Back to Journals » Eye and Brain » Volume 15
Normal-Tension Glaucoma: A Glymphopathy?
Received 8 January 2023
Accepted for publication 7 March 2023
Published 6 April 2023 Volume 2023:15 Pages 37—44
DOI https://doi.org/10.2147/EB.S401306
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Margaret Wong-Riley
Peter Wostyn,1 Hanspeter Esriel Killer2,3
1Department of Psychiatry, PC Sint-Amandus, Beernem, Belgium; 2Department of Biomedicine, University of Basel, Basel, Switzerland; 3Augenärzte Zentrum Aarau, Aarau, Switzerland
Correspondence: Peter Wostyn, Department of Psychiatry, PC Sint-Amandus, Reigerlostraat 10, Beernem, 8730, Belgium, Tel +32-472713719, Fax +32-50-819720, Email [email protected]
Abstract: Glaucoma is one of the main causes of irreversible blindness in the world. The most common form, primary open-angle glaucoma, is an optic neuropathy that is characterized by a progressive loss of retinal ganglion cells and their axons, leading to structural changes in the optic nerve head and associated visual field defects. Elevated intraocular pressure remains the most important modifiable risk factor for primary open-angle glaucoma. However, a significant proportion of patients develop glaucomatous damage in the absence of increased intraocular pressure, a condition known as normal-tension glaucoma (NTG). The pathophysiology underlying NTG remains unclear. Several studies have revealed that vascular and cerebrospinal fluid (CSF) factors may play significant roles in the development of NTG. Vascular failure caused by functional or structural abnormalities, and compartmentation of the optic nerve subarachnoid space with disturbed CSF dynamics have been shown to be associated with NTG. In the present article, based on the concept of the glymphatic system and observations in patients with NTG, we hypothesize that failure of fluid transport via the glymphatic pathway in the optic nerve may be involved in the pathogenesis of some if not many cases of NTG. According to this hypothesis, vascular and CSF factors may share reduced glymphatic transport and perivascular waste clearance in the optic nerve as a final common pathway leading to the development of NTG. In addition, we speculate that some cases of NTG may reflect glymphatic dysfunction in natural brain aging and central nervous system diseases, such as Alzheimer’s disease. Clearly, further studies are needed to gain additional insight into the relative contribution of these factors and conditions to reduced glymphatic transport in the optic nerve.
Keywords: cerebrospinal fluid, glymphatic system, normal-tension glaucoma, optic nerve, perivascular spaces, vascular failure
Introduction
Glaucoma is one of the main causes of irreversible blindness in the world.1–3 The most common form, primary open-angle glaucoma (POAG), is an optic neuropathy that is characterized by a progressive loss of retinal ganglion cells (RGCs) and their axons, leading to structural changes in the optic nerve head and associated visual field defects.4 Elevated intraocular pressure (IOP) remains the most important modifiable risk factor for POAG.5 IOP is determined by the balance between the production and drainage of aqueous humor (AH).6 AH is produced by the ciliary body and is drained from the eye by conventional trabecular meshwork and unconventional uveoscleral outflow pathways.6–8 More recently, evidence indicated the presence of distinct lymphatic channels in the human ciliary body, and suggested that AH drainage occurs at least partially through this novel “uveolymphatic” outflow pathway.8,9 Increased IOP is, however, not present in all cases of glaucoma. Indeed, a significant proportion of patients develop glaucomatous damage in the absence of elevated IOP, a condition known as normal-tension glaucoma (NTG).5 It has been reported that NTG accounts for 20% to 90% of all patients with POAG, with percentages appearing to vary according to race.5 The pathophysiology underlying NTG remains unclear. Several studies have revealed that non-IOP factors, including vascular and cerebrospinal fluid (CSF) factors, may play significant roles in the development of NTG.10–12
In the present article, based on the concept of the glymphatic system and observations in patients with NTG, we hypothesize that failure of fluid transport via the glymphatic pathway in the optic nerve may be involved in the pathogenesis of some if not many cases of NTG. According to this hypothesis, such NTG cases may result from glymphatic dysfunction, leading to reduced removal of metabolic waste products in the optic nerve, ultimately resulting in glaucomatous damage.
