Back to Journals » International Journal of General Medicine » Volume 14
Nomogram for Predicting Overall Survival in Acral Lentiginous Melanoma: A Population‐based Study
Authors Yin T, Zhao Y , Yang Y, Xu H, Zheng D, Lyu J , Fu G
Received 27 August 2021
Accepted for publication 1 December 2021
Published 16 December 2021 Volume 2021:14 Pages 9841—9851
DOI https://doi.org/10.2147/IJGM.S336443
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Tingting Yin,1,* Yuhui Zhao,1,* Ying Yang,1 Huaxiu Xu,1 Dongxiang Zheng,2 Jun Lyu,3 Guanglei Fu4
1School of Nursing, Jinan University, Guangzhou, People’s Republic of China; 2Department of Neurology, The First Affiliated Hospital of Jinan University, Guangzhou, People’s Republic of China; 3Clinical Research Department, The First Affiliated Hospital of Jinan University, Guangzhou, People’s Republic of China; 4Infectious Disease Department, The First Affiliated Hospital of Jinan University, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jun Lyu
Clinical Research Department, The First Affiliated Hospital of Jinan University, Guangzhou, People’s Republic of China
Tel +86 13922274169
Email [email protected]
Guanglei Fu
Infectious Disease Department, The First Affiliated Hospital of Jinan University, Guangzhou, People’s Republic of China
Tel +86 18922217029
Email [email protected]
Background: The objective of this study was to establish a nomogram for predicting the overall survival (OS) of patients with acral lentiginous melanoma (ALM).
Materials and Methods: The study sample was selected from 1785 patients diagnosed with ALM from 2004 to 2015 in the Surveillance, Epidemiology, and End Results (SEER) database, and R software was used to divide patients into the training cohort and validation cohort at a ratio of 7: 3. Stepwise selection method in the Cox regression model was used in the training cohort to select predictive variables to construct the nomogram, and model validation parameters were used in the validation cohort to evaluate the performance of the nomogram.
Results: The nomogram showed that age at diagnosis had the greatest impact on OS in patients with ALM, followed by AJCC stage, surgical treatment, SEER stage, sex, race, and marital status. The index of concordance, area under the receiver operating characteristic curve, calibration plots, net reclassification improvement, integrated discrimination improvement, and decision curve analysis demonstrate the good performance of this nomogram.
Conclusion: The prognostic value of the nomogram is superior to that of the AJCC staging system alone, and it helps clinicians to better predict 3-, 5-, and 8-year OS in patients with ALM.
Keywords: acral lentiginous melanoma, nomogram, overall survival, SEER
Introduction
Cutaneous melanoma (CM) is a tumor caused by malignant changes in melanocytes, mostly in the skin, but also visible in the mucous membrane and internal organs.1 Acral lentiginous melanoma (ALM) is a rare melanoma subtype.2 It is mainly found in the acral parts without hair covering such as the palms of the hands, soles of the feet, and nail bed, and it accounts for about 2%–3% of all melanomas.3–5 Although the incidence of ALM is low, the early symptoms of ALM are not obvious compared with other types of CM, which may lead to delayed medical treatment for patients or misdiagnosis by medical staff.6,7 Therefore, most patients have developed advanced cancer when ALM is diagnosed.8 In addition, some studies have shown that over the next few decades, the incidence of ALM will continue to increase, and patients with ALM may have a shorter life cycle.9 Currently, ALM remains a particularly important subtype of CM worldwide.
