Back to Journals » Patient Preference and Adherence » Volume 14
Non-Adherence to Pharmacotherapy: A Prospective Multicentre Study About Its Incidence and Its Causes Perceived by Chronic Pain Patients
Authors Sampaio R , Azevedo LF , Dias CC, Castro Lopes JM
Received 30 September 2019
Accepted for publication 17 December 2019
Published 19 February 2020 Volume 2020:14 Pages 321—332
DOI https://doi.org/10.2147/PPA.S232577
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Rute Sampaio, 1, 2 Luís Filipe Azevedo, 3– 5 Cláudia Camila Dias, 4, 5 José M Castro Lopes 1–3
1Departamento de Biomedicina, Faculdade de Medicina da Universidade do Porto, Porto, Portugal; 2Instituto de Biologia Molecular e Celular (IBMC), Universidade do Porto, Porto, Portugal; 3Centro Nacional de Observação em Dor - OBSERVDOR, Porto, Portugal; 4Departamento de Medicina da Comunidade, Informação e Decisão em Saúde, Faculdade de Medicina da Universidade do Porto (MEDCIDS), Porto, Portugal; 5Centro de Investigação Em Tecnologias e em Serviços de Saúde (CINTESIS), Porto, Portugal
Correspondence: Rute Sampaio
Faculty of Medicine of Porto University, Department of Biomedicine – 5 th Floor, Alameda Hernani Monteiro, Porto 4200-319, Portugal
Email [email protected]
Objective: Pharmacological interventions remain the cornerstone of chronic pain treatment; however, nearly 40% of the prescription medicines are not taken as prescribed. The present study aims at understanding and describing non-adherence from the perspective of chronic pain patients during a 1-year follow-up study.
Methods: A cohort of 950 consecutive patients referred to a first consultation in Multidisciplinary Chronic Pain Clinics was followed with a standardized protocol for 1 year. This included assessment of pain characteristics; prescribed medication; therapeutic adherence; effectiveness of treatment, non-adherence and its perceived reasons; clinical outcomes and quality of life. We used a mixed methods approach, including qualitative and quantitative analyses.
Results: Forty-nine percent of the 562 patients who responded to all assessments during follow-up were adherent after 1 year of chronic pain treatment. The core associations between each “non-adherence reason” and Anatomical Therapeutic Chemical Code (ATC) group were perceived side effects (p=0.019) and delayed start (p=0.022) for narcotic analgesics (opioids); perceived non-efficacy (p=0.017) and delayed start (p=0.004) for antiepileptics and anticonvulsants; perceived low necessity (p=0.041) and delayed start (p=0.036) for analgesics antipyretics; change in prescriptions because of a new clinical condition for antidepressants (p=0.024); high concerns (p=0.045) and change in prescriptions because of a new clinical condition (p< 0.001) for non-steroidal anti-inflammatory drugs; delayed start (p=0.016) and financial constraints (p=0.018) for other medications.
Discussion: This study emphasizes the patient’s perspective regarding non-adherence to pharmacological treatment of chronic pain, providing valuable and novel information to be used in future interventions to help patients make an informed choice about their adherence behavior.
Keywords: chronic pain, pharmacological treatment, non-adherence, patient’s perspective
Introduction
Therapeutic adherence was initially defined as “the extent to which a person’s behaviour (in terms of taking medications, following diets, or executing lifestyle changes) coincides with medical or health advice”,1 and it is a commonly used term, which considers the active role played by the patient in managing his or her disease.2 In chronic conditions, particularly, therapeutic interventions require a special commitment on the part of the patient, family or significant others, demanding a sharing of meanings and treatment decision-making. Concerted efforts have been made to understand adherence difficulties and identify predictors of non-adherence.3 In fact, there is currently a growing interest on the impact of non-adherence to pharmacological pain treatments; and the value of non-adherence monitoring is clearly emphasized in a recent systematic review,4 in both routine clinical practice and research.5 However, adherence behaviours are diverse and heterogeneous, so their concrete and objective determination is difficult.6
A complex combination of therapeutic interventions is often used for the proper management of chronic pain, but pharmacological interventions remain the cornerstone of its treatment,7 with, e.g., over 76% of the Portuguese population suffering from chronic pain using pharmacotherapy.8 Despite the effectiveness of pain medicines, recent data point to about 40% of the prescription medicines not taken as prescribed.4 There are many possible factors related to pharmacotherapy that may influence non-adherence, such as the complexity of the therapeutic regimen, lack of efficacy, side effects, duration of treatment, concomitant treatment regimens, various changes in prescriptions during treatment, immediate availability of the health-care professionals and the associated economic costs.1 Therefore, non-adherence to medication is conceptualized giving the reported taxonomy as “the process by which patients do not take their medications as prescribed”9 and it can occur in three interrelated distinct phases (initiation, implementation, and persistence) according to the ESPACOMP Medication Adherence Reporting Guideline (EMERGE).10 Moreover, there is a consensual definition in the literature of non-adherence according to the intentional and unintentional dimensions of the decision-making process,11,12 which can co-occur or overlap,11,13 leading to the underutilization or overuse of prescribed medication. Hence, non-adherence is often a hidden problem.14 Health behaviours are complex and result of multiple factors and their intricate and unpredictable interactions. Each experience is based on a meaning build up from varied and different perspectives or representations. Therefore, it seems to be worthwhile understanding the main reasons that influence the decision-making process when patients are non-adherent to their pain treatment.14,15 In this conceptual framework, and since there is still very limited evidence regarding the importance of therapeutic adherence in pain management,4 the present study aimed to describe adherence patterns and understand non-adherence from pain patient’s during 1 year of pharmacologic pain treatment; and investigate if there is any link between specific drugs prescribed for chronic pain management and perceived reasons for non-adherence.
