,26 However, Th17.1 lymphocytes have not been reported previously in TAK.
Another recently described population of Th17 lymphocytes express programmed cell death 1 (PD1, classically considered as a marker of T lymphocyte exhaustion).27 However, it is now recognized that PD1+ CD4+ T lymphocytes as well as PD1+ Th17 lymphocytes secrete the regulatory, pro-fibrotic cytokine TGF-β1, and associate with lung fibrosis in sarcoidosis and idiopathic Pulmonary fibrosis (IPF). Celada et al demonstrated higher circulating PD1+ lymphocytes in the peripheral blood and lung tissue of patients with IPF than healthy controls. Peripheral circulating T lymphocytes secreted more TGF-β1 in IPF and sarcoidosis than healthy controls. Such T lymphocytes secreting TGF-β1 also expressed higher levels of PD1. Th17 lymphocytes expressing PD1 were identified as the major source of TGF-β1.28 PD1+ CD4+ T lymphocytes and PD1+ Th17 lymphocytes have not been reported in TAK. Recent literature also suggests that circulating markers of fibrosis associate with vascular damage in TAK.29 It is reasonable to hypothesize that PD1+ CD4+ T lymphocytes and PD1+ Th17 lymphocytes might drive vascular fibrosis in TAK. Other T lymphocyte populations that have been recently implicated in the pathogenesis of TAK include cytotoxic T lymphocytes as well as dysfunctional regulatory T lymphocytes (Treg).30,31
It is logical to hypothesize that the corticosteroid resistance demonstrated by Th17 lymphocytes in TAK might be due to Th17.1 lymphocytes since Th17.1 lymphocytes also express p-gp.32,33 Additionally, PD1+ Th17 lymphocytes might drive vascular fibrosis in TAK. In this context, we aimed to evaluate T helper lymphocyte profile and related cytokines in the peripheral blood of patients with TAK.
Materials and Methods
Ethics Approval and Participant Recruitment
The present study complies with the Declaration of Helsinki. The study was approved by the Institute Ethics Committee of Sanjay Gandhi Postgraduate Institute of Medical Sciences (SGPGIMS), Lucknow [document submission numbers PGI/BE/497/2017 (22nd June 2017) and PGI/BE/310/2019 (16th April 2019)]. Fifty-seven patients with TAK, 21 healthy controls and 11 patients with sarcoidosis (disease control group, where the role of Th17, PD1+ CD4+ T lymphocytes and PD1+ Th17 lymphocytes had been described before) were recruited between September 2017 and October 2021 into the study after obtaining written informed consent for study participation.28,34 Patients were recruited from the out-patient departments or wards of the Departments of Clinical Immunology and Rheumatology, Cardiology, Nephrology, Surgical Gastroenterology, Medical Gastroenterology and Pulmonary Medicine of SGPGIMS, Lucknow. Healthy controls were frequency matched for age and sex with TAK.
Objectives
The primary objective of the study was to compare Th17 lymphocyte populations between patients with TAK and healthy controls. Secondary objectives included comparison of the circulating T helper lymphocyte profile (Th1, Th2, Th17, Th17.1, PD1+ CD4+, PD1+ Th17 and Treg lymphocytes, individually as well as normalized in ratio to Treg lymphocytes), serum cytokines (IFN-γ, IL-4, IL-6, IL-17A, IL-23, IL-1β, TNF-α, IL-10, TGF-β1) and cytokines secreted from culture supernatant of PBMCs between TAK and healthy controls as well as between TAK and sarcoidosis. Other secondary objectives involved culture of PBMCs from participants followed by analysis of the cytokines IFN-γ, IL-4, IL-6, IL-17A, IL-23, IL-1β, TNF-α, IL-10 and TGF-β1 in the culture supernatant following stimulation of the T-cell receptor with anti-CD3/CD28 and its modulation by the addition of immunosuppressive medication tacrolimus or anti-fibrotic agent tadalafil singly or in combination. Sub-group comparisons were performed between patients with TAK with active or inactive disease, and between patients with TAK on immunosuppressive medication at study enrollment with those that were immunosuppressive medication-naïve. A subset of patients with active TAK who were immunosuppressive medication-naïve were longitudinally followed up to compare T helper lymphocyte profile and cytokines before and after immunosuppression.
Inclusion and Exclusion Criteria
Adult patients (≥18 years of age) with TAK fulfilling the American College of Rheumatology 1990 classification criteria for TAK or the 2012 Chapel Hill Consensus Conference definition of TAK were recruited into the study.9,10 Those patients who had active disease and were not on immunosuppressive medications were longitudinally followed up and sampled after initiation of immunosuppressive therapy. Consenting patients were recruited consecutively. Healthy controls were frequency-matched for age and sex with the patients of TAK. A further disease control group of sarcoidosis diagnosed by clinical presentation, supporting radiology with or without histopathological confirmation of granulomatous inflammation were also included. Patients who were critically ill and therefore unable to provide informed consent or those belonging to vulnerable populations such as pregnant women or children were excluded from the study.
