Back to Journals » Cancer Management and Research » Volume 12
Novel Prognostic Nomograms for Predicting Early and Late Recurrence of Hepatocellular Carcinoma After Curative Hepatectomy
Received 11 December 2019
Accepted for publication 25 February 2020
Published 9 March 2020 Volume 2020:12 Pages 1693—1712
DOI https://doi.org/10.2147/CMAR.S241959
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Yong Teng
Wei Xu, Ruineng Li, Fei Liu
Department of Hepatobiliary Surgery, Hunan Provincial People’s Hospital, The First Hospital Affiliated with Hunan Normal University, Changsha 410005, People’s Republic of China
Correspondence: Wei Xu
Department of Hepatobiliary Surgery, Hunan Provincial People’s Hospital, The First Hospital Affiliated with Hunan Normal University, No. 61 West Jiefang Road, Changsha 410005, People’s Republic of China
Tel +8613873159491
Fax +8673182278012
Email [email protected]
Aim: Hepatectomy is the main curative method for patients with hepatocellular carcinoma (HCC) in China. Unfortunately, high recurrence rate after hepatectomy poses negative impact on the prognosis of patients. This study aimed to develop prognostic nomograms to predict early recurrence (ER) and late recurrence (LR) of HCC after curative hepatectomy.
Patients and Methods: Total of 318 HCC patients undergoing curative hepatectomy from January 2012 to January 2018 were retrospectively recruited. Potential risk factors that were significant for predicting ER and LR in univariate analysis were selected for multivariate survival model analysis using the backward stepwise method. Risk factors identified in multivariate analysis were used to develop nomograms to predict ER and LR. The nomogram was internally validated using 2,000 bootstrap samples from 75% of the original data.
Results: Among 318 patients, 164 showed postoperative recurrence, of which 140 and 24 had ER (≤ 2 years) and LR (> 2 years), respectively. Multivariate analysis showed that age, Hong Kong Liver Cancer Stage, albumin-bilirubin, METAVIR fibrosis grade, and microvascular invasion were risk factors of ER for HCC after curative hepatectomy. The AUC of the ROC curve for ER in the development set (D-set) was 0.888 while that in the validation set (V-set) was 0.812. Neutrophil/lymphocyte ratio and glypican-3 (+) were risk factors for LR in HCC patients after curative hepatectomy. The AUC of the ROC curve for LR predictive nomogram that integrated all independent predictors was 0.831. The AUC of the ROC curve for LR in the D-set was 0.833, while that for LR in the V-set was 0.733. The C-index and AUC of ROC for the proposed nomograms were more satisfactory than three conventional HCC staging systems used in this study.
Conclusion: We developed novel nomograms to predict ER and LR of HCC patients after curative hepatectomy for clinical use to individualize follow-up and therapeutic strategies.
Keywords: hepatocellular carcinoma, hepatectomy, risk factor, prognosis, nomogram
Introduction
As the sixth most common malignancy and the third most leading cause of tumor-related death, hepatocellular carcinoma (HCC) accounts for more than 90% of all hepato-malignancies worldwide.1,2 Patients with HCC have poor prognosis, with just 12.1% surviving after 5 years.3 Therefore, HCC represents a major health challenge.4 The standard therapeutic strategies for patients with HCC without metastasis are surgical approaches. Hepatectomy is the main therapeutic method used for patients with HCC in the Asia-Pacific region, especially for patients in China, due to the shortage of donor organs.3 However, there is a high recurrence rate after hepatectomy, which impacts the survival of patients with HCC. The cumulative recurrence within a 5-year period ranges from 50% to 70%.5
It is proposed that early recurrence (ER) and late recurrence (LR) can be distinguished by the 2-year postoperative time point following curative resection of HCC.6,7 ER is associated with the pathological factors of aggressive tumors, including large tumor size, microvascular invasion (MVI), high tumor staging, microsatellite lesions, and high levels of serum alpha-fetoprotein (AFP). ER has been attributed to intrahepatic occult metastases or the multicenter metastasis of primary tumors.6–10 In contrast, LR is caused by the formation of “de novo” tumors in the context of hepatitis and cirrhosis.2,6,7,11 Various risk factors for HCC recurrence after hepatectomy have been reported, but the results are often conflicting. Thus, an efficient model to predict recurrence after curative hepatectomy is urgently needed to develop individualized follow-up, early intervention, and improvements to prognosis.
Existing models/scoring systems are considered to have good predictive function, but they have shortcomings.12–40 First, there is no standardized way of distinguishing ER and LR after hepatectomy in terms of recurrence time, which ranges from 0.5 years to 5 years after surgery.14,15,20,29,35,36 In some cases, predictive analyses are performed using an indicator of disease-free survival (DFS).12,14,16–19,21–27,31–34 Some investigators have suggested using the time to recurrence, rather than recurrence-free survival, as the endpoint to predict and evaluate ER after HCC resection.41 Second, these systems tend to only be used for specific populations, limiting interpretation or the detection of consistent patterns across groups. For example, some predictive systems have been used for patients with portal vein tumor thrombus (PVTT)12,23,25,26,29,31,32,35 and bile duct tumor thrombus (BDTT),31 for which curative hepatectomy is not suitable.42 Third, scoring systems emphasize the weight of all risk factors in tumor recurrence; however, objective assessments of liver status are not available when using the liver fibrosis/cirrhosis in Child-Turcotte-Pugh (CTP) scoring system12,14 or the Model for end-stage liver disease (MELD) scoring system.12,33 There is not even qualitative evaluation of liver fibrosis/cirrhosis.15,18,19,24,25,29,35–37 Therefore, this study aimed to develop novel prognostic nomograms characterized by easily collected and objective clinical data for ER and LR after curative hepatectomy.