Methods
Procedures and Participants
This is a prospective, observational and longitudinal study that included 950 consecutive patients, referred to a first consultation in one of the five pain clinics in Porto metropolitan area. A standardized protocol was followed that included a first face-to-face interview performed by two trained health psychologists’ and with the attending physician and nurse collaboration; and three-time specific telephone interviews – 7 days (T7d), 6 months (T6m) and 12 months (T12m) after baseline interview (T0). A total of 562 patients that have been on chronic pain treatment throughout 1 year responded to all assessments throughout the follow-up and were included in the therapeutic adherence analysis. During the follow-ups, a total of 133 patients died and 20 refused to participate in the study (Figure 1.)
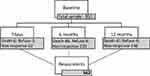 |
Figure 1 Flowchart of the study design and follow-up times. |
The size of the sample ensures the possibility of subgroup analysis and the maintenance of an acceptable power level in the hypothesis test, according to formal sample size calculations performed in the initial study planning. Considering the primary outcome variable of the study, the sample size (700–750) was calculated in order to ensure proportional estimation with a margin of error of 3.5% and a 95% confidence level. The possibility of estimating association measures in the form of odds ratio (OR) of magnitude equal to or greater than 2, with a confidence level of 95% and a power of 80%, was also taken into account, assuming a frequency of the relevant events of 5%.
The exclusion criteria were the inability to communicate in Portuguese language and the presence of psychiatric and cognitive disorders precluding the interviews. The study was carried out in accordance with the applicable laws and regulations, namely the authorization of the National Data Protection Committee and the authorization of each Ethic Committee of all hospitals involved (Centro Hospitalar do Porto – Hospital de Santo António; Centro Hospitalar de São João, E.P.E. - Hospital de São João; Centro Hospitalar Vila Nova de Gaia/Espinho - Hospital Eduardo Santos Silva; Instituto Português de Oncologia do Porto, E.P.E.; e Unidade Local de Saúde de Matosinhos, E.P.E. - Hospital Pedro Hispano). The study was carried out by researchers of the Faculty of Medicine of the University of Porto and the National Observatory for Pain – NOPain (Centro Nacional de Observação em Dor – OBSERVDOR), and taking into consideration the number of institutions and professionals involved in data collection, measures for quality promotion were implemented and professional–patient privilege was ensured formally. All patients were informed about the study objectives and all selection and data collection procedures; all their questions regarding the study were properly answered; and finally, they were invited to sign an informed consent form, which explicitly included authorization for the telephone contacts after the first interview.
Data on pharmacological prescription were obtained and recorded regarding the generic names of the drugs only. Medicines were grouped according to WHO’s Anatomical Therapeutic Chemical Classification System (ACT). Safety data, namely on adverse events, were collected, and they were also reported by the attending physicians to the appropriate national pharmaco-surveillance authorities.
Data Collection
We aimed to use a mixed methods approach, including quantitative and qualitative analyses; thus, data were collected using questionnaires scored and ranged according to their specific scales and patient’s open responses using qualitative research principles of Content Analysis.16 At baseline (T0) data collection consisted of clinical questions to be completed by the attending physician and an interview to participant patients that included: a sociodemographic questionnaire; Portuguese versions of validated questionnaires to assess pain, anxiety, depressive symptoms and quality of life (see below). The same measures were repeated after 7 days (T7d), 6 months (T6m) and 12 months (T12m), and a structured questionnaire to evaluate treatment prescriptions was added and repeated along the T7d to T12m. Given the lack of research in the field of non-adherence to pharmacotherapy in chronic pain, a qualitative methodology was used to explore this topic. Accordingly, open-end questions concerning the reasons for non-adherence were asked, aiming to identify key relationships to enhance our understanding of the non-adherence behaviour. Moreover, the research team felt the need to deepen the theme of non-adherence, by listening to patients individually, analysing their narratives to better understand the reasons for non-adherence to each ACT group medication, and capture the individual perspective.
Instruments
Clinical Questionnaire
Data about diagnosis and co-morbidities, pain history, body mass index (BMI), pharmacologic prescriptions and non-pharmacologic interventions were obtained using a structured clinical questionnaire, with the physician collaboration. Pain diagnosis was classified according to the recent Chronic Pain Syndromes Classification by IASP Taskforce on Pain Classification for ICD-11.17
Sociodemographic Questionnaire
Information about sex, age, marital status, household, education level, professional situation, job impairment was gathered using this questionnaire.
