Back to Journals » Clinical and Experimental Gastroenterology » Volume 15
Non-Locatable Internal Opening in Anal Fistula Associated with Acute Abscess and Its Definitive Management by Garg Protocol
Authors Yagnik VD , Kaur B, Dawka S , Sohal A , Menon GR, Garg P
Received 16 May 2022
Accepted for publication 20 September 2022
Published 26 September 2022 Volume 2022:15 Pages 189—198
DOI https://doi.org/10.2147/CEG.S374848
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Andreas M. Kaiser
Vipul D Yagnik,1,* Baljit Kaur,2 Sushil Dawka,3 Aalam Sohal,4 Geetha R Menon,5 Pankaj Garg6,7,*
1Department of Surgical Gastroenterology, Nishtha Surgical Hospital and Research Center, Patan, Gujarat, India; 2Department of Radiology, SSRD Magnetic Resonance Imaging Institute, Chandigarh, India; 3Department of Surgery, SSR Medical College, Belle Rive, Mauritius; 4Department of Internal Medicine, University of California San Francisco (UCSF), Fresno, CA, USA; 5Department of Statistics, Indian Council of Medical Research, New Delhi, India; 6Department of Colorectal Surgery, Indus International Hospital, Mohali, Punjab, India; 7Department of Colorectal Surgery, Garg Fistula Research Institute, Panchkula, Haryana, India
*These authors contributed equally to this work
Correspondence: Pankaj Garg, Department of Colorectal Surgery, Indus International Hospital, Mohali, Punjab, India, Email [email protected]
Background: Definitive management of acute fistula-abscess (anal fistulas associated with acute abscess) is gaining popularity against the two-staged approach (early abscess drainage with deferred fistula management). However, locating an internal opening (IO) in acute fistula-abscess can be difficult. A recent protocol (Garg protocol) has been shown to be effective in managing anal fistulas with non-locatable IO.
Purpose: To test the efficacy of the Garg protocol in managing acute fistula-abscess with non-locatable IO.
Methods: Patients with acute fistula-abscess operated by a definitive procedure were included. A preoperative MRI was done in all patients. Patients in whom the IO was non-locatable after clinical, MRI, and intraoperative examination were managed by the three-step Garg protocol. Garg protocol: 1) Reassessment of MRI; 2) In non-horseshoe fistulas, the IO was assumed to be at the point where the fistula tract reached closest to the sphincter-complex; 3) In horseshoe fistulas, the IO was assumed to be located in the midline (anterior or posterior as per the horseshoe location). Low fistulas were treated by fistulotomy and high fistulas by a sphincter-sparing procedure. The long-term healing rate and change in continence (Vaizey scores) were evaluated.
Results: A total of 201 patients with acute fistula-abscess were operated over six years, and 19 were lost to follow-up. A total of 182 patients (154-males) were followed up (median-37 months). The IO was locatable in 133/182 (73.1%) (control group) and was non-locatable in 49/182 (26.9%) (study group). The study group was managed as per the Garg protocol. The age, sex-ratio, and fistula parameters were comparable in both groups. The long-term healing rate was 112/133(84.2%) in the IO-locatable group and 43/49 (87.8%) in the IO-non-locatable group (p=0.64, not-significant). The objective continence scores did not change significantly after surgery in both groups.
Conclusion: Acute fistula-abscess with non-locatable IO can be managed successfully by the Garg protocol without any risk of incontinence.
Keywords: anal fistula, fistulotomy, incontinence, surgery, recurrence, abscess
Introduction
Anal fistula management is challenging because of the high rate of recurrence and the risk of incontinence due to sphincter damage.1 Recurrence after surgery is not uncommon, often requiring multiple operations.1 The high recurrence rate is also associated with the unpredictability of the time to recurrence and of the factors responsible. These points add to the physical and psychological trauma of the patient.2
The two challenges are (1) the definitive treatment of anal fistula associated with acute abscess (acute fistula-abscess) and (2) managing fistulas in which the internal opening (IO) is non-locatable (IO non-locatable). The challenge is magnified when these problems co-exist - “IO non-locatable” in patients with acute fistula-abscess. There is no literature on the management of such patients and this scenario is analyzed in this study.