Materials and Methods
Patients
All 318 patients with HCC that underwent radical hepatectomy in Hunan Provincial People’s Hospital (The First Hospital Affiliated with Hunan Normal University) from January 2012 to January 2018 were recruited. Inclusion and exclusion criteria of patients with HCC after curative hepatectomy were based on the published literature.42 Inclusion criteria were: (a) Eastern Cooperative Oncology Group (ECOG) Performance Status score 0 to 1 in the absence of macroscopic hepatic vein tumor thrombus, macroscopic bile duct tumor thrombus, extrahepatic spread, and/or distant metastases; (b) patients with preoperative status child-pugh A or B or with child-pugh ≥ B that recovered from child-pugh C after short-term therapy; (c) exact pathological diagnosis of HCC; and (d) patients with completely resected HCC (R0). Exclusion criteria were: (a) patients with other cancers in combination to HCC; (b) patients with cardiovascular, pulmonary disease or liver cirrhosis; (c) incomplete clinicopathological reports and follow-up data; and (d) perioperative death or postoperative death within 90 days of hospitalization. Patient data were retrospectively extracted from the medical records, which included demographic information, preoperative laboratory data, surgical results, pathological data, postoperative complications, recurrence and survival information, and oncologic outcomes. These data points were gathered and presented in Table 1. This study conformed to the Helsinki principle and was approved by the Ethics Committee of Hunan Provincial People’s Hospital. Every participant gave written informed consent.
 |  |  |  |  |  |
Table 1 Patient Characteristics and Univariate Analysis of Postoperative Recurrence |
Preoperative Examination
Preoperative examinations were performed, including routine blood test, biochemistry test, serum alpha-fetoprotein (AFP) level, abdominal ultrasound, computed tomography (CT) and/or magnetic resonance imaging (MRI). Diagnosis criteria were based on the published literature.43 HCC was confirmed by (a) typical imaging characteristics of HCC being detected by at least two types of diagnostic equipment and at least two radiologists; (b) imaging examination combined with serum AFP > 400 μg/L, and/or (c) indisputable cytological and histological evidence.
Indicators of Fibrosis/Cirrhosis
The indicators of liver fibrosis/cirrhosis included (a) Aspartate aminotransferase-to platelet ratio (APRI):44 APRI=[(AST(IU/L)/ULN of AST) ×100]/(platelet count×109/L). (b) Fibrosis index based on four factors (FIB-4):45 Fib-4=[age (years)×AST (IU/L)]/[platelet count (109/L)×(ALT (IU/L)1/2)]. (c) Albumin-bilirubin (ALBI):46 ALBI= −0.085×(albumin (g/L))+(log10 bilirubin (mmol/L)×0.66), and ALBI classification: grade 1, ALBI<−2.60; grade 2 (between −2.60<ALBI and −1.39); grade 3, ALBI (above −1.39). (d) Gamma-glutamyl transpeptidase (GGT)-to platelet ratio (GPR):47 GPR = [(GGT (IU/L)/ULN of GGT) ×100]/[platelet count (109/L)]. (e) Portal hypertension (PH), which is diagnosed by reference criteria according to the published literature:43 (1) esophageal and gastric varices are is detected by endoscopic examination, or (2) CT and (or) MRI examination show the enlargement of the spleen and laboratory examination shows platelet (PLT)<100X109/L. The degree of esophageal and gastric varices was determined by examination of imaging and/or endoscopy. The degree of PH was classified as: none, mild (slight esophagogastric varices), moderate (obvious esophagogastric varices without “red wale” signs), and severe (obvious esophagogastric varices with “red wale” signs). (f) The METAVIR score48 was used to assess the status of the non-tumor parts of the liver. (1) Liver tissue fibrosis was graded as F0: no fibrosis; F1: portal area fibrosis extends to the portal vein area, but no fibrous interval forms; F2: portal area fibrosis extends to most portal vein areas, with a few fiber intervals forming; F3: most fibrous spaces are formed, but without hardened nodules; and F4: cirrhosis. (2) Liver tissue inflammation activity: A0: no activity; A1: mild activity; A2: moderate activity; and A3: severe activity.
Tumor Characteristics, Staging, and Pathology
Tumor size was determined from the maximum tumor diameter (measuring unit: centimeters) of the specimen during pathological examination. The number of tumor tissues was grouped as 1, 2, 3, and ≥4 (containing satellite nodules). Tumor encapsulation was assessed as the presence of intact capsules or absence of capsules. American Joint Committee on Cancer (AJCC) 8th edition TNM staging system,49 Barcelona Clinic Liver Cancer (BCLC) system,50 and Hong Kong Liver Cancer (HKLC) classification system51 were adopted to delineate tumor staging. Postoperative pathology was confirmed by at least two pathologists. Tumor differentiation was graded by the Edmondson-Steiner system.52 A tumor satellite, whether it was detected macroscopically or microscopically, was defined as a lesion that was separated from the primary tumor, and had a tumor diameter and distance from the main tumor of no more than 2 cm. Microvascular invasion (MVI) was diagnosed as the presence of tumor emboli in a vascular space lined by endothelial cells under a microscope, based on “the evidence-based practice guidelines for standardized pathological diagnosis of primary liver cancer in China: 2015 update”.42 MVI was classified as M0: no MVI; M1 (low-risk group): ≤5 MVI in para-cancerous liver tissues; M2 (high-risk group): >5 MVI, or MVI in distant para-carcinoma liver tissue (Chinese Society of Liver Cancer et al 201542).
Surgical Therapies
This study followed the established criteria for Couinaud liver segmenting. Surgical methods containing laparotomy and laparoscopic hepatectomy were randomly performed on patients with HCC by experienced surgeons. The indications and type of operation for hepatectomy were usually based on tumor location, remnant liver volume, and the hepatic functional reserve assessed by Child-Turcotte-Pugh classification. Curative hepatectomy was defined as the complete resection of all tumor nodules without involving any major branch of the portal or hepatic veins. Anatomic hepatectomy was characterized as any type of complete excision of at least one segment based on Couinaud’s classification in the published literature.53 Nonanatomic hepatectomy referred to wedge resection and tumor enucleation. Clinical “resectional margin distance” was defined as the shortest distance measured from the liver resection margin to the edge of the tumor. An appropriate resection margin length was used if the ideal anatomical resection could not be performed. A negative resection margin was used if the recommended intraoperative resection margin length ≥2 cm was not possible. Postoperative complications within 90 days after surgery were classified as grade I–V based on the Clavien-Dindo classification system.54
Follow-Up
All patients were followed up by telephone inquiry or special clinic re-examination. The date of surgery was defined as initiation time. The follow-up was censored on December 31, 2018. The time to recurrence (TTR) was termed the endpoint as the time from date of surgery to the date of first relapse detected on imaging, and was recorded with overall survival (OS) and disease-free survival (DFS). OS was defined as the interval between the date of surgery and the date of patient death, or the date of the last follow-up. DFS was the interval between the date of surgery and the date when tumor recurrence was diagnosed, or the date of the last follow-up, or the date of patient death. Ultrasound and serum AFP levels were postoperatively examined every 3 months within 2 years of diagnosis, and every 6 months after 2 years. Enhanced CT and/or MRI, as well as pathology examination, was performed if the ultrasound showed new liver lesions and/or if serum AFP showed progressive elevation. ER was defined as recurrence time ≤2 years, and LR was defined as >2 years after curative hepatectomy. The site of recurrence (intra- or extrahepatic) and the size and the number of recurrent nodules were evaluated. Patients with recurrence of HCC were treated with various therapies, including re-hepatectomy, microwave ablation, transcatheter arterial chemoembolization (TACE), immuno-targeted drugs such as sorafenib, radiotherapy, or best supportive care.