Brief Pain Inventory – BPI18
The BPI is a simple and short questionnaire composed of 15 items aiming to assess two scales: pain intensity and pain interference. The pain intensity scale contains 4 pain intensity items of maximum, minimum, on average, and right now, measured with an 11-point numeric rating scale, ranging from 0 (no pain) to 10 (the worst pain possible). The pain interference scale is composed by 7 items of patient’s pain-related interference regarding general activities, mood, walking ability, normal work, relations with other people, sleep and enjoyment of life, measured also with an 11-point scale, ranging from 0 (no interference) to 10 (extreme interference). BPI is translated in 10 different languages, including a Portuguese version,18 and has been shown to have excellent psychometric properties. Therefore, it is an instrument recommended for clinical and epidemiological research and highly consensual on the guidelines for pain assessment.19
Hospital Anxiety and Depression Scale20
HADS, used at baseline, comprises two subscales, one for measuring anxiety and other for depression, with seven items each, using a four-point (0–3) response category. On both scales, a score bellow or equal to 7 is interpreted as normal; between 8 and 10 is borderline; and equal or above 11 corresponds to anxiety and depression symptomology. At time 7 days, time 6 months and time 12 months, depressive and anxiety state were assessed by asking one question for each state in a Likert scale between 0 =never feeling anxious and depressive to 5=always feeling, anxious and depressive in the last month.
Medication Adherence Rating Scale
(©Professor Rob Horne) – MARS evaluates non-adherence in a non-threating way, where questions are posed as a negative statement to minimize social desirability bias.21 Responses are recorded in a 5-point Likert scale, ranging from 1 (always) to 5 (never) and only one item (9) is inverted. Higher scores indicate higher adherence. The Portuguese version of scale – MARS-P9 – has been shown good psychometric properties and to be an adequate instrument for Portuguese researchers to assess the adherence patterns during the management of chronic pain.22 MARS-P9 contains eight items assessing intentional aspects of non-adherence (e.g., “Only use medication when feeling pain”) and one assessing unintentional nonadherence (e.g., “Forget to use medication”).22
Structured Questionnaire to Assess Treatment
With direct questions and open-end questions. Direct questions evaluate ongoing pharmacotherapy, the effectiveness of treatment and non-adherence. Open-end questions evaluate the perceived reasons for non-adherence.
Data Analysis
We used a mixed methods approach, including quantitative and qualitative analyses, described in more detail in the following paragraphs.
A descriptive analysis of the overall characteristics of the sample was performed. Categorical variables were described as absolute (n) and relative (%) frequencies; mean and standard deviation (SD) summarized continuous variables. Non-adherence was defined as an affirmative answer to one or two of the following direct questions: “Is there any medicine that you have decided not to take?” and “Is there any medicine that you have decided not to take as prescribed?”. To compare the two groups (adherents and non-adherents) on the variables under analysis, Chi-square, Mann–Whitney and T-test statistical tests were performed at each assessment moment, according to the variables’ distribution.
In order to understand more deeply the reasons for non-adherence, qualitative and statistical analyses were performed. The ongoing pharmacotherapy was recorded and each medicine which was not taken or not taken as prescribed was grouped according the Anatomical Therapeutic Chemical Classification System (ATC), which has resulted in seven ATC groups: Narcotic Analgesics (Opioids), Antiepileptics and anticonvulsants, Antidepressants, Analgesics antipyretics, Topical non-steroidal anti-inflammatory drugs, Non-steroidal anti-inflammatory drugs and Other medication. An open-end question was used to record specific reasons for non-adherence and each reason of non-adherence for each ACT was transcribed verbatim – “What is the reason/why did you decide not to take (ACT specific) or … not to take it as prescribed?”. Records from the open-end questions were analysed using categorization analysis, which implies a data reduction technique by means of coding and thematic organization from Content Analysis.16 It consists of determining underlying reasons behind the data to clarify the voice of the patients. The researchers used predefined categories based on the empirical knowledge about the main reasons for non-adhere to medications1,4,14 to obtain additional interpretive possibilities that go beyond the statistical inferences. At the end of this process, the registration units and content units were identified and reduced to manageable representations as “Non-adherence reasons”. This information is presented and structured below in section 3.4. To ensure the robustness and credibility of analysis, a peer review involving data analysis by two experts was undertaken.
Finally, to capture the associations between each ACT classification and each “Non-adherence reasons”, a separate Chi-square analysis was performed.
Data were analysed using the IBM SPSS Statistics version 24.0. Results were considered to be significant for p-values <0.05.