Disease Activity Assessment
Disease activity assessment utilized a composite of Indian Takayasu Clinical Activity Score (ITAS2010) of one or more, active extravascular disease such as uveitis or erythema nodosum, evidence of metabolic activity to suggest active large vessel vasculitis on positron emission tomography computerized tomography or evidence of clinical progression and angiographic worsening of disease extent on serial imaging.1,11,35,36
Antibodies Used for T-Lymphocyte Stimulation and Intracellular Staining
CD3 Monoclonal Antibody (Cat No.16–0037-85, eBioSciencesTM), Purified NA/LE Mouse Anti-human CD28 (Cat No. 555725), anti-human CD4 BV510 (Cat. No. 563094), anti-human CD279 (PD1) BV421 (Cat. No. 562516), anti- Human IFN-γ PE-Cy7 (Cat. No. 557844), anti-human IL-4 APC (Cat. No. 554486), anti-human IL-17A Alexa Fluor 488 (Cat. No. 560488), anti-human CD25 PE-Cy7 (Cat. No. 557741), anti-human FoxP3 Alexa Fluor 647 (Cat. No. 560045), anti-human CD4 FITC (Cat. No. 555346) were all purchased from Pharmingen BD Biosciences.
Activation of CD4+ Lymphocytes and in-vitro Culture
Cytotoxicity and viability of the cells were assessed as per previously standardized protocol.37 Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Hypaque density gradient centrifugation. The cells were counted by using haemocytometer and then cells were re-suspended (106 cells/mL) in complete RPMI medium (Roswell Park Memorial Institute; Cat No-R6504), containing 10% fetal bovine serum (FBS; Cat No-10270106) with 1% antibiotic. The cells were cultured in 6-well plates which were pre-coated (for 24 hr at 37°C with 5% CO2 in incubator) with anti-CD3 (2.5 μg/mL final concentration in 1mL) monoclonal antibody. Subsequently mouse anti-human CD28 mAb was added (5 μg/mL). Thereafter, cells were treated with tacrolimus (10mM; Cat. No. F4679), tadalafil (PDE-5 inhibitor-10Mm; Cat.No. SML1877) or combination of both tacrolimus and tadalafil for 48 hours. After completion of incubation, the cultures were transferred to micro centrifuge tubes and centrifuged to collect the culture supernatant for cytokine analysis.
Flow Cytometry Analysis
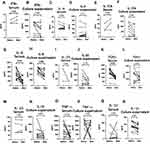
Intracellular staining of T helper lymphocyte subsets was done after 48-hour incubation of whole blood in complete RPMI culture media by the activation of CD3 and CD28 monoclonal antibodies. After that, red blood corpuscles (RBC) were lysed by using 1X RBC Lysis Buffer for 10 minutes at room temperature. Cells were incubated with CD4 (FITC for T regulatory, BV510 for other T lymphocyte populations), anti-human CD279 (PD1) and anti human CD25 (PE-Cy7) for surface stain and subsequently cells were fixed and permeabilized by using Cytofix/Cytoperm W/Golgi Stop (554715, BD Biosciences). For intracellular T helper lymphocyte subset staining, cells were incubated with anti-human IL-4 (APC), anti-human IFN-γ (PE-Cy7), anti-human IL-17A (Alexa Fluor 488) and anti-human FoxP3 (Alexa Fluor 647) for T-regulatory lymphocytes. Cells were finally resuspended in 500 μL of 1X PBS to acquisition on a BD FACS Canto II (BD BioSciences, USA) or BD FACSLyricTM (BD Biosciences,USA) flow cytometer. Th1 lymphocytes were identified as CD4+ IFN-γ+, Th2 lymphocytes were CD4+ IL-4+, Th17 lymphocytes were CD4+ IL-17A+, Th17.1 lymphocytes were CD4+ IL17A+ IFN-γ+, PD1+ T helper lymphocytes were CD4+ PD1+, PD1+Th17 lymphocytes were CD4+ IL-17A+ PD1+ and T regulatory lymphocytes were CD4+ CD25+ FoxP3+. Figure 1 provides representative flow cytometry plots for each of the T helper lymphocyte populations.
Cytokine Profile
The levels of IFN-γ [Catalogue number (Cat. No.) DY285B], TNF-α (Cat. no. DY210), IL-17 (Cat. no. DY317), IL-6 (Cat. no. DY203), IL-4 (Cat. no. DY204), IL-10 (Cat. no. DY417), IL-1β (Cat. no. DY401), IL-23 (Cat. no. DY1290) and TGF-β1 (Cat. no. DY240) in serum and culture supernatant of TAK patients, disease control group and in healthy donors were determined using commercially available enzyme-linked immunosorbent assays (ELISA, from R&D Systems, USA and Canada) according to the manufacturer’s instructions. Biotek ELISA reader ELX800 (Biotek, VT, USA) was used to obtain the optical density of each sample at wavelength of 450 nm.
Sample Size Calculation and Statistical Analysis
Sample size calculation was based on the primary objective of comparison of Th17 lymphocyte population between patients with TAK and healthy control using an online sample size calculator.38 In a previous study conducted by us, mean Th17 lymphocytes (as a percentage of CD4+ T lymphocytes) in patients with TAK was 2.87±2.07% and 0.87±0.61% in healthy controls.16 The pooled effect size was 2% and pooled standard deviation was 1.65%. Assuming a ratio of 8:3 between patients with TAK and healthy controls with α error (level of significance) of 0.05 and study power of 80%, the minimum sample size calculated as 22 patients with TAK and 8 healthy controls. In our study, we recruited patients with TAK and healthy controls in the same proportion as above (57 TAK and 21 healthy controls).
Statistical analyses were conducted using Prism 9 for macOS (version 9.3.1). The presented data was summarized using medians with interquartile range (25th quartile – 75th quartile). Proportions were compared using Chi-square test or Fisher’s exact test. Non-parametric tests were used for comparison viz. Mann–Whitney test for unpaired samples and Wilcoxon matched-pairs signed rank test for paired samples. A p value of ≤0.05 was considered as statistically significant. Whenever multiple comparisons were performed for a set of related variables, the p values were further corrected for multiple testing using the Bonferroni-Sidak method. These analyses were conducted using Prism 9 for macOS (version 9.3.1). Logistic regression analyses were conducted using Stata/IC 16.1 for Mac to identify multivariable-adjusted associations for disease activity in TAK taking into account C-reactive protein and either T lymphocyte populations or cytokines in different regression models.
