Back to Journals » Lung Cancer: Targets and Therapy » Volume 9
Non-small cell to small cell lung cancer on PD-1 inhibitors: two cases on potential histologic transformation
Authors Abdallah N , Nagasaka M , Abdulfatah E, Shi D, Wozniak AJ, Sukari A
Received 10 May 2018
Accepted for publication 17 July 2018
Published 25 October 2018 Volume 2018:9 Pages 85—90
DOI https://doi.org/10.2147/LCTT.S173724
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sai-Hong Ignatius Ou
Nadine Abdallah,1 Misako Nagasaka,2,3 Eman Abdulfatah,4 Dongping Shi,4 Antoinette J Wozniak,2 Ammar Sukari2
1Department of Internal Medicine, Wayne State University, Detroit, MI 48201, USA; 2Department of Oncology, Barbara Ann Karmanos Cancer Institute, Wayne State University School of Medicine, Detroit, MI 48201, USA; 3Department of Advanced Medical Innovation, St. Marianna University Graduate School of Medicine, Kawasaki, Kanagawa, Japan; 4Department of Pathology, School of Medicine, Wayne State University, Detroit, MI 48201, USA
Introduction: Histologic transformation from non-small cell lung cancer (NSCLC) to small cell lung cancer (SCLC) is a well-recognized mechanism of resistance in EGFR-mutant adenocarcinoma upon treatment with TKIs, but rarely reported with programmed death1 (PD-1) inhibitors. We report two cases of potential transformation during treatment with PD-1 inhibitors.
Case presentations: Case 1, a 65-year-old man was diagnosed with stage IVa lung adenocarcinoma on pleural fluid cytology. He received six cycles of carboplatin and pemetrexed, then maintained on pemetrexed. He had disease progression after nine cycles of pemetrexed and was switched to nivolumab. He progressed after five cycles of nivolumab. Core biopsy of the lung mass revealed SCLC. Case 2, a 68-year-old man was diagnosed with two primary NSCLCs and underwent resection. He had recurrence after several months and was treated with four cycles of carboplatin, paclitaxel, and pembrolizumab on clinical trial, with partial response. He was continued on pembrolizumab and had disease progression after 30 cycles. Biopsy of the new lesions showed SCLC.
Discussion: Histologic transformation from NSCLC to SCLC can be explained by the presence of a common cell precursor. Proposed molecular mechanisms include loss of RB1, TP53 mutations, and MYC amplification. The distinction between transformation and mixed histology tumors is challenging, especially when pathologic material used for the initial diagnosis is limited. The possibility of a second metachronous primary lung cancer cannot be excluded in our cases.
Conclusion: Histologic transformation with PD-1 inhibitors could be under-recognized. Disease progression should prompt re-biopsy to uncover new histology and change in treatment. Future studies are needed to elucidate mechanisms and predictors of transformation.
Keywords: transformation, small cell lung cancer, non-small cell lung cancer, PD-1 inhibitor, resistance, checkpoint inhibitor
Introduction
Lung cancer is the leading cause of cancer-related death worldwide.1 It is broadly classified into two histologic subtypes: non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). NSCLC accounts for 85% of cases and includes squamous cell carcinoma, adenocarcinoma, and large cell carcinoma, while SCLC accounts for the remaining 15%. The main treatment options for advanced stage NSCLC are chemotherapy, usually platinum-based doublet therapy and molecularly targeted therapies in eligible patients. Recently, programmed death1 (PD-1)/programmed death ligand (PDL-1) inhibitors, such as pembrolizumab, nivolumab, and atezolizumab, have also become integral parts of both squamous and non-squamous NSCLC treatment in both the first- and second-line settings.2–5 The development of resistance is one of the main challenges of cancer treatment. Histologic transformation to SCLC is a known mechanism of resistance in EGFR-mutant adenocarcinoma on treatment with TKIs. Reports of similar transformation occurring independent of EGFR inhibitor use and of EGFR mutational status suggest that this mechanism may occur in other settings as well. However, the distinction between transformation, and mixed histology tumors missed on initial pathologic diagnosis, remains a challenge. We report two cases of potential transformation of advanced stage NSCLC to SCLC during treatment with PD-1 inhibitors.
