Back to Journals » International Journal of General Medicine » Volume 14
Nonspecific ST-Segment and T-Wave (NS-STT) on Electrocardiogram is Associated with Increasing the Incidence of Perioperative Deep Vein Thrombosis in Patients with Lower Extremity Fracture Under 75 Years Old
Authors Ren C, Li M, Ma T, Xu YB, Li Z, Xue HZ, Wang Q, Lu Y , Sun L, Zhang K
Received 30 September 2021
Accepted for publication 11 November 2021
Published 23 November 2021 Volume 2021:14 Pages 8631—8641
DOI https://doi.org/10.2147/IJGM.S335243
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Cheng Ren,* Ming Li,* Teng Ma, Yi-Bo Xu, Zhong Li, Han-Zhong Xue, Qian Wang, Yao Lu, Liang Sun, Kun Zhang
Department of Orthopedic Trauma, Honghui Hospital, Xi’an Jiaotong University, Xi’an, Shaanxi Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Kun Zhang
Department of Orthopedic Trauma, Honghui Hospital, Xi’an Jiaotong University, Xi’an, Shaanxi Province, People’s Republic of China
Email [email protected]
Objective: This study aims to explore the clinical correlation between nonspecific ST-segment or T-wave (NS-STT) changes and perioperative deep vein thrombosis (DVT) in patients with lower extremity fractures.
Methods: One thousand four hundred sixty-nine consecutive patients who suffered lower extremity fractures were screened at Xi’an Honghui Hospital between Feb 2016 and Nov 2018. According to the included and excluded criteria, the patients were included in this retrospective study. After collecting the electrocardiogram baseline, the patients were divided into the NS-STT group and the non-NS-STT group. After comparing the demographic and clinical characteristics, multivariate logistic regression models were used to identify the role of NS-STT changes on perioperative DVT. All analyses were performed with R and EmpowerStats software.
Results: Nine hundred and sixty-eight patients were included in the study. Ninety-seven patients (10.02%) had NS-STT changes on the electrocardiogram at admission. A total of 303 patients (31.30%) developed perioperative DVT in lower extremities. The univariate analysis showed that NS-STT segment changes were correlated with perioperative DVT significantly (OR = 3.45, 95% CI: 2.25– 5.30, P < 0.0001). In addition, age ≥ 50 (P < 0.0001), female (OR = 1.50, 95% CI: 1.14– 1.97, P = 0.0038), hypertension (OR = 1.54, 95% CI: 1.08– 2.20, P = 0.0161), blood transfusion (OR = 1.78, 95% CI: 1.34– 2.37, P < 0.0001), joint prosthesis (OR = 3.26, 95% CI: 2.21– 4.81, P < 0.0001), and blood loss ≥ 300 mL (OR = 2.12, 95% CI: 1.50– 3.01, P < 0.0001) were associated with perioperative DVT in lower extremities. We identified the confounding factors of age, gender, classification of internal implants, operation time, blood loss, and infusion. After adjustment for potential confounding factors, NS-STT changes were associated with perioperative DVT (OR = 2.13, 95% CI: 1.33– 3.42; P = 0.0017). The sensitive analysis showed that the result was stable.
Conclusion: The NS-STT changes on electrocardiograms are associated with an increase in the incidence of perioperative DVT by 2.13-fold in patients with lower extremity fractures under 75 years old. In clinical practice, surgeons should pay more attention to these patients.
Keywords: nonspecific ST-segment and T-wave, NS-STT, electrocardiogram, DVT, lower extremity fracture, logistic regression
Introduction
Deep vein thrombosis (DVT) is an essential complication in patients with lower extremities fractures. However, previous studies have paid more attention to the thrombosis after fracture but ignored the thrombosis after surgery. In patients with lower extremities fracture, the prevalence of postoperative DVT is often higher than the data before surgery.1 The incidence of postoperative DVT could reach 61.3%.2 It is also found that 28% of patients would have new DVT after the operation, and most of the thrombosis was preoperative DVT and persisted to post-operation in a recent study.3
The perioperative DVT could influence the process of rehabilitation and recovery. Notably, a freshly formed thrombosis is loosely attached to the vein wall, and it is easy to fall off and cause a pulmonary embolism. In addition, the leg swelling, skin changes, pain, and chronic ulceration would affect the patients’ psychology. Therefore, perioperative DVT has a higher risk of falling off during functional exercise and should be paid attention to.