Conventionally, in patients with acute fistula-abscess, the abscess is drained to bring sepsis under control and the fistula is tackled later at a second operation. However, evidence shows that definitive management of acute anal fistula-abscess at the first operation is possible with comparable success and no risk to continence.1–3 Understandably, definitive management, if performed successfully, would mitigate suffering and decrease morbidity significantly.
There are several reasons for the recurrence of anal fistulas.4–6 A few common reasons are inability to properly image and then manage high tracts (as in supralevator fistulas), unidentified or missed tracts, IO non-locatable, presence of horseshoe or multiple tracts, associated pathology like Crohn’s disease, tuberculosis etc. Among all these known reasons, inability to accurately find the internal opening has been identified as the single most important reason responsible for fistula recurrence.4–6
Acute Fistula-Abscess Can Present in Different Ways
- Anorectal abscess developing in a patient known to have an anal fistula: The patient may or may not have been operated previously for the fistula
- Anorectal abscess as the first presentation: There are two possibilities:
- Communication with the anus seen on MRI ie, internal opening locatable (Fistula confirmed)
- No fistula tract or internal opening locatable (Fistula not confirmed): In this subset, there is a possibility that an anal fistula may not develop).
Incidentally, in acute fistula-abscess patients, the likelihood of the IO being non-locatable is higher than in routine fistulas. This happens because inflammation makes clinical examination difficult, and swelling around the internal opening may temporarily occlude it. This has been identified as a major bottleneck in the definitive management of acute anal fistula-abscess.3 A recent protocol (Garg protocol) was shown to be effective in managing anal fistulas with “IO non-locatable”.2,7 In this study, we analyzed the efficacy of the Garg protocol employed in a cohort of acute fistula-abscess with IO non-locatable which were managed definitively during the first operation.
Materials and Methods
Patients with acute fistula-abscess operated over a six-year period between 2015 and 2021 and who underwent definitive management at the first surgery were included. Indus International Hospital-Institute Ethics Committee (IIH-IEC) granted approval for the study (approval number EC/IIH-IEH/SP6). The patients were informed about the purpose of the study, written informed consent was taken and the study was conducted in accordance with the Declaration of Helsinki.
In this study, all different types of acute fistula-abscess patients (anorectal abscess as the first presentation, or developing in a patient with known anal fistula) were managed by definitive surgery and were included. Preoperative MRI was done in all the patients. This was done as our center is a referral center for anal fistulas due to which the proportion of complex fistulas is higher. Moreover, as MRI scan is quite economical in India (60–80 USD), patients can easily afford it. Endoanal ultrasonography was not done as the authors’ experience in MRI is extensive but minimal in ultrasonography. Localization of the IO was attempted in three steps- clinical examination (point of maximum induration on per rectal examination, though this was not possible in many patients), preoperative MRI assessment, and intraoperative examination under anesthesia (visual inspection, injection of povidone-iodine solution through the external opening and noting its egress from the IO inside the anal canal).
The patients in whom the IO was located after these three steps were labelled as “IO-locatable” and were included in the control group. On the other hand, the patients in whom the IO was not locatable after these three steps were labelled as “IO-non-locatable”. These patients were included in the study group and were managed as per the Garg protocol.7
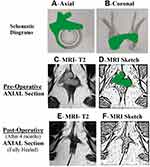
Garg Protocol:2,7 A three-step protocol was followed. First, the already done MRI was reassessed. Second, in non-horseshoe fistulas, the site where the fistula was physically nearest to the internal sphincter was noted. It was assumed that the internal-opening was located at that position only and the fistula was treated accordingly (Figures 1 and 2). Third, in horseshoe (HS) fistulas with no clear internal-opening, it was assumed that the internal-opening was located in the midline [for fistulas with anterior HS tract, the IO was assumed to be anterior midline (Figures 1 and 3) and similarly for posterior HS tract, the IO was assumed to be posterior midline (Figures 1 and 4) and the fistulas were managed accordingly].7
 |
Figure 1 Flow-chart: Management of acute fistula-abscess patients. |
Low fistulas (involving <1/3 of the external anal sphincter) were treated by fistulotomy and high fistulas (involving >1/3 of the external anal sphincter) were managed by a sphincter-sparing procedure. The sphincter-sparing procedure utilized in this study was TROPIS (transanal opening of intersphincteric space) as the operating team had more experience with this procedure.