The American Joint Committee on Cancer (AJCC) staging manual established the criteria for the Tumor Node Metastasis (TNM) staging system, which is widely used in clinical practice to evaluate prognosis and make treatment decisions.10 However, there is evidence that patients with the same pathological grade or clinical stage may have different prognosis and ultimate survival,11 and the separate application may affect the prediction of overall survival,12 which means that although TNM staging is the golden standard for oncology prognosis, it cannot accurately and reliably predict patient survival. Another reason might be that the survey of the prognosis for patients was made from the perspective of anatomy alone, without considering other variables affecting the prognosis, such as patients’ age, gender, race, diagnosis treatment, etc. Consequently, ignoring these important potential factors may reduce the accuracy of patient survival prediction.13 Therefore, new prognostic tools need to be constructed to improve the accuracy of predicting the survival probability of patients with ALM.14,15
The SEER database is one of the largest and most representative tumor registries in North America. It has collected a large amount of data related to evidence-based medicine, covering nearly 30% of the population in the United States,16 providing systematic evidence support and valuable first-hand information for clinicians’ evidence-based practice and clinical medical research. In order to accurately and reliably predict the overall survival, researchers have raised the idea of using a prediction model of nomogram. The nomogram is based on multi-factor regression analysis. First, it integrates multiple prediction indicators and then draws them on the same plane in proportion with a graduated line segment to express the relationship between variables in the prediction model. The basic idea is that by building a multi-factor regression model, a score is assigned to each value level of each risk factor in the model according to its contribution to the outcome variable. Then, the scores can be added together to obtain the total score, and the predicted value of the individual outcome event is calculated by the function conversion relationship between the total score and the probability of the outcome event.17 Since there is no nomogram on ALM is available yet, in this study, data from the Surveillance, Epidemiology, and End Results (SEER) database were used to construct a prediction model for the 3-, 5-, and 8-year overall survival (OS) of patients with ALM, and several model validation parameters were used to verify the prediction accuracy of the nomogram.
Materials and Methods
Search Strategy
This study analyzed data obtained from the SEER database (covering 18 registries), and selected and identified eligible patients using SEER*Stat 8.3.5 (https://seer.cancer.gov/).18 The study included patients who met the ICD-O-3 (third revision of the International Classification of Diseases for Oncology) and histological code 8744/3. Patients who were diagnosed only on autopsy or death certificates, patients not diagnosed under a microscope, and patients with incomplete or unavailable case information were excluded. Of note, we used the sixth edition of the AJCC staging system, which was released in 2004, so we limited our search between 2004 and 2015. See Figure 1.
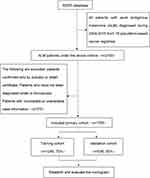 |
Figure 1 Flowchart of the selection of the studies in the meta-analysis. |
Variable Selection and Assignment
The selected variables included (1) age at diagnosis (a continuous variable), (2) gender (1 = male, 2 = female), (3) race (1 = white, 2 = black, 3 = others), (4) marital status (1 = married, 2 = unmarried, 3 = divorced/separated/widowed [DSW]), (5) AJCC stage (1 = stage I, 2 = stage II, 3 = stage III, 4 = stage IV), (6) SEER stage (1 = localized, 2 = regional, 3 = distant), (7) site (1 = head and neck, 2 = trunk, 3 = upper limb, 4 = lower limb, 5 = others), (8) laterality (1 = left, 2 = right, 3 = not a paired site), (9) surgical treatment (1 = yes [surgery was performed], 2 = no [patient died before surgery was recommended, surgery was not recommended, surgery was recommended but the patient refused]), and (10) health status (0 = life, 1 = death).
Statistical Analysis
Establishment of the Nomogram
All of the above factors were analyzed descriptively in this study. The continuous variable was expressed as median and quartile spacing, while the categorical variable was expressed as percentage. The predictive variables were selected using stepwise selection method in the Cox regression model in the training cohort,19 and the 3-, 5-, and 8-year OS rates for patients with ALM were constructed on the basis of the identified variables.
Validation of the Nomogram
The internal validation method, index of concordance (C-index), and area under the receiver operating characteristic curve (AUC) were used to evaluate the predictive capability of the model. A calibration plot was used to evaluate the consistency between the predicted probability and the actual results. Net reclassification improvement (NRI) and integrated discrimination improvement (IDI) were used to identify improvements achieved using the nomogram. Decision curve analysis (DCA) was used to evaluate the clinical validity of the predictive model.
Results
Characteristics of the Included Patients
A total of 1785 patients who met the criteria were included in the study, including 1249 in the training cohort and 536 in the validation cohort. Median age at diagnosis was 65 years in the training cohort and 66 years in the validation cohort, with a quartile interval of 54 to 76 years in both cohorts. There were slightly more female patients than male patients. The majority of patients were white, married, and AJCC stage I. More than 60% of patients were limited to local metastases. About 79% of patients had tumors located in the lower extremity. The number of patients with tumors on the left side was equal to that on the right. About 98% of patients underwent surgery. See Table 1 for details.