Statistical Analysis
SPSS 23.0 (IBM Corporation, Armonk, New York, USA) was used for the analyses, and a P value of <0.05 was considered to be statistically significant. Cox-regression analysis was used to estimate the hazard ratio (HR) with a 95% confidence interval (95% CI) for each of the potential risk factors (P < 0.05 was set as significant). Univariate analysis was applied for risk factors with preliminary estimation. Variable selection methods with three steps (forward, backward, and stepwise; inclusion and exclusion criteria of type I error = 0.1 based on likelihood ratio tests) were adopted in the multivariate analysis. Potential risk factors for predicting ER and LR in univariate analyses that were considered significant (P < 0.05) were selected for the multivariate analysis using the backward stepwise method, with a P-value threshold of <0.05. R (version 3.4.1) with rms software package version 5.1–1 (http://www.R-project.orghttp://CRAN.R-project.org/package=rms) was used. Risk factors detected from the multivariate analysis were used to construct nomograms to predict ER and LR, respectively. AUC values (95% CI) of nomograms for both ER and LR were obtained. Using Bootstrap analysis, 75% of all patients were randomly selected 2000 times for internal verification. Harrell’s concordance index (C-index) analysis was used to evaluate the consistency probability between predicted cohorts and observed cohorts. The C-index range extended from 0.5 (no discriminant ability) to 1.0 (perfect discriminant ability), and P < 0.05 was considered significant. A calibration curve was plotted to estimate the prognostic performance of the nomograms for ER and LR. The AUC of ROC and C-index analysis was used to compare the predictive power of nomograms with three conventional HCC staging systems/models (the 8th AJCC-TNM staging system, BCLC system, and HKLC system).
Results
Patients
Total of 318 patients out of 586 recruited HCC patients met the eligibility criteria. Patient characteristics are presented in Table 1. Of the patients excluded, 73 cases had tumor thrombus of the main/branch portal vein, 21 cases had tumor thrombus of the bile duct, three cases had positive surgical margins, 31 cases had incomplete clinical data, five cases died within 90 d of hospitalization, and the follow-up on 135 cases was not available. The median follow-up of eligible patients was 30.1 months (range: 3.2–105.7 months).
Recurrence and Survival
Total of 164 patients with HCC had postoperative recurrence by the end of the follow-up period. The median time to recurrence (TTR) was 10.6 months (range: 1.0–153.0 months). Out of the 140 patients with ER, 107 cases had intrahepatic recurrence, 24 cases had synchronous intrahepatic recurrence and extrahepatic metastases, including metastases to the pulmonary area (n = 14), peritoneal cavity (n = 6), lymph node (n = 1), bone (n = 1), right adrenal gland (n = 1), and brain (n = 1). Nine patients had extrahepatic metastases only, including metastases to the peritoneal cavity (n = 3), pulmonary (n = 4), lymph node (n = 1), and bone (n = 1). Out of the 24 patients with LR, 21 cases (87.5%, 21/24) had intrahepatic recurrence only, two cases had extrahepatic metastases only, including right adrenal gland + brain (n = 1), chest wall + mediastinal lymph nodes (n = 1), and one case (4.2%, 1/24) had intrahepatic and extrahepatic recurrence with metastases to the pulmonary (n = 1).
Patients with ER received the following therapies: 18 cases received re-resection, 76 cases received TACE, 10 cases received immunotargeted drugs, eight cases received microwave ablation, two cases received radiotherapy, and 26 cases received best supportive care. Patients with LR received the following therapies: eight cases received re-resection, eight cases received TACE, one case received microwave ablation, seven cases received best supportive care. Cumulative recurrence rates of 1, 2, 3, 4, and 5 years were 29.9% (95/318), 44.0% (140/318), 47.8% (152/318), 49.7% (158/318), and 51.6% (164/318), respectively. Disease-free survival (DFS) rates of 1, 2, 3, 4, and 5 years were 66.7% (212/318), 45.9% (146/318), 26.7% (85/318), 12.9% (41/318), and 6.6% (21/318), respectively. Overall survival (OS) rates of 1, 2, 3, 4, and 5 years were 88.7% (282/318), 64.8% (206/318), 36.8% (117/318), 17.6% (56/318), and 9.8% (31/318), respectively.
Risk Factors for Recurrence and Prognostic Nomograms
Univariate analysis showed that 25 factors were correlated with ER and three factors were correlated with LR (Table 1). Multivariate analysis showed that patient age, HKLC stage, ALBI, METAVIR fibrosis grade, and MVI were risk factors for ER (Table 2), while the neutrophil to lymphocyte ratio (NLR) and glypican-3 (+) were risk factors for LR (Table 3).
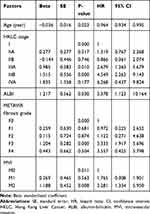 |
Table 2 Multivariate Analysis for Postoperative ER |
 |
Table 3 Multivariate Analysis for Postoperative LR |
Nomograms for Predicting ER
The AUC of ROC for the nomogram integrating all independent predictors for ER was 0.860 (Figure 1A). A prognostic nomogram was established according to the results of the multivariate analysis (Figure 1B). A score was generated for each risk factor by drawing an upward line to the corresponding scoring axis. The predictive probability of ER was represented by the total score accumulated from each score positioned on the total-score axis.
Internal Validation Results
Using Bootstrap analysis, 75% of all patients were randomly selected 2000 times for internal validation. The AUC of ROC for ER in the development set (D-set) was 0.888, while that in the validation set (V-set) was 0.812 (Figure 1C). The calibration plots predicted ER well (Figure 1D). In our internal validations, the bootstrap-corrected C-index to predict ER in the nomogram was 0.7794 (95% CI, 0.6456–0.9132). The calibration plots showed strong consistency between the prediction cohort and the observation cohort, confirming the repeatability and reliability of this nomogram.