In the TROPIS procedure, the external anal sphincter was completely preserved and the fistula tracts on both sides of the external anal sphincter were managed separately.2,8–10 The tract inside the external anal sphincter (fistula tract in the intersphincteric space) and the internal fistula opening were laid open into the anal canal through the transanal route. This wound was not sutured and was allowed to heal by secondary intention. The fistula tract outside (lateral) to the external anal sphincter was thoroughly curetted and cleaned.2,8 A soft drainage tube was inserted into this tract and sutured to the skin. Once the intra-anal wound had healed in about 8–12 weeks, the tube in the external tract was removed.2,8–12
The long-term healing rates were tabulated and compared in both the groups. The continence scores were evaluated objectively (by Vaizey scores)13 preoperatively and on long-term follow-up. The difference between the preoperative and the postoperative continence scores was compared in both the study and the control groups.
Statistical Analysis
The StatsDirect software for statistics was used (StatsDirect Ltd Merseyside, UK). Fisher’s exact test or chi-squared test was used to compare categorical variables. For normally distributed data, Student’s t-test was used for the continuous variables in two samples. For samples more than two, ANOVA test was used. In data which was not normally distributed, for paired samples, Wilcoxon signed-rank test was used and for unpaired samples, Mann–Whitney U-test was used. The cut-off point for significance was set at p<0.05.
Results
A total of 201 patients with acute fistula-abscess were operated over a six-year period. Of these, 19 patients were lost to follow-up; 182 patients (M/F: 154/28) were followed up for a median period of 37 months (range: 4–73 months). The internal opening (IO) was locatable in 133/182 (72.1%) (control group) and was non-locatable in 49/182 (26.9%) patients (study group). The age, sex-ratio, and fistula parameters (multiple tracts, recurrent fistulas, complex fistulas and supralevator fistulas) were comparable in both the groups (Table 1). Amongst 49 patients in the study group (IO non-locatable), non-horseshoe fistulas were present in 35/49 (Figures 1 and 2) and horseshoe fistulas were present in 14/49 patients. The horseshoe was posterior in 12/14 (Figure 4) and anterior in 2/14 (Figure 3) patients. The long-term success rate was 112/133 (84.2%) in the IO-locatable group and 43/49 (87.8%) in the IO-non-locatable group (p=0.64, not-significant, Fisher’s exact test) (Table 2). The objective continence scores did not change significantly after surgery in both the groups (Table 2). The change in mean continence scores (postoperative – preoperative scores) in the IO-non-locatable group was 0.0612±0.31 while the change in mean continence scores (postoperative – preoperative scores) in the IO-locatable group was 0.0977±0.54 (p=0.66, not significant, Fisher’s exact test) (Table 2).
 |
Table 1 Patient Parameters |
 |
Table 2 Results |
In this cohort, 87 (65.4%) patients in the control group and 39 (79.6%) patients in the study group were known patients of anal fistula (Table 3). The number of patients presenting for the first time as anorectal abscess was 46 (34.6%) in the control group and 10 (20.4%) in the study group. Among the 46 patients in the control group presenting for the first time as anorectal abscess, the fistula tract and the internal opening inside the anus could be detected in 26 patients but no fistula could be detected in 20 patients (Table 3). On the other hand, in the 10 patients in the study group presenting for the first time as anorectal abscess, no fistula could be detected in any of the patients (Table 3). Thus, a total of 30/182 (16.5%) patients presented for the first time with acute anorectal abscess with no demonstrable fistula. These patients were also managed as per the Garg protocol (Table 3).
 |
Table 3 Different Ways of Presentation of Anorectal Abscess |
Discussion
This study has highlighted the efficacy of the Garg protocol in definitive management of anal fistulas associated with acute abscess (acute fistula-abscess) in which, additionally, the internal opening was not locatable (IO-non-locatable). Not only was the healing rate in the IO-non-locatable group comparable to the IO-locatable group, the change in continence levels was also not significantly different. This is the first study on the definitive management of these complex fistulas (acute fistula-abscess with IO-non-locatable) and may provide a means to reduce the morbidity associated with these fistulas.