Results
Sociodemographic Characterization
Table 1 presents the sociodemographic characteristics of the 562 patients included, 69% of which were women. The mean age was 58.6 (sd=14.2), 41% lived with their husband/wife and 31% with their children. Most patients completed four or fewer years of education (50%) and only 12% completed secondary education level. Approximately 45% of the patients were retired, 30% had a full-time job, 12% were unemployed and 53% revealed difficulties and limitations dealing with their daily job. The majority reported having family support (77%).
 |
Table 1 Sociodemographic Characterization of the Patients (n=562) |
Clinical Characterization
Table 2 shows the clinical characterization of the patients. According to the Chronic Pain Syndromes Classification by IASP,17 the distribution of diagnostic categories was musculoskeletal (55%), neuropathic (22%), chronic primary pain (7%), post-surgical or post-traumatic (13.0%), visceral (3.0%), oncologic (17%) and headache and orofacial (1%). Most patients presented a single pain diagnostic category (81%).
 |
Table 2 Clinical Characterization of the Patients (n=562) |
Evolution of the Adherence Pattern and Differences Between Adherents and Non-Adherents During 1 Year of Chronic Pain Treatment
Figure 2 illustrates the adherence behaviour patterns during 1 year of chronic pain treatment. At T7d, almost 37% of the patients were non-adherent, i.e., responded “yes” to one or the two direct questions: “Is there any medicine that you have decided not to take?” and “Is there any medicine that you have decided not to take as prescribed?”. This percentage increased significantly to 47% at 6 months (T6m) and to 51% after 1-year treatment (T12m).
 |
Figure 2 Evolution of the non-adherence along 1-year treatment (n=562). |
Table 3 presents the results for the MARS scale, revealing significant statistical differences between adherents and non-adherents on self-rated adherence in each of the three assessment moments. Significant differences were also observed between adherents and non-adherents for the intentional component of non-adherence at all-time points, while for the unintentional non-adherence a significant statistical result was observed only at T7d (Table 3).
 |
Table 3 Comparison Between Adherent and Non-Adherent Patients, Concerning Self-Rated Adherence (MARS), Emotional State (HADS) and Pain (BPI) at the Time Points Evaluated |
Regarding the pain severity and interference measured by the BPI questionnaire, there were no statistically significant differences between adherents and non-adherents in any of the three assessment moments (Table 3).
Depressive and anxiety symptoms with clinical meaning (with HADS scores >11) occurred in between 38% and 50% of the patients in both groups. At T7d, depressive symptoms were more frequent in non-adherents than in adherents (51% vs 41%, p=0.011), but no other significant differences were observed concerning anxiety and depressive symptoms (Table 3).
Content Analysis
Thirteen “Non-adherence reasons” units emerged, namely: low necessity; high concerns; side effects; poor communication between health-care professionals (HCP) and patients; difficulties managing medication; non-efficacy; financial constraints; pharmacy issues; change in prescriptions by other HCP/because of a new clinical condition; delayed start; increasing dose or adding other medicine; finished medicine; not otherwise specified.
Low necessity refers to intentional reasons that reveal perceptions of non-necessity to take the drug or lowering the dose of the prescription. High concerns, another intentional reason, includes affirmations of non-taking the medication because of previous concerns about possible side effects, possible interactions, or other preoccupations revealed by the patient, despite the inexistence of previous personal experience with the adverse event. On the contrary, side effects refer to real and objective existing experiences perceived by the patient as side effects due to a specific drug, with or without an HCP confirmation. Poor communication between HCP and patients includes affirmations of unfamiliarity about any aspect concerning the prescription. Difficulties managing medication relates to not being aware about how to take the medicines or difficulties including them on their daily routine. Non-efficacy is an intentional reason related with affirmations of not taking the medicines because of the perception of absence of effect. Financial constraints reveal patients not buying medication justified with financial reasons. Pharmacy issues refer to mistakes or unavailability of medicines at the pharmacy, which resulted in the patient not obtaining the medicine. Change in prescriptions by other HCP/because of a new clinical condition refers to an objective alteration on the prescription by other HCP. Delayed start refers to an intentional reason revealed by the patient about not acquiring the medicine and a waiting intention immediately after the prescription. Increasing dose or adding other medicine refers to an intentional reason of increasing a dose not prescribed or including another medicine also not prescribed. Finished medicine refers to the fact that the medicine as prescribed has finished and the patient do not intend to acquire another refill or another alternative medicine. Finally, not otherwise specified refers to affirmations which are uncertain or objectively “not knowing” responses. The percentages of each of the “non-adherence reasons” with respect to ATC groups are presented in Figure 3.
Associations Between “Non-Adherence Reasons” and ATC Groups
Figure 3 presents the significant associations between the main “non-adherence reasons” and ATC groups. Perceived side effects (p= 0.019) and delayed start (p=0.022) are significantly associated with intentional non-adherence to Narcotic Analgesics (Opioids); the perceived non-efficacy (p= 0.017) and delayed start (p= 0.004) are significantly associated with intentional non-adherence to Antiepileptics and anticonvulsants; the perceived low necessity (p= 0.041) and delayed start (p= 0.036) are significantly associated with intentional non-adherence to Analgesics antipyretics; change in prescriptions by other HCP/because of a new clinical condition is significantly associated with unintentional non-adherence to Antidepressants (p= 0.024); high concerns (p= 0.045) and change in prescriptions by other HCP/because of a new clinical condition concerns (p<0.001) are significantly associated with intentional and unintentional non-adherence, respectively, to Non-steroidal anti-inflammatory drugs; finally, delayed start (p= 0.016) and financial constraints (p= 0.018) are significantly associated with intentional non-adherence to Other Medications.