The etiology of perioperative DVT following lower extremity fracture is usually multifactorial, and most of the factors were related to surgery, including blood loss, operation time, and so on.1 Although the perioperative DVT caused by surgery have increasingly been attracting attention recently, few researchers are concerned about the effects of the preoperative factor on perioperative DVT.
Traditionally, ST-segment or T-wave changes on the electrocardiogram are known predictors of cardiovascular mortality.4 However, physiological nonspecific ST-segment or T-wave (NS-STT) is also very common and can be seen in any lead of the electrocardiogram. Most clinicians regard isolated, minor, or NS-STT abnormalities to be incidental, often transient, and benign findings in asymptomatic patients.5 In fact, it is reported that NS-STT changes are related to inflammation diseases.6 The inflammatory response is involved in thrombus formation, thrombus organization, and the vein recanalization process, and the link between inflammation and DVT has been identified.7–9 Additionally, the relationship between arrhythmogenesis, inflammation, and coagulation was established early.10 Thus, the evidence indicates that physiological NS-STT may relate to thrombosis formation.
As far as we know, no preceding work or report has considered the effects of NS-STT changes on perioperative thrombosis. This study aims to explore the clinical correlation between NS-STT changes and perioperative DVT in patients with lower extremity fractures.
Materials and Methods
Study Population
Clinical and surgical data for the cases reviewed were obtained from original medical records. The ethics committee of Xi’an Honghui Hospital approved this retrospective study (No. 2014026). As patient identity remained anonymous, and the requirement for informed consent was waived due to the observational nature of the study, as reported elsewhere.11,12 All human-related procedures were in concordance with the 1964 Helsinki Declaration and its later amendments.
One thousand four hundred sixty-nine consecutive patients suffered lower extremity fracture at Xi’an Honghui Hospital between Feb 2016 and Nov 2018. The inclusion criteria were as follows: 1). Age of ≥18 years; 2) Fresh occurring lower extremity fractures that were receiving surgical treatment; 3) Availability of electrocardiogram; 4) Completing the ultrasonography examination at admission and peri-operation. We excluded data from a patient with coronary heart disease symptoms (n = 28), and with age >75 years (n = 320) and patients receiving the closed reduction and internal fixation (n = 153), and the remaining 968 cases formed the final analysis population finally.
When at admission, the nurses recorded the individual electrocardiogram electrical signals. Professional physicians, diagnosing the reports more than 50,000 cases every year, reported the electrocardiogram results. Their reports were independent and undisturbed. Two trained physicians analyzed each ECG to keep the consistency and resolved disagreements through discussion, and unresolved disagreements were discussed with a third senior physician. The criteria of NS-STT changes included subtle straightening of the ST segment, actual ST-segment depression or elevation, flattening of the T wave, biphasic T waves, or T wave inversion. According to these criteria, a total of 97 patients experienced NS-STT changes following the fracture.
We defined perioperative DVT as the thrombosis occurring before or after the operation, defecting by ultrasonography. Ultrasonography was used to diagnose DVT. Three trained operators performed vascular ultrasonography with a bedside machine. The diagnostic criterion of fresh thrombosis was the presence of a constant intraluminal filling defect.13 All the patients received the first examination in both lower extremities when at admission.
When the nurses collected electrocardiogram and blood samples at admission, the surgeons assessed the thromboembolism risk for every patient using the Risk Assessment Profile for Thromboembolism score.14 For the patients without contraindications, low-molecular-weight heparin (LMWH; 3800 IU/0.4 mL, once per day; Fraxiparine, Glaxo Wellcome Production, GlaxoSmithKline) was subcutaneously injected to prevent DVT, according to the guidelines in CHEST 2012.15 The anticoagulant therapy was discontinued 12 h before operation and resumed 24 h after operation. In addition, we used a mechanical pressure pump (20 min, twice per day) to promote blood reflux. For the patients without thrombosis, LMWH was continuously subcutaneously injected. For the patients with thrombosis, physicians from the vascular surgery department prescribed the DVT treatment, and LMWH (3800 IU/0.4 mL, twice per day; Fraxiparine, Glaxo Wellcome Production, GlaxoSmithKline) was subcutaneously injected. If needed, we used an inferior vena cava filter to prevent fatal pulmonary embolism before the operation. After the operation, the protocol of anticoagulant strategy was continued. The patients received the second ultrasonography examination in both lower extremities on the third to fifth day after the operation.
