Back to Journals » Pharmacogenomics and Personalized Medicine » Volume 14
No Association Between Pharmacogenomics Variants and Hospital and Emergency Department Utilization: A Mayo Clinic Biobank Retrospective Study
Authors Takahashi PY, Ryu E, Bielinski SJ , Hathcock M, Jenkins GD, Cerhan JR , Olson JE
Received 12 September 2020
Accepted for publication 29 December 2020
Published 11 February 2021 Volume 2021:14 Pages 229—237
DOI https://doi.org/10.2147/PGPM.S281645
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Martin H Bluth
Paul Y Takahashi, 1 Euijung Ryu, 2 Suzette J Bielinski, 3 Matthew Hathcock, 2 Gregory D Jenkins, 2 James R Cerhan, 3 Janet E Olson 3
1Division of Community Internal Medicine, Department of Internal Medicine, Mayo Clinic, Rochester, MN, USA; 2Division of Biomedical Statistics and Informatics, Department of Health Sciences Research, Mayo Clinic, Rochester, MN, USA; 3Division of Epidemiology, Department of Health Sciences Research, Mayo Clinic, Rochester, MN, USA
Correspondence: Paul Y Takahashi
Division of Community Internal Medicine, Department of Internal Medicine, Mayo Clinic, 200 First Street SW, Rochester, MN, 55905, USA
Tel +1-507-284-2511
Fax +1-507-266-2297
Email [email protected]
Background: The use of pharmacogenomics data is increasing in clinical practice. However, it is unknown if pharmacogenomics data can be used more broadly to predict outcomes like hospitalization or emergency department (ED) visit. We aim to determine the association between selected pharmacogenomics phenotypes and hospital utilization outcomes (hospitalization and ED visits).
Methods: This cohort study utilized 10,078 patients from the Mayo Clinic Biobank in the RIGHT protocol with sequence and interpreted phenotypes for 10 selected pharmacogenes including CYP2D6, CYP2C9, CYP2C19, CYP3A5, HLA B 5701, HLA B 5702, HLA B 5801, TPMT, SLCO1B1, and DPYD. The primary outcome was hospitalization with ED visits as a secondary outcome. We used Cox proportional hazards model to test the association between each pharmacogenomics phenotype and the risk of the outcomes.
Results: During the follow-up period (median [in years] = 7.3), 13% (n=1354) and 8% (n=813) of the subjects experienced hospitalization and ED visits, respectively. Compared to subjects who did not experience hospitalization, hospitalized patients were older (median age [in years]: 67 vs 65), poorer self-rated health (15% vs 4.7% for fair/poor), and higher disease burden (median number of chronic conditions: 7 vs 4) at baseline. There was no association of hospitalization with any of the pharmacogenomics phenotypes. The pharmacogenomics phenotypes were not associated with disease burden, a well-established risk factor for hospital utilization outcomes. Similar findings were observed for patients with ED visits during the follow-up period.
Conclusion: We found no association of 10 well-established pharmacogenomics phenotypes with either hospitalization or ED visits in this relatively large biobank population and outside the context of specific drug use related to these genes. Traditional risk factors for hospitalization like age and self-rated health were much more likely to predict hospitalization and/or ED visits than this pharmacogenomics information.
Keywords: pharmacogenomics, emergency department, hospitalization
A Letter to the Editor has been published for this article.
A Response to Letter has been published for this article.
Introduction
Healthcare organizations utilize risk stratification as an important tool for managing population health.2,3 In the ambulatory practice, clinicians determine high-risk patients by their age4 as a primary predictor of risk for hospitalization. Risk prediction instruments utilize other risk factors for hospitalization including comorbid health conditions,5–7 and self-reported physical health.4,8 Other important risk factors for health outcomes include socioeconomic status9 and previous hospital utilization.5 Following the determination of high-risk status, healthcare organizations might provide different levels of care to best suit the risk stratification of the patient.10 While the traditional methods of risk stratification for hospital use are effective,11 further refinement of risk would improve our ability to better identify patients at high risk. Pharmacogenomics is one potential clinical factor that may help predict adverse health outcomes given the association between medication and adverse events like emergency department visits.12 To date, personalized medicine using genomic information has not been used in risk-stratification tools for health outcomes including hospitalization.
While pharmacogenomics has not been specifically evaluated for healthcare utilization, the use or misuse of medications can certainly place patients at risk for hospitalization or ED use. Polypharmacy may place patients at risk for adverse health outcomes like 30-day hospital readmission13 or hospitalization in patients receiving home care.14 Patients experience an increased risk of ED visits when they take pain medications, diabetic medications, or anticoagulants.15 The American Geriatrics Society published guidelines on medications that are potentially hazardous for older adults.16 Providers recognize common inappropriate medications like anticholinergic medications, opioid pain medications, sedative/hypnotic medications among other medications.16
The metabolism of medications could potentially affect efficacy and toxicity17 and may impact the majority of patients. A recent study tested only five pharmacogenomics (PGx) and yet found that 99% of patients had one or more actionable results.18 In our previous study of 1013 patients with pharmacogenomics for CYP2D6, we found 8% of patients possessed a poor metabolizer phenotype and 8% had ultra-rapid metabolizer phenotypes.19 In further work in this same population, we showed that the extremes of metabolism phenotype (poor or ultra-rapid) were associated with a higher risk of hospitalization.20 However, this has not been shown in all studies that have examined pharmacogenomics phenotypes and hospitalization. In a study of 729 patients with pre-emptive VKORC1 and CYP2C9 PGx genes, pharmacogenomics-guided warfarin dosing did not lead to a reduction in hospitalization or mortality.21 In addition, the identification of new alleles in pharmacogenes plus improved variant functional characterization has led to new variant classifications.22,23 In this study, we undertook a larger examination of the association of pharmacogenomics phenotype agnostic of specific medication usage and the utilization outcomes of hospitalization and ED visits using the most recent variant classifications. Further, we explored the association between pharmacogenomics and disease burden because as many as 50% of patients react inadequately to prescribed drugs24 which can lead to poorly treated health conditions.