Comparison of the Nomogram to Alternative Staging Models as a Predictor of ER
The AUC under the ROC of the proposed ER nomogram was 0.860 (95% CI: 0.814–0.935), and was more satisfactory than the AUCs of the three representative conventional HCC staging systems/models; specifically 0.685 (95% CI: 0.625–0.746) for the eighth AJCC-TNM staging system, 0.678 (95% CI: 0.617–0.739) for the HKLC staging system and 0.655 (95% CI: 0.592–0.717) for the BCLC system (Figure 1E). The C-index of our proposed ER nomogram [0.7794 (95% CI: 0.6456–0.9132)] was much higher than those of the three representative conventional HCC staging systems/models; specifically: 0.5913 (95% CI: 0.5481–0.6279) for the eighth AJCC-TNM staging system, 0.5954 (95% CI: 0.5378–0.6184) for the BCLC system, and 0.6226 (95% CI: 0.5623–0.6458) for the HKLC staging system.
Nomograms for Predicting LR and Their Predictive Performance
The AUC under the ROC for the nomogram that integrated all independent predictors for LR was 0.831 (Figure 2A). A prognostic nomogram was established based on the results of the multivariate analysis (Figure 2B). A score was generated for each risk factor by drawing an upward line to the corresponding scoring axis. The predictive probability of LR was represented by the total score accumulated from each score positioned on the total-score axis.
Internal Validation Results
Using Bootstrap analysis, 75% of all patients were randomly selected 2000 times for internal validation. The AUC of the ROC for LR predicted in the D-set was 0.833, while that in the V-set was 0.733 (Figure 2C). The calibration plots had a strong predictive ability for LR (Figure 2D). In our internal validation, the bootstrap-corrected C-index to predict LR in the nomogram LR was 0.7364 (95% CI, 0.6854–0.7844). The calibration plots showed strong consistency between the prediction cohort and the observation cohort, validating the repeatability and reliability of this nomogram.
Comparison of the Nomogram to Alternative Clinical Staging Systems as a Predictor of LR
The AUC under the ROC of the proposed LR nomogram was 0.831 (95% CI: 0.802–0.903), which was more satisfactory than the three representative conventional HCC staging systems/models; specifically, 0.424 (95% CI: 0.322–0.526) for the eighth AJCC-TNM staging system, 0.422 (95% CI: 0.313–0.532) for the BCLC system, and 0.405 (95% CI: 0.290–0.520) for the HKLC staging system (Figure 2E). The C-index of our proposed LR nomogram was 0.7364 (95% CI: 0.6854–0.7844), and was much higher than those of the three representative conventional HCC staging systems/models; specifically, 0.4900 (95% CI: 0.4324–0.5956) for the eighth AJCC-TNM staging system, 0.5510 (95% CI: 0.4799–0.6482) for the BCLC system, and 0.5403 (95% CI: 0.4825–0.6156) for the HKLC staging system.
Discussion
This study developed novel nomograms to predict postoperative ER and LR for individual patients with HCC after curative hepatectomy. In China, most HCC patients are treated with curative hepatectomy. These easy-to-use graphical nomograms contain typical clinical variables (preoperative and pathological characteristics) that have the potential to improve the prognostic function of staging systems from the group-level to an individual level. Within this framework, it would be possible to identify patients who are at high risk of recurrence after curative surgery, allowing clinicians to provide appropriate surveillance to detect recurrence as early as possible.
Predictive performance was verified using an internal validation cohort, with a C-index of 0.7794 (95% CI, 0.6456–0.9132) for ER and a C-index of 0.7364 (95% CI, 0.6854–0.7844) for LR. The AUCs of our novel predictive nomograms for ER and LR were 0.860 and 0.831, respectively. These results were more satisfactory than previously reported predictive models/scoring systems (Tables 4 and 5). Our results confirm previous studies suggesting that ER and LR are separate clinical entities resulting from different risk factors. Specifically, intrahepatic recurrence remains the main cause of ER/LR, with a low proportion of repeat operations. However, it is difficult to distinguish ER and LR based on the clinical or biological characteristics of HCC.55 As a simple alternative, our novel prognostic nomograms for predicting ER and LR after curative hepatectomy, have the advantage in that they: (a) are suitable for all types of patients with HCC after resection, not just one specific group; (b) clearly distinguish ER and LR after operation, and could be used in place of DFS to predict postoperative recurrence; (c) take descriptions of tumor characteristics into account, reasonably select indicators to evaluate basic liver status, and incorporate postoperative pathological indicators.
 |
Table 4 Nomogram to Predict DFS or Recurrence After Hepatectomy in the Published Literature |
 |
Table 5 Scoring System to Predict DFS or Recurrence After Hepatectomy in the Published Literature |
A multi-center study in China showed that patient gender, liver fibrosis/cirrhosis, and several, initial stage aggressive tumor characteristics represented risk factors for patients with HCC after hepatectomy.56 However, differences in fibrosis/cirrhosis and microvascular invasion were only described as presence and absence. Some investigators showed that liver fibrosis/cirrhosis is an important risk factor for HCC recurrence after surgery.2 The well-known risk factors for ER in patients with HCC were also confirmed by our multivariate analysis. Evaluation of the prognostic significance of indicators that reflect liver fibrosis/cirrhosis was reasonable for patients with HCC after hepatectomy. However, liver reserve function tends to be overlooked or minimally used by existing staging systems. CTP classification is widely used to assess liver reserve in patients with cirrhosis, and has been incorporated in many HCC staging systems. A model for end-stage liver disease (MELD) score was used to assess the severity of liver dysfunction. Therefore, HCC with chronic liver disease status (fibrosis/cirrhosis) was evaluated in detail using several preoperative non-invasive indicators and the postoperative METAVIR score for assessing the status of non-tumor parts of the liver in our study. This approach was used to clarify how the status of chronic liver disease would impact ER and LR in patients with HCC.