 |
Table 1 Baseline Characteristics of Patients with ALM |
Variable Screening
After univariate Cox regression analysis, multivariate Cox regression analysis was performed on age at diagnosis, sex, race, marital status, AJCC stage, SEER stage, and surgical treatment. The results indicate that age at diagnosis (hazard ratio [HR] = 1.044, P < 0.001), black race (HR = 1.590 vs white race, P = 0.002), unmarried (HR = 1.421 vs married, P = 0.031), DSW (HR = 1.374 vs married, P = 0.006), AJCC stage II (HR = 2.246 vs AJCC stage I, P < 0.001), AJCC stage III (HR = 4.013 vs AJCC stage I, P < 0.001), AJCC stage IV (HR = 4.988 vs AJCC stage I, P < 0.001), distant metastasis (HR = 2.351 vs localized, P = 0.006), and no surgical treatment (HR = 3.533 vs surgical treatment, P < 0.001) were risk factors for survival in patients with ALM, while female (HR = 0.579 vs male, P < 0.001) was a protective factor for survival in patients with ALM. See Table 2 for details.
 |
Table 2 Variables Selected by Multivariate Cox Regression Analysis (Training Cohort) |
Construction of the Nomogram
On the basis of the results of multivariate Cox regression analysis, we established a nomogram that ultimately included age at diagnosis, sex, race, marital status, AJCC stage, SEER stage, and surgical treatment to predict 3-, 5-, and 8-year OS for patients with ALM. As can be seen from the nomogram (Figure 2), age at diagnosis had the greatest impact on OS in patients with ALM, followed by AJCC stage, surgical status, SEER stage, sex, race, and marital status.
 |
Figure 2 Nomogram of ALM. |
Evaluation of the Nomogram
After establishing the nomogram, we used several model validation parameters to evaluate its performance. First, the C-index of the nomogram (0.772 for the training cohort and 0.755 for the validation cohort) was higher than that of the AJCC staging system (0.697 for the training cohort and 0.677 for the validation cohort). Second, the AUC values of the nomogram at 3, 5, and 8 years (0.803, 0.823, and 0.835 in the training cohort and 0.772, 0.766, and 0.814 in the validation cohort) were higher than those of the AJCC staging system at 3, 5, and 8 years (0.736, 0.739, and 0.716 in the training cohort and 0.714, 0.683, and 0.713 in the validation cohort) (Figure 3). Third, the calibration plots show that the calibration curves for 3-, 5-, and 8-year OS of the nomogram are very close to the standard 45° diagonal (Figure 4). Fourth, the NRI values at 3, 5, and 8 years were 0.524 (95% CI = 0.328–0.632), 0.616 (95% CI = 0.437–0.721), and 0.690 (95% CI = 0.576–0.835) in the training cohort and 0.479 (95% CI = 0.226–0.655), 0.602 (95% CI = 0.386–0.760), and 0.668 (95% CI = 0.372–0.867) in the validation cohort, respectively. Fifth, the 3-, 5-, and 8-year IDI rates were 0.085, 0.110, and 0.125 in the training cohort and 0.093, 0.125, and 0.138 in the validation cohort, respectively (P < 0.001). Sixth, the 3-, 5-, and 8-year DCA generated a net benefit in the training and validation cohorts, and the net benefit generated by the 3-, 5-, and 8-year DCA was higher than that of the staging system (Figure 5). Therefore, the above verification results show that this nomogram has good predictive performance.
 |
Figure 5 DCA. (A, C, and E) represent the DCA of 3-, 5-, and 8-year OS in the training cohort, while (B, D, and F) represent the DCA of 3-, 5-, and 8-year OS in the validation cohort, respectively. |
Discussion
ALM is the most common histopathologic subtype of CM in the acral area. Increasing evidence shows that ALM has poorer prognosis and higher mortality rate compared with other types of melanoma and has many unknown causes.20,21 Therefore, effective treatment plans for patients with ALM are still lacking. Nomograms are widely used in oncology to predict disease outcomes and meet the needs of clinicians to provide individualized treatment for patients.22,23 Although the AJCC staging system has a significant predictive power for the prognosis of patients with ALM, it does not include some important demographic characteristics and potential risk factors, such as age, race, marital status, and surgical treatment. Therefore, this study used the SEER database to obtain case data and developed a nomogram prediction model to predict the individualized OS of patients with ALM at 3, 5, and 8 years. In addition, this study verified the prediction model and found that it has better prediction performance. Compared with the AJCC staging system, this prediction model can provide more accurate data, so as to help medical staff predict the prognosis of diseases.