Acute anorectal abscesses of cryptoglandular origin are conventionally managed by incision and drainage. The fistula is then managed by a definitive procedure at a later date because the risk of incontinence due to sphincter injury is potentially higher when definitive surgery (especially fistulotomy) is done in the presence of an acute abscess.14,15 Additionally, it is assumed that some proportion of abscesses might not develop an anal fistula at all and therefore treating all abscesses with definitive fistula surgery might overtreat this subset of patients.16 Another dilemma in acute abscess is that the internal opening may not be accurately located in up to 88% of patients.14,15,17,18 This makes the management of these patients even more challenging.
As discussed above, anorectal abscess can either occur in a patient known to have an anal fistula or it may be the first presentation (Table 3). In the latter category, a fistula may be confirmed (communication with anal canal seen) or not (no communication with anal canal) (Table 3). It is only in the last category (anorectal abscess with no obvious fistula) that a fistula may not develop in future and definitive management would overtreat these. In the present study, 83.5% (150/182) patients had anorectal abscess with confirmed fistula and a definitive procedure, if feasible safely and successfully, is a logical proposition (Table 3). On the other hand, 16.5% (30/182) patients had anorectal abscess with no obvious fistula (Table 3). The concern has been raised about overtreatment (as a fistula may not develop in the future) in this subset of patients.2,15,16,19–22 However, there are reasons supporting management with definitive fistula surgery during the initial presentation even in these patients. First, fistula development has been reported in up to 73% of patients undergoing only abscess drainage especially for high intersphincteric and high transsphincteric abscesses.15,16,19–22 Second, if these patients subsequently require definitive fistula surgery, the fistula is generally more complex.3,15,16,19–22 Third, the time required for recovery from a simple drainage procedure is comparable to that from definitive fistula surgery.2 Therefore, if definitive fistula surgery can be safely conducted during acute abscess presentation, it would reduce morbidity in patients in whom the fistula would eventually develop.2
Against this background, the evidence that definitive management of acute fistula-abscess was feasible and safe assumed importance.2,3,14,17 But in this, a major bottleneck was management of those patients in whom the internal opening was non-locatable (IO-non-locatable).14,15,17,18
The management of routine fistulas (without associated abscess) with non-locatable IO has been considered quite challenging as the healing rate in such fistulas had been dismal. It was shown than >50% of these patients were at risk of fistula recurrence after surgery.4 The relative risk (RR) of fistula recurrence was 20-times higher in IO-non-locatable fistulas as compared to IO-locatable fistulas.5 In a recent meta-analysis, amongst several risk factors responsible for fistula recurrence, the IO being non-locatable was associated with the maximum risk of fistula recurrence.6 This meta-analysis highlighted that the relative risk (RR) of fistula recurrence in different scenario was 8.54 (IO-non-locatable), 4.77 (high transsphincteric fistula), 4.77 (presence of multiple tracts), 1.92 (associated horseshoe tract), and 1.52 (recurrent fistula).6 Therefore, when both factors, acute abscess and IO-non-locatable were present together, the management was difficult and had to be done with care. Therefore, assessing the validity of the Garg protocol in this subset of patients was quite pertinent.
The incidence of non-locatable IO in acute abscess-fistula patients is higher than in routine fistulas.3 In the present study, the incidence is 26.9%. There are several reasons for the higher incidence of the IO being non-locatable in acute abscess-fistulas.3,5 The most common reason seems to be blockage of the internal opening by fecal material, granulation tissue or surrounding inflamed tissues due to abscess.5 Another reason is that the method of injecting a colored solution into the external opening and noting its egress from the internal opening does not work well in acute abscess as in many cases, a large abscess cavity does not allow enough pressure to build up for the colored fluid to emerge from the internal opening.2 Also, clinical examination before anesthesia is usually not possible due to marked inflammation and tenderness.5
The management of lateral horseshoe abscesses (from 12 to 6 or 6 to 12 o’clock) was more challenging. In cases where the IO was locatable (control group), the TROPIS procedure (laying opening of intersphincteric space) was performed at the site of the IO. However, in cases where the IO was non-locatable (study group), the IO was assumed to be at the location where the induration was maximum and/or the point where the fistula tract was closest to the anal canal. The TROPIS procedure (laying opening of intersphincteric space) was performed at that point. From experience we can say that the IO was in the midline (posterior or anterior) in most cases in patients with lateral horseshoe abscesses with no obvious locatable IO.