Discussion
The Brain Glymphatic System
The “glymphatic system” was first identified in mice by a research team led by Iliff and Nedergaard in 2012.13 The authors discovered a brain-wide clearance system that uses perivascular spaces and that facilitates CSF-interstitial fluid (ISF) exchange. According to the glymphatic system model, subarachnoid CSF enters the brain via periarterial channels, while interstitial solutes, including amyloid-β (Aβ), are drained out of the brain along perivenous pathways for ultimate drainage towards the cervical lymphatic system (Figure 1).13,14 The pulsatility of the arterial wall is a major driver of periarterial CSF inflow.15–17 The subsequent CSF-ISF exchange is dependent on aquaporin-4 (AQP4) water channels which are densely present at the astrocytic endfeet ensheathing the cerebral vasculature.15
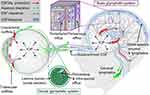 |
Figure 1 The brain and ocular glymphatic systems. Macroscopic overview of the brain and ocular glymphatic systems, emphasizing the role played by pressure gradients, hydrostatic barriers, and lymphatic drainage, shown in the context of known pathways for aqueous humor and cerebrospinal fluid (CSF) efflux. Abbreviations: ICP, intracranial pressure; IOP, intraocular pressure. Notes: Reproduced from Rangroo Thrane V, Hynnekleiv L, Wang X, Thrane AS, Krohn J, Nedergaard M. Twists and turns of ocular glymphatic clearance – new study reveals surprising findings in glaucoma. Acta Ophthalmol. 2021;99(2):e283-e284. Creative Commons.14 |
The Ocular Glymphatic System
The optic nerve is a central nervous system (CNS) white matter tract that is enveloped by all three meningeal layers and is surrounded by CSF in the subarachnoid space (SAS).18,19 The SASs of the brain and the optic nerve are normally contiguous, allowing CSF to circulate between the intracranial and optic nerve SASs.
In 2015, we published a hypothesis article, providing arguments for the existence of a glymphatic system in the optic nerve.20 Given the key role that the glymphatic system may play in the elimination of interstitial solutes from the brain, confirmation of such a perivascular transport system in the optic nerve could have great importance for our understanding of how solutes are removed from the ISF in the optic nerve, and could lead to impactful advances in our knowledge of the pathogenesis of glaucoma.20 Subsequently, we provided the first histological evidence for the presence of a perivascular pathway within the human optic nerve after injection of India ink into the optic nerve SAS.21,22 In this study of post-mortem human eyes, the globe and its optic nerve were removed and the optic nerve was ligated proximal to the optic chiasm before administration of India ink into the optic nerve SAS. High-injection pressure was carefully avoided in order to prevent artifacts. A very striking accumulation of India ink was seen in well-delineated perivascular spaces of the optic nerve and in collagen fiber bundles that are continuous with these spaces surrounding blood vessels (Figure 2).21,22
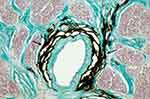 |
Figure 2 Optic nerve cross-section after injection of India ink into the optic nerve subarachnoid space. Distribution of ink (indicated by arrows) in the surroundings of the central retinal artery showing the complex slit-like space (Holmes-Luxol, 400x). Notes: Adapted from Wostyn P, De Groot V, Van Dam D, Audenaert K, De Deyn PP, Killer HE. The glymphatic system: a new player in ocular diseases? Invest Ophthalmol Vis Sci. 2016;57(13):5426–5427. Creative Commons.21 |
Interestingly, more recent studies have provided further evidence for entry of CSF into the optic nerve via a glymphatic pathway.23–26 In mice, following injection of fluorescent tracer into the CSF, Mathieu and colleagues23 demonstrated the entry of CSF into the optic nerve via spaces surrounding blood vessels, bordered by AQP4-positive astrocytic endfeet. AQP4 had already previously been found on astrocytic endfeet in the rat optic nerve.27 Additionally, a recent study conducted by Wang and colleagues25 confirmed the glymphatic transport in the retrograde direction, from the brain towards the eye, and identified a novel anterograde ocular glymphatic clearance system for removal of fluid and metabolites from the intraocular space via the proximal optic nerve in mice and rats (Figure 1). The authors extended the results from the study by Mathieu and colleagues23 by showing that influx of CSF tracer occurs along periarterial and pericapillary routes.25 In addition to this retrograde glymphatic CSF inflow path into the optic nerve, the authors demonstrated that intraocularly administered tracers like Aβ entered RGC axons, and then were cleared via a perivenous route in the optic nerve and subsequently drained by dural lymphatic vessels surrounding the optic nerve to cervical lymph nodes.25 Lymphatics in the dura mater of the human optic nerve were first described by Gausas and colleagues28 and Killer and colleagues in 1999.29 Similar to the brain’s glymphatic