Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Nonulcerated Necrobiosis Lipoidica Successfully Treated with Tapinarof: A Case Report
Authors Palomares SJ, Farberg AS
Received 4 March 2023
Accepted for publication 25 May 2023
Published 29 May 2023 Volume 2023:16 Pages 1373—1376
DOI https://doi.org/10.2147/CCID.S408070
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Samantha J Palomares, Aaron S Farberg
Bare Dermatology, Dallas, TX, USA
Correspondence: Aaron S Farberg, Tel +1 847 721 2725, Email [email protected]
Abstract: Necrobiosis lipoidica (NL) is a chronic granulomatous disorder of the skin which usually presents with red papules and plaques on the lower extremities. Diabetes mellitus has been found to be associated with NL, but the pathophysiology of the disease is unknown. Based on a Doppler flowmetry study showing increased blood flow at NL lesions and the macrophage upregulation of granulomatous disorders, it is reasonable to conclude that there is an inflammatory component to it. NL is extremely challenging to manage. The initial treatment of choice is usually topical or intralesional corticosteroids, and if this fails to work, many dermatologists depend on the small number of case reports for more treatment options. We present a pre-diabetic patient with nonulcerative NL who was successfully treated with the first-in-class therapeutic aryl hydrocarbon receptor (AHR)-modulating agent tapinarof cream (VTAMA, Dermavant). Following the case presentation is a discussion of this topical novel agent and its unique anti-inflammatory mechanism of action. Tapinarof specifically binds to and activates AHR leading to downregulation of TNF-α/IL-23/IL-17 and inhibition of IL-4/IL-13 mediated STAT6 activation. Anti-TNF-α agents and JAK-inhibitors have also been found to be beneficial in treating NL; tapinarof seems to target both these pathways without the risk of their serious adverse reactions.
Keywords: aryl hydrocarbon receptor, AHR, granulomatous disease, JAK-STAT pathway, macrophage activation disorder, necrobiosis lipoidica diabeticorum, therapeutic aryl hydrocarbon receptor-modulating agent, TAMA, tumor necrosis factor-alpha antagonists, TNF-α antagonists
Introduction
Necrobiosis lipoidica (NL) is a chronic granulomatous skin disorder typically manifesting as erythematous papules and plaques on the pretibial region of the lower extremities. Patients usually present asymptomatic, however, pruritus, dysesthesia or pain at the site of the lesions may be reported.1 It has been associated with diabetes mellitus, hence its former name necrobiosis lipoidica diabeticorum, but the exact pathogenesis is unknown. Common complications of the disease are ulceration, and rarely NL can transform into squamous cell carcinoma.2 It is important to note trauma can incite the development of ulcerations in NL, and patients should be educated accordingly.3 NL has been proven to be difficult to treat despite the various treatment modalities available. Herein we report a case of NL that was successfully treated with tapinarof cream.
Case Report
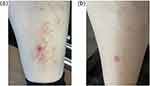
A 44-year-old woman presented to our office with a four-month history of a pruritic rash that had been treated with triamcinolone cream for three weeks without clinical improvements. Exam revealed pink papules and plaques with some central atrophy on the left pretibial region (Figure 1a). Clinical examination and medical history were negative, except for prediabetes with a hemoglobin A1C of 5.7% shortly afterwards.
A deep scoop shave biopsy was then obtained from the left shin and sent for hematoxylin and eosin (H&E) stain. The pathology report showed a diffuse infiltrate of histiocytes in a palisaded pattern extending from the upper dermis to the base of the specimen, as well as degenerated collagen, and an infiltrate of lymphocytes and plasma cells. These findings were compatible with the diagnosis of necrobiosis lipoidica. Treatment was initiated with tapinarof 1% cream (VTAMA, Dermavant) twice daily, and there was improvement over one-month of use. At this time, she reported reduction of pruritus, and all but two lesions were resolved; one of which was the healing biopsy site (Figure 1b). Atrophic scars were seen in the place of resolved lesions. The medication was well tolerated by the patient, and no adverse reactions occurred. The patient was directed to wean use of tapinarof with the goal of application once daily on the weekends to keep from reoccurrence. She transitioned to once daily application for two weeks and then to every other day. After ten days of every other day application of tapinarof, new lesions developed. Twice daily application was resumed and within one week, the new lesions resolved, and the patient began a slower wean of tapinarof.