Case presentation
Case 1
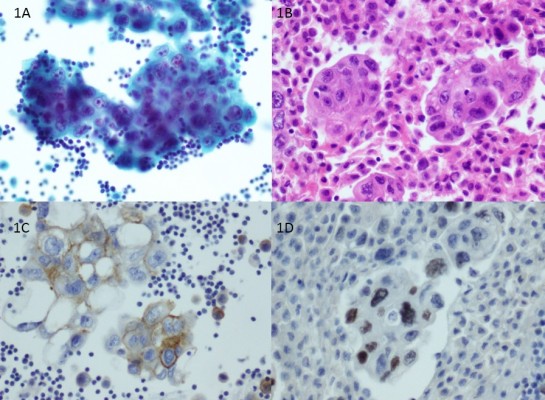
A 65-year-old man with a 35-pack-year smoking history, presented with shortness of breath, cough, and chest pain. He was treated empirically for pneumonia without improvement; therefore, a chest X-ray was done, revealing left pleural effusion. Thoracentesis was performed, and fluid cytology was positive for malignant cells consistent with adenocarcinoma (Figure 1A–D). Chest computed tomographic (CT) scan showed re-accumulation of pleural fluid, with near complete collapse of the left lung. Positron emission tomography (PET)/CT scan showed an ill-defined hypermetabolic mass in the left hilar region occluding the left mainstem bronchus, hypermetabolic lesions (SUV 6.6) on both sides of the mediastinum and a large left-sided pleural effusion. MRI brain did not show metastasis. He was diagnosed clinically with stage IVa lung adenocarcinoma. Liquid biopsy test utilizing Guardant 360 showed EGFR wild-type adenocarcinoma and no ALK translocation. Given the urgency to start treatment, he was started on palliative chemotherapy with carboplatin and pemetrexed, and tissue biopsy was not obtained. Scans performed after two cycles showed partial response, with further response after four cycles. Scans after six cycles showed stable disease, so he was switched to maintenance therapy with single-agent pemetrexed. Further response was seen on CT scan after six cycles of pemetrexed. However, restaging PET/CT after nine cycles showed disease progression with increase in size of the residual left lower lobe nodule, new subcentimeter left infrahilar nodules, and an enlarged prevascular lymph node. He was switched to treatment with nivolumab. Scans after the fifth cycle revealed disease progression with increase in size of the left lower lobe nodular mass and several mediastinal lymph nodes. CT-guided core biopsy of the left lung mass was done showing tumor cells positive for synaptophysin, CD56, and CAM 5.2 and negative for chromogranin immunostains, which was consistent with SCLC (Figure 2A–C). Based on this, he was started on treatment with carboplatin and etoposide. Scans after two cycles showed response in the left lower lobe lung nodule and mediastinal lymph nodes.
Case 2
A 68-year-old man presented with cough. CT scan confirmed the presence of an irregular pleural-based mass measuring 3.1×4.2×4 cm in the right upper lobe. PET scan re-demonstrated the primary lesion and in addition revealed local chest wall invasion with erosion of the surrounding rib at that level. Another distinct 1.3 cm left apex nodule was also identified. CT-guided biopsy of the right upper lobe mass from November 2013 showed moderately differentiated squamous cell carcinoma. Pathology from wedge resection of the left apex nodule had immunohistochemical stains positive for AE1/AE3 and negative for TTF-1, Napsin A, CK5/6, and p63, supporting the diagnosis of poorly differentiated non-small cell carcinoma. This was morphologically non-identical to the right lobe primary. He was, therefore, thought to have two primaries at early stage and underwent a right upper lobectomy. The patient declined adjuvant chemotherapy.
Subsequently, new nodules appeared in the left lung on follow-up CT scan in May 2014. These were FDG avid on PET scan, with a pleural-based 1.5×1.1 cm mass (SUV 4.9) in left upper lobe, 2.3×2.4 cm mass (SUV 7.8) in apex of left lung adjacent to the surgical site, and subcarinal lymph node measuring 1.7 cm (SUV 3.5). He enrolled on a clinical trial utilizing carboplatin + paclitaxel with pembrolizumab (MK3475) every 3 weeks for a total of four cycles. Upon completion of four cycles, partial response was observed with reduction in the size of the left pleural masses. He was continued on pembrolizumab alone every 3 weeks completing a total of 30 cycles. During this 2-year time frame, periodic surveillance scans demonstrated stable disease. However, during a clinic visit for his 31st cycle, follow-up CT scan showed interval appearance of right para-tracheal and right hilar lymphadenopathy. The detection of new breakthrough lesions while on pembrolizumab dictated the need for a re-biopsy of the new disease. Surprisingly, pathology revealed richly cellular malignant cells with occasional crush artifact. Immunohistochemical stains were positive for CD56, synaptophysin, chromogranin, and CAM5.2, consistent with small cell lung carcinoma. From July 2016, he underwent four cycles of carboplatin and etoposide followed by definitive radiation to the chest. He continues to do clinically well without evidence of disease ~18 months since his diagnosis of lung cancer.