Our study showed that the basic indicators used to evaluate the status of liver disease (such as ALBI and chronic liver disease staging) are risk factors for ER after curative resection. Various non-invasive models have been developed to assess liver fibrosis in patients with HBV or hepatitis C virus (HCV).47,57 For example, APRI and Fib-4 have been recommended as non-invasive indicators under WHO guidelines to diagnose liver fibrosis/cirrhosis in patients with chronic HBV infection.58 However, these indicators were not statistically significant in the univariate analysis of HCC recurrence after hepatectomy in our study. Furthermore, we evaluated how pathological staging and inflammatory activity of chronic liver disease affect HCC recurrence after surgery. We used METAVIR fibrosis grade F0 as reference indicator, with HR only being statistically significant for staging F3 out of F1-4 and staging F0. Unexpectedly, ALBI (B =1.217) had a more significant effect than pathological chronic liver disease staging. ALBI was graded as a simple and objective indicator of evaluating liver function.46 ALBI is also significantly correlated with higher recurrence rates after hepatectomy, and can be used to determine patient prognosis.46,59,60 However, ALBI grade was not statistically significant in the univariate analysis, because patients of the same ALBI grade showed different liver functions in our study. Most HCC patients that underwent hepatectomy in this study were CTP grade A; therefore, the indicator of ALBI that was included in the nomogram to predict ER did not add extra weight in this study.
This study revealed significant heterogeneity in inflammation and fibrosis in different regions of the liver, thus assessing inflammation and fibrosis staging in non-tumor liver areas after hepatectomy might not reflect overall liver status. Previous studies also showed that the severity of liver fibrosis of HCC patients might not be effectively diagnosed by the monitoring models of chronic liver disease without HCC.61 In the current study, chronic liver disease staging F0 was used as reference indicator, with statistical significance in HR only being shown when comparing staging F3 out of F1-4 and staging F0. Thus, the actual risk of liver cirrhosis on postoperative ER requires the development of a monitoring model for HCC patients that have liver cirrhosis. The predictive function of these indicators for HCC recurrence and the need for routinely monitoring fibrosis in the follow-up require further evaluation. Routine liver biopsies might not be needed to monitor postoperative patients with HCC during follow-up, even though such invasive examination serves as a gold standard for assessing liver status.
Various HCC staging systems use different biological characteristics, including tumor diameter/number and vascular invasion, as variables to classify cancer classification. However, these factors are not sufficient to predict the postoperative status of patients after hepatectomy. Thus, we used the most representative systems to describe oncological characteristics, including the 8th AJCC-TNM staging system, BCLC system, and HKLC classification system. These predictive systems primarily use tumor status, tumor size, tumor number, vascular invasion, and serum AFP, failing to provide an accurate assessment of the status of underlying liver disease. Hepatitis B virus (HBV) infection is the main cause of HCC in China.3 The proportion of HCC patients with HBV in the current study represented 85.5% (272/318) of all patients. Furthermore, HCC patients with HBV after postoperative recurrence accounted for 91.25% (146/164) of all patients. HKLC system was established for a large population of HBV-related patients with HCC.51 It was more accurate than the BCLC staging system to predict patient prognosis.62–64 The AUC of ROC for proposed nomograms was better than the three conventional HCC staging systems/models (the 8th AJCC-TNM staging system, BCLC system, and HKLC system). However, the multivariate analysis showed no difference in HR when comparing the HKLC-IIA/IIB stage with the HKLC-I stage. In comparison, there was a significant difference in HR when comparing the HKLC-IIIA/IIIB stage with the HKLC-I stage. In addition, the negative correlation between tumor size and other adverse critical prognostic factors (such as vascular invasion, poorly differentiated tumors, and multiple lesions) was reported in a large cohort study.65 The importance of tumor size as a prognostic factor for HCC is highly controversial,65,66 affirming that postoperative ER after hepatectomy cannot be solely predicted on tumor characteristics for HCC.
As an important component of the AJCC-TNM system, microvascular invasion (MVI) is considered to represent a histological indicator of HCC recurrence and long-term survival after hepatectomy.67,68 However, MVI is overlooked by existing staging systems. As shown in Tables 4 and 5, in current predictive models or scoring systems of HCC recurrence after hepatectomy, MVI is simply classified as present or absent.13,16,17,19,21–28,30,32,34 Some studies do not even evaluate MVI.12,14,15,18,29,31,33,36,39 Considering the potential role of MVI in predicting the recurrence of HCC in patients following hepatectomy, MVI absence (M0) was used as a reference indicator in our study. Of note, strong significance was obtained for HR when comparing MVI M2 and MVI M0. In addition, small HCC (≤3 cm) may be detected with MVI, and multinodular HCC with ≤3 nodules might arise from MVI, leading to the formation of satellite nodules. Consequently, the biological behavior of actual tumors was not pragmatically reflected by the early-stage in these patients.68 Therefore, future studies are needed to verify the role of MVI in predicting HCC recurrence in patients following hepatectomy.
Our study showed that LR might be associated with inflammation and glypican-3 positive expression. A previous study showed that inflammation contributes to the development and prognosis of HCC.69 Neutrophils produce chemokines and cytokines, which promote the proliferation, angiogenesis, invasion, and metastasis of tumors. On the other hand, lymphocytes produce cytotoxic factors that enhance antitumor immune responses.70 Increased platelet/lymphocyte ratio (PLR) is associated with a higher recurrence rate and poorer prognosis of patients with HCC after hepatectomy.69 Our results supported that neutrophil/lymphocyte ratio (NLR) could be used as an independent prognostic factor for HCC after hepatectomy.71 However, some studies showed that NLR is not associated with HCC recurrence and DFS after surgery.72,73 These differences might be attributed to the fact that current studies on prognosis of liver cancer patients lack supporting data on neutrophil and lymphocyte subgroups. These groups might maintain the balance between tumor-promoting and antitumor actions, along with their relationship with the platelet and tumor microenvironment. Thus, this group should be selected as clinical therapeutic targets to improve patient prognosis in future research.
The overexpression of GPC3 is significantly correlated with highly invasive activity of tumor cells.74,75 GPC3 is significantly correlated with poor prognosis of patients with HCC.74,75 Furthermore, GPC3 can be detected in the blood samples of HCC patients.75 GPC3 is valuable for monitoring overall survival rates (OS) and disease-free survival (DFS) for patients with HCC. Our internal validation analysis showed that the AUC of the ROC curve in the internal validation cohort was 0.812 for D-set and 0.733 for V-set), respectively. The values of AUC and even the predictive power of nomograms could be improved if more large-scale multicenter studies that incorporate GPC3 and inflammation factors could be performed in the future.