system, Wang and colleagues25 found that efflux of intraocularly administered Aβ was facilitated by AQP4 in both the retina and the optic nerve. Furthermore, the authors demonstrated that efflux via this pathway is driven by the high-to-low pressure gradient between the IOP and intracranial pressure, and by light-induced pupil constriction.25 In addition, a recent study by Jacobsen and colleagues26 utilized magnetic resonance imaging (MRI) along with intrathecally administered gadobutrol, serving as a CSF tracer, to visualize human visual pathway structures. The authors demonstrated enrichment of CSF tracer within the optic nerve, optic chiasm, optic tract, and primary visual cortex. There was only a nonsignificant signal increase in the vitreous body.26 Based on their findings, the authors postulated the existence of a glymphatic circulation in the human visual pathway. Notably, as compared with the middle and posterior parts of the optic nerve, the authors observed substantial gadobutrol enrichment in the retrobulbar part, corresponding to the entrance of the central retinal artery.26 According to the authors, this area could represent a major periarterial route for CSF tracer entry from the optic nerve SAS into the interstitium, analogous to the main routes of entry for CSF tracer molecules into the human brain parenchyma that have been reported to be along large artery trunks at the brain surface.
Vascular Factors in NTG Patients and Their Influence on Glymphatic Function
Multiple vascular risk factors including systemic hypotension and hypertension, diabetes mellitus, peripheral vascular disease, Raynaud syndrome, and migraine headache have been shown to be associated with NTG.10 The vascular theory of glaucomatous optic neuropathy (GON) in NTG considers it as a consequence of insufficient blood supply due to risk factors that reduce ocular blood flow and ocular perfusion pressure, leading to RGC death.30 Here, we propose an additional alternate mechanism to explain how vascular factors may contribute to the pathogenesis of NTG. We hypothesize that these vascular factors may increase the risk of developing NTG, at least in part, by failure of fluid transport via the optic nerve glymphatic circulation.
In mice, Mestre and colleagues31 found that acute arterial hypertension leads to a decrease in perivascular pumping, likely resulting from altered vessel wall dynamics, reducing the net CSF flow in brain perivascular spaces. The authors proposed that stiffening of artery walls could account for the decrease in CSF flow, suggesting that the same mechanism may be involved in chronic hypertension and arteriosclerosis.31 In arteriosclerosis, the walls of arteries become more rigid, the pulsations of the pulsation-driven “perivascular pump” decrease in amplitude, and perivascular fluid transport is reduced.32 Diabetes mellitus, systemic hypertension, and peripheral vascular disease are traditionally believed to cause vascular failure through arteriosclerosis resulting in an increase in vascular resistance to blood flow.10 It seems reasonable to assume that systemic arterial stiffening may also affect the central retinal artery to some extent, which could consequently result in a decrease in perivascular pumping efficiency in the optic nerve. Therefore, we believe that impaired glymphatic transport in the optic nerve could be a potential mechanism for NTG pathogenesis in patients with diabetes mellitus, systemic hypertension, and peripheral vascular disease.
Further, both Raynaud syndrome and migraines have been described as vasospastic disorders.10 Given that arterial vasospasm may cause a secondary decrease of arterial pulsations, and given that arterial pulsatility is a key force driving perivascular CSF influx, spasm of the central retinal artery could decrease net CSF flow in the optic nerve perivascular spaces in these diseases.
Finally, systemic hypotension, especially a nocturnal dip in blood pressure, has been shown to be associated with increased risk of NTG.10 To the best of our knowledge, no previous research has investigated the relationship between hypotension and glymphatic activity. However, increased choroid plexus blood flow and a higher CSF secretion rate have been observed in hypertensive rats.33 If systemic hypotension results in reduced CSF production, this decreased volume of CSF could hypothetically cause reduction of glymphatic CSF influx along the perivascular spaces of the optic nerve.
We suggest that the above vascular factors may contribute to reduced glymphatic transport and perivascular waste clearance in the optic nerve, and thereby may play a role in the pathogenesis of NTG. The crucial role of adequate optic nerve CSF flow has previously been demonstrated in sheep.34 Experimental segregation of CSF, induced by distal optic nerve SAS ligation, led to marked loss of optic nerve axons, providing evidence for a potential harmful effect of CSF stasis on the optic nerve.34 Impaired glymphatic CSF flow in the optic nerve may, therefore, lead to glaucomatous damage.