Through multivariate Cox regression analysis, this study found that age at diagnosis, sex, race, marital status, AJCC stage, SEER stage, and surgical treatment were all influential factors affecting the survival of patients with ALM. In this study, the median age at diagnosis in the training cohort and validation cohort was 65 and 66 years, respectively, and the interval between quartiles was 54–76 years old, which was similar to the results of Csányi’s study, indicating that ALM mainly affected the elderly.24 It has been confirmed that elderly melanoma patients have a distinct natural history of disease in AJCC stages I, II, and III, with higher morbidity and mortality,25 Therefore, the most likely reason for the influence of age on patient overall survival in this study was the inclusion of a large number of ALM patients with AJCC I, II, and III stage. In addition, there may be other reasons for this result, such as the decreased immune capacity of elderly patients, the increase of chronic inflammatory markers, and the elderly patients are more susceptible to more aggressive tumor invasion, which lead to the decreased survival rate.26–29 A previous study30 indicated that ALM is more common in females compared with other subtypes of melanoma. However, females increase the independent prognostic factors for OS in ALM. The conclusions are consistent with the results, showing that females are a protective factor compared with males. ALM is the main type of melanoma in colored races.31 Studies have found that ALM occurs less frequently in white people, accounting for only 4%–10% of skin melanoma, but it has a higher proportion in Hispanic, Asian, African, and other people of color.32–35 Of note, the study also found that being unmarried and DSW were risk factors affecting the prognosis of patients with ALM. Marriage may have a positive impact on the early diagnosis of malignant tumors.36 Therefore, unmarried and DSW patients are more likely to develop advanced cancer disease. The analysis may be because spouses of married patients may find suspicious or changing moles on their body parts that are not easily observed, or married patients will get more social or material support in the process of disease treatment.37 The study also found that AJCC stage, SEER stage, and surgical treatment affected the survival rate of patients with ALM: the higher the AJCC stage, the lower the survival rate. In the SEER stage, distant metastasis is a risk factor compared with local metastasis, while regional metastasis has no significance in this study. In addition, surgical treatment is the primary effective treatment for patients with ALM.38 In this study, the HR for patients who did not receive surgical treatment was more than 3.5%. Therefore, the nomogram prediction model in this study included not only the AJCC staging system, but also patient demographic characteristics and other clinical parameters. In this nomogram, clinicians can use the total score of these seven factors to predict the OS of an individual patient and make decisions that are more likely to improve their outcome.
Several common model validation parameters, including C-index, AUC, calibration plot, NRI, IDI, and DCA, were used to evaluate the performance of the conventional AJCC staging system after the construction of the nomogram. In the training cohort and validation cohort, the C-index and AUC were used to evaluate the discrimination performance, and the parameters of the nomogram were higher than those of the AJCC staging system. In this study, the calibration plots approximate to the 45° line, indicating that the prediction of the nomogram has been well calibrated. NRI and IDI are more sensitive indicators than the C-index. NRI is usually used to compare the predictive ability of the two models, while IDI can be used to reflect the overall improvement of the new model.39 The NRI showed that the proportion of 3-, 5-, and 8-year survival probability correctly classified in the nomogram increased by 52.4%, 61.6%, and 69.0% in the training cohort and 47.9%, 60.2%, and 66.8%% in the validation cohort, respectively (P < 0.001). Compared with the AJCC staging system, the IDI showed that the predictive ability of 3-, 5-, and 8-year survival probability was 8.5%, 11.0%, and 12.5% better in the training cohort and 9.3%, 12.5%, and 13.8% better in the validation cohort, respectively (P < 0.001). Therefore, the verification results of NRI and IDI show that the nomogram has better predictive performance than the AJCC staging system. DCA is used to evaluate predictive models by calculating their clinical net benefits.40 In this study, the DCA of 3-, 5-, and 8-year survival probability for the nomogram was higher than that of the AJCC staging system, indicating a greater net benefit from the nomogram and thus a better clinical validity of the new model.