Results
Characteristics of Study Participants
Fifty-seven patients with TAK [median age 35 (26–45) years; 40 females and 17 males], 21 healthy controls [median age 32 (29.5–35) years; 15 females and 6 males], and 11 granulomatous disease control patients with sarcoidosis [median age 54 (46–56) years; 7 females and 4 males] were recruited. The age and gender of patients with TAK and healthy controls were comparable (p=0.616 for Mann–Whitney test for age, p=0.914 for Chi-square test for sex). Patients with sarcoidosis were older although of similar distribution of sex when compared with TAK (p<0.001 for Mann–Whitney test for age, p=0.727 for Fisher’s exact test for sex).
Vascular bruits, hypertension, pulse loss, pulse inequality and constitutional symptoms were the most prevalent clinical features. The commonest angiographic subtype (Numano’s) was Type V (58%) followed by Type I (19%).39 Twenty-four patients (42%) were treatment-naïve, whereas 30 (53%) had active disease (Table 1). Sixteen immunosuppressive treatment-naïve TAK with active disease were serially followed up to analyze T lymphocyte populations and cytokines before and after immunosuppressive therapy. The median duration of follow-up for these patients was 0.21 (0.13–2.52) years. All these patients had been treated with corticosteroids (corticosteroids were ongoing in 15/16 at the time of follow-up sample collection). Nine of the patients were on tacrolimus alone, two were on a combination of tacrolimus and methotrexate, whereas one was on methotrexate alone. At the time of follow-up sample collection, TAK was clinically inactive in 14/16 patients.
 |
Table 1 Characteristics of Patients with Takayasu Arteritis |
Cross-Sectional Analyses
T Lymphocyte Populations
Th1, Th2, Th17, PD1+CD4+ and Treg populations were increased in TAK when compared with healthy controls (individually as well as normalized to Treg lymphocytes). Novel Th17 populations viz. Th17.1 and PD1+Th17 lymphocytes were also elevated in TAK when compared with healthy controls (individually as well as normalized to Treg lymphocytes). Compared to the disease control group of sarcoidosis, patients with TAK had higher Th2, PD1+CD4+ and Treg populations (individually as well as normalized to Treg lymphocytes). Th17 and Th1 lymphocytes were higher in TAK than in sarcoidosis individually but not when normalized against Treg lymphocytes (Figure 2).
Cytokines
The cytokines IFN-γ, IL-4, IL-17A, IL-23, IL-1β and IL-10 were undetectable in serum in most patients and controls. IL-6 was higher in serum of patients with TAK when compared with healthy controls or sarcoidosis. TNF-α was higher in serum of TAK patients than healthy controls, but was similar to sarcoidosis. TGF-β1 was similar in patients with TAK when compared with sarcoidosis or healthy controls (Figure 3).
Previous studies have reported similar circulating serum cytokine levels between patients with TAK and healthy controls, possibly because serum cytokines do not accurately represent vascular wall inflammation in TAK.40 Therefore, we also compared the level of cytokines in culture supernatant of PBMCs from patients with TAK, healthy controls and sarcoidosis after stimulating them with anti-CD3/anti-CD28. In the culture supernatant, IFN-γ, IL-17A, IL-10, IL-6, TNF-α and IL-1β were elevated in TAK when compared with healthy controls. However, only IL-6, TNF-α and IL-1β were elevated in TAK when compared with sarcoidosis (Figure 3).
Comparison Between Active and Inactive TAK
Patients with active TAK had higher circulating Th17 lymphocytes (individually as well as normalized to Treg lymphocytes) when compared with those with inactive disease. When taken alone, Th17.1 lymphocytes were increased in active TAK than in inactive TAK but were not significantly different when normalized against Treg lymphocytes. None of the cytokines in serum or in culture supernatant were significantly different between active and inactive TAK (Table 2).
 |
Table 2 Comparison of T Helper Lymphocyte Profiles and Cytokines Between Patients with Takayasu Arteritis with Active and Inactive Disease |
Logistic regression analyses were undertaken to identify multivariable-adjusted associations of disease activity. ITAS2010 was excluded from logistic regression model as it demonstrated a perfect correlation with disease activity (it was a part of the disease activity assessment). In a logistic regression model adjusted for C-reactive protein, Th1, Th2, Th17 and Treg lymphocyte populations, only Th17 lymphocytes remained significantly associated with disease activity (p=0.008) (Table 3). The other logistic regression models using Th17.1 or PD1+Th17 lymphocytes in place of Th17 lymphocytes did not show any significant adjusted associations with disease activity for any of the covariates in the model. The logistic regression models using serum cytokines included only C-reactive protein with IL-6, TNF-α and TGF-β1 as covariates, since the other cytokines were mostly undetectable in serum of TAK. This model showed no significant adjusted associations with disease activity (Table 3). It was not possible to construct an adequately powered logistic regression model for C-reactive protein and cytokines in culture supernatant of PBMCs due to data sparsity.41
 |
Table 3 Logistic Regression Analysis for Associations of Disease Activity in Takayasu Arteritis |
Comparison Between TAK on Immunosuppressive Treatment with Those Not on Such Treatment
None of the T lymphocyte populations studied, serum cytokines or cytokines in culture supernatant were significantly different between patients of TAK receiving or not receiving immunosuppressive treatment (Table 4).