Statistics Analysis
Firstly, we compared the data distribution of each covariate between the NS-STT group and the non-NS-STT group, using the t-test or Kruskal–Wallis rank sum test for continuous variables and c2 tests for categorical data. Secondly, univariate and subgroup analysis and multivariate logistic regression models were used to examine whether NS-STT changes on electrocardiogram and other covariates had an independent effect on perioperative occurring lower extremity DVT following lower extremities fracture separately. Thirdly, we explored the relationship between NS-STT changes on electrocardiogram and perioperative DVT in patients with lower extremity fracture by the smoothing plot, with an adjustment for potential confounders. All analyses were performed with R (http://www.R-project.org) and EmpowerStats software (www.empowerstats.com, X&Y solutions, Inc. Boston, MA).
Results
Patient Characteristics
Among the 968 patients included in the study, 97 (10.02%) underwent NS-STT changes on electrocardiogram at admission. The demographic and clinical characteristics, including comorbidities, factors associated with injuries and operation are shown in Table 1. The mean age of the NS-STT group was 65.05±8.41, compared to 47.93±15.34 in the non-NS-STT group (P < 0.001). As for the fracture type, there were more femur fractures in NS-STT group than non-NS-STT group. In the comorbidities, there were more hypertension in NS-STT group and more associated injuries in non-NS-STT group. In addition, in the factors related to the operation, the operation time and operative blood loss in NS-STT group were more than non-NS-STT group. Still, fewer patients were using a tourniquet in NS-STT group. More patients were using an intramedullary nail and joint prosthesis in NS-STT group.
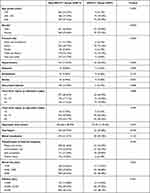 |
Table 1 The Demographic and Clinical Characteristics |
A total of 303 patients (31.30%) developed perioperative DVT in lower extremities in this study. The univariate regression analysis showed that ST-T segment changes on ECG significantly correlated with perioperative DVT (OR = 3.45, 95% CI: 2.25–5.30, P < 0.0001). In addition, age ≥50 years, female, hypertension, blood transfusion, joint prosthesis, blood loss ≥300 mL might also be associated with perioperative DVT in lower extremities (Table 2).
 |
Table 2 Effects of Risk Factors on Perioperative DVT by Univariate Analysis |
The subgroup analysis of risk factors contributing to perioperative DVT was shown in Table 3. In these factors, the results in different subgroups were comparable.
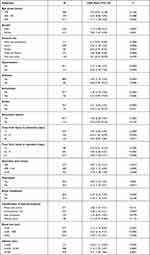 |
Table 3 Subgroup Analysis of Potential Risk Factors Contributing into Perioperative DVT |
At the beginning of the analysis, we identified two confounding factors of age and classification of internal implants. To identify other important confounding factors, we explore the potential factors. Because age, operation time, blood loss, and infusion were continuous variables, the smoothing curve fitting of these factors between perioperative DVT was drawn, shown in Figure 1. As for the curve relationship to perioperative DVT, these factors were considered into the following adjust factors. In addition, we also explore the relationship to NS-STT changes, and we found that the gender (reference to male, female (OR = 4.05, 95% CI: 2.54–6.44, P < 0.0001)) was an essential factor. Thus, we identified the factors needed to control.
 |
Figure 1 The curve correlation between age (A), operation time (B), blood loss (C) and infusion (D) and perioperative DVT. |
After adjustment of potential confounding factors, the independent effect of NS-STT changes on perioperative DVT was confirmed, shown in Table 4. In the models I to IV, the results were close to each other. Also, to explore the stability of the result, we continued to complete the sensitivity analysis. When the subgroups were divided as above-identified factors one by one, the results in different subgroups were very close. Therefore, NS-STT changes were associated with perioperative DVT (OR = 2.13, 95% CI 1.33–3.42; P = 0.0017).
 |
Table 4 Relationship Between NS-STT Changes on Electrocardiogram and Perioperative DVT |
Discussion
In this study, we have found that NT-STT change on electrocardiogram is associated with an increase in the incidence of perioperative DVT by 2.13-fold in patients with lower extremity fracture under 75 years old. In clinical practice, when there is an NT-STT change at admission in patients with lower extremity fracture under 75 years old, surgeons should pay more attention to prevent the perioperative DVT. As we know, it is the first correlation analysis that illustrates the relationship between characteristics of electrocardiograms and thrombosis.