In this study, the nomogram established based on SEER database has certain advantages especially related to its practicability. First, nomogram, which is adopted to integrate different prognosis and decisive variables, can generate individual numerical probability of clinical events, such as death or relapse; it is tailored for individual patients, making it easier to find Patients with poor overall survival as early as possible in the evaluation duration, which is superior to the clinician’s judgment.41 Second, when there is no clear clinical guidance, the nomogram provides a quick and convenient way to solve complex situation.42 Third, the parameters involved in the nomogram are easy to obtain and evaluate, which means that there is no cost burden associated with complex tests in clinical use.43 Fourth, visual nomogram can enhance patients’ understanding, help doctors and patients communicate from a medical perspective, and further promote cooperation between patients and doctors, so as to achieve better treatment effects.44 Additionally, the results of this study are conducive to the timely detection of patients with low overall survival, but it is still worth considering whether the results of the nomogram score can be used as the basis for intervention to improve the overall survival of ALM patients.
This study based on the SEER database to investigate a large number of people has certain advantages, but also has some limitations. First, it is a retrospective study, which may cause potential selection bias and information bias. Secondly, the evolution at the time of diagnosis was not considered in this study, since some following reasons involved in. SEER database fails to access some important information, such as patients’ radiotherapy and chemotherapy information, pathological information, treatment time information, surgical method information, etc., which may have an important impact on the course of disease development of patients; Then, TNM stage was not taken as a separate variable in the study, only AJCC stage was used as a variable, which simplified the model, but may sacrifice some accuracy in the prediction of survival rate in the nomogram; The last is the limitation of the nomogram itself, ie, only factors that can be obtained and measured can be considered, and data should be assumed to be static with time.13,45 Thirdly, the study did not perform external validation for the nomogram, and using only internal validation could lead to overfitting of the new model. Therefore, in future studies, we will further study the SEER database to find more information on clinical disease development, so as to make full use of the database resources, and we also plan to add more predictors, use prospective data to establish a nomogram, and use external validation methods to verify the model’s effectiveness, so as to compensate for these limitations as much as possible.
Conclusion
This study is the first to establish a comprehensive ALM nomogram based on the SEER database and evaluate it using a range of indicators. The validation results showed that the nomogram was a powerful predictor. Compared to the AJCC staging system alone, both the C-index and calibration curves of the nomogram performed well and predicted better overall survival at 3-, 5-, and 8- years for ALM patients, but since we did not externally validate it, the accuracy of the model remains to be determined. The nomogram can be used as a supplement to the AJCC staging system for disease prognosis. Therefore, the clinical use of nomogram and AJCC staging system in conjunction may lead to more accurate assessment results. The nomogram may give further reference information for clinicians to provide personalized prognosis for patients with ALM. In addition, in the next phase of the study, we will attend to collect prospective data on ALM by, exploring and utilizing unknown prognostic factors to optimize the nomogram, thus verifying the model’s effectiveness with the help of external validation methods.
Data Sharing Statement
All procedures performed in the present study were in accordance with the principles outlined in the 1964 Helsinki Declaration and its later amendments. This study was exempted from obtaining informed consents by the institutional research committee of School of Nursing in Jinan University because SEER research data is publicly available and all patient data are de-identified. The data sets generated and/or analyzed during the current study are available in the SEER database (https://seer. cancer.gov/).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Tingting Yin and Yuhui Zhao are co-first authors for this study. The authors have no conflicts of interest to declare.
References
1. Michielin O, van Akkooi ACJ, Ascierto PA, et al. Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2019;30:1884–1901. doi:10.1093/annonc/mdz411
2. Goydos JS, Shoen SL. Acral lentiginous melanoma. Cancer Treat Res. 2016;167:321–329.
3. Hall KH, Rapini RP. Acral lentiginous melanoma: 1. Introduction. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2021.
4. Carter TM, Strassle PD, Ollila DW, et al. Does acral lentiginous melanoma subtype account for differences in patterns of care in Black patients? Am J Surg. 2021;221:706–711. doi:10.1016/j.amjsurg.2020.12.040
5. Tod B, Visser W, de Wet J, et al. Clinicopathological features and associations in a series of South African acral melanomas. Pigment Cell Melanoma Res. 2021;00:1–3.