Predictive systems/models must be able to detect the risk factors of ER and LR accurately. Moreover, these systems need to be simple and based on easily available clinical data. The novel nomograms proposed in our study meet these baseline requirements, providing the opportunity to gain a prognostic evaluation for ER and LR after curative hepatectomy. The current study has limitations such as the lack of large-scale prospective data, and the loss of follow-up on some patients. Therefore, the proposed nomograms require external validation in more multicenter prospective samples. In addition, our prognostic nomogram might not be applicable to HCC patients with HCV-infection, because most of our patients had HBV-infection.
In conclusion, risk factors of ER and LR for patients with HCC after curative hepatectomy can be predicted by the novel prognostic nomograms. Our novel monitoring models are of clinical use for individualized follow-up and therapeutic strategies, and could potentially improve prognosis and prolong survival of patients with HCC.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This study was supported by funds from the Hunan Provincial Department of Education of China (Project No. 17C0969), and the Hunan Provincial Health Commission of China (Project No. 20200074).
Disclosure
The authors declare that they have no competing of interests in this work.
References
1. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi:10.3322/caac.21262
2. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi:10.3322/caac.21338
3. Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11(4):317–370.
4. Zeng H, Chen W, Zheng R, et al. Changing cancer survival in China during 2003-15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Health. 2018;6(5):e555–e567. doi:10.1016/S2214-109X(18)30127-X
5. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi:10.3322/caac.21387
6. Poon RT, Fan ST, Ng IO, et al. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer. 2000;89(3):500–507. doi:10.1002/1097-0142(20000801)89:3<500::AID-CNCR4>3.0.CO;2-O
7. Poon RT. Differentiating early and late recurrences after resection of HCC in cirrhotic patients: implications on surveillance, prevention, and treatment strategies. Ann Surg Oncol. 2009;16(4):792–794. doi:10.1245/s10434-009-0330-y
8. Cheng Z, Yang P, Qu S, et al. Risk factors and management for early and late intrahepatic recurrence of solitary hepatocellular carcinoma after curative resection. HPB (Oxford). 2015;17(5):422–427. doi:10.1111/hpb.12367
9. Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology. 2016;150(4):835–853. doi:10.1053/j.gastro.2015.12.041
10. Shimoda M, Tago K, Shiraki T, et al. Risk factors for early recurrence of single lesion hepatocellular carcinoma after curative resection. World J Surg. 2016;40(10):2466–2471. doi:10.1007/s00268-016-3529-7
11. Zheng J, Chou JF, Gonen M, et al. Prediction of hepatocellular carcinoma recurrence beyond Milan criteria after resection: validation of a clinical risk score in an international cohort. Ann Surg. 2017;266(4):693–701. doi:10.1097/SLA.0000000000002360
12. Cho CS, Gonen M, Shia J, et al. A novel prognostic nomogram is more accurate than conventional staging systems for predicting survival after resection of hepatocellular carcinoma. J Am Coll Surg. 2008;206(2):281–291. doi:10.1016/j.jamcollsurg.2007.07.031
13. Li J, Liu Y, Yan Z, et al. A nomogram predicting pulmonary metastasis of hepatocellular carcinoma following partial hepatectomy. Br J Cancer. 2014;110(5):1110–1117. doi:10.1038/bjc.2014.19
14. Ang SF, Ng ES, Li H, et al. The Singapore Liver Cancer Recurrence (SLICER) score for relapse prediction in patients with surgically resected hepatocellular carcinoma. PLoS One. 2015;10(4):e0118658. doi:10.1371/journal.pone.0118658
15. Shim JH, Jun MJ, Han S, et al. Prognostic nomograms for prediction of recurrence and survival after curative liver resection for hepatocellular carcinoma. Ann Surg. 2015;261(5):939–946. doi:10.1097/SLA.0000000000000747
16. Shen J, He L, Li C, et al. Prognostic nomograms for patients with resectable hepatocellular carcinoma incorporating systemic inflammation and tumor characteristics. Oncotarget. 2016;7(49):80783–80793. doi:10.18632/oncotarget.13038
17. Li J, Zhou J, Yang PH, et al. Nomograms for survival prediction in patients undergoing liver resection for hepatitis B virus related early stage hepatocellular carcinoma. Eur J Cancer. 2016;62:86–95. doi:10.1016/j.ejca.2016.04.011
18. Torzilli G, Donadon M, Belghiti J, et al. Predicting individual survival after hepatectomy for hepatocellular carcinoma: a novel nomogram from the “HCC East & West Study Group”. J Gastrointest Surg. 2016;20(6):1154–1162. doi:10.1007/s11605-016-3132-0
19. Fu YP, Ni XC, Yi Y, et al. A novel and validated inflammation-based score (IBS) predicts survival in patients with hepatocellular carcinoma following curative surgical resection: a STROBE-compliant article. Medicine (Baltimore). 2016;95(7):e2784. doi:10.1097/MD.0000000000002784