Discussion
The pathogenesis of NL is unknown. Due to its association with diabetes, it has been thought to be related to diabetic microangiopathy. This theory has been challenged by a Doppler flowmetry study showing increased blood flow in NL lesions, suggesting it to be more of an inflammatory process.4 Furthermore, NL has been categorized as a macrophage activation disorder along with sarcoidosis, granuloma annulare, crohn’s disease, and hemophagocytic lymphohistiocytosis. Within these granulomatous disorders, macrophages are upregulated and produce IL-6, IL-12, IL-18, IL-23, TNF-α, and T cell chemokines.5 In addition, low levels of JAK-STAT signaling have been found in NL. In a histologic case series, activation of STAT1 and STAT3 were discovered in all eleven cases of NL examined.6 Based on these findings, we think it is reasonable to target the downregulation of TNF-α and the JAK-STAT pathway when considering treatment of NL.
There have been several case reports using TNF-α antagonists (infliximab, etanercept, adalimumab) to successfully treat NL. Basoulis et al reported a case of ulcerative NL successfully treated with infliximab and reviewed the literature of cases of NL treated with TNF-α antagonists.7 Turning our attention to the JAK-STAT pathway, there are a few case reports of patients with NL successfully treated with JAK inhibitors. Janßen et al showed healing of a patient with ulcerated NL with tofacitinib and a hair-containing punch graft transplantation after initially failing therapy with the TNF-α inhibitor adalimumab.8 Damsky et al achieved a better response with the dual therapy of tofacitinib with intralesional corticosteroids compared to monotherapy with either.6 They hypothesized that the synergistic response was the resultant of inhibition of both JAK-dependent and JAK-independent (ie TNF-α) cytokines. Lee et al reported a case using ruxolitinib to treat polycythemia vera, which resulted in the resolution of the patient’s concomitant ulcerative NL.9 Using TNF-α antagonists and JAK-inhibitors is not without risk. There is a black box warning on infliximab and tofacitinib of increased risk of serious infections that could lead to hospitalization or death. Infections include acute tuberculosis, reactivation of latent tuberculosis, invasive fungal infections and opportunistic infections.10,11
In our patient, we selected the novel therapeutic aryl hydrocarbon receptor (AHR)-modulating agent (TAMA)—tapinarof 1% cream. AHR is a ligand-dependent transcription factor that helps regulate skin homeostasis.12 Tapinarof selectively binds to AHR, leading to the downregulation of proinflammatory cytokines, antioxidant activity, and the promotion of skin barrier normalization through regulation of skin barrier protein expression, including filaggrin and loricrin.12 More specifically, there is a suppression of TNF-α/IL-23/IL-17 and inhibition of IL-4/IL-13 mediated STAT6 activation.13
We believe that tapinarof’s downregulation of TNF-α and IL-23, which are produced by macrophages upregulated by NL, and deactivation of STAT6 is what makes it a good option for the treatment of NL. Though there has been some successful treatment with TNF-α antagonists and JAK inhibitors, there is more risk associated with their use. In contrast, there are no serious adverse events nor contraindications with tapinarof.14,15 It is well tolerated with adverse reactions being folliculitis (20%), nasopharyngitis (11%), contact dermatitis (7%), headache (4%), pruritus (3%), and influenza (2%).15 Therefore, we believe that topical tapinarof could be a good option when it comes to the treatment of NL.
Conclusion
NL is a rare disorder affecting the skin. It can have a significant impact on quality of life due to the aesthetic concerns, symptoms of pain and pruritus, and the common complication of ulceration. Even though the pathogenesis of NL is unclear, it is reasonable to conclude that there is an inflammatory component to it. Tapinarof is a selective AHR agonist with impressive anti-inflammatory effects through the downregulation of TNF-α/IL-23/IL-17 and inhibition of IL-4/IL-13 mediated STAT6 activation. This topical agent is a non-steroidal cream and was well tolerated in clinical trials with a few adverse reactions. Since therapeutic options for NL are limited due to missing guidelines and randomized controlled trials, it is of high clinical impact to report about new and successful approaches. Tapinarof showed therapeutic benefit for our patient with NL and may prove to successfully treat more patients with NL.
