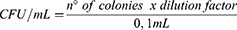Back to Journals » Medical Devices: Evidence and Research » Volume 17
Non Clinical Model to Assess the Mechanism of Action of a Combined Hyaluronic Acid, Chondroitin Sulfate and Calcium Chloride: HA+CS+CaCl2 Solution on a 3D Human Reconstructed Bladder Epithelium
Authors Brambilla L, Frangione V , Meloni M
Received 16 September 2023
Accepted for publication 10 January 2024
Published 30 January 2024 Volume 2024:17 Pages 47—58
DOI https://doi.org/10.2147/MDER.S433261
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Laura Brambilla,1 Valeria Frangione,2 Marisa Meloni1
1VitroScreen, in vitro Research Laboratory, Milan, Italy; 2IBSA Institut Biochimique SA, Pambio-Noranco, Switzerland
Correspondence: Marisa Meloni, VitroScreen, Via Mosè Bianchi, 103, Milan, 20149, Italy, Tel +39 02 89077608, Email [email protected]
Purpose: Medical Device Regulation (EU) 2017/745 requires the principal mode of action (MoA) to be demonstrated by experimental data. The MoA of Ialuril® Prefill (combined as HA+CS+CaCl2: sodium hyaluronate 1.6%, sodium chondroitin sulphate 2% w/v and calcium chloride 0.87%) Class III medical device, indicated for intravesical instillation to reduce urinary tract infections, has been evaluated on a 3D reconstructed human bladder epithelium (HBE).
Methods: Three experimental designs; i) E. coli strain selection (DSM 103538, DSM 1103) to investigate the HA+CS+CaCl2 properties in modifying bacterial growth in liquid broth (CFU 4h and 24h) at 80%, 50% and 25% concentrations; ii) evaluation of film forming properties on HBE after 15 min exposure by quantifying caffeine permeation across the epithelium; iii) capacity to counteract E. coli adhesion and biofilm formation on colonized HBE by viable counts and ultrastructural analysis by scanning electron microscopy (SEM) using ciprofloxacin as the reference antimicrobial molecule.
Results: No significant differences were observed in bacterial viability for both the E. coli strains. HA+CS+CaCl2 reduced caffeine permeation of 51.7% and 38.1% at 1h and 2h, respectively and determined a significant decrease in caffeine permeation rate at both timepoints supporting HA+CS+CaCl2 capacity to firmly adhere to the bladder epithelium creating a physical barrier on the surface. The viable counts in HBE treated tissues then infected with E. coli resulted not different from the negative control suggesting that the device did not inhibit E. coli growth. SEM images showed homogenous product distribution over the HBE surface and confirmed the capacity of HA+CS+CaCl2 to adhere to the bladder epithelium, counteracting biofilm formation.
Conclusion: The results support the capacity of HA+CS+CaCl2 to counteract bacterial invasion by using a physico-mechanical mode of action: this medical device represents a valid alternative to antibiotics in the treatment of recurrent UTIs.
Keywords: recurrent cystitis, urinary tract infections, E. coli UPEC, E. coli biofilm, hyaluronic acid, chondroitin sulphate, film forming properties, anti-bacterial adhesion, reconstructed 3D human bladder epithelium
Introduction
Intravesical instillations of glycosaminoglycans (GAGs) represent a valid therapeutical approach for different pathologies, such as bladder pain syndrome/interstitial cystitis (BPS/IC), chemical induced cystitis (including BCG therapy), radiation induced cystitis (irradiation of pelvic tumors) and recurrent urinary tract infections (UTIs). In particular, UTIs are one of the most prevalent infections both in the community (only second to respiratory tract’s) and in hospital settings, affecting 50–60% of adult women in their lifetime.1 Risk factors associated with UTIs include female gender, age, individual history of UTI, sexual activity, and medical comorbidities. UTIs are mainly caused by bacteria, although fungi and some viruses have also been implicated. Among bacteria, Gram-negative bacteria of the Enterobacteriaceae family, including Escherichia coli (E. coli), Klebsiella, Enterobacter, Proteus species, etc., are mostly involved. In particular, some E. coli strains with distinctive features have been identified as causing most UTIs: they have been designated uropathogenic E. coli (UPEC) and used as models to better understand host-pathogen interactions during urinary tract pathogenesis.2–5 These strains possess diverse virulence-associated factors (VFs) that assist them in attaching to, invading, and injuring the host, and include adhesins, toxins, protectins, and biofilm production.6,7 In