To date, studies have investigated so many risk factors for developing perioperative DVT after lower extremity fracture. It is now clear that in addition to Virchow’s triad (blood flow disturbance, hypercoagulability, and vessel wall changes), inflammation has a crucial role in triggering DVT.8 ST and T wave changes may represent cardiac pathology or be a normal variant. NS-STT abnormalities are associated with benign factors such as food ingestion, posture, emotional stressors, hyperventilation, electrolyte disturbances, and use of antiarrhythmic or psychotropic drugs, in addition to being prevalent in middle-aged men, women, and blacks.16–28 Furthermore, inflammation might also be associated with NS-STT on electrocardiograms.6 Therefore, we explore the relationship between perioperative DVT and NS-STT changes on electrocardiograms.
In including patients, we only limit fresh occurring lower extremity fractures that received surgical treatment to control the baseline of included patients and avoid the confounding factors from delayed fracture and conservative treatment. In addition, we identified two crucial factors. As we know, ST and T wave changes had a close relationship to coronary heart disease.29,30 It is a robust confounding factor to our result. We excluded patients with coronary heart disease symptoms and patients with age >75 years in this study. The percentage of coronary heart disease of age >75 years was nearly 40% (127/320) in the fracture patients. Thus, we excluded the patients with pathological ST and T wave changes, and most of the rest patients were physiological NS-STT. In addition, the ways of reduction (open reduction or closed reduction) were another confounding factor.31,32 As most of the patients received the open procedure, we excluded the patients receiving the closed reduction and internal fixation. The age, operation time, and operative blood loss in NS-STT group were more than non-NS-STT group in the demographic and clinical characteristics. Also, the percentage of female, femur fracture, hypertension, and associated injuries in NS-STT group were higher than non-NS-STT group, but the portion of using the tourniquet was lower in NS-STT group. These characteristics were different in the two groups.
The mean age in NS-STT group was 65.05. The age was near the boundary of older people. In the previous studies, elderly age was related to hypertension,33 associated injuries,34 and hip fracture.35 A higher incidence of hip fracture means lower percentage of using tourniquets. Thus, age was the crucial factor to control. In the subgroups of age ≥60 years, the NT-STT change on electrocardiogram was associated with perioperative DVT. There was no relationship in subgroups of age <60 years. We considered the reason was the samples in subgroups of age <60 years were small. In addition, we also found the female took 72.16% of patients in the NS-STT group, compared to 39.04% in non-NS-STT group, that the gender was related to NS-STT changes on electrocardiogram. In the previous studies, the female related to hip fracture36 has been identified. Thus, gender was another confounding factor. In the subgroups, the males (OR = 4.14) had a closer relationship between NT-STT change and perioperative DVT than females (OR = 2.83).
In the subgroup analysis and sensitivity analysis, the results in different subgroups were close to each other. Thus, we finally found that patients with NS-STT changes on electrocardiogram were associated with an increase in the incidence of perioperative DVT by 2.13-fold in patients with lower extremity fracture under 75 years. In clinical practice, when a patient with NS-STT changes is at admission, surgeons should be paid more attention to DVT formation in the lower extremity and screen the ultrasonography more.
Although our analysis suggested that NS-STT changes at admission are associated with perioperative DVT, this study has several limitations that should be noted. Firstly, the result is suitable for the patients under 75 years and excluding the diagnosis of coronary heart disease, so the patients with age more than 75 years or coronary heart disease could not adopt this conclusion. Secondly, the causes of NS-STT changes were too much: including functional and physiologic variants, myocardial ischemia, any cardiomyopathy, myocarditis, pericarditis, post-cardiac surgery, pulmonary emboli or intrinsic pulmonary disease, fever, anemia, acidosis or alkalosis, electrolyte or other metabolic abnormalities, endocrine abnormalities, endogenous catecholamines, acute abdominal process, cerebrovascular accidents. We think the critical point linking NS-STT changes and perioperative DVT is inflammation, but the evidence was insufficient. It is needed to research the relationship in the future. Thirdly, due to the limitations of a retrospective study, possible selection bias and the sample size from a single center might have been presented in this study.
Conclusions
The NS-STT changes on electrocardiograms are associated with an increase in the incidence of perioperative DVT by 2.13-fold in patients with lower extremity fractures under 75 years old. In clinical practice, surgeons should pay more attention to these patients.
Abbreviations
NS-STT, nonspecific ST-segment or T-wave; DVT, deep vein thrombosis.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to data privacy but are available from the corresponding author on reasonable request.
Ethics Approval
The study was approved by the ethical board of Xi’an Honghui Hospital (No. 2014026).