 |
Table 4 Comparison of T Helper Lymphocyte Profiles and Cytokines Between Patients with Takayasu Arteritis Who Were Immunosuppressive Treatment-Naïve and Those on Immunosuppressive Therapy |
In-vitro Stimulation Experiments on PBMCs from TAK and Modulation by Tacrolimus and Tadalafil
In-vitro stimulation experiments were performed on isolated PBMCs from 52 patients with TAK. All the cytokines studied were significantly increased following stimulation with anti-CD3/anti-CD28 when compared with baseline. Further treatment with tacrolimus significantly reduced the levels of all cytokines except TGF-β1 when compared with the stimulated state. Tadalafil alone significantly reduced the levels of IL-6, IL-1β and IL-10 when compared with the stimulated state. A combination of tacrolimus and tadalafil demonstrated a synergistic effect to further significantly reduce the levels of IL-17A, IL-6, IL-1β and IL-10 from the stimulated state when compared with tacrolimus alone (Figure 4).
Longitudinal Analyses
Comparison Between Patients with TAK Before and After Immunosuppressive Therapy
Th1, Th2, Th17, PD1+ CD4+ T lymphocytes, Th17.1 and PD1+ Th17 lymphocytes all reduced significantly following immunosuppressive therapy individually but not when normalized against Treg lymphocytes. Normalised Th17, Th17.1 and PD1+Th17 lymphocyte populations tended to reduce following immunosuppressive therapy but did not achieve statistical significance when p values were corrected for multiple testing [p value unadjusted (adjusted) for Wilcoxon matched-pairs signed rank test 0.074 (0.369), 0.055 (0.290) and 0.034 (0.188), respectively] (Figure 5).
Serum cytokines IL-6 and TGF-β1 significantly reduced following immunosuppressive therapy. In culture supernatant, IL-6 significantly reduced following immunosuppressive therapy (Figure 6).
Discussion
Our study identified higher circulating overall Th17 lymphocytes and novel Th17 lymphocyte populations viz. Th17.1 and PD1+Th17 lymphocytes in TAK when compared with healthy controls. PD1+CD4+ T lymphocytes were elevated in TAK when compared with disease control of sarcoidosis.28 Cytokines secreted by adaptive [IFN-γ (by Th1 and Th17.1), IL-17A (by Th17), IL-10 (by Treg), when compared with healthy controls] and innate immune cells (IL-6, TNF-α and IL-1β, when compared with healthy controls or sarcoidosis) were elevated in TAK. Th17 and Th17.1 lymphocyte populations associated with active TAK on univariable analyses. However, only Th17 lymphocytes remained associated with active TAK after multivariable-adjusted logistic regression analyses. In-vitro experiments on isolated PBMCs from TAK identified a reduction in all the inflammatory cytokines following treatment with tacrolimus. While the reduction of cytokines associated with inflammatory T lymphocyte populations with tacrolimus treatment can be expected, the reduction in the regulatory cytokine IL-10 (predominantly secreted by T regulatory lymphocytes) was intriguing, possibly related to pan-T lymphocyte suppressant effect of tacrolimus by reducing IL-2 secretion.42 Interestingly, a synergistic effect of further significant reduction in IL-17A, IL-6, IL-1β and IL-10 was noted when tadalafil was added to tacrolimus. Serial follow-up samples from immunosuppressive-naïve TAK identified a reduction in cytokines involved in Th17 polarization ie IL-6 and TGF-β1 in serum, and IL-6 in culture supernatant following immunosuppressive therapy.
Saadoun et al reported increased circulating Th17 lymphocytes and greater secretion of Th17-related cytokines from cultured PBMCs of TAK when compared with healthy controls. Moreover, treatment of in-vitro cultured PBMCs from healthy controls with sera from active TAK induced greater Th17 polarization than sera from inactive TAK or from healthy controls. TAK had higher IL-17A expression in three aortic tissue samples.15 Misra et al reported increased circulating Th17 lymphocytes, serum IL-17A and IL-23 in TAK versus healthy controls, however, these did not associate with active disease.16 Goel et al reported similar levels of IL-17 and IL-23 in patients with TAK with and without active disease.40 Our findings affirm the elevation of circulating Th17 lymphocytes in TAK. To the best of our knowledge, we have reported for the first time elevation of recently identified Th17 populations viz. Th17.1 and PD1+ Th17 lymphocytes, as well as overall PD1+CD4+ T lymphocytes in TAK.
Both Saadoun et al and Misra et al observed the Th17 lymphocytes and cytokines failed to reduce following glucocorticoid therapy in TAK, in contrast to corticosteroid-responsive Th17 lymphocytes in GCA.15,16,43 Increased circulating Th17.1 lymphocytes might explain corticosteroid resistance of Th17 lymphocytes in TAK. Literature suggests that Th17.1 lymphocytes secrete IFN-γ in addition to IL-17A and express the efflux protein p-glycoprotein, which might be responsible for corticosteroid resistance.19 In line with previous observations by Saadoun et al and contrary to those of Misra et al, Th17 lymphocyte population associated with active TAK.15,16 This discrepancy with Misra et al might relate to the use of only clinical disease activity scales in the former study, whereas, the present study also used imaging modalities including positron emission tomography computerized tomography.16 Such discrepancy might also relate to the considerably larger sample size of the present study when compared with Misra et al.16 In the present study, Th17, Th17.1 and PD1+ Th17 lymphocytes appeared to reduce following immunosuppressive therapy, contrary to prior observations of Saadoun et al and Misra et al15,16 Eleven of our sixteen patients were on tacrolimus along with corticosteroids. Tacrolimus inhibits the expression and function of p-glycoprotein in other treatment-refractory immune-mediated inflammatory diseases such as rheumatoid arthritis.44 The reduction in Th17 as well as Th17.1 lymphocytes in our patients following treatment with corticosteroids and tacrolimus supports our hypothesis that expression of p-glycoprotein might underlie corticosteroid resistance of Th17 lymphocytes in TAK. These findings also highlight the potential for the evaluation of tacrolimus as an immunosuppressive agent in TAK in future clinical trials. As a proof of concept, tacrolimus reduced the levels of inflammatory cytokines (including those involved in the Th17-IL-17 axis viz. IL-6 and IL-17A) from cultured, in-vitro stimulated PBMCs of TAK in our study.