CSF Factors in NTG Patients and Their Influence on Glymphatic Function
In mice, Mathieu and colleagues23 provided evidence for CSF entry into the optic nerve via spaces surrounding blood vessels, bordered by astrocytic endfeet containing AQP4 water channels. CSF entry was observed up to and including the glia lamina, representing the mouse equivalent of the lamina cribrosa in humans. The authors hypothesized that CSF flow through the optic nerve’s glymphatic pathway may contribute to neurotoxin clearance in the retrolaminar and laminar optic nerve, which might have potential implications for glaucoma pathogenesis.23
In a more recent article, Mathieu and colleagues24 found that CSF entry into the SAS and perivascular spaces of the optic nerve is impaired after tracer injection into the CSF of 10-month-old DBA/2J glaucoma mice. Five of eight glaucoma mice showed absent CSF tracer in the SAS and in the optic nerve, while two of eight mice showed minimal tracer, and one of eight mice no difference compared with controls. Absent CSF tracer was observed in the laminar optic nerve in all female glaucoma mice (n = 4/4) and in one of four males.24 In this context, it is intriguing to note that sex-related differences have been reported in DBA/2J glaucoma mice, with females manifesting a more severe disease phenotype and earlier onset than males.35 The findings of an abnormal optic nerve glymphatic circulation in glaucoma24 provide support for our previously proposed glymphatic hypothesis of glaucoma.20,22,36 Mathieu and colleagues24 noted that the reduced perivascular inflow of CSF into the optic nerve in these DBA/2J glaucoma mice appears to result from blockage of the flow of CSF to the SAS surrounding the optic nerve, in contrast to localized obstruction of the perivascular inflow route. Indeed, the authors did not see any cases where CSF tracer was present in the SAS of the optic nerve, but not in the perivascular spaces of the same nerve.24 Their findings suggest that localized obstruction of CSF inflow through perivascular spaces is unlikely to account for reduced CSF inflow to the optic nerve, and point to the importance of an intact CSF circulation in the optic nerve SAS, without which glymphatic perivascular entry of CSF into the optic nerve may be impaired. One possible explanation for blocked perioptic CSF flow, proposed by the authors, could be a decreased pressure gradient between the intracranial SAS and the termination of the optic nerve SAS. Given that lymphatics of the dura mater or orbit may be a CSF outflow route from the SAS termination of the optic nerve, the authors proposed that if this CSF drainage into lymphatics is impaired in glaucoma, this may result in blockage of CSF inflow to the optic nerve SAS since the pressure gradient no longer facilitates CSF flow from the intracranial to the orbital CSF spaces.24
Interestingly, disturbed CSF dynamics within the SAS of the optic nerve have been observed in patients with NTG.12,37 In a study of 18 NTG patients with progressive bilateral glaucoma who underwent computed tomographic (CT) cisternography, Killer and colleagues12 found a significantly higher density of contrast-loaded CSF in the intracranial spaces compared with its density in the perioptic SAS. This difference was not observed in the control group without glaucoma. According to the authors, these findings suggest that in NTG patients, the SAS of the optic nerve can develop into an isolated CSF compartment with different CSF dynamics, a different CSF composition, and probably a different CSF pressure.12 In addition, Pircher and colleagues37 recently performed a retrospective analysis of CT cisternographies in 56 patients with NTG. The authors found a gradual decrease in contrast-loaded CSF towards the retrobulbar segment in NTG, while in control subjects without NTG, no such decrease was demonstrated.37 It was suggested that compromised CSF dynamics and CSF turnover within the optic nerve SAS may contribute to the pathophysiology of NTG.37
Recently, Pircher and colleagues38 conducted a retrospective biochemical analysis of CSF in 13 NTG patients with optic nerve sheath compartmentation. This study demonstrated a significant higher concentration of the CSF protein lipocalin-type prostaglandin D synthase (L-PGDS) in the compartmentalized optic nerve SAS compared to the concentration in the lumbar SAS.38 These findings support the concept that biochemical changes within the optic nerve SAS may contribute to the pathogenesis of GON in NTG patients with optic nerve sheath compartmentation.38 It has been suggested that the build-up of biologically highly active substances, such as L-PGDS, could have deleterious effects on mitochondria and axons of the optic nerve.34 As noted above, a marked loss of optic nerve axons has been observed in sheep following experimental compartmentation of the SAS of the optic nerve.34 Mitochondria are found in highest concentration in axons of an unmyelinated region of the human optic nerve right behind the lamina cribrosa.34 Mitochondria play a critical role in the production of adenosine triphosphate (ATP), which is required for cell survival.39 Given that the unmyelinated segment of the human optic nerve has a much higher mitochondrial enzyme activity to support higher energy demands, the immediate retrobulbar part of the optic nerve may be particularly susceptible to harmful effects.34 In an in vitro model, Xin and colleagues40 demonstrated a drastic reduction in the production of ATP by astrocyte mitochondria following exposure to increased levels of L-PGDS. Obviously, besides L-PGDS, other CSF components with high biological activity could also have toxic effects on the optic nerve.