Discussion
In these two cases, small cell histology was found upon re-biopsy, which was prompted by radiologic signs of disease progression. The use of checkpoint inhibitors can be associated with “pseudo-progression”, where initial signs of progression appear on imaging with increase in tumor size and/or development of new lesions, followed by radiologic responses with continued treatment. This is an uncommon phenomenon reported at 0%–5% in NSCLC, and mostly occurs within a few weeks of treatment, although it can rarely be seen months later.6,7 It is thought to be mediated by an inflammatory infiltration of the tumor.7 In our cases, progression was seen beyond the initial weeks of PD-1 inhibitor initiation. In addition, the immune-related RECIST criteria were used, and progression was confirmed with serial imaging, and therefore likely represented true progression.
Histologic transformation of NSCLC to SCLC has mostly been recognized as a mechanism of resistance adopted by EGFR-mutant adenocarcinomas in the setting of EGFR-TKI use, which is reported at a frequency of about 3%–14%.8,9 Less commonly, histologic transformation has also been reported with EGFR-mutant tumors in the absence of or prior to TKI treatment and in tumors with wild-type EGFR.10,11 In addition, resistance has been reported upon treatment with other agents such as crizotinib.12 There are only few published cases reporting histologic transformation upon treatment with the PD-1 inhibitors: Imakita et al reported NSCLC to SCLC transformation during nivolumab treatment,13 while a case of transformation from squamous cell carcinoma to adenocarcinoma during nivolumab treatment was reported by Nagasaka et al.14 Similarly, histologic transformation has also been reported with pembrolizumab treatment.15
The molecular events leading to transformation have not been fully elucidated. It has been proposed that alveolar type II cells may be a common precursor, allowing plasticity in transformation when certain molecular events occur like loss of RB1 and lack of EGFR protein expression.16 RB1 inactivation has been shown to be essential but insufficient for the development of SCLC.16 Other molecular mechanisms involved in transformation include TP53 mutations and MYC amplification. It is postulated that ASCL1 overexpression, triggered by NOTCH pathway alterations, promotes retinoblastoma tumor suppressor protein phosphorylation, which leads to its inactivation and provides a survival advantage for p53-mutated cells.17
Another possibility in both cases is that the initial tumor was of combined histology and the small cell component was missed on initial pathology. Combined SCLC is defined by the WHO as SCLC combined with adenocarcinoma, large cell, squamous cell, spindle cell, or giant cell carcinomas.17,18 The frequency of combined histology tumors has been reported in two large studies by Adelstein et al and Magnum et al as 10% and 2%, respectively.19,20 However, the identification of combined histology at diagnosis may be limited in small tissue or cytology specimens, and only found later upon surgical resection, or repeat biopsy. Nicholson et al identified mixed histology in 28% of surgically resected specimens of patients diagnosed with SCLC, with large cell carcinoma being the most common (16%), followed by adenocarcinoma (9%) and squamous cell carcinoma (3%).21 Similarly, Zhang et al reported combined histology in 30.4% of surgically resected specimens of SCLC patients.22
In our two cases, the limited specimens obtained by cytology and core biopsy, respectively, may have not provided sufficient pathologic material to identify mixed histology. In addition, treatment with chemotherapy may have led to responses in the dominant non-small cell component, allowing better identification of the small cell component on biopsy done later. The distinction between combined tumor histology and transformation may be difficult and has been a subject of debate in previous reports, especially with EGFR-TKI-treated tumors. In our first case, the initial response of the tumor to carboplatin and pemetrexed, and progression upon treatment with nivolumab, suggests that the tumor transformed later to small cell histology as a mechanism of resistance, although initial diagnosis of mixed histology cannot be ruled out given the limited specimen at the time of diagnosis and development of a second primary is also possible. In the second case, disease progression with new histology of SCLC after 2 years of stable disease on pembrolizumab treatment favors the hypothesis of histologic transformation or a second primary rather than initial combined histology. The diagnosis of a second metachronous primary lung cancer is considered when tumors are of distinct histology, or if they are of similar histology separated by at least 4 years of disease-free interval and in the absence of metastatic disease.23 The development of second primary lung tumors has been estimated to occur at a rate of 1%–2% per year for patients with NSCLC and 6% for SCLC after successful treatment, with the majority of cases occurring after 5 years.24,25 This possibility cannot be excluded in our cases.