particular adhesins, which appear as hair-like fibers called fimbriae (or pili), facilitate the colonization with E. coli in the urinary tract by attaching to host epithelial cells. This attachment promotes the persistence of the organism in the bladder and serves as a reservoir for ascending infection in the urinary tract.8 However intracellular reservoirs of bladder epithelium have been discovered more recently.9–11 Various adhesins have been identified and are classified mainly according to receptor specificity such as P-fimbriae, which, along with type 1 fimbriae, are particularly important in bladder colonization.12 Antimicrobials are the first-line therapy for uncomplicated UTIs, aiming to alleviate symptoms and reduce recurrence.13 However, the increase in antimicrobial resistance at clinical level is stimulating the use of alternative therapeutic approaches that can selectively treat and prevent UTIs as suggested by the European Association of Urology14 (EAU) and the American Urological Association15 (AUA), recommending the appropriate use of antimicrobials ensuring cost-effective therapy whilst minimizing microbial resistance. Alternative approaches for non-antibiotic prevention therapies, include glycomimetic antiadhesives, immunomodulatory therapies, and competition with probiotics.1 The efficacy and the safety of these non-antibiotic approaches need to be demonstrated with appropriate randomized controlled trials, including the evidence of their mode of action for a proper product qualification as requested by the medical device regulatory framework.16–18
Ialuril® prefill (IBSA) is a class III medical device for intravesical instillation indicated to replenish the glycosaminoglycan (GAG) layer of the bladder epithelium in cases in which their loss causes frequent and recurring cystitis. It contains a 50 mL sterile solution of sodium hyaluronate (1.6% w/v – 800 mg/50 mL), sodium chondroitin sulphate (2% w/v – 1 g/50 mL) and calcium chloride (0.87% – 440 mg/50 mL). The intravesical instillation aids in the reduction of urinary tract infections (UTIs) incidence in patients with recurrent UTIs (rUTIs).
The aim of this research project was to investigate the physico-mechanical mode of action of a medical device (MD) that consists of a combined hyaluronic acid, chondroitin sulfate and calcium chloride HA+CS+CaCl2 solution (Ialuril®) at bladder epithelium level by using an in vitro pre-clinical experimental approach.
A commercially available 3D reconstructed human bladder epithelium (HBE) has been applied as a biologically relevant test system to investigate HA+CS+CaCl2 properties in forming a physical barrier on the epithelium, counteracting this mean E. coli adhesion and biofilm formation on the living tissue without showing a direct antimicrobial efficacy on E. coli, which is the mode of action of antibiotics.
Three different experimental designs have been defined with the following aims:
- To select the E. coli strain (DSM 103538, DSM 1103) and to investigate the HA+CS+CaCl2 properties in modifying bacterial growth by a microdilution assay in liquid broth and resulting CFU (colony forming unit) considering three different concentrations of the product (80%, 50% and 25%).
- To evaluate the film forming properties of HA+CS+CaCl2, applied topically as such on the bladder epithelium and its capacity to induce a temporary modification of caffeine permeation across the epithelium due to the product acting as a protective physical barrier (“film”).
- To investigate the anti-bacterial adhesion properties of HA+CS+CaCl2, applied as such, and the pili and biofilm formation prevention on E. coli DSM1103 colonized HBE for 4 h by performing viable counts at tissue level and ultrastructural analysis by scanning electron microscopy (SEM).
These protocols contribute to support product qualification and classification according to the Medical Device Regulation (EU) 2017/745 (MDR) and MDCG 2022–5 on borderline products by providing state-of-the-art evidence on the physico-mechanical mechanism of action exerted by HA+CS+CaCl2 on the bladder epithelium, showing an interesting alternative product, mirroring the performance of antibiotics.
Materials and Methods
Test Items
HA+CS+CaCl2 prefill (IBSA) contains sodium hyaluronate 1.6%, sodium chondroitin sulfate 2% and calcium chloride 0.87%. Ciprofloxacin (≥98) (Sigma-Aldrich) was used as a reference at the concentration of 0.312 μg/mL. White vaseline (Sella srl, Schio Italy) was used as a positive control in the film forming assay.