Given that the perivascular inflow of CSF into the optic nerve may depend on an intact CSF circulation in the optic nerve SAS, it would be interesting to investigate whether the optic nerve glymphatic circulation is disturbed in NTG patients with compartmentation of the optic nerve sheath. This could be accomplished by means of MRI with intrathecal administration of a CSF tracer. Delayed tracer enrichment within the optic nerve would suggest that glymphatic entry of CSF into the optic nerve is reduced in these NTG patients. Given that neurotoxins may accumulate as a result of impaired glymphatic flow, this finding might be of high importance to better understand the pathogenesis of GON in NTG.
Given that there exists a glymphatic circulation within the optic nerve, and given that this perivascular transport system is similar and likely continuous with the glymphatic pathway in the rest of the CNS, we further speculate that some NTG cases may actually reflect glymphatic dysfunction in natural brain aging41 and disorders of the CNS, such as Alzheimer’s disease (AD).42,43 This is in line with the age-specific prevalence pattern of NTG,44 and the association reported between NTG and AD.45
Finally, it should be noted that pathological changes in ocular glymphatic transport may also play a role in high-tension glaucoma. Disruption of ocular glymphatic flow has been demonstrated in murine models of high IOP glaucoma. Using two distinct murine models of glaucoma and chronic ocular hypertension, Wang and colleagues25 found that ocular glymphatic flow was severely disrupted. Intravitreally injected Aβ tracer leaked from the eye via defects in the lamina barrier instead of intra-axonal efflux. The authors hypothesized that the reduction in intra-axonal flow might lead to accumulation of metabolic waste products like Aβ within RGCs, leading to dysfunction and cell death.25 Moreover, as noted above, Mathieu and colleagues24 showed that glymphatic entry of CSF into the optic nerve was reduced in 10-month-old DBA/2J glaucoma mice. IOP was significantly higher in these glaucoma mice compared with age-matched controls.24 The authors concluded that this new CSF-related mechanism might be relevant to understanding optic nerve damage in glaucoma.
Conclusion
In this article, we present a hypothesis according to which vascular and CSF factors may increase the risk of developing NTG, at least in part, by reduced glymphatic transport and perivascular waste clearance in the optic nerve. A variety of vascular-associated conditions including systemic hypertension, peripheral vascular disease, diabetes mellitus, Raynaud syndrome, and migraine headache may restrict arterial pulsatility, resulting in reduced glymphatic function in the optic nerve, ultimately leading to GON. Systemic hypotension, another known risk factor for NTG, may hypothetically reduce CSF production, which may have a negative impact on glymphatic transport in the optic nerve. Compromised CSF dynamics within the SAS of the optic nerve may also contribute to the pathogenesis of NTG by reducing glymphatic flow in the optic nerve. As such, these vascular and CSF factors may share reduced perivascular waste clearance in the optic nerve as a final common pathway leading to the development of NTG. In addition, we speculate that some cases of NTG may reflect glymphatic dysfunction in natural brain aging and disorders of the CNS, such as AD. Clearly, further studies are needed to gain additional insight into the relative contribution of these factors and conditions to reduced glymphatic transport in the optic nerve.
Abbreviations
Aβ, amyloid-β; AD, Alzheimer’s disease; AH, aqueous humor; AQP4, aquaporin-4; ATP, adenosine triphosphate; CNS, central nervous system; CSF, cerebrospinal fluid; CT, computed tomography; GON, glaucomatous optic neuropathy; ICP, intracranial pressure; IOP, intraocular pressure; ISF, interstitial fluid; L-PGDS, lipocalin-type prostaglandin D synthase; MRI, magnetic resonance imaging; NTG, normal-tension glaucoma; POAG, primary open-angle glaucoma; RGC, retinal ganglion cell; SAS, subarachnoid space.