Vascular wall inflammation in TAK resolves by fibrosis resulting in vascular stenosis.6 It is possible that PD1+CD4+ and PD1+ Th17 lymphocytes might drive vascular fibrosis in TAK. Our novel observation of an increased population of PD1+CD4+ and PD1+ Th17 lymphocytes in TAK when compared with healthy controls or with disease control group of sarcoidosis might have relevance from a therapeutic viewpoint. Interestingly, in-vitro experiments on cultured PBMCs from TAK identified a synergistic effect of reduction of IL-17A, IL-6, IL-1β and IL-10 when tadalafil was added to tacrolimus. Our previous experiments had identified the anti-fibrotic effect of tadalafil on cultured human adult dermal fibroblasts from scleroderma (a disease which causes systemic fibrosis in skin, lung and other internal organs).45 At present, no immunosuppressive drug has been proven to be effective in clinical trials of TAK.46 Tocilizumab (IL-6 receptor blocker) and abatacept (T lymphocyte co-stimulation blocker via agonism of the cytotoxic T lymphocyte antigen 4) failed to meet primary endpoints in randomized controlled trials.47,48 Based on observational studies, anti-tumor necrosis factor-alpha (anti-TNF) agents and tocilizumab achieve clinical response in nearly three-fourth patients, although their effect on angiographic stabilization is less defined.49 Sub-optimal angiographic response with currently used immunosuppressive therapies in TAK lends credence to the hypothesis that adding an anti-fibrotic therapy to immunosuppressive therapy might favorably modulate vascular remodeling by reducing fibrosis consequent to inflammation-associated vascular injury. Costly biological therapies are inaccessible in vast swathes of the globe including India. The use of tacrolimus in patients with TAK was based on our favourable experience with the use of this drug in other diseases associated with corticosteroid resistance such as steroid-resistant nephrotic syndrome.50 Results from the in-vitro experiments conducted on PBMCs in our study demonstrate the potential for the combination of tacrolimus (or other immunosuppressants) with anti-fibrotic tadalafil (or with other anti-fibrotic agents such as pirfenidone or nintedanib, or other immunosuppressive agents with anti-fibrotic potential such as tocilizumab).51–53 These require further exploration as a therapeutic regimen in TAK in clinical trials before translation into clinical practice. Figure 7 depicts potential roles played by Th17 lymphocytes in driving vascular inflammation of TAK, including granuloma formation by secreting IFN-γ from Th17.1 lymphocytes.19,54
There were limitations to our study. Peripheral blood populations of immune cells and circulating cytokines need not necessarily reflect ongoing vascular wall inflammation. This is an inherent limitation of TAK studies, since the site of inflammation viz. large vessels are largely inaccessible for direct histopathological examination. We attempted to address this issue by culturing in-vitro PBMCs from patients with TAK and stimulating the T-cell receptor with anti-CD3/ anti-CD28 to study the behavior of such activated cells. We also evaluated potential therapeutic modalities in vitro by using tacrolimus or tadalafil alone or in combination in the PBMC cultures. Another limitation was that only sixteen patients with active TAK who were immunosuppressive-medication naïve were followed up longitudinally. However, TAK is a rare disease, and the ongoing coronavirus disease 19 pandemic further hampered recruitment of treatment-naïve TAK. A third limitation was that we have not isolated Th17.1 and Th17 PD1+ T lymphocytes from peripheral blood and examined their expression of p-gp (Th17.1) and TGF-β1 (Th17 PD1+), since these experiments were beyond the scope of the present study. The disease control group included sarcoidosis of older age than TAK, however, sarcoidosis inherently affects older age groups.55 Another suitable control group might have been GCA (which is a large vessel vasculitis predominantly affecting older individuals). This was not feasible as GCA is uncommon in India.56 A strength of our study was disease activity assessment using imaging modalities including positron emission tomography computerized tomography in addition to clinical scales. Discordance between traditionally used inflammatory markers (erythrocyte sedimentation rate and C-reactive protein) and vascular inflammation in TAK necessitates the use of multiple modalities to accurately assess disease activity in TAK.36,57,58 A disease control group of sarcoidosis (which causes lung and major organ fibrosis) was particularly relevant due to the previous description of PD1+CD4+ and PD1+Th17 lymphocytes as drivers of fibrosis in sarcoidosis.28 Our study utilized multiple approaches to study pathophysiological processes, viz. T lymphocyte populations, serum cytokines and cytokines in culture supernatant from PBMCs as well as in-vitro evaluation of therapeutic modalities on PBMC cultures.
The multifarious roles played by Th17 lymphocytes in human disease merit their further evaluation in TAK. Isolating T lymphocytes from peripheral blood of TAK and studying their gene expression profile using transcriptomics could provide further clues to pathological processes operational in TAK. Such an approach has been successfully used to characterize T regulatory dysfunction in GCA.59 In the light of in-vitro experiments supporting the potential use of tacrolimus and tadalafil in TAK, future clinical trials in TAK might consider evaluating tacrolimus along with tadalafil or other anti-fibrotic therapies (nintedanib, pirfenidone).