Test System
Human bladder epithelium (HBE) is produced by Episkin SA (Lyon, France): it is a 3D reconstructed human bladder epithelium originated from RT-112 urinary bladder transitional human carcinoma cells cultured on an inert polycarbonate filter with airlift technology, in a chemically defined medium. It is cultured for 5–7 days, reaching a mean thickness of 55–90 μm and it is viable for 1 week. The model is proposed for research use and it has been characterized by the manufacturer for its capacity to form an epithelial barrier confirming the expression of keratin 17, keratin 20 and CD44 as biomarkers of the organization of extracellular matrix and it has been further characterized for the expression and localization of Cadherin 1 and Claudin 4 (data not shown). The 3D bladder epithelium reproduces the inner mucosa of the bladder, a transition epithelium that covers the first tract of the ureters: this epithelium is impermeable to counteract urine re-absorption. The batch was tested for the absence of HIV, hepatitis B, hepatitis C and mycoplasma, and the maintenance medium was tested for sterility. The inserts containing the tissues at day 5 were placed at room temperature in a multi-well plate filled with an agarose nutrient solution in which they were embedded for shipment.
After arrival, the HBEs were removed from the agarose nutrient solution under a sterile airflow cabin. The inserts were rapidly transferred to 6-well plates previously filled with specific medium without antibiotics (1 mL/well) (Episkin SA, Lyon, France) at room temperature and incubated at 37 °C, 5% CO2 and saturated humidity. The test was performed 2 days after the arrival, with tissues kept in maintenance medium till the end of the experiment.
Bacterial Strains
Escherichia coli is the most abundantly isolated, Gram-negative bacterium responsible for urinary tract infections (UTIs). It is able to produce several soluble, metabolically active and toxic molecules, such as pore forming toxins and proteases. Its adherence ability is mediated by biofilm production, which confers on E. coli a higher resistance to the antimicrobial substances access and improved adherence to host tissues.
In Table 1 the two uropathogenic strains used for the experiments provided by the German collection of microorganisms and cell cultures GmbH (DSMZ) are described.
 |
Table 1 E. Coli Strains Characterization |
Before the assay, each bacterial strain was inoculated on their medium (TSYE broth or agar) and incubated statically under the required growth conditions (at 37 °C in aerobic conditions for 24–48 h) to check their normal colony morphology and to use fresh cultures.
Chemicals for Microdilution
For bacterial culture: nutrient broth (code: 70149, Sigma-Aldrich), nutrient agar (code: PO5025A, Thermo Scientific), tryptone soy yeast extract broth (TSYEB, tryptic soy broth 30 g/L BD, yeast extract 3 g/L, Sigma-Aldrich), tryptone soy yeast extract agar (TSYEA, tryptic soy agar 30 g/L BD, yeast extract 3 g/L, Sigma-Aldrich) and sterile saline solution (sodium chloride 0.9%, EUROSPITAL) were used to resuspend bacterial culture.
Simulated urine (SU) was prepared with 7.1 g of urea, 1.5 g of creatinine, 1 g of ammonium citrate, 4 g of sodium chloride, 0.825 g of potassium chloride, 0.25 g of potassium bisulfate, 0.1 g of magnesium sulfate, 0.875 g of monobasic potassium phosphate and 0.25 g of potassium bicarbonate were dissolved in 250 mL of ultrapure water. All the compounds used were from Sigma-Aldrich. Ultrapure water was added to reach a final volume of 500 mL. The final pH of SU was 5.00. The SU was used within 24 hours from preparation.22
Microdilution Assay Method
The microdilution test was performed on the two Escherichia coli uropathogenic strains to investigate the mechanism of action of the product and to pick out the most appropriate strain to be used in the anti-adhesion and biofilm formation on HBE. HA+CS+CaCl2 was tested at three different concentrations (25%, 50% and 80%).
Test Mixture Preparation
The bacterial cultures were resuspended in SU solution to simulate the physiological use conditions; their OD600nm were checked by means of a spectrophotometer (UV/VIS ONDA TOUCH UV-21, ONDA SPECTROPHOTOMETER Italy) and the final concentration was adjusted to obtain a concentration of 107 CFU/mL.