The Garg protocol is based on logical principles.7 Non-locatable IO usually does not mean that there is no communication between the fistula and the anal canal.7 In most cases, anatomical and physiological factors are responsible for the IO being non-locatable, as discussed in previous paragraphs. Therefore, the second step of the protocol is reasonable: in non-horseshoe fistulas, the site where the fistula was closest to the internal sphincter was assumed to be the location of the internal-opening, and the fistula was treated accordingly.7 In a fistula with non-locatable IO, if a fistula tract could be seen crossing the ischiorectal fossa and reaching the external anal sphincter at a single definite point, then logically the fistula would be expected to enter the anal canal at that point only (Figure 2). Similarly, horseshoe tracts usually open in the midline and it is logical that anterior horseshoe or posterior horseshoe fistulas would open in respective midline positions (Figures 3 and 4).7 The Garg protocol validated and documented the efficacy of these principles in a large cohort of patients with non-locatable IO.7 Now, its extension to acute abscess-fistula patients has added further cogency to the protocol. Needless to mention, adequate surgical expertise and radiological support to accurately follow the Garg protocol are prerequisites for successful and safe management of these patients (acute abscess fistula with non-locatable IO).2
The findings of this study corroborating the efficacy of the Garg protocol in management of acute anorectal abscesses with non-locatable IO have important clinical implications. This protocol is the first in the literature to provide a clear management outline to operating surgeons for tackling this difficult clinical condition. The protocol not only enhances healing rates in anal fistulas but also decreases morbidity by reducing the requirement of further operations. Not uncommonly, while trying to locate the IO in order to insert a loose draining seton, a false iatrogenic IO is created which further complicates the condition. The Garg protocol effectively helps prevent this.
There are a few limitations of this study. First, it was a retrospective study with a relatively small sample size. Second, though continence was evaluated objectively by Vaizey’s scores, anal manometry would have added further value to the study. Third, the inclusion of quality of life and psychological impact of treatment parameters would have added another dimension to the study.
Conclusion
The incidence of non-locatable IO was 26.9% in patients with acute abscess. This is the first study in which the management of acute abscess-fistula patients with non-locatable IO has been highlighted. The Garg protocol was found to be highly effective on long-term follow-up in managing these patients (non-locatable IO) with a healing rate comparable to locatable-IO. The change in mean continence scores between patients with non-locatable IO managed as per the Garg protocol and patients in whom the IO was locatable was not significantly different. Further prospective comparative studies are needed to corroborate the findings of this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Loder PB, Zahid A. Immediate treatment of anal fistula presenting with acute abscess: is it time to revisit? Dis Colon Rectum. 2021;64(4):371–372. doi:10.1097/DCR.0000000000001925
2. Garg P, Kaur B, Goyal A, Yagnik VD, Dawka S, Menon GR. Lessons learned from an audit of 1250 anal fistula patients operated at a single center: a retrospective review. World J Gastrointest Surg. 2021;13(4):340–354. doi:10.4240/wjgs.v13.i4.340
3. Rojanasakul A, Booning N, Huimin L, Pongpirul K, Sahakitrungruang C. Intersphincteric exploration with ligation of intersphincteric fistula tract or attempted closure of internal opening for acute anorectal abscesses. Dis Colon Rectum. 2021;64(4):438–445. doi:10.1097/DCR.0000000000001867
4. Garcia-Aguilar J, Belmonte C, Wong WD, Goldberg SM, Madoff RD. Anal fistula surgery. Factors associated with recurrence and incontinence. Dis Colon Rectum. 1996;39(7):723–729. doi:10.1007/BF02054434