Transformation to SCLC, as a mechanism of resistance, is based on the premise that SCLC is less susceptible to checkpoint inhibitor treatment. At this time, there are limited studies for checkpoint inhibitors in SCLC. The role of the CTLA-4 inhibitor ipilimumab in extensive-disease SCLC was studied in a Phase II clinical trial, which showed improved immune-related progression-free survival when ipilimumab was combined with first-line platinum-based chemotherapy in a phased fashion.26 However, in the confirmatory Phase III clinical trial, there was no improvement in overall survival.27 Studies targeting the PD-1/PDL-1 axis showed more promising results. The Phase I/II CheckMate 032 trial showed antitumor activity with nivolumab, alone and in combination with ipilimumab in recurrent SCLC.28,29 Pembrolizumab was studied in the Phase I KEYNOTE-028 trial, which showed an overall response rate of 33% in PDL-1, expressing recurrent ES-SCLC.30 However, the KEYNOTE-028 study has been criticized for its small sample size (N=24) and wide CI of the RR 33% (95% CI 16%–55%). While the option of nivolumab with or without ipilimumab has been incorporated into the second-line treatment choices for SCLC on National Comprehensive Cancer Network guidelines, the evidence on efficacy of PD-1/PDL-1 inhibitors in SCLC remains limited.
Theoretically, the high mutational burden associated with SCLC, and association with tobacco exposure, support a role of immunotherapy in SCLC.31,32 In addition, the immunogenicity of SCLC is suggested by its association with paraneoplastic autoimmune syndromes such as Lambert Eaton. However, SCLC has been shown to have decreased intra-tumoral PDL-1 expression, which is a known predictor of response to PD-1 inhibitors.33 In addition, SCLC tumors exhibit a lower expression of MHC-1 antigens and decreased tumor-infiltrating lymphocytes. Together, these factors may decrease the probability of response to single-agent checkpoint inhibitor use in SCLC.34 Several clinical trials are currently ongoing, and results are awaited to elucidate whether there is a place for checkpoint inhibitors in SCLC treatment.29
Conclusion
Phenotypic transformation to SCLC is a potential mechanism of resistance to immunotherapy adopted by NSCLC. This phenomenon could be under-recognized, as repeat biopsy is not always routinely done upon disease progression with treatment. However, clinicians should be aware of this, especially in patients who lose responsiveness after a period of treatment, thus prompting repeat biopsy. This has important clinical implications because the treatments options for NSCLC and SCLC are different. Future studies are needed to shed light on the predictors of transformation and molecular mechanisms, thereby allowing early recognition and use of alternate therapies. In addition, clinicians should be aware of the possibility of combined histology, especially when pathologic material used for the initial diagnosis is limited or insufficient.
Informed consent
Written informed consent was obtained from the patients to have the case details and any accompanying images published.