Each test mixture was prepared in a tube, according to the following schemes and Table 2: mixing 8 mL of each bacterial suspension (107 CFU/mL) in culture medium (2x concentrated) with 32 mL of test item at 80%, mixing 20 mL of each microbial suspension (107 CFU/mL) in the culture medium (standard concentrated) with 20 mL of the test item at 50% or 25%.
 |
Table 2 Ialuril® Dilutions Preparations for Microdilution Test |
Additional control mixtures were prepared adding the bacterial suspensions to their culture media and ciprofloxacin solution (0.0312 µg/mL), respectively as negative control and reference to validate the study.
All the obtained suspensions were then incubated at 37 °C in aerobic conditions and allowed to grow for up to 24 hours. The growth rate was checked, performing viable counts compared to the reference or negative control.
Bacterial Viable Counts
An aliquot from each mixture described in the paragraph before was taken after 4 h and 24 h for the viable counts performed by serial decimal dilutions in sterile saline solution. They were plated by spread method onto agar plates and incubated at bacterial growth conditions (37 °C, aerobic conditions).
The following formula was used to calculate the CFU/mL:
Film Forming Protocol
The film forming protocol developed on epidermis19 was adapted to HBE.
Bladder Epithelium Treatment
At arrival in the laboratory, culture inserts were placed in 6-well plates previously filled with 1 mL/well saline solution (basolateral compartment, receptor fluid). All the series were evaluated in triplicate. Fifty microliters of HA+CS+CaCl2 and white vaseline, used as the positive control (PC), were applied directly on the bladder epithelium surface for an exposure of 15 min at room temperature. Negative control (NC) tissues were untreated.
After treatment with HA+CS+CaCl2 and white vaseline, 100 μL of 0.5% w/v caffeine solution (1 mg caffeine/cm2) was applied to the apical compartment. Caffeine penetration was monitored by collecting the receptor fluids (1 mL) from the basolateral compartment at 1 h and 2 h of exposure.
Analytical Method for Caffeine Quantification
The concentration of caffeine in the 0.5% reference solution and in the collected receptor fluid samples was quantified via UPLC method. Receptor fluid samples were stored at 4 °C before UPLC/MS analysis. The caffeine concentration was determined by using a 1290 Infinity II LC System (AGILENT Santa Clara, Ca. USA) equipped with a C18 reversed-phase column (ACQUITY UPLCBEH-C18, 1.7 μm, 100×2.1 mm, WATERS CORPORATION, Ma. USA) set at 25 °C. A 5 μL sample was injected for isocratic elution at 0.25 mL/min. The composition of the eluent was 80% water/20% methanol. The wavelength was set at 273 nm. Standard calibration curves for caffeine (0.1 and 1000 mg/L) were used.
The results are expressed in μg and % of the diffused caffeine in comparison to the applied dose as well as in comparison to the negative control.
Statistical Analysis
One-way ANOVA followed by a post hoc Tukey HSD test was performed using GraphPad Prism, version 9.2.0 (GraphPad Software, San Diego, Ca. USA). The product and the white vaseline were compared with the untreated negative control, and statistically significant results were reported as p-values.
Anti-Bacterial Adhesion on the 3D Bladder Epithelium
E. coli DSM1103 (cystitis isolate) was selected as the most relevant strain to assess the HA+CS+CaCl2 properties to counteract its adhesion to the bladder epithelium.
The day of the colonization, E. coli DSM1103, previously grown in nutrient agar, was resuspended in SU solution to simulate the bladder epithelium microenvironment, and its concentration was checked by means of a spectrophotometer adjusting the inoculum level to 106–107 CFU/mL. The inoculum level was also checked, performing the viable counts with the appropriate 10-fold dilutions prepared in sterile saline solution and spreading them onto nutrient agar.
The day of the test, tissues (HBE) were pre-treated on the apical part (directly spread on tissue surface) with 50 μL of HA+CS+CaCl2 or reference (ciprofloxacin, 0.312 μg/mL solution) during 4 h as reported in Figure 1. Not treated and not colonized HBE were used as negative control (NC) while non treated but colonized HBE served as positive control.
 |
Figure 1 Scheme of the anti-bacterial adhesion assay on HBE. |
At the end of the exposure time (4 h) the residual volume of product or reference was removed and 30 μL of bacterial suspension were added on tissue apical surface and incubated at 37 °C with CO2 5%. After 2 hours (E. coli DSM1103 replication time), the inoculum excess was removed from each apical surface and tissues were re-incubated for another 2 hours (4 h total).