Author Contributions
Both authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas. P.W. drafted the article. H.E.K. critically reviewed the article. Both authors agreed on the journal to which the article will be submitted; reviewed and agreed on all versions of the article before submission, during revision, the final version accepted for publication, and any significant changes introduced at the proofing stage; and agreed to take responsibility and be accountable for the contents of the article.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996;80(5):389–393.
2. Resnikoff S, Pascolini D, Etya’ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82(11):844–851.
3. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267.
4. Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363(9422):1711–1720.
5. Harada T, Harada C, Nakamura K, et al. The potential role of glutamate transporters in the pathogenesis of normal tension glaucoma. J Clin Invest. 2007;117(7):1763–1770.
6. Civan MM, Macknight AD. The ins and outs of aqueous humour secretion. Exp Eye Res. 2004;78(3):625–631.
7. Kim M, Johnston MG, Gupta N, Moore S, Yücel YH. A model to measure lymphatic drainage from the eye. Exp Eye Res. 2011;93(5):586–591.
8. Yücel YH, Johnston MG, Ly T, et al. Identification of lymphatics in the ciliary body of the human eye: a novel “uveolymphatic” outflow pathway. Exp Eye Res. 2009;89(5):810–819.
9. Yucel Y, Gupta N. Lymphatic drainage from the eye: a new target for therapy. Prog Brain Res. 2015;220:185–198.
10. Funk RO, Hodge DO, Kohli D, Roddy GW. Multiple systemic vascular risk factors are associated with low-tension glaucoma. J Glaucoma. 2022;31(1):15–22.
11. Berdahl JP, Allingham RR. Intracranial pressure and glaucoma. Curr Opin Ophthalmol. 2010;21(2):106–111.
12. Killer HE, Miller NR, Flammer J, et al. Cerebrospinal fluid exchange in the optic nerve in normal-tension glaucoma. Br J Ophthalmol. 2012;96(4):544–548.
13. Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4(147):147ra111.
14. Rangroo Thrane V, Hynnekleiv L, Wang X, Thrane AS, Krohn J, Nedergaard M. Twists and turns of ocular glymphatic clearance – new study reveals surprising findings in glaucoma. Acta Ophthalmol. 2021;99(2):e283–e284.
15. Jessen NA, Munk AS, Lundgaard I, Nedergaard M. The Glymphatic System: a Beginner’s Guide. Neurochem Res. 2015;40(12):2583–2599.
16. Iliff JJ, Wang M, Zeppenfeld DM, et al. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J Neurosci. 2013;33(46):18190–18199.
17. Mogensen FL, Delle C, Nedergaard M. The glymphatic system (en)during inflammation. Int J Mol Sci. 2021;22(14):7491.
18. London A, Benhar I, Schwartz M. The retina as a window to the brain - from eye research to CNS disorders. Nat Rev Neurol. 2013;9(1):44–53.
19. Killer HE, Jaggi GP, Flammer J, Miller NR, Huber AR. The optic nerve: a new window into cerebrospinal fluid composition? Brain. 2006;129(Pt 4):1027–1030.
20. Wostyn P, Van Dam D, Audenaert K, Killer HE, De Deyn PP, De Groot V. A new glaucoma hypothesis: a role of glymphatic system dysfunction. Fluids Barriers CNS. 2015;12:16.
21. Wostyn P, De Groot V, Van Dam D, Audenaert K, De Deyn PP, Killer HE. The glymphatic system: a new player in ocular diseases? Invest Ophthalmol Vis Sci. 2016;57(13):5426–5427.
22. Wostyn P, Killer HE, De Deyn PP. Glymphatic stasis at the site of the lamina cribrosa as a potential mechanism underlying open-angle glaucoma. Clin Exp Ophthalmol. 2017;45(5):539–547.
23. Mathieu E, Gupta N, Ahari A, Zhou X, Hanna J, Yücel YH. Evidence for cerebrospinal fluid entry into the optic nerve via a glymphatic pathway. Invest Ophthalmol Vis Sci. 2017;58(11):4784–4791.
24. Mathieu E, Gupta N, Paczka-Giorgi LA, et al. Reduced Cerebrospinal Fluid Inflow to the Optic Nerve in Glaucoma. Invest Ophthalmol Vis Sci. 2018;59(15):5876–5884.