20. Feng LH, Dong H, Lau WY, et al. Novel microvascular invasion-based prognostic nomograms to predict survival outcomes in patients after R0 resection for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2017;143(2):293–303. doi:10.1007/s00432-016-2286-1
21. Shen J, He L, Li C, et al. Nomograms to predict the individual survival of patients with solitary hepatocellular carcinoma after hepatectomy. Gut Liver. 2017;11(5):684–692. doi:10.5009/gnl16465
22. Fu YP, Yi Y, Huang JL, et al. Prognostic nomograms stratify survival of patients with hepatocellular carcinoma without portal vein tumor thrombosis after curative resection. Oncologist. 2017;22(5):561–569. doi:10.1634/theoncologist.2016-0231
23. Zhang W, Tan Y, Shen S, et al. Prognostic nomogram for hepatocellular carcinoma in adolescent and young adult patients after hepatectomy. Oncotarget. 2017;8(63):106393–106404. doi:10.18632/oncotarget.18192
24. Luo XY, Du CY, Wei XF, et al. The study of nomogram based on Ishak inflammation score for recurrence of hepatocellular carcinoma after curative resection. Zhonghua Wai Ke Za Zhi. 2018;56(2):124–129. doi:10.3760/cma.j.issn.0529-5815.2018.02.009
25. Gan W, Huang JL, Zhang MX, et al. New nomogram predicts the recurrence of hepatocellular carcinoma in patients with negative preoperative serum AFP subjected to curative resection. J Surg Oncol. 2018;117(7):1540–1547. doi:10.1002/jso.25046
26. Gan W, Yi Y, Fu Y, et al. Fibrinogen and C-reactive protein score is a prognostic index for patients with hepatocellular carcinoma undergoing curative resection: a prognostic nomogram study. J Cancer. 2018;9(1):148–156. doi:10.7150/jca.22246
27. Zheng BH, Liu LZ, Zhang ZZ, et al. Radiomics score: a potential prognostic imaging feature for postoperative survival of solitary HCC patients. BMC Cancer. 2018;18(1):1148. doi:10.1186/s12885-018-5024-z
28. Chan AWH, Zhong J, Berhane S, et al. Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J Hepatol. 2018;69(6):1284–1293. doi:10.1016/j.jhep.2018.08.027
29. Hu Y, You S, Yang Z, et al. Nomogram predicting survival of hepatocellular carcinoma with portal vein tumour thrombus after curative resection. ANZ J Surg. 2019;89(1–2):E20–E25. doi:10.1111/ans.2019.89.issue-1-2
30. Chen S, Gao Y, Li Z, et al. A nomogram predicting extrahepatic metastases for patients with adjuvant transarterial chemoembolization after hepatectomy. J Cancer. 2018;9(22):4223–4233. doi:10.7150/jca.25886
31. Kim JM, Kwon CHD, Joh JW, et al. Nomograms in hepatectomy patients with hepatitis B virus-related hepatocellular carcinoma. J Gastrointest Surg. 2019;23(8):1559–1567. doi:10.1007/s11605-018-04074-z
32. Wang Y, Sun K, Shen J, et al. Novel prognostic nomograms based on inflammation-related markers for patients with hepatocellular carcinoma underwent hepatectomy. Cancer Res Treat. 2019;51(4):1464–1478. doi:10.4143/crt.2018.657
33. Ho SY, Hsu CY, Liu PH, et al. Albumin-bilirubin (ALBI) grade-based nomogram to predict tumor recurrence in patients with hepatocellular carcinoma. Eur J Surg Oncol. 2019;45(5):776–781. doi:10.1016/j.ejso.2018.10.541
34. Li Y, Ma B, Yin Z, et al. Competing endogenous RNA network and prognostic nomograms for hepatocellular carcinoma patients who underwent R0 resection. J Cell Physiol. 2019;234(11):20342–20353. doi:10.1002/jcp.28634
35. Zhang XP, Chen ZH, Zhou TF, et al. A nomogram to predict early postoperative recurrence of hepatocellular carcinoma with portal vein tumour thrombus after R0 liver resection: A large-scale, multicenter study. Eur J Surg Oncol. 2019;45(9):1644–1651. doi:10.1016/j.ejso.2019.03.043
36. Zhang Z, Jiang H, Chen J, et al. Hepatocellular carcinoma: radiomics nomogram on gadoxetic acid-enhanced MR imaging for early postoperative recurrence prediction. Cancer Imaging. 2019;19(1):22. doi:10.1186/s40644-019-0209-5
37. Dekervel J, Popovic D, van Malenstein H, et al. A Global Risk Score (GRS) to simultaneously predict early and late tumor recurrence risk after resection of hepatocellular carcinoma. Transl Oncol. 2016;9(2):139–146. doi:10.1016/j.tranon.2016.02.003
38. Hung IF, Wong DK, Poon RT, et al. Risk factors and post-resection independent predictive score for the recurrence of hepatitis B-related hepatocellular carcinoma. PLoS One. 2016;11(2):e0148493. doi:10.1371/journal.pone.0148493
39. Zou H, Zhu CZ, Wang C, et al. Recurrence of Barcelona Clinic Liver Cancer Stage A hepatocellular carcinoma after hepatectomy. Am J Med Sci. 2017;354(3):262–267. doi:10.1016/j.amjms.2017.05.014
40. Tokumitsu Y, Sakamoto K, Tokuhisa Y, et al. A new prognostic model for hepatocellular carcinoma recurrence after curative hepatectomy. Oncol Lett. 2018;15(4):4411–4422. doi:10.3892/ol.2018.7821
41. Yan WT, Quan B, Xing H, et al. Time to recurrence, but not recurrence-free survival, should be the endpoint used to predict early recurrence after HCC resection. J Hepatol. 2019;70(3):570–571. doi:10.1016/j.jhep.2018.10.025
42. Zhou J, Sun HC, Wang Z, et al. Guidelines for diagnosis and treatment of primary liver cancer in China (2017 Edition). Liver Cancer. 2018;7(3):235–260. doi:10.1159/000488035
43. Bruix J, Sherman M. American association for the study of liver diseases. Management of Hepatocellular Carcinoma: an Update. Hepatology. 2011;53(3):1020–1022.