6. Madankumar R, Gumaste PV, Martires K, et al. Acral melanocytic lesions in the United States: prevalence, awareness, and dermoscopic patterns in skin-of-color and non-Hispanic white patients. J Am Acad Dermatol. 2016;74(4):724–730. doi:10.1016/j.jaad.2015.11.035
7. Markinson BC, Stowers JM, Black A, et al. The misdiagnosis of acral lentiginous melanoma: three case presentations. J Am Podiatr Med Assoc. 2019;109:166–171. doi:10.7547/17-038
8. Redi U, Marruzzo G, Lovero S, et al. Acral lentiginous melanoma: a retrospective study. J Cosmet Dermatol. 2021;20(6):1813–1820. doi:10.1111/jocd.13737
9. Nakamura Y, Fujisawa Y. Diagnosis and management of acral lentiginous melanoma. Curr Treat Options Oncol. 2018;19(8):42. doi:10.1007/s11864-018-0560-y
10. Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67:93–99.
11. Zhang Y, Hong YK, Zhuang DW, et al. Bladder cancer survival nomogram: development and validation of a prediction tool, using the SEER and TCGA databases. Medicine (Baltimore). 2019;98:e17725. doi:10.1097/MD.0000000000017725
12. Yang J, Pan Z, Zhao F, et al. A nomogram for predicting survival in patients with nodular melanoma: a population-based study. Medicine (Baltimore). 2019;98:e16059. doi:10.1097/MD.0000000000016059
13. Balachandran VP, Gonen M, Smith JJ, et al. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16:e173–e180. doi:10.1016/S1470-2045(14)71116-7
14. Hu CY, Pan ZY, Yang J, et al. Nomograms for predicting long-term overall survival and cancer-specific survival in lip squamous cell carcinoma: a population-based study. Cancer Med. 2019;8:4032–4042. doi:10.1002/cam4.2260
15. Weiss SA, Hanniford D, Hernando E, et al. Revisiting determinants of prognosis in cutaneous melanoma. Cancer. 2015;121:4108–4123. doi:10.1002/cncr.29634
16. Liu Z, Xu Y, Xu G, et al. Nomogram for predicting overall survival in colorectal cancer with distant metastasis. BMC Gastroenterol. 2021;21:103. doi:10.1186/s12876-021-01692-x
17. Chen DM, Zhang RC, Zhang Q, et al. Analysis of prognostic factors in children with Wilms tumor and construction of predictive nomogram. J China Pediatr Blood Cancer. 2021;26:137–142.
18. Yang J, Li YJ, Liu QQ, et al. Brief introduction of medical database and data mining technology in big data era. J Evid Based Med. 2020;13(1):57–69. doi:10.1111/jebm.12373
19. Wu WT, Li YJ, Feng AZ, et al. Data mining in clinical big data: the frequently used databases, steps, and methodological models. Military Med Res. 2021;8(1):44. doi:10.1186/s40779-021-00338-z
20. Lobl MB, Santos C, Clarey D, et al. Treatments and associated outcomes of acral lentiginous melanoma: a review. J Am Acad Dermatol. 2020;83:230–234. doi:10.1016/j.jaad.2019.11.021
21. Tas F, Erturk K. Limb melanomas: acral melanomas have worse survival. J Dermatolog Treat. 2021;1–8. doi:10.1080/09546634.2021.1877248
22. Guo Q, Wu M, Li H, et al. Development and validation of a prognostic nomogram for myocardial infarction patients in intensive care units: a retrospective cohort study. BMJ Open. 2020;10(12):e040291. doi:10.1136/bmjopen-2020-040291
23. Wang W, Jin ZR, Wu GL, et al. A nomogram to predict prognosis of patients with large hepatocellular carcinoma: a study based on SEER database. Chin J Bases Clin General Surg. 2021;1–8:4564.