Disclosure
The authors report no conflicts of interest in this work
References
Ferlay JSI, Ervik M, Dikshit R, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11; 2012. | ||
Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. | ||
Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. | ||
Horn L, Spigel DR, Vokes EE, et al. Nivolumab versus docetaxel in previously treated patients with advanced non-small-cell lung cancer: two-year outcomes from two randomized, open-label, phase III trials (CheckMate 017 and CheckMate 057). J Clin Oncol. 2017;35(35):3924–3933. | ||
Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. | ||
Beer L, Hochmair M, Prosch H. Pitfalls in the radiological response assessment of immunotherapy. Memo. 2018;11(2):138–143. | ||
Katz SI, Hammer M, Bagley SJ, et al. Radiologic pseudoprogression during Anti-PD-1 therapy for advanced non-small cell lung cancer. J Thorac Oncol. 2018;13(7):978–986. | ||
Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19(8):2240–2247. | ||
Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3(75):75ra26. | ||
Norkowski E, Ghigna MR, Lacroix L, et al. Small-cell carcinoma in the setting of pulmonary adenocarcinoma: new insights in the era of molecular pathology. J Thorac Oncol. 2013;8(10):1265–1271. | ||
Ahn S, Hwang SH, Han J, et al. Transformation to small cell lung cancer of pulmonary adenocarcinoma: clinicopathologic analysis of six cases. J Pathol Transl Med. 2016;50(4):258–263. | ||
Miyamoto S, Ikushima S, Ono R, et al. Transformation to small-cell lung cancer as a mechanism of acquired resistance to crizotinib and alectinib. Jpn J Clin Oncol. 2016;46(2):170–173. | ||
Imakita T, Fujita K, Kanai O, Terashima T, Mio T. Small cell lung cancer transformation during immunotherapy with nivolumab: a case report. Respir Med Case Rep. 2017;21:52–55. | ||
Nagasaka M, Pansare RS, Abdulfatah E, Guan H, Tranchida P, Gadgeel SM. Histologic transformation in NSCLC with PD-1 therapy. J Thorac Oncol. 2017;12(9):e133–e134. | ||
Hsu CL, Chen KY, Kuo SW, Chang YL. Histologic transformation in a patient with lung cancer treated with chemotherapy and pembrolizumab. J Thorac Oncol. 2017;12(6):e75–e76. | ||
Oser MG, Niederst MJ, Sequist LV, Engelman JA. Transformation from non-small-cell lung cancer to small-cell lung cancer: molecular drivers and cells of origin. Lancet Oncol. 2015;16(4):e165–e172. | ||
Dorantes-Heredia R, Ruiz-Morales JM, Cano-García F. Histopathological transformation to small-cell lung carcinoma in non-small cell lung carcinoma tumors. Transl Lung Cancer Res. 2016;5(4):401–412. | ||
Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10(9):1243–1260. | ||
Mangum MD, Greco FA, Hainsworth JD, Hande KR, Johnson DH. Combined small-cell and non-small-cell lung cancer. J Clin Oncol. 1989;7(5):607–612. | ||
Adelstein DJ, Tomashefski JF, Snow NJ, Horrigan TP, Hines JD. Mixed small cell and non-small cell lung cancer. Chest. 1986;89(5):699–704. | ||
Nicholson SA, Beasley MB, Brambilla E, et al. Small cell lung carcinoma (SCLC): a clinicopathologic study of 100 cases with surgical specimens. Am J Surg Pathol. 2002;26(9):1184–1197. | ||
Zhang C, Yang H, Zhao H, et al. Clinical outcomes of surgically resected combined small cell lung cancer: a two-institutional experience. J Thorac Dis. 2017;9(1):151–158. | ||
Shen KR, Meyers BF, Larner JM, Jones DR, American College of Chest Physicians. Special treatment issues in lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007;132(3 Suppl):290S–305. | ||
Sánchez de Cos Escuín J, Rodríguez López DP, Utrabo Delgado I, Gallego Domínguez R, Sojo González MA, Hernández Valle M. Disease recurrence and second tumors in long-term survivors of lung cancer. Arch Bronconeumol. 2016;52(4):183–188. | ||
Johnson BE. Second lung cancers in patients after treatment for an initial lung cancer. J Natl Cancer Inst. 1998;90(18):1335–1345. | ||
Reck M, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol. 2013;24(1):75–83. | ||
Reck M, Luft A, Szczesna A, et al. Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J Clin Oncol. 2016;34(31):3740–3748. | ||
Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016;17(7):883–895. | ||
Pakkala S, Owonikoko TK. Immune checkpoint inhibitors in small cell lung cancer. J Thorac Dis. 2018;10(Suppl 3):S460–S467. | ||
Ott PA, Elez E, Hiret S, et al. Pembrolizumab in patients with extensive-stage small-cell lung cancer: results from the phase Ib KEYNOTE-028 study. J Clin Oncol. 2017;35(34):3823–3829. | ||
Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–128. | ||
Chalmers ZR, Connelly CF, Fabrizio D, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9(1):34. | ||
Schultheis AM, Scheel AH, Ozretić L, et al. PD-L1 expression in small cell neuroendocrine carcinomas. Eur J Cancer. 2015;51(3):421–426. | ||
Schmid S, Früh M. Immune checkpoint inhibitors and small cell lung cancer: what’s new? J Thorac Dis. 2018;10(Suppl 13):S1503–S1508. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