44. Kao WY, Chiou YY, Hung HH, et al. Risk factors for long-term prognosis in hepatocellular carcinoma after radiofrequency ablation therapy: the clinical implication of aspartate aminotransferase-platelet ratio index. Eur J Gastroenterol Hepatol. 2011;23(6):528–536. doi:10.1097/MEG.0b013e328346d529
45. Toyoda H, Kumada T, Tada T, et al. A laboratory marker, FIB-4 index, as a predictor for long-term outcomes of hepatocellular carcinoma patients after curative hepatic resection. Surgery. 2015;157(4):699–707. doi:10.1016/j.surg.2014.10.022
46. Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33(6):550–558. doi:10.1200/JCO.2014.57.9151
47. Lemoine M, Shimakawa Y, Nayagam S, et al. The gamma-glutamyl transpeptidase to platelet ratio (GPR) predicts significant liver fibrosis and cirrhosis in patients with chronic HBV infection in West Africa. Gut. 2016;65(8):1369–1376. doi:10.1136/gutjnl-2015-309260
48. Bedossa P. Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C The French METAVIR Cooperative Study Group. Hepatology. 1994;20(1 Pt 1):15–20. doi:10.1002/(ISSN)1527-3350
49. Kamarajah SK, Frankel TL, Sonnenday C, et al. Critical evaluation of the American Joint Commission on Cancer (AJCC) 8th edition staging system for patients with Hepatocellular Carcinoma (HCC): a surveillance, epidemiology, end results (SEER) analysis. J Surg Oncol. 2018;117(4):644–650. doi:10.1002/jso.24908
50. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314. doi:10.1016/S0140-6736(18)30010-2
51. Yau T, Tang VY, Yao TJ, et al. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology. 2014;146(7):1691–1700. e3. doi:10.1053/j.gastro.2014.02.032
52. Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7(3):462–503. doi:10.1002/1097-0142(195405)7:3<462::AID-CNCR2820070308>3.0.CO;2-E
53. Shindoh J, Hasegawa K, Inoue Y, et al. Risk factors of post-operative recurrence and adequate surgical approach to improve long-term outcomes of hepatocellular carcinoma. HPB (Oxford). 2013;15(1):31–39. doi:10.1111/j.1477-2574.2012.00552.x
54. DeOliveira ML, Winter JM, Schafer M, et al. Assessment of complications after pancreatic surgery: A novel grading system applied to 633 patients undergoing pancreaticoduodenectomy. Ann Surg. 2006;244(6):931–937. doi:10.1097/01.sla.0000246856.03918.9a
55. Furuta M, Ueno M, Fujimoto A, et al. Whole genome sequencing discriminates hepatocellular carcinoma with intrahepatic metastasis from multi-centric tumors. J Hepatol. 2017;66(2):363–373. doi:10.1016/j.jhep.2016.09.021
56. Xu XF, Xing H, Han J, et al. Risk factors, patterns, and outcomes of late recurrence after liver resection for hepatocellular carcinoma: a multicenter study from China. JAMA Surg. 2019;154(3):209–217. doi:10.1001/jamasurg.2018.4334
57. Czul F, Bhamidimarri KR. Noninvasive markers to assess liver fibrosis. J Clin Gastroenterol. 2016;50(6):445–457. doi:10.1097/MCG.0000000000000534
58. World Health Organization. Guidelines for the Prevention, Care and Treatment of Persons with Chronic Hepatitis B Infection. Switzerland: World Health Organization, Geneva; 2015.
59. Hiraoka A, Kumada T, Michitaka K, et al. Usefulness of albumin-bilirubin grade for evaluation of prognosis of 2584 Japanese patients with hepatocellular carcinoma. J Gastroenterol Hepatol. 2016;31(5):1031–1036. doi:10.1111/jgh.13250
60. Mx L, Zhao H, Bi XY, et al. Prognostic value of the albumin-bilirubin grade in patients with hepatocellular carcinoma: validation in a Chinese cohort. Hepatol Res. 2017;47(8):731–741. doi:10.1111/hepr.12796
61. Ho SY, Liu PH, Hsu CY, et al. Current noninvasive liver reserve models do not predict histological fibrosis severity in hepatocellular carcinoma. Sci Rep. 2018;8(1):15074. doi:10.1038/s41598-018-33536-2
62. Zhong JH, Ke Y, Gong WF, et al. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg. 2014;260(2):329–340. doi:10.1097/SLA.0000000000000236
63. Yan X, Fu X, Cai C, et al. Validation of models in patients with hepatocellular carcinoma: comparison of Hong Kong Liver Cancer with Barcelona Clinic Liver Cancer staging system in a Chinese cohort. Eur J Gastroenterol Hepatol. 2015;27(10):1180–1186. doi:10.1097/MEG.0000000000000418
64. Lee YS, Seo YS, Kim JH, et al. Can more aggressive treatment improve prognosis in patients with hepatocellular carcinoma? A direct comparison of the Hong Kong Liver Cancer and Barcelona Clinic Liver Cancer Algorithms. Gut Liver. 2018;12(1):94–101. doi:10.5009/gnl17040
65. Zhang H, Yuan SX, Dai SY, et al. Tumor size does not independently affect long-term survival after curative resection of solitary hepatocellular carcinoma without macroscopic vascular invasion. World J Surg. 2014;38(4):947–957. doi:10.1007/s00268-013-2365-2
66. Yang A, Xiao W, Chen D, et al. The power of tumor sizes in predicting the survival of solitary hepatocellular carcinoma patients. Cancer Med. 2018;7(12):6040–6050. doi:10.1002/cam4.2018.7.issue-12
67. Rodríguez-perálvarez M, Luong TV, Andreana L, et al. A systematic review of microvascular invasion in hepatocellular carcinoma: diagnostic and prognostic variability. Ann Surg Oncol. 2013;20(1):325–339. doi:10.1245/s10434-012-2513-1
68. Chen ZH, Zhang XP, Wang H, et al. Effect of microvascular invasion on the postoperative long-term prognosis of solitary small HCC: a systematic review and meta-analysis. HPB (Oxford). 2019;21(8):935–944. doi:10.1016/j.hpb.2019.02.003
69. Cong WM, Wu MC. New insights into molecular diagnostic pathology of primary liver cancer: advances and challenges. Cancer Lett. 2015;368(1):14–19. doi:10.1016/j.canlet.2015.07.043
70. Zhao Y, Si G, Zhu F, et al. Prognostic role of platelet to lymphocyte ratio in hepatocellular carcinoma: a systematic review and meta-analysis. Oncotarget. 2017;8(14):22854–22862. doi:10.18632/oncotarget.22557
71. Lakshmi Narendra B, Eshvendar Reddy K, Shantikumar S, et al. Immune system: a double-edged sword in cancer. Inflamm Res. 2013;62(9):823–834. doi:10.1007/s00011-013-0645-9
72. Wang Y, Peng C, Cheng Z, et al. The prognostic significance of preoperative neutrophil-lymphocyte ratio in patients with hepatocellular carcinoma receiving hepatectomy: A systematic review and meta-analysis. Int J Surg. 2018;55:73–80. doi:10.1016/j.ijsu.2018.05.022
73. Uchinaka E, Amisaki M, Morimoto M, et al. Utility and limitation of preoperative neutrophil lymphocyte ratio as a prognostic factor in hepatocellular carcinoma. Yonago Acta Med. 2018;61(4):197–203. doi:10.33160/yam.2018.12.002
74. Zhang J, Zhang M, Ma H, et al. Overexpression of glypican-3 is a predictor of poor prognosis in hepatocellular carcinoma: an updated meta-analysis. Medicine (Baltimore). 2018;97(24):e11130. doi:10.1097/MD.0000000000011130
75. Hamaoka M, Kobayashi T, Tanaka Y, et al. Clinical significance of glypican-3-positive circulating tumor cells of hepatocellular carcinoma patients: a prospective study. PLoS One. 2019;14(5):e0217586. doi:10.1371/journal.pone.0217586
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