Discussion
In this study, new potential ways of looking at the problem of non-adherence to medication in chronic pain were undertaken.8,23,24 The last decade reflected an increased interest on medication non-adherence.4,9 Notwithstanding, the operational definition of non-adherence remains unclear25,26 and often conceptualized as a fixed behavioural construct assessed by unstandardized methods.5,9,27 To help overcoming this gap, the authors conceptualized the adherence as a dynamic process (initiation, implementation and persistence) that needs to be monitored longitudinally, as proposed by the European consensus on adherence taxonomy and terminology9 and by European Society for Patient Adherence, COMpliance, and Persistence (ESPACOM).10 Pursuing this, non-adherence behaviour to a specific drug reflects an intentional or unintentional behaviour (or both) of no drug usage, miss dose usage or over usage. It is of particular interest to emphasize Butow and Sharpe’s statement that non-adherence should be understandable within the patient’s world view in a non-judgmental approach.28 Following this, these authors encourage studies to document reasons for non-adherence and recommend consultations that consider patient’s views to improve adherence.28 Moreover, the importance of understanding adherence to multiple complex regimens, differentially and in daily life, in patients with multimorbidity was recently emphasized.10
Direct and dichotomous (yes/no) questions about adherence were posed to those who decide what to do with each one of the prescription(s) received – the patients. Also, a specific adapted non-adherence questionnaire, the MARS-P9, was used to obtain standardized results for non-adherence behaviour,22 but not as a unique measure of non-adherence because there is the possibility of underestimation of this behaviour.14 Therefore, the authors advocate a multimethod approach to measure the adherence behaviour, in the absence of a consensus rate of enough adherence to prevent poor outcomes in chronic pain.7 This is based on directly questioning the patients and self-report measurement, since when a person is stating his/her own non-adherence behaviour he/she is usually telling the truth.29 At the same time, and although toxicological screenings give objective information on medication intake comparable with the most frequently used self-reported measurements, they fail at giving information about behavioural change patterns over long time periods.25
Our results confirmed that non-adherence to medication is common in chronic pain patients,1,25 underuse being reported to be higher than overuse (mean of 29.9% against 13.7%).4 Contrary to the conception that mainly asymptomatic conditions have a negative impact on adherence,26 symptomatic conditions (like chronic pain) have also rates of non-adherence around 50%.30 Following the same pattern, adherence remains an ongoing problem in other health conditions that can affect clinical outcomes, in both symptomatic and asymptomatic conditions. A good example are the rates of 7-59% non-adherence to oral medication in type 1 diabetes, and 62-64% for insulin non-adherence, among type 2 diabetes.31 Likewise, hypertension and diabetes coexist with reported non-adherence rates to antihypertensive therapy among diabetes patients linked to cardiovascular morbidity and mortality,32 confirming the wide range of the problem of non-adherence.
In our cohort, non-adherence was about 37% after 7 days’ treatment and increased during the 12-month evaluation period to over 50%, compromising the persistence with treatments as it has been reported.1,33 In spite of the high MARS-P9 scores on general adherence, MARS-P9 showed differences between the two groups, adherents and non-adherents, during the follow-up period of 1 year. MARS-P9 scale has also distinguished adherents from non-adherents in what concerns intentional non-adherence, but did not differentiate the unintentional non-adherence between the two groups. This is partially due to the fact, as observed in other studies,21,34 MARS-P9 only assesses unintentional non-adherence with one item that reflects forgetfulness. This may be an important non-adherence reason in asymptomatic diseases but not so important in a symptomatic condition such as pain.
Concerning outcome variables, pain intensity and interference were not related with the adherence behaviour, consistent with Broekmans et al meta-analysis.25 These authors also emphasized the limited efficacy of pain medicines to treat persistent pain, which compromises by itself adherence.25 Interestingly, non-adherents presented a higher percentage of clinical depressive symptoms compared with adherents at t7d, but no other significant differences between the two adherence pattern groups were observed concerning anxiety and depressive symptoms at T6m and T12m. Probably, if pain and depression would be seen as an interactive nature and not as separated dimensions,35 the differences between the two groups would be clearer.
The association between general beliefs about medicines and adherence has been difficult to establish. Accordingly, the importance of encouraging patients to express their personal views about medicines as a basis for an enhanced and personalized information process has been emphasized.36 To our knowledge, this is the first study describing adherence patterns and evaluating non-adherence from the perspective of chronic pain patients during 1-year of pharmacologic pain treatment. Moreover, research concerning the persistence with medication is limited so far26 and reflects a huge problem across multiple chronic conditions. The qualitative analysis of the perceptions and beliefs applied in this study allowed the understanding of the patients interpretations on how they think and cope with their pain medication treatment.16 Pre-categorization facilitates the alignment of the open-ended research questions and their interpretation. This extensive work analysis resulted in 13 categories that may complement and provide a more useful conceptual framework for understanding the non-adherence behaviour during chronic pain management. This is consistent with the increasing recognition of the importance of concerns and beliefs regarding medication and its association with non-adherence behaviour, beyond side effects.7 Indeed, of the 13 “Non-adherence reasons” identified, only two were clearly unintentional, namely pharmacy issues and change in prescriptions by other HCP/because of a new clinical condition, confirming the predominance of intentionality in non-adherence.12,37 Although being advocated as one of the major contributors to non-adherence to chronic disease medication,13,38 forgetfulness did not arise from the content analysis as a reason for non-adhere to chronic pain medication. Indeed, forgetfulness has not been considered to date a determinant for medication non-adherence in chronic pain patients,4 which may be related with the persistent presence of the unpleasant symptom.