5. Sygut A, Mik M, Trzcinski R, Dziki A. How the location of the internal opening of anal fistulas affect the treatment results of primary transsphincteric fistulas. Langenbecks Arch Surg. 2010;395(8):1055–1059. doi:10.1007/s00423-009-0562-0
6. Mei Z, Wang Q, Zhang Y, et al. Risk Factors for Recurrence after anal fistula surgery: a meta-analysis. Int J Surg. 2019;69:153–164. doi:10.1016/j.ijsu.2019.08.003
7. Garg P, Kaur B, Singla K, Menon GR, Yagnik VD, Simple A. Protocol to effectively manage anal fistulas with no obvious internal opening. Clin Exp Gastroenterol. 2021;14:33–44. doi:10.2147/CEG.S291909
8. Garg P, Kaur B, Menon GR. Transanal opening of the intersphincteric space: a novel sphincter-sparing procedure to treat 325 high complex anal fistulas with long-term follow-up. Colorectal Dis. 2021;23(5):1213–1224. doi:10.1111/codi.15555
9. Li YB, Chen JH, Wang MD, et al. Transanal opening of intersphincteric space for fistula-in-ano. Am Surg. 2021:3134821989048. doi:10.1177/0003134821989048
10. Huang B, Wang X, Zhou D, et al. Treating highly complex anal fistula with a new method of combined intraoperative endoanal ultrasonography (IOEAUS) and transanal opening of intersphincteric space (TROPIS). Videosurg Other Miniinv Tech. 2021;16(1):697–703. doi:10.5114/wiitm.2021.104368
11. Yan J, Ma L. Clinical effect of tunnel-like fistulectomy plus draining seton combined with incision of internal opening of anal fistula (TFSIA) in the treatment of high trans-sphincteric anal fistula. Med Sci Monit. 2020;26:e918228. doi:10.12659/MSM.918228
12. Ji L, Zhang Y, Xu L, Wei J, Weng L, Jiang J. Advances in the treatment of anal fistula: a mini-review of recent five-year clinical studies. Front Surg. 2020;7:586891. doi:10.3389/fsurg.2020.586891
13. Vaizey CJ, Carapeti E, Cahill JA, Kamm MA. Prospective comparison of faecal incontinence grading systems. Gut. 1999;44(1):77–80. doi:10.1136/gut.44.1.77
14. Benjelloun EB, Jarrar A, El Rhazi K, Souiki T, Ousadden A, Ait Taleb K. Acute abscess with fistula: long-term results justify drainage and fistulotomy. Updates Surg. 2013;65(3):207–211. doi:10.1007/s13304-013-0218-z
15. Quah HM, Tang CL, Eu KW, Chan SY, Samuel M. Meta-analysis of randomized clinical trials comparing drainage alone vs primary sphincter-cutting procedures for anorectal abscess-fistula. Int J Colorectal Dis. 2006;21(6):602–609. doi:10.1007/s00384-005-0060-y
16. Lunniss PJ, Phillips RK. Surgical assessment of acute anorectal sepsis is a better predictor of fistula than microbiological analysis. Br J Surg. 1994;81(3):368–369. doi:10.1002/bjs.1800810314
17. Knoefel WT, Hosch SB, Hoyer B, Izbicki JR. The initial approach to anorectal abscesses: fistulotomy is safe and reduces the chance of recurrences. Dig Surg. 2000;17(3):274–278. doi:10.1159/000018847
18. Tang CL, Chew SP, Seow-Choen F. Prospective randomized trial of drainage alone vs. drainage and fistulotomy for acute perianal abscesses with proven internal opening. Dis Colon Rectum. 1996;39(12):1415–1417. doi:10.1007/BF02054531
19. Mocanu V, Dang JT, Ladak F, et al. Antibiotic use in prevention of anal fistulas following incision and drainage of anorectal abscesses: a systematic review and meta-analysis. Am J Surg. 2019;217(5):910–917. doi:10.1016/j.amjsurg.2019.01.015
20. Sozener U, Gedik E, Kessaf Aslar A, et al. Does adjuvant antibiotic treatment after drainage of anorectal abscess prevent development of anal fistulas? A randomized, placebo-controlled, double-blind, multicenter study. Dis Colon Rectum. 2011;54(8):923–929. doi:10.1097/DCR.0b013e31821cc1f9
21. Lohsiriwat V, Yodying H, Lohsiriwat D. Incidence and factors influencing the development of fistula-in-ano after incision and drainage of perianal abscesses. J Med Assoc Thai. 2010;93(1):61–65.
22. Hamadani A, Haigh PI, Liu IL, Abbas MA. Who is at risk for developing chronic anal fistula or recurrent anal sepsis after initial perianal abscess? Dis Colon Rectum. 2009;52(2):217–221. doi:10.1007/DCR.0b013e31819a5c52
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.