The residual of E. coli DSM1103 viable rate was checked by viable counts by spread method onto agar plates (CFU/tissue) and SEM analysis compared to the reference.
E. Coli DSM1103 Viable Count on HBE
At the end of the colonization period (4 h), the tissues were collected for viable counts: Apical part: 0.3 mL of sterile saline solution was added to the apical compartment and then removed and collected in 2 mL vials. Homogenate: tissues were separated from the plastic insert with a scalpel, placed into a vial containing 0.5 mL of sterile saline solution and processed by a Minilysis homogenizer (3 cycles of 30 sec, lowest power) (VWR, USA).
The residual bacterial viable counts (CFU) were determined in the apical fraction (non-adherent) and on tissue homogenates (adherent) using the spread plate method onto agar plates.
Scanning Electron Microscopy (SEM)
SEM analyses were performed by Service Biotech srl (Naples, Italy). Samples for SEM were fixed by fixative buffer solution (proprietary composition Service Biotech srl). The fixed samples were washed in 0,1 M sodium cacodylate buffer, pH 7.4 and then carried out in 1% osmium tetraoxide (OsO4) in the same buffer (2 h at RT). They were dehydrated in ascending grades of ethanol at room temperature and hexamethyldisilazane overnight.
The samples were placed on pins with carbon tabs coated with a layer of gold using the Polaron Equipment limited SEM coating unit E5100 and then transferred to the SEM electron microscope for viewing and photography. Thanks to a digital camera, the scanning electron microscopy analysis allows an examination of the epithelial surface ultrastructure that gives information in terms of bacterial phenotype, density, attachment to the epithelium, and formation of biofilm.
Results and Discussion
E. Coli Viable Counts on Microdilution Assay
In Table 3 are reported the results of the viable counts (as per formula 1) performed to assess HA+CS+CaCl2 activity at three different concentrations on two Escherichia coli uropathogenic strains (DSM 103538, DSM 1103). The growth rates were compared to the negative control (ie culture media, in bold).
 |
Table 3 Bacterial Viable Counts (Expressed as Log Values) at 4 h and 24 h |
No significant differences were observed in bacterial viability for both the E. coli strains tested both at 4 h and 24 h. Due to the more representative origin of the uropathogenic E. coli strain ATCC25922, DSM1103 (cystitis isolate), this strain was selected for the anti-adhesion efficacy test on the bladder epithelium.
Film Forming on HBE
The results of caffeine quantification in the receptor compartment at 1 h and 2 h are presented in Figure 2. The results are expressed as the caffeine percentage compared to the amount of caffeine applied (510 μg) as a function of time. As expected, the positive control has reduced caffeine passage through the bladder epithelium of 89.4% and 76.8% at 1 h and 2 h, respectively allowing us to validate the experimental run.
In the HA+CS+CaCl2 treated tissues caffeine permeation after 1 h was significantly reduced (p< 0.001) compared to the untreated control: the percentage of permeated caffeine for HA+CS+CaCl2 was found to be 15.6%, showing a reduction in caffeine penetration of 51.7%, compared to the untreated control for which a permeation of 32.3% was quantified. At 2 h, the percentage of permeated caffeine for HA+CS+CaCl2 was found to be 32.2%, showing a reduction in caffeine penetration of 38.1%, compared to the untreated control.
The caffeine kinetics through the HBE at 1 h and 2 h is reported in Figure 3A and B, respectively. Compared to the negative control, HA+CS+CaCl2 showed a significant decrease in the caffeine permeation rate at both timepoints (p< 0.001).
Anti-Bacterial Adhesion Efficacy on HBE
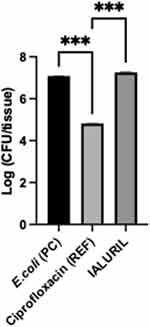
The results of the viable counts performed on the apical and homogenate fractions are reported in Figure 4.
The results of the reference (ciprofloxacin) allowed a validation of the experimental run, demonstrating a significant inhibition (reduction >2Log, p < 0.001) of E. coli growth during 4 h and confirming its antimicrobial activity.
Based on the results of viable counts in HBEs treated during 4 h with HA+CS+CaCl2 and then infected during 4 h with E. coli, no inhibition of E. coli growth was observed as the total viable count resulted in the same as the negative control (untreated).