The second novelty introduced by the present study was the establishment of significant associations between qualitatively categorized reasons for non-adherence and specific ACT drug groups prescribed for chronic pain management. While the inexistence of previous studies does not allow documenting the associations obtained, they could contribute to target specific concerns and barriers which may facilitate adherence, even if only indirectly. In this context, it is of notice that patients tend to justify non-adherence to Narcotic Analgesics (Opioids) with concerns about their side effects, which probably justifies a delayed start. The Portuguese reality about opioids consumption and abuse is far from what happens in other European countries and the USA, with only 4.37% of chronic pain patients reporting opioids utilization, and less than 1% using strong opioids.39 In this scenario, increase in dose did not come up as a non-adherence reason, neither for opioids nor for other pharmacological groups. Regarding Analgesics antipyretics, the belief about non-efficacy emerged as the major reason for not being adherent. Probably, the immediate availability of this medication and a general consumption without medical surveillance leads to a disbelief about their real effectiveness. In fact, in a study conducted with Taiwanese patients, answering questions about analgesics resulted in a decrease of barriers to analgesic use and an increase of adherence.40 Concerning antidepressants, there are an increasing number of new classes for chronic pain treatment. However, evidence from the best clinical profile and effectiveness is still scarce,41 and the highly variable nature of responses to all drugs42 could justify the prescription changes in this pharmacological group by HCP. Another interesting result reveals that chronic pain patients rationalise their non-adherence to Antiepileptics and anticonvulsants with non-efficacy reasons, concurrent with the conclusion of a small benefit over placebo in reducing pain and sleep problems, in a recent systematic review.43 Although side effects were not associated with the use of Non-steroidal anti-inflammatory drugs, in the present study chronic pain patients revealed high concerns about their consumption due to their awareness of the possible side effects of these drugs. Maybe the appalling equation “less pain–more deaths/morbidity”44 reflects this belief. Finally, it is a matter of concern if chronic pain patients are neglecting treating comorbidities by choosing their medication, and justifying their non-adherence to other medicines (not for chronic pain treatment) with financial constraints and delaying the treatment initiation.
This study must be interpreted taking into account its main limitations. First, although using a large sample and a real-world perspective, the socio-demographic characteristics have to be taken into account, namely the low literacy level and older ages. It is worth to mention that a medium of 110 patients did not complete the follow-up assessments which may underestimate the prevalence of non-adherence. Also, the authors did not distinguish non-oncologic versus oncologic pain in terms of adherence, as there is no evidence about factors predicting adherence differently in each chronic pain patient group.45 In addition, patients were recruited and followed in Specialized Chronic Pain Clinics, and this certainly precludes the generalization of results to primary health care and the general population. Fifth, patients are prescribed with a heterogeneous set of medications in terms of Pharmacologic-Therapeutic Classification. Moreover, we focused exclusively on drugs, as our previous study revealed that only 17.2% of patients were not prescribed with pain medicines.46
Our study has several strengths also worth mentioning. It was a “real-world” observational prospective study regarding therapeutic adherence to chronic pain medication, with 12-months follow-up period and a large sample. With this research, we describe a simple self-report measure in clinical practice, together with questions about objective patterns of treatment use and reasons for non-compliance, to provide reliable information about non-adherence patterns from the patient perspective during chronic pain treatment.
The present research contributes to the increasing evidence of the importance of the patients’ reasons, including their beliefs and concerns, in non-adherence to pharmacological treatment. In addition, the main reasons for non-adherence underlined by the patients’ voice47 were related with specific the ACT classification of pain medicines. Therefore, the authors believe that identifying patients at risk of non-adherence to specific pain medicines, based on patients’ beliefs, could give reliable information to develop new target interventions to prevent and reduce non-adherence behaviours.
Acknowledgments
Rute Sampaio work was financed by Project NORTE-01-0145-FEDER-000008 (Porto Neurosciences and Neurologic Disease Research Initiative at I3S) that is financed by the North Portugal Regional Operational Programme (NORTE 2020), under the PORTUGAL 2020 Partnership Agreement, and through the European Regional Development Fund (ERDF). The work of Luís Filipe Azevedo and Cláudia Camila Dias was partially financed by the Project “NORTE-01-0145-FEDER-000016” (NanoSTIMA), also financed by the North Portugal Regional Operational Programme (NORTE 2020), under the PORTUGAL 2020 Partnership Agreement, and through the European Regional Development Fund (ERDF).
Disclosure
The authors have no conflicts of interest to disclose in this work.
References
1. World Health Organization. Adherence to Long-Term Therapies. Evidence for Action. Geneva, Switzerland: World Health Organization. 199; 2003.