Conclusion
Th17 lymphocytes and their sub-populations of Th17.1 and PD1+ Th17 lymphocytes are elevated in TAK. Th17 lymphocytes also associate with disease activity in TAK. In-vitro experiments on cultured PBMCs from TAK suggest the need to further evaluate a combination of immunosuppressive medication with tadalafil or other anti-fibrotic agents in clinical trials of TAK.
Prior Conference Presentations
Part of the work in this paper was presented as an oral presentation at the Indian Rheumatology Association (IRACON) Online Virtual conference in December 2021.
Ethics Approval and Informed Consent
The present study complies with the Declaration of Helsinki. The study was approved by the Institute Ethics Committee of Sanjay Gandhi Postgraduate Institute of Medical Sciences (Sgpgims), Lucknow [document submission numbers PGI/BE/497/2017 (22nd June 2017) and PGI/BE/310/2019 (16th April 2019)]. All subjects were included into the study after obtaining written informed consent for participation.
Acknowledgments
Durga Prasanna Misra would like to acknowledge the support of his late father Dr. Sasanka Sekhar Misra for his academic endeavours.
Funding
This work was supported by a research grant from the Indian Council of Medical Research (ICMR) – grant number 5/4/1-2/2019-NCD-II – to Durga Prasanna Misra.
Disclosure
Dr Durga Prasanna Misra reports grants from Indian Council of Medical Research, during the conduct of the study. The authors report no other potential conflicts of interest in this work.
References
1. Misra DP, Wakhlu A, Agarwal V, Danda D. Recent advances in the management of Takayasu arteritis. Int J Rheum Dis. 2019;22(Suppl 1):60–68. doi:10.1111/1756-185X.13285
2. Watts RA, Hatemi G, Burns JC, Mohammad AJ. Global epidemiology of vasculitis. Nat Rev Rheumatol. 2022;18(1):22–34. doi:10.1038/s41584-021-00718-8
3. Mirouse A, Biard L, Comarmond C, et al. Overall survival and mortality risk factors in Takayasu’s arteritis: a multicenter study of 318 patients. J Autoimmun. 2019;96:35–39. doi:10.1016/j.jaut.2018.08.001
4. Comarmond C, Biard L, Lambert M, et al. Long-term outcomes and prognostic factors of complications in Takayasu arteritis: a multicenter study of 318 patients. Circulation. 2017;136(12):1114–1122. doi:10.1161/CIRCULATIONAHA.116.027094
5. Misra DP, Rathore U, Patro P, Agarwal V, Sharma A. Patient-reported outcome measures in Takayasu arteritis: a systematic review and meta-analysis. Rheumatol Ther. 2021;8(3):1073–1093. doi:10.1007/s40744-021-00355-3
6. Arnaud L, Haroche J, Mathian A, Gorochov G, Amoura Z. Pathogenesis of Takayasu’s arteritis: a 2011 update. Autoimmun Rev. 2011;11(1):61–67. doi:10.1016/j.autrev.2011.08.001
7. Rathore U, Thakare DR, Patro P, Agarwal V, Sharma A, Misra DP. A systematic review of clinical and preclinical evidences for Janus kinase inhibitors in large vessel vasculitis. Clin Rheumatol. 2022;41(1):33–44. doi:10.1007/s10067-021-05973-4
8. Zhang H, Watanabe R, Berry GJ, Tian L, Goronzy JJ, Weyand CM. Inhibition of JAK-STAT signaling suppresses pathogenic immune responses in medium and large vessel vasculitis. Circulation. 2018;137(18):1934–1948. doi:10.1161/CIRCULATIONAHA.117.030423
9. Arend WP, Michel BA, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum. 1990;33(8):1129–1134. doi:10.1002/art.1780330811
10. Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65(1):1–11. doi:10.1002/art.37715
11. Misra DP, Misra R. Assessment of disease activity in Takayasu’s arteritis. Indian J Rheumatol. 2015;10(5):S43- S47. doi:10.1016/j.injr.2015.08.006
12. Uppal SS, Verma S. Analysis of the clinical profile, autoimmune phenomena and T cell subsets (CD4 and CD8) in Takayasu’s arteritis: a hospital-based study. Clin Exp Rheumatol. 2003;21(6 Suppl 32):S112–116.