Ultrastructural Analysis by SEM
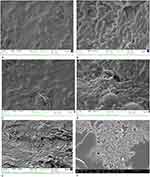
The SEM analysis allowed an investigation, at ultrastructural level, of the bladder epithelium surface, giving information in terms of product distribution, bacterial phenotype, density, adhesion mechanism to the epithelium (pili formation) and biofilm.
In Figure 5 an efficient colonization and bacterial-epithelium interaction at 4 h is shown: pili (fimbriae) were visible in the E. coli DSM1103 wall (Figure 5A and B). E. coli appears organized in biofilm phenotype macro-aggregates that still show a replicative capacity.
In the samples pre-treated with HA+CS+CaCl2 (Figure 5C and D) bacterial density seems no different compared to the colonized control and a thick film of amorphous electron dense material (corresponding to Ialuril®) is visible as a homogenous product distribution within the surface.
In the samples pre-treated with HA+CS+CaCl2 it is possible to visualize the inhibition of E. coli pili formation (Figure 5D): pedestal structures are no more visible near the test item matrix. This evidence confirms HA+CS+CaCl2 specific properties to selectively counteract E. coli adhesion mechanisms on bladder epithelium.
Conclusion
In vitro reconstructed human epithelia models are closer in term of morphology (multistratified epithelium), biochemical and physiological properties to in vivo human tissues and represent the most promising alternative to animals, ex vivo explants and submerged cell monolayers to access efficacy, mechanism of action and safety of topically applied products in different fields, including the medical device sector.23–30
The experimental approach adopted in this pre-clinical project was based on the use of a 3D reconstructed model of bladder epithelium chosen for its biological relevance as biological barrier it reproduces the inner mucosa of the bladder, a transition epithelium that covers the first tract of the ureters and is impermeable to counteract urine re-absorption. The main goal was to demonstrate that the test item was able to create an additional mechanical-physical barrier against E. coli adhesion without a direct interaction with the bacterium itself in terms of antimicrobial activity. It has been first demonstrated that the E. coli UPC cystitis isolated strain, DSM1103, in reconstituted urine was able to colonize the epithelium surface, mirroring a bladder microenvironment during 24 h (7.09 Log CFU/tissue). SEM ultrastructural analysis has been applied as a non quantitative readout to confirm experimental conditions it has been possible to visualize bacterial distribution on the epithelium surface and its specific adhesion mechanism, mainly based on the formation of fimbriae on cell walls and the establishment of a biofilm adherent to the epithelium.
This innovative test system (bladder epithelium colonized with E. coli UPC, DSM1103 in reconstituted urine) was applied to investigate the properties of HA+CS+CaCl2 to form an additional mechanical-physical barrier counteracting E. coli overgrowth and to reduce its capacity to adhere to the bladder epithelium.
The mechanism of action demonstrated by the medical device HA+CS+CaCl2 can be defined as physico-mechanical, based on the medical device's peculiar properties: it has been shown to firmly adhere to the bladder epithelium and to create a protective physical barrier (“film”) inducing a temporary modification of tissue permeability (reduction of 51.7% and 38.1%, compared to the untreated control, respectively at 1 h and 2 h) to an hydrosoluble probe (caffeine) without showing a significant antibacterial efficacy (Log reduction calculated versus the NC were not significantly different) confirming no effect on bacterial growth and bacterial viability. On the contrary, treatment with ciprofloxacin induced a significant inhibition (reduction >2Log, p<0.001) of E. coli growth during 4 h confirming its antibiotic activity. SEM ultrastructural analysis has given qualitative information about the bacterial-epithelium interaction in the bladder epithelium previously treated during 4 h with Ialuril®: in the E. coli wall the formation of pili (fimbriae) appears inhibited as well as the biofilm formation.
The experimental data of the viable counts and the qualitative analysis based on SEM globally support the effective capacity of HA+CS+CaCl2 to counteract the bacterial invasion by a physico-mechanical mode of action: this medical device represents an alternative to antibiotics in the treatment of recurrent UTIs.
Abbreviations
CFU, colony forming unit; HBE, human bladder epithelium; MDR, medical device regulation (EU) 2017/745; MD, medical device; NC, negative control; Ph.I.M, pharmacological, immunological or metabolic; PC, positive control; SEM, scanning electron microscopy; SBMDs, substance-based medical devices; UTIs, urinary tract infections.