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Social Development Foundation of Shaanxi Province under grant 2017ZDXM-SF-009 and 2019ZDLSF01-09.
Disclosure
The authors report no conflicts of interest.
References
1. Zhang B, Wang P, Fei C, et al. Perioperative deep vein thrombosis in patients with lower extremity fractures: an observational study. Clin Appl Thromb Hemost. 2020;26:1076029620930272.
2. Terao M, Ozaki T, Sato T. Diagnosis of deep vein thrombosis after operation for fracture of the proximal femur: comparative study of ultrasonography and venography. J Orthop Sc. 2006;11(2):146–153. doi:10.1007/s00776-005-0997-2
3. Fu Y, Liu P, Xu X, et al. Deep vein thrombosis in the lower extremities after femoral neck fracture: a retrospective observational study. J Orthop Surg. 2020;28(1):2309499019901172. doi:10.1177/2309499019901172
4. Mozos I, Caraba A. Electrocardiographic predictors of cardiovascular mortality. Dis Markers. 2015;2015:727401. doi:10.1155/2015/727401
5. Badheka AO, Rathod A, Marzouka GR, et al. Isolated nonspecific ST-segment and T-wave abnormalities in a cross-sectional United States population and mortality (from NHANES III). Am J Cardiol. 2012;110(4):521–525. doi:10.1016/j.amjcard.2012.04.023
6. Geraldino-Pardilla L, Gartshteyn Y, Piña P, et al. ECG non-specific ST-T and QTc abnormalities in patients with systemic lupus erythematosus compared with rheumatoid arthritis. Lupus Sci Med. 2016;3(1):e000168. doi:10.1136/lupus-2016-000168
7. Colling M, Tourdot B, Kanthi Y. Inflammation, infection and venous thromboembolism. Circ Res. 2021;128(12):2017–2036. doi:10.1161/CIRCRESAHA.121.318225
8. Borgel D, Bianchini E, Lasne D, et al. Inflammation in deep vein thrombosis: a therapeutic target?. Hematology. 2019;24(1):742–750. doi:10.1080/16078454.2019.1687144
9. Alipanahzadeh H, Ghulamreza R, Shokouhian M, et al. Deep vein thrombosis: a less noticed complication in hematologic malignancies and immunologic disorders. J Thromb Thrombolysis. 2020;50(2):318–329. doi:10.1007/s11239-019-02005-6
10. Culić V. Inflammation, coagulation, weather and arrhythmogenesis: is there a linkage?. Int J Cardiol. 2014;176(1):289–293. doi:10.1016/j.ijcard.2014.06.078
11. Moreno G, Rodríguez A, Reyes L, et al. Corticosteroid treatment in critically ill patients with severe influenza pneumonia: a propensity score matching study. Intensive Care Med. 2018;44(9):1470–1482. doi:10.1007/s00134-018-5332-4
12. Hong J, Tong Y, He J, et al. Association between tumor molecular subtype, clinical stage and axillary pathological response in breast cancer patients undergoing complete pathological remission after neoadjuvant chemotherapy: potential implications for de-escalation of axillary surgery. Ther Adv Med Oncol. 2021;13:1758835921996673. doi:10.1177/1758835921996673
13. Mantoni M. Ultrasound of limb veins. Eur Radiol. 2001;11(9):1557–1562. doi:10.1007/s003300101016
14. Greenfield LJ, Proctor MC, Rodriguez JL, et al. Posttrauma thromboembolism prophylaxis. J Trauma. 1997;42(1):100–103. doi:10.1097/00005373-199701000-00017
15. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e419S–e496S. doi:10.1378/chest.11-2301
16. Kumar A, Lloyd-Jones D. Clinical significance of minor nonspecific ST-segment and T-wave abnormalities in asymptomatic subjects: a systematic review. Cardiol Rev. 2007;15(3):133–142. doi:10.1097/01.crd.0000249382.65955.14
17. Greenland P, Xie X, Liu K, et al. Impact of minor electrocardiographic ST-segment and/or T-wave abnormalities on cardiovascular mortality during long-term follow-up. Am J Cardiol. 2003;91(9):1068–1074. doi:10.1016/S0002-9149(03)00150-4
18. Bartel A, Heyden S, Tyroler H, et al. Electrocardiographic predictors of coronary heart disease. Arch Intern Med. 1971;128(6):929–937. doi:10.1001/archinte.1971.00310240083010
19. Miall W, Del Campo E, Fodor J, et al. Longitudinal study of heart disease in a Jamaican rural population. I. Prevalence, with special reference to ECG findings. Bull World Health Organ. 1972;46(4):429–441.