24. Csányi I, Houshmand N, Szűcs M, et al. Acral lentiginous melanoma: a single-centre retrospective review of four decades in East-Central Europe. J Eur Acad Dermatol Venereol. 2020;34(9):2004–2010. doi:10.1111/jdv.16227
25. Balch CM, Soong SJ, Gershenwald JE, et al. Age as a prognostic factor in patients with localized melanoma and regional metastases. Ann Surg Oncol. 2013;20(12):3961–3968. doi:10.1245/s10434-013-3100-9
26. Macdonald JB, Dueck AC, Gray RJ, et al. Malignant melanoma in the elderly: different regional disease and poorer prognosis. J Cancer. 2011;2:538–543. doi:10.7150/jca.2.538
27. Chao C, Martin RC
28. Pollack LA, Li J, Berkowitz Z, et al. Melanoma survival in the United States, 1992 to 2005. J Am Acad Dermatol. 2011;65:S78–S86. doi:10.1016/j.jaad.2011.05.030
29. Enninga EAL, Moser JC, Weaver AL, et al. Survival of cutaneous melanoma based on sex, age, and stage in the United States, 1992-2011. Cancer Med. 2017;6(10):2203–2212. doi:10.1002/cam4.1152
30. Behbahani S, Malerba S, Samie FH. Acral lentiginous melanoma: clinicopathological characteristics and survival outcomes in the US National Cancer Database 2004–2016. Br J Dermatol. 2020;183(5):952–954. doi:10.1111/bjd.19211
31. Han B, Hur K, Ohn J, et al. Acral lentiginous melanoma in situ: dermoscopic features and management strategy. Sci Rep. 2020;10:20503. doi:10.1038/s41598-020-77425-z
32. Lichte V, Breuninger H, Metzler G, et al. Acral lentiginous melanoma: conventional histology vs. three-dimensional histology. Br J Dermatol. 2009;160:591–599. doi:10.1111/j.1365-2133.2008.08954.x
33. Darmawan CC, Jo G, Montenegro SE, et al. Early detection of acral melanoma: a review of clinical, dermoscopic, histopathologic, and molecular characteristics. J Am Acad Dermatol. 2019;81:805–812. doi:10.1016/j.jaad.2019.01.081
34. Howard M, Xie C, Wee E, et al. Acral lentiginous melanoma: clinicopathologic and survival differences according to tumour location. Australas J Dermatol. 2020;61:312–317. doi:10.1111/ajd.13310
35. Asgari MM, Shen L, Sokil MM, et al. Prognostic factors and survival in acral lentiginous melanoma. Br J Dermatol. 2017;177:428–435. doi:10.1111/bjd.15600
36. Buja A, Lago L, Lago S, et al. Marital status and stage of cancer at diagnosis: a systematic review. Eur J Cancer Care. 2018;27:e12755. doi:10.1111/ecc.12755
37. McLaughlin JM, Fisher JL, Paskett ED, McLaughlin JM, Fisher JL, Paskett ED. Marital status and stage at diagnosis of cutaneous melanoma: results from the Surveillance Epidemiology and End Results (SEER) program, 1973‐2006. Cancer. 2011;117:1984–1993. doi:10.1002/cncr.25726
38. Brazen BC, Gray T, Farsi M, et al. Acral lentiginous melanoma: a rare variant with unique diagnostic challenges. Cureus. 2020;12:e8424. doi:10.7759/cureus.8424
39. Li C, Yang J, Xu F, et al. A prognostic nomogram for the cancer-specific survival of patients with upper-tract urothelial carcinoma based on the Surveillance, Epidemiology, and End Results Database. BMC Cancer. 2020;20:534. doi:10.1186/s12885-020-07019-5
40. Xiao WJ, Lu Q, Yao XD, et al. Application of decision curve analysis and evaluation of clinical prediction model. Chin J Health Stat. 2012;29:460–461.
41. Iasonos A, Schrag D, Raj GV, et al. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26:1364–1370. doi:10.1200/JCO.2007.12.9791
42. Lu YJ, Wang H, Fang LY, et al. A nomogram for predicting overall survival in patients with uterine leiomyosarcoma: a SEER population-based study. Future Oncol. 2020;16(10):573–584. doi:10.2217/fon-2019-0674
43. Chen L, Wang Y, Zhao K, et al. Postoperative nomogram for predicting cancer-specific and overall survival among patients with medullary thyroid cancer. Int J Endocrinol. 2020;2020:8888677. doi:10.1155/2020/8888677
44. Kattan MW, Marasco J. What is a real nomogram? Semin Oncol. 2010;37:23–26. doi:10.1053/j.seminoncol.2009.12.003
45. Diao JD, Wu CJ, Cui HX, et al. Nomogram predicting overall survival of rectal squamous cell carcinomas patients based on the SEER database: a population-based STROBE cohort study. Medicine (Baltimore). 2019;98:e17916. doi:10.1097/MD.0000000000017916
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