2. Sampaio R, Pereira MG, Winck JC. A new characterization of adherence patterns to auto-adjusting positive airway pressure in severe obstructive sleep apnea syndrome: clinical and psychological determinants. Sleep Breath. 2013;17(4):1145–1158. doi:10.1007/s11325-013-0814-7
3. Dunbar-Jacob J, Mortimer-Stephens MK. Treatment adherence in chronic disease. J Clin Epidemiol. 2001;54(Suppl 1):S57–60. doi:10.1016/S0895-4356(01)00457-7
4. Timmerman L, Stronks DL, Groeneweg JG, Huygen FJ. Prevalence and determinants of medication non-adherence in chronic pain patients: a systematic review. Acta Anaesthesiol Scand. 2016;60(4):416–431.
5. Lehmann A, Aslani P, Ahmed R, et al. Assessing medication adherence: options to consider. Int J Clin Pharm. 2014;36(1):55–69. doi:10.1007/s11096-013-9865-x
6. Bond WS, Hussar DA. Detection methods and strategies for improving medication compliance. Am J Hosp Pharm. 1991;48(9):1978–1988.
7. Rosser BA, McCracken LM, Velleman SC, et al. Concerns about medication and medication adherence in patients with chronic pain recruited from general practice. Pain. 2011;152(5):1201–1205. doi:10.1016/j.pain.2011.01.053
8. Azevedo LF, Costa-Pereira A, Mendonça L, et al. Epidemiology of chronic pain: a population-based nationwide study on its prevalence, characteristics and associated disability in Portugal. Med Care. 2013;51(10):859–869. doi:10.1097/MLR.0b013e3182a53e4e
9. Vrijens B, De Geest S, Hughes DA, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012;73(5):691–705. doi:10.1111/bcp.2012.73.issue-5
10. De Geest S, Zullig LL, Dunbar-Jacob J, et al. ESPACOMP Medication Adherence Reporting Guideline (EMERGE). Ann Intern Med. 2018;169(1):30–35. doi:10.7326/M18-0543
11. Lehane E, McCarthy G. Intentional and unintentional medication non-adherence: a comprehensive framework for clinical research and practice? A discussion paper. Int J Nurs Stud. 2007;44(8):1468–1477. doi:10.1016/j.ijnurstu.2006.07.010
12. Mukhtar O, Weinman J, Jackson SH. Intentional non-adherence to medications by older adults. Drugs Aging. 2014;31(3):149–157. doi:10.1007/s40266-014-0153-9
13. Gadkari AS, McHorney CA. Unintentional non-adherence to chronic prescription medications: how unintentional is it really? BMC Health Serv Res. 2012;12:98. doi:10.1186/1472-6963-12-98
14. Horne R, Chapman SCE, Parham R, et al. Understanding patients’ adherence-related beliefs about medicines prescribed for long-term conditions: a meta-analytic review of the Necessity-Concerns Framework. PLoS One. 2013;8(12):e80633. doi:10.1371/journal.pone.0080633
15. Leventhal H, Zimmerman R, Gutmann M. Compliance: a self-regulation perspective. In: Gentry D, editor. Handbook of Behaviour Medicine. New York: Pergamon Press; 1984:369–434.
16. Bardin L. Análise De Conteúdo. Lisboa: Edic¸ões; 1977:70.
17. International Association for the study of Pain. Revision of the International Classification of Diseases (ICD-11). [cited 2017]; Available from: http://www.iasp-pain.org/Advocacy/icd.aspx?ItemNumber=5234&navItemNumber=5236.
18. Azevedo LF, Dias C, Agualusa L, Lemos L, Romão J. Translation, cultural adaptation and multicentric validation study of chronic pain screening and impact assessment instruments [Tradução, adaptação cultural e estudo multicêntrico de validação de instrumentos para rastreio e avaliação do impacto da dor crónica]. Dor. 2007;15(4):6–56.
19. Caraceni A, Cherny N, Fainsinger R, et al. Pain measurement tools and methods in clinical research in palliative care: recommendations of an Expert Working Group of the European Association of Palliative Care. J Pain Symptom Manage. 2002;23(3):239–255. doi:10.1016/S0885-3924(01)00409-2
20. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi:10.1111/acp.1983.67.issue-6
21. Mora PA, Berkowitz A, Contrada RJ, et al. Factor structure and longitudinal invariance of the medical adherence report scale-asthma. Psychol Health. 2011;26(6):713–727. doi:10.1080/08870446.2010.490585
22. Sampaio R, Azevedo LF, Dias CC, Horne R, Castro Lopes JM. Portuguese version of the Medication Adherence Report Scale (MARS-9): validation in a population of chronic pain patients. J Eval Clin Pract. 2019;25(2):346–352.