20. Strogatz D, Tyroler H, Watkins L, et al. Electrocardiographic abnormalities and mortality among middle-aged black men and white men of Evans County, Georgia. J Chronic Dis. 1987;40(2):149–155. doi:10.1016/0021-9681(87)90066-X
21. Sutherland S, Gazes P, Keil J, et al. Electrocardiographic abnormalities and 30-year mortality among white and black men of the Charleston Heart Study. Circulation. 1993;88(6):2685–2692. doi:10.1161/01.CIR.88.6.2685
22. Rose G, Baxter P, Reid D, et al. Prevalence and prognosis of electrocardiographic findings in middle-aged men. Br Heart J. 1978;40(6):636–643. doi:10.1136/hrt.40.6.636
23. Higgins I, Kannel W, Dawber T. The electrocardiogram in epidemiological studies: reproducibility, validity, and international comparison. Br J Prev Soc Med. 1965;19:53–68. doi:10.1136/jech.19.2.53
24. Ostrander L, Brandt R, Kjelsberg M, et al. Electrocardiographic findings among the adult population of a total natural community, Tecumseh, Michigan. Circulation. 1965;31(6):888–898. doi:10.1161/01.CIR.31.6.888
25. Cullen K, Murphy B, Cumpston G. Electrocardiograms in the Busselton population. Aust N Z J Psychiatry. 1974;4(4):325–330. doi:10.1111/j.1445-5994.1974.tb03199.x
26. Ostör E, Schnohr P, Jensen G, et al. Electrocardiographic findings and their association with mortality in the Copenhagen City Heart Study. Eur Heart J. 1981;2(4):317–328. doi:10.1093/oxfordjournals.eurheartj.a061212
27. Reunanen A, Aromaa A, Pyörälä K, et al. The Social Insurance Institution’s coronary heart disease study. Baseline data and 5-year mortality experience. Acta Med Scand Suppl. 1983;673:1–120.
28. Kumar A, Prineas R, Arnold A, et al. Prevalence, prognosis, and implications of isolated minor nonspecific ST-segment and T-wave abnormalities in older adults: cardiovascular health study. Circulation. 2008;118(25):2790–2796. doi:10.1161/CIRCULATIONAHA.108.772541
29. Farhan HL, Hassan KS, Al-Belushi A, et al. Diagnostic value of electrocardiographic T wave inversion in lead aVL in diagnosing coronary artery disease in patients with chronic stable angina. Oman Med J. 2010;25(2):124–127. doi:10.5001/omj.2010.33
30. Birnbaum Y, Wilson JM, Fiol M, et al. ECG diagnosis and classification of acute coronary syndromes. Ann Noninvasive Electrocardiol. 2014;19(1):4–14. doi:10.1111/anec.12130
31. Jupiter D, Saenz F, Mileski W, et al. Acute deep venous thrombosis and pulmonary embolism in foot and ankle trauma in the national trauma data bank: an update and reanalysis. J Foot Ankle Surg. 2019;58(6):1152–1162. doi:10.1053/j.jfas.2019.03.011
32. Weinraub G, Newport I, Kim B, et al. Outcomes following Open Reduction Internal Fixation of ankle fractures (ORIF) by Podiatric surgeons. J Foot Ankle Surg. 2021;60(5):960–963. doi:10.1053/j.jfas.2021.04.004
33. Wen J, Su M. A randomized trial of Tai Chi on preventing hypertension and hyperlipidemia in middle-aged and elderly patients. Int J Environ Res Public Health. 2021;18(10):5480. doi:10.3390/ijerph18105480
34. Almegbel F, Alotaibi I, Alhusain F, et al. Period prevalence, risk factors and consequent injuries of falling among the Saudi elderly living in Riyadh, Saudi Arabia: a cross-sectional study. BMJ Open. 2018;8(1):e019063. doi:10.1136/bmjopen-2017-019063
35. Prestmo A, Hagen G, Sletvold O, et al. Comprehensive geriatric care for patients with hip fractures: a prospective, randomised, controlled trial. Lancet. 2015;385(9978):1623–1633.
36. George J, Sharma V, Farooque K, et al. Injury mechanisms of hip fractures in India. Hip Pelvis. 2021;33(2):62–70. doi:10.5371/hp.2021.33.2.62
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