13. Piggott K, Deng J, Warrington K, et al. Blocking the NOTCH pathway inhibits vascular inflammation in large-vessel vasculitis. Circulation. 2011;123(3):309–318. doi:10.1161/CIRCULATIONAHA.110.936203
14. Tian Y, Li J, Tian X, Zeng X. Using the co-expression network of T cell-activation-related genes to assess the disease activity in Takayasu’s arteritis patients. Arthritis Res Ther. 2021;23(1):303. doi:10.1186/s13075-021-02636-2
15. Saadoun D, Garrido M, Comarmond C, et al. Th1 and Th17 cytokines drive inflammation in Takayasu arteritis. Arthritis Rheumatol. 2015;67(5):1353–1360. doi:10.1002/art.39037
16. Misra DP, Chaurasia S, Misra R. Increased circulating Th17 cells, serum IL-17A, and IL-23 in Takayasu Arteritis. Autoimmune Dis. 2016;2016:7841718. doi:10.1155/2016/7841718
17. Tesmer LA, Lundy SK, Sarkar S, Fox DA. Th17 cells in human disease. Immunol Rev. 2008;223:87–113. doi:10.1111/j.1600-065X.2008.00628.x
18. Zúñiga LA, Jain R, Haines C, Cua DJ. Th17 cell development: from the cradle to the grave. Immunol Rev. 2013;252(1):78–88. doi:10.1111/imr.12036
19. Ramesh R, Kozhaya L, McKevitt K, et al. Pro-inflammatory human Th17 cells selectively express P-glycoprotein and are refractory to glucocorticoids. J Exp Med. 2014;211(1):89–104. doi:10.1084/jem.20130301
20. Bordon Y. T cells: spotting the troublemakers. Nat Rev Immunol. 2014;14(2):64–65. doi:10.1038/nri3610
21. Prasad S, Tripathi D, Rai MK, Aggarwal S, Mittal B, Agarwal V. Multidrug resistance protein-1 expression, function and polymorphisms in patients with rheumatoid arthritis not responding to methotrexate. Int J Rheum Dis. 2014;17(8):878–886. doi:10.1111/1756-185X.12362
22. Kansal A, Tripathi D, Rai MK, Agarwal V. Persistent expression and function of P-glycoprotein on peripheral blood lymphocytes identifies corticosteroid resistance in patients with systemic lupus erythematosus. Clin Rheumatol. 2016;35(2):341–349. doi:10.1007/s10067-015-3079-7
23. Edavalath S, Rai MK, Gupta V, et al. Tacrolimus induces remission in refractory and relapsing lupus nephritis by decreasing P‐glycoprotein expression and function on peripheral blood lymphocytes. Rheumatol Int. 2022. Epub ahead of print. doi:10.1007/s00296-021-05057-1
24. Tristão FSM, Rocha FA, Carlos D, et al. Th17-inducing cytokines IL-6 and IL-23 are crucial for granuloma formation during experimental paracoccidioidomycosis. Front Immunol. 2017;8:949. doi:10.3389/fimmu.2017.00949
25. Ramstein J, Broos CE, Simpson LJ, et al. IFN-γ-producing T-Helper 17.1 cells are increased in sarcoidosis and are more prevalent than T-Helper Type 1 cells. Am J Respir Crit Care Med. 2016;193(11):1281–1291. doi:10.1164/rccm.201507-1499OC
26. Broos CE, Koth LL, van Nimwegen M, et al. Increased T-helper 17.1 cells in sarcoidosis mediastinal lymph nodes. Eur Respir J. 2018;51:3. doi:10.1183/13993003.01124-2017
27. Jiang Y, Li Y, Zhu B. T-cell exhaustion in the tumor microenvironment. Cell Death Dis. 2015;6(6):. doi:10.1038/cddis.2015.162.
28. Celada LJ, Kropski JA, Herazo-Maya JD, et al. PD-1 up-regulation on CD4(+) T cells promotes pulmonary fibrosis through STAT3-mediated IL-17A and TGF-β1 production. Sci Transl Med. 2018;10:460. doi:10.1126/scitranslmed.aar8356
29. Stojanovic M, Raskovic S, Milivojevic V, et al. Enhanced liver fibrosis score as a biomarker for vascular damage assessment in patients with takayasu arteritis-a pilot study. J Cardiovasc Dev Dis. 2021;8(12):187. doi:10.3390/jcdd8120187
30. Li T, Gao N, Cui W, et al. The role of CD8(+) Granzyme B(+) T cells in the pathogenesis of Takayasu’s arteritis. Clin Rheumatol. 2021;41(1):167. doi:10.1007/s10067-021-05903-4.
31. Gao N, Cui W, Zhao LM, Li TT, Zhang JH, Pan LL. Contribution of Th2-like Treg cells to the pathogenesis of Takayasu’s arteritis. Clin Exp Rheumatol. 2020;38 Suppl 124(2):48–54.
32. Abdallah HM, Al-Abd AM, El-Dine RS, El-Halawany AM. P-glycoprotein inhibitors of natural origin as potential tumor chemo-sensitizers: a review. J Adv Res. 2015;6(1):45–62. doi:10.1016/j.jare.2014.11.008
33. Lum BL, Gosland MP. MDR expression in normal tissues: pharmacologic implications for the clinical use of P-glycoprotein inhibitors. Hematol Oncol Clin North Am. 1995;9(2):319–336. doi:10.1016/S0889-8588(18)30097-2
34. Jain A, Singh H, Nath A, et al. Distinct T-cell immunophenotypic signature in a subset of sarcoidosis patients with arthritis. J R Coll Physicians Edinb. 2020;50(3):226–232. doi:10.4997/JRCPE.2020.304
35. Misra R, Danda D, Rajappa SM, et al. Development and initial validation of the Indian Takayasu Clinical Activity Score (ITAS2010). Rheumatology (Oxford). 2013;52(10):1795–1801. doi:10.1093/rheumatology/ket128
36. Campochiaro C, Misra DP. PET in Takayasu arteritis: onwards and upwards towards a future of robust multimodality disease activity assessment? Rheumatology (Oxford). 2021. doi:10.1093/rheumatology/keab644