23. Azevedo LF, Costa-Pereira A, Mendonça L, et al. Epidemiology of chronic pain: a population-based nationwide study on its prevalence, characteristics and associated disability in Portugal. J Pain. 2012;13(8):773–783. doi:10.1016/j.jpain.2012.05.012
24. MacPherson RD. New directions in pain management. Drugs Today (Barc). 2002;38(2):135–145. doi:10.1358/dot.2002.38.2.668325
25. Broekmans S, Dobbels F, Milisen K, et al. Medication adherence in patients with chronic non-malignant pain: is there a problem? Eur J Pain. 2009;13(2):115–123. doi:10.1016/j.ejpain.2008.02.010
26. Kardas P, Lewek P, Matyjaszczyk M. Determinants of patient adherence: a review of systematic reviews. Front Pharmacol. 2013;4:91. doi:10.3389/fphar.2013.00091
27. Alsalman AJ, Smith WR. Expanding the framework of assessing adherence and medication-taking behavior. J Pain Palliat Care Pharmacother. 2013;27(2):114–124. doi:10.3109/15360288.2013.765532
28. Butow P, Sharpe L. The impact of communication on adherence in pain management. Pain. 2013;154(Suppl 1):S101–7. doi:10.1016/j.pain.2013.07.048
29. Cramer J, Mattson R. Monitoring compliance with antiepileptic drug therapy,In: Cramer J, Spilker B, editors. Patient Compliance in Medical Practice and Clinical Trials. New York: Raven Press; 1991.
30. Haynes RB, McKibbon KA, Kanani R. Systematic review of randomised trials of interventions to assist patients to follow prescriptions for medications. Lancet. 1996;348(9024):383–386. doi:10.1016/S0140-6736(96)01073-2
31. Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care. 2004;27(5):1218–1224. doi:10.2337/diacare.27.5.1218
32. Abughosh SM, Wang X, Serna O, et al. A pharmacist telephone intervention to identify adherence barriers and improve adherence among nonadherent patients with comorbid hypertension and diabetes in a medicare advantage plan. J Manag Care Spec Pharm. 2016;22(1):63–73. doi:10.18553/jmcp.2016.22.1.63
33. Benner JS, Glynn RJ, Mogun H, et al. Long-term persistence in use of statin therapy in elderly patients. JAMA. 2002;288(4):455–461. doi:10.1001/jama.288.4.455
34. Salt E, Hall L, Peden AR, et al. Psychometric properties of three medication adherence scales in patients with rheumatoid arthritis. J Nurs Meas. 2012;20(1):59–72. doi:10.1891/1061-3749.20.1.59
35. Woo AKM. Depression and anxiety in pain. Reviews in Pain. 2010;4(1):8–12. doi:10.1177/204946371000400103
36. Mardby AC, Akerlind I, Jorgensen T. Beliefs about medicines and self-reported adherence among pharmacy clients. Patient Educ Couns. 2007;69(1–3):158–164. doi:10.1016/j.pec.2007.08.011
37. Clifford S, Barber N, Horne R. Understanding different beliefs held by adherers, unintentional nonadherers, and intentional nonadherers: application of the necessity-concerns framework. J Psychosom Res. 2008;64(1):41–46. doi:10.1016/j.jpsychores.2007.05.004
38. Choudhry NK, Krumme AA, Ercole PM, et al. Effect of reminder devices on medication adherence: the REMIND randomized clinical trial. JAMA Intern Med. 2017;177(5):624–631.
39. Azevedo LF, Costa-Pereira A, Mendonça L, et al. A population-based study on chronic pain and the use of opioids in Portugal. PAIN®. 2013;154(12):2844–2852. doi:10.1016/j.pain.2013.08.022
40. Lin CC, Chou P-L, Wu S-L, et al. Long-term effectiveness of a patient and family pain education program on overcoming barriers to management of cancer pain. Pain. 2006;122(3):271–281. doi:10.1016/j.pain.2006.01.039
41. Mika J, Zychowska M, Makuch W, Rojewska E, Przewlocka B. Neuronal and immunological basis of action of antidepressants in chronic pain - clinical and experimental studies. Pharmacol Rep. 2013;65(6):1611–1621. doi:10.1016/S1734-1140(13)71522-6
42. Trouvin AP, Perrot S, Lloret-Linares C. Efficacy of venlafaxine in neuropathic pain: a narrative review of optimized treatment. Clin Ther. 2017;39(6):1104–1122. doi:10.1016/j.clinthera.2017.05.347
43. Uceyler N, Sommer C, Walitt B, Häuser W. WITHDRAWN: anticonvulsants for fibromyalgia. Cochrane Database Syst Rev. 2017;10:Cd010782.
44. Wehling M. Non-steroidal anti-inflammatory drug use in chronic pain conditions with special emphasis on the elderly and patients with relevant comorbidities: management and mitigation of risks and adverse effects. Eur J Clin Pharmacol. 2014;70(10):1159–1172. doi:10.1007/s00228-014-1734-6
45. Millan-Millan MJ, Reinoso-Barbero F, Díaz-Miguel MP, et al. [Clinical characteristics of children with chronic pain in a pediatric pain unit: oncologic pain versus non-oncologic pain]. An Pediatr (Barc). 2003;58(4):296–301. doi:10.1016/s1695-4033(03)78061-8
46. Gouvinhas C, Veiga D, Mendonça L, et al. Interventional pain management in multidisciplinary chronic pain clinics: a prospective multicenter cohort study with one-year follow-up. Pain Res Treat. 2017;2017:8402413.
47. Basch E. The missing voice of patients in drug-safety reporting. N Engl J Med. 2010;362(10):865–869. doi:10.1056/NEJMp0911494
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