25. Wang X, Lou N, Eberhardt A, et al. An ocular glymphatic clearance system removes β-amyloid from the rodent eye. Sci Transl Med. 2020;12(536):eaaw3210.
26. Jacobsen HH, Ringstad G, Jørstad ØK, Moe MC, Sandell T, Eide PK. The human visual pathway communicates directly with the subarachnoid space. Invest Ophthalmol Vis Sci. 2019;60(7):2773–2780.
27. Nagelhus EA, Veruki ML, Torp R, et al. Aquaporin-4 water channel protein in the rat retina and optic nerve: polarized expression in Müller cells and fibrous astrocytes. J Neurosci. 1998;18(7):2506–2519.
28. Gausas RE, Gonnering RS, Lemke BN, Dortzbach RK, Sherman DD. Identification of human orbital lymphatics. Ophthal Plast Reconstr Surg. 1999;15(4):252–259.
29. Killer HE, Laeng HR, Groscurth P. Lymphatic capillaries in the meninges of the human optic nerve. J Neuroophthalmol. 1999;19(4):222–228.
30. Flammer J, Orgül S, Costa VP, et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002;21(4):359–393.
31. Mestre H, Tithof J, Du T, et al. Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat Commun. 2018;9(1):4878.
32. Hadaczek P, Yamashita Y, Mirek H, et al. The “perivascular pump” driven by arterial pulsation is a powerful mechanism for the distribution of therapeutic molecules within the brain. Mol Ther. 2006;14(1):69–78.
33. Al-Sarraf H, Philip L. Effect of hypertension on the integrity of blood brain and blood CSF barriers, cerebral blood flow and CSF secretion in the rat. Brain Res. 2003;975(1–2):179–188.
34. Jaggi GP, Harlev M, Ziegler U, Dotan S, Miller NR, Killer HE. Cerebrospinal fluid segregation optic neuropathy: an experimental model and a hypothesis. Br J Ophthalmol. 2010;94(8):1088–1093.
35. Libby RT, Anderson MG, Pang IH, et al. Inherited glaucoma in DBA/2J mice: pertinent disease features for studying the neurodegeneration. Vis Neurosci. 2005;22(5):637–648.
36. Wostyn P, De Groot V, Van Dam D, Audenaert K, Killer HE, De Deyn PP. The glymphatic hypothesis of glaucoma: a unifying concept incorporating vascular, biomechanical, and biochemical aspects of the disease. Biomed Res Int. 2017;2017:5123148.
37. Pircher A, Montali M, Wostyn P, et al. Impaired cerebrospinal fluid dynamics along the entire optic nerve in normal-tension glaucoma. Acta Ophthalmol. 2018;96(5):e562–e569.
38. Pircher A, Neutzner A, Montali M, et al. Lipocalin-type prostaglandin D synthase concentration gradients in the cerebrospinal fluid in normal-tension glaucoma patients with optic nerve sheath compartmentation. Eye Brain. 2021;13:89–97.
39. Bristow EA, Griffiths PG, Andrews RM, Johnson MA, Turnbull DM. The distribution of mitochondrial activity in relation to optic nerve structure. Arch Ophthalmol. 2002;120(6):791–796.
40. Xin X, Huber A, Meyer P, et al. L-PGDS (betatrace protein) inhibits astrocyte proliferation and mitochondrial ATP production in vitro. J Mol Neurosci. 2009;39(3):366–371.
41. Kress BT, Iliff JJ, Xia M, et al. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol. 2014;76(6):845–861.
42. Peng W, Achariyar TM, Li B, et al. Suppression of glymphatic fluid transport in a mouse model of Alzheimer’s disease. Neurobiol Dis. 2016;93:215–225.
43. Taoka T, Masutani Y, Kawai H, et al. Evaluation of glymphatic system activity with the diffusion MR technique: diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer’s disease cases. Jpn J Radiol. 2017;35(4):172–178.
44. Mi XS, Yuan TF, So KF. The current research status of normal tension glaucoma. Clin Interv Aging. 2014;9:1563–1571.
45. Chen YY, Lai YJ, Yen YF, et al. Association between normal tension glaucoma and the risk of Alzheimer’s disease: a nationwide population-based cohort study in Taiwan. BMJ Open. 2018;8(11):e022987.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