Ethics
This in vitro study doesn’t require ethics committee approval because it has been conducted on 3D human reconstructed bladder epithelium.
Acknowledgments
The authors acknowledge the technical support of Dr. Salvatore del Prete for SEM analysis and fruitful images discussion (Service Biotech srl (Naples, Italy) and the excellent support of Dr. Laura Ceriotti, VitroScreen SrL for drafting the manuscript.
Funding
This study was financially supported by IBSA Institut Biochimique SA, Switzerland.
Disclosure
VF is an employee of IBSA. The authors report no other conflicts of interest in this work.
References
1. Klein RD, Hultgren SJ. Urinary tract infections: microbial pathogenesis, host-pathogen interactions and new treatment strategies. Nat Rev Microbiol. 2020;18(4):211–226. doi:10.1038/s41579-020-0324-0
2. Foxman B, Brown P. Epidemiology of urinary tract infections: transmission and risk factors, incidence, and costs. Infect Dis Clin North Am. 2003;17(2):227–241. doi:10.1016/s0891-5520(03)00005-9
3. Hunstad DA, Justice SS, Hung CS, Lauer SR, Hultgren SJ. Suppression of bladder epithelial cytokine responses by uropathogenic Escherichia coli. Infect Immun. 2005;73(7):3999–4006. doi:10.1128/IAI.73.7.3999-4006.2005
4. Billips BK, Forrestal SG, Rycyk MT, Johnson JR, Klumpp DJ, Schaeffer AJ. Modulation of host innate immune response in the bladder by uropathogenic Escherichia coli. Infect Immun. 2007;75(11):5353–5360. doi:10.1128/IAI.00922-07
5. Billips BK, Schaeffer AJ, Klumpp DJ. Molecular basis of uropathogenic Escherichia coli evasion of the innate immune response in the bladder. Infect Immun. 2008;76(9):3891–3900. doi:10.1128/IAI.00069-08
6. Kaper JB, Nataro JP, Mobley HL. Pathogenic Escherichia coli. Nat Rev Microbiol. 2004;2(2):123–140. doi:10.1038/nrmicro818
7. Kudinha T The Pathogenesis of Escherichia coli Urinary Tract Infection [Internet]. Escherichia coli - Recent Advances on Physiology, Pathogenesis and Biotechnological Applications. InTech; 2017.
8. Mobley HL, Island MD, Massad G. Virulence determinants of uropathogenic Escherichia coli and Proteus mirabilis. Kidney Int Suppl. 1994;47:S129–S136.
9. Schilling JD, Lorenz RG, Hultgren SJ. Effect of trimethoprim-sulfamethoxazole on recurrent bacteriuria and bacterial persistence in mice infected with uropathogenic Escherichia coli. Infect Immun. 2002;70(12):7042–7049. doi:10.1128/IAI.70.12.7042-7049.2002
10. Rosen DA, Hooton TM, Stamm WE, Humphrey PA, Hultgren SJ. Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med. 2007;4(12):e329. doi:10.1371/journal.pmed.0040329
11. Mulvey MA, Schilling JD, Hultgren SJ. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect Immun. 2001;69(7):4572–4579. doi:10.1128/IAI.69.7.4572-4579.2001
12. Stærk K, Khandige S, Kolmos HJ, Møller-Jensen J, Andersen TE. Uropathogenic Escherichia coli express type 1 fimbriae only in surface adherent populations under physiological growth conditions. J Infect Dis. 2016;213(3):386–394. doi:10.1093/infdis/jiv422
13. Gupta K, Grigoryan L, Trautner B. Urinary tract infection. Ann Intern Med. 2017;167(7):ITC49–ITC64. doi:10.7326/AITC201710030