Abbreviations
AHR, Aryl hydrocarbon receptor; IL-4, interleukin-4; IL-13, interleukin-13; IL-17, interleukin-17; IL-23, interleukin-23; JAK, Janus kinase; NL, necrobiosis lipoidica; TNF-α antagonist, tumor necrosis factor α antagonist; STAT, signal transducers and activators of transcription.
Consent
Written informed consent for publication of their details was obtained from the patient. Institutional approval was not required to publish the case details.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lowitt MH, Dover JS. Necrobiosis lipoidica. J Am Acad Dermatol. 1991;25(5):735–748. doi:10.1016/S0190-9622(08)80961-9
2. Reid SD, Ladizinski B, Lee K, Baibergenova A, Alavi A. Update on necrobiosis lipoidica: a review of etiology, diagnosis, and treatment options. J Am Acad Dermatol. 2013;69(5):783–791. doi:10.1016/j.jaad.2013.05.034
3. Muller SA, Winkelmann RK. Necrobiosis lipoidica diabeticorum: a clinical and pathological investigation of 171 cases. Arch Dermatol. 1966;93(3):272–281. doi:10.1001/archderm.1966.01600210008002
4. Ngo B, Wigington G, Hayes K, et al. Skin blood flow in necrobiosis lipoidica diabeticorum. Int J Dermatol. 2008;47(4):354–358. doi:10.1111/j.1365-4632.2008.03549.x
5. Wang A, Singh K, Ibrahim W, King B, Damsky W. The promise of JAK inhibitors for treatment of sarcoidosis and other inflammatory disorders with macrophage activation: a review of the literature. Yale J Biol Med. 2020;93(1):187–195.
6. Damsky W, Singh K, Galan A, King B. Treatment of necrobiosis lipoidica with combination Janus kinase inhibition and intralesional corticosteroid. JAAD Case Rep. 2020;6(2):133–135. doi:10.1016/j.jdcr.2019.11.016
7. Basoulis D, Fragiadaki K, Tentolouris N, Sfikakis PP, Kokkinos A. Anti-TNFα treatment for recalcitrant ulcerative necrobiosis lipoidica diabeticorum: a case report and review of the literature. Metabolism. 2016;65(4):569–573. doi:10.1016/j.metabol.2015.12.014
8. Janßen S, Jansen TM. Ulcerated necrobiosis lipoidica successfully treated with tofacitinib. Int J Dermatol. 2022;61(6):739–741. doi:10.1111/ijd.15960
9. Lee JJ, English JC. Improvement in ulcerative necrobiosis lipoidica after Janus kinase–inhibitor therapy for polycythemia vera. JAMA Dermatol. 2018;154(6):733–734. doi:10.1001/jamadermatol.2018.0756
10. Remicade (infliximab) [package insert]. Horsham, PA: Janssen Biotech, Inc; 2021.
11. Xeljanz (tofacitinib) [package insert]. New York, NY: Pfizer Labs; 2022.
12. Bissonnette R, Stein Gold L, Rubenstein DS, Tallman AM, Armstrong A. Tapinarof in the treatment of psoriasis: a review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent. J Am Acad Dermatol. 2021;84(4):1059–1067. doi:10.1016/j.jaad.2020.10.085
13. Furue M, Hashimoto-Hachiya A, Tsuji G. Aryl hydrocarbon receptor in atopic dermatitis and psoriasis. Int J Mol Sci. 2019;20(21):5424. doi:10.3390/ijms20215424
14. Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385(24):2219–2229. doi:10.1056/NEJMoa2103629
15. VTAMA (tapinarof) [package insert]. Long Beach, CA: Dermavant Sciences, Inc; 2022.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.