37. Singh H, Agarwal V, Chaturvedi S, Misra DP, Jaiswal AK, Prasad N. Reciprocal relationship between HDAC2 and P-Glycoprotein/MRP-1 and their role in steroid resistance in childhood nephrotic syndrome. Front Pharmacol. 2019;10:558. doi:10.3389/fphar.2019.00558
38. Sample size calculator using means. Available from: https://sample-size.net/sample-size-means/. Accessed
39. Hata A, Noda M, Moriwaki R, Numano F. Angiographic findings of Takayasu arteritis: new classification. Int J Cardiol. 1996;54(Suppl):S155–163. doi:10.1016/S0167-5273(96)02813-6
40. Goel R, Kabeerdoss J, Ram B, et al. Serum cytokine profile in asian Indian patients with takayasu arteritis and its association with disease activity. Open Rheumatol J. 2017;11:23–29. doi:10.2174/1874312901711010023
41. Jenkins DG, Quintana-Ascencio PF. A solution to minimum sample size for regressions. PLoS One. 2020;15(2):e0229345. doi:10.1371/journal.pone.0229345
42. Miroux C, Morales O, Ghazal K, et al. In vitro effects of cyclosporine A and tacrolimus on regulatory T-cell proliferation and function. Transplantation. 2012;94(2):123–131. doi:10.1097/TP.0b013e3182590d8f
43. Deng J, Younge BR, Olshen RA, Goronzy JJ, Weyand CM. Th17 and Th1 T-cell responses in giant cell arteritis. Circulation. 2010;121(7):906–915. doi:10.1161/CIRCULATIONAHA.109.872903
44. Suzuki K, Saito K, Tsujimura S, et al. Tacrolimus, a calcineurin inhibitor, overcomes treatment unresponsiveness mediated by P-glycoprotein on lymphocytes in refractory rheumatoid arthritis. J Rheumatol. 2010;37(3):512–520. doi:10.3899/jrheum.090048
45. Chaturvedi S, Rai M, Singh H, et al. Dual inhibition by phosphodiesterase 5 and 5-HT2B Inhibitor leads to near complete amelioration of fibrotic potential of human adult dermal fibroblasts isolated from a scleroderma patient. Indian J Rheumatol. 2021;16(1):43–48. doi:10.4103/injr.injr_169_19
46. Misra DP, Sharma A, Kadhiravan T, Negi VS. A scoping review of the use of non-biologic disease modifying anti-rheumatic drugs in the management of large vessel vasculitis. Autoimmun Rev. 2017;16(2):179–191. doi:10.1016/j.autrev.2016.12.009
47. Langford CA, Cuthbertson D, Ytterberg SR, et al. A randomized, double-blind trial of Abatacept (CTLA-4Ig) for the treatment of Takayasu Arteritis. Arthritis Rheumatol. 2017;69(4):846–853. doi:10.1002/art.40037
48. Nakaoka Y, Isobe M, Takei S, et al. Efficacy and safety of tocilizumab in patients with refractory Takayasu arteritis: results from a randomised, double-blind, placebo-controlled, Phase 3 trial in Japan (the TAKT study). Ann Rheum Dis. 2018;77(3):348–354. doi:10.1136/annrheumdis-2017-211878
49. Misra DP, Rathore U, Patro P, Agarwal V, Sharma A. Disease-modifying anti-rheumatic drugs for the management of Takayasu arteritis—a systematic review and meta-analysis. Clin Rheumatol. 2021;40(11):4391–4416. doi:10.1007/s10067-021-05743-2
50. Singh H, Prasad N, Misra DP, Jaiswal AK, Agarwal V. P-glycoprotein and/or histone deacetylase 2 regulates steroid responsiveness in childhood nephrotic syndrome. Indian J Rheumatol. 2020;15(1):5–10. doi:10.4103/injr.injr_126_19
51. Acharya N, Sharma SK, Mishra D, Dhooria S, Dhir V, Jain S. Efficacy and safety of pirfenidone in systemic sclerosis-related interstitial lung disease-a randomised controlled trial. Rheumatol Int. 2020;40(5):703–710. doi:10.1007/s00296-020-04565-w
52. Distler O, Highland KB, Gahlemann M, et al. Nintedanib for systemic sclerosis–associated interstitial lung disease. N Engl J Med. 2019;380(26):2518–2528. doi:10.1056/NEJMoa1903076
53. Khanna D, Lin CJF, Furst DE, et al. Tocilizumab in systemic sclerosis: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2020;8(10):963–974. doi:10.1016/S2213-2600(20)30318-0
54. Lammas DA, De Heer E, Edgar JD, et al. Heterogeneity in the granulomatous response to mycobacterial infection in patients with defined genetic mutations in the interleukin 12-dependent interferon-gamma production pathway. Int J Exp Pathol. 2002;83(1):1–20. doi:10.1046/j.1365-2613.2002.00216.x
55. Arkema EV, Cozier YC. Epidemiology of sarcoidosis: current findings and future directions. Ther Adv Chronic Dis. 2018;9(11):227–240. doi:10.1177/2040622318790197
56. Gonzalez-Gay MA, Martinez-Dubois C, Agudo M, Pompei O, Blanco R, Llorca J. Giant cell arteritis: epidemiology, diagnosis, and management. Curr Rheumatol Rep. 2010;12(6):436–442. doi:10.1007/s11926-010-0135-9
57. Hoffman GS. Takayasu arteritis: lessons from the American National Institutes of Health experience. Int J Cardiol. 1996;54(Suppl):S99–102. doi:10.1016/S0167-5273(96)88778-X
58. Incerti E, Tombetti E, Fallanca F, et al. 18F-FDG PET reveals unique features of large vessel inflammation in patients with Takayasu’s arteritis. Eur J Nucl Med Mol Imaging. 2017;44(7):1109–1118. doi:10.1007/s00259-017-3639-y
59. Adriawan IR, Atschekzei F, Dittrich-Breiholz O, et al. Novel aspects of regulatory T cell dysfunction as a therapeutic target in giant cell arteritis. Ann Rheum Dis. 2022;81(1):124–131. doi:10.1136/annrheumdis-2021-220955
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.