14. EAU Guidelines on Urological Infections. presented at the EAU annual congress Milan, Italy 2023; 2023.
15. Anger JT, Bixler BR, Holmes RS, Lee UJ, Santiago-Lastra Y, Selph SS. Updates to recurrent uncomplicated urinary tract infections in women: AUA/CUA/SUFU guideline. J Urol. 2022;208(3):536–541. doi:10.1097/JU.0000000000002860
16. Damiano R, Quarto G, Bava I, et al. Prevention of recurrent urinary tract infections by intravesical administration of hyaluronic acid and chondroitin sulphate: a placebo-controlled randomised trial [published correction appears in Eur Urol. 2011 Jul;60(1):193]. Eur Urol. 2011;59(4):645–651. doi:10.1016/j.eururo.2010.12.039
17. De Vita D, Giordano S. Effectiveness of intravesical hyaluronic acid/chondroitin sulfate in recurrent bacterial cystitis: a randomized study. Int Urogynecol J. 2012;23(12):1707–1713. doi:10.1007/s00192-012-1794-z
18. Goddard JC, Janssen DAW. Intravesical hyaluronic acid and chondroitin sulfate for recurrent urinary tract infections: systematic review and meta-analysis. Int Urogynecol J. 2018;29(7):933–942. doi:10.1007/s00192-017-3508-z
19. Wiles TJ, Kulesus RR, Mulvey MA. Origins and virulence mechanisms of uropathogenic Escherichia coli. Exp Mol Pathol. 2008;85(1):11–19. doi:10.1016/j.yexmp.2008.03.007
20. Hilbert DW, Pascal KE, Libby EK, Mordechai E, Adelson ME, Trama JP. Uropathogenic Escherichia coli dominantly suppress the innate immune response of bladder epithelial cells by a lipopolysaccharide- and Toll-like receptor 4-independent pathway. Microbes Infect. 2008;10(2):114–121. doi:10.1016/j.micinf.2007.10.012
21. Maldonado-Barragán A, Mshana SE, Keenan K, et al. Predominance of multidrug-resistant (MDR) bacteria causing urinary tract infections (UTIs) among symptomatic patients in East Africa: a call for action. medRxiv. 2023. doi:10.1101/2023.06.13.23291274
22. Penn R, Ward BJ, Strande L, Maurer M. Review of synthetic human faeces and faecal sludge for sanitation and wastewater research. Water Res. 2018;132:222–240.
23. Casiraghi A, Ranzini F, Musazzi UM, Franzè S, Meloni M, Minghetti P. In vitro method to evaluate the barrier properties of medical devices for cutaneous use. Regul Toxicol Pharmacol. 2017;90:42–50. doi:10.1016/j.yrtph.2017.08.007
24. Gordon S, Daneshian M, Bouwstra J, et al. Non-animal models of epithelial barriers (skin, intestine and lung) in research, industrial applications and regulatory toxicology. ALTEX. 2015;32(4):327–378. doi:10.14573/altex.1510051
25. Ayehunie S, Wang YY, Landry T, Bogojevic S, Cone RA. Hyperosmolal vaginal lubricants markedly reduce epithelial barrier properties in a three-dimensional vaginal epithelium model [published correction appears in Toxicol Rep. 2020 Dec 25;8:62–63]. Toxicol Rep. 2017;(5):134–140. doi:10.1016/j.toxrep.2017.12.011
26. De Jong WH, Hoffmann S, Lee M, et al. Round robin study to evaluate the reconstructed human epidermis (RhE) model as an in vitro skin irritation test for detection of irritant activity in medical device extracts. Toxicol In Vitro. 2018;50:439–449. doi:10.1016/j.tiv.2018.01.001
27. Meloni M, Balzaretti S, Ceriotti L. Medical devices biocompatibility assessment on HCE: evidences of delayed cytotoxicity of preserved compared to preservative free eye drops. Regul Toxicol Pharmacol. 2019;106:81–89. doi:10.1016/j.yrtph.2019.04.022
28. Sica VP, Friberg MA, Teufel AG, et al. Safety assessment scheme for menstrual cups and application for the evaluation of a menstrual cup comprised of medical grade silicone. EBioMedicine. 2022;86:104339. doi:10.1016/j.ebiom.2022.104339
29. Cárdenas-Calderón C, Veloso-Giménez V, González T, et al. Development of an implantable three-dimensional model of a functional pathogenic multispecies biofilm to study infected wounds. Sci Rep. 2022;12(1):21846. doi:10.1038/s41598-022-25569-5
30. De Servi B, Ranzini F. Protective efficacy of antidiarrheal agents in a permeability model of Escherichia coli-infected CacoGoblet® cells. Future Microbiol. 2017;12:1449–1455. doi:10.2217/fmb-2016-0195
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.