Methods and Materials
Study Subjects
This is a retrospective cohort study. All participants in this study were enrolled in the RIGHT expansion study as described previously by Bielinski et al.1 Briefly, adult subjects were selected from the Mayo Clinic Biobank (MCB)25 for use of the Mayo Clinic for their healthcare and offered the opportunity to receive pharmacogenomic testing plus deposition of key results in the Mayo Clinic electronic health record (EHR). A total of 18,199 adult subjects were invited and 10,085 enrolled. Subjects who had subsequently withdrawn consent from the MCB or the RIGHT 10K study were excluded, leaving 10,078 in these analyses. The majority of RIGHT 10K participants lived within the Rochester Epidemiology Project (REP) catchment area where we can access comprehensive electronic health records (EHRs) information including hospitalization and ED visit information.26 The Mayo Clinic IRB and the Olmsted Medical Center IRB reviewed and approved the study protocol. The study was conducted in accordance with the Declaration of Helsinki. The study was also reviewed and approved by the Mayo Clinic Biobank access committee and the RIGHT access committee.
Outcomes
We searched the EHR systems of Mayo Clinic and Olmsted Medical Center to ascertain hospitalization and/or ED visits. Follow-up time began on the date of consent to the Mayo Clinic Biobank (April 2009 through April 2017). Follow-up time ended when participants exited the study which was either the date when PGx gene information was available in participants’ EHR or the date of death date, whichever came first. The median time for the patients to exit the study was 7.3 years to study exit. Index date was defined as the first date (or the first date if multiple outcomes occurred) associated with ICD 9 or 10 codes for each outcome. Hospitalization included a stay that was overnight and excluded outpatient hospital services (ie, colonoscopy and outpatient surgery). We used the REP data linkage system to identify participants with each outcome. The medical cause for readmission was identified using ICD9 and ICD 10 codes and was mapped to Phecodes.27,28
Predictors
We obtained sociodemographic characteristics from the EHR which included age, sex, and race (white race versus non-white race) and the MCB questionnaire for educational level and self-rated perceived general health. Educational level was reported as high school or less, some college or 4-year college degree, advanced degree, or missing. We reported the classification of patients’ self-reported perceived general health as excellent/very good, good, fair/poor, or missing. We obtained chronic condition information at the time of the biobank consent using the Department of Health and Human Services guidelines for determining chronic conditions29 which utilized previously methodologies and added them to define disease burden.30 The conditions were derived from ICD 9 or 10 billing codes for all participants.31 The team quantified socioeconomic status using the HOUSES index which uses housing characteristics.32 We placed the HOUSES index scores into quartiles for comparison consistent with our previous work with higher quartiles with higher socioeconomic status.9
For pharmacogenomics phenotypes, we utilized the records of those patients with PGx data within the biobank. The PGx phenotype was obtained from PGx sequencing data from the RIGHT protocol. These PGx genes were selected based upon the best clinical practice advisories and decision support for clinicians. We reported all phenotypes in Supplementary Table 3; however, because of the small numbers of phenotypes and outcomes, we categorized phenotypes into normal, abnormally fast, or abnormally slow. For DPYD, we reported as normal, intermediate or poor. For HLA, we reported present or absent.
Statistical Analyses
Socio-demographic data were summarized as counts and percentages or medians and 25–75%tile as appropriate, stratified by status of each outcome. Differences in risk of hospitalization from PGx phenotypes were assessed by modeling time from biobank consent to hospitalization predicted by PGx phenotypes using Cox proportional hazards models. We analyzed the primary endpoint of hospitalization using Likelihood ratio tests based on comparing Cox models with and without the individual PGx phenotype predictors. We also tested whether PGx phenotype is associated with disease burden and/or sex, known risk factors for risk of hospitalization outcomes, by using Kruskal–Wallis test and Chi-square test, respectively. In a similar fashion, we used Cox proportional hazard model for ED visit outcome.
Results
Of 10,078 patients, the average age of the patients at the MCB enrollment was 66 years with (IQR: 54 to 74; Table 1). Consistent with prior literature, patients who were hospitalized compared to non-hospitalized, were older (median 67 years versus 65 in non-hospitalized, p value <0.001) and were more likely to have high school or less education (22% versus 13%, p value <0.001). Female gender was also less likely to be hospitalized compared to males (OR 0.76 (95% CI: 0.68,0.84)). Patients of white race were also less likely to be hospitalized compared to non-white race (OR 0.73 (95% CI 0.56,0.96)). The rate of hospitalization and ED visit at 5 years were 9.3% and 5.4%, respectively. The hospitalized patients were more likely to report poor/fair health (15%) compared to non-hospitalized patients (5%) (p value<0.001). Those hospitalized tended to have lower socioeconomic status measure by HOUSES (18% vs 12% for the lowest quartile in the local population, p value <0.001), which is similar to the trend associated with education level. (Table 2) When looking at ED visit as the primary outcome, we found similar associations between ED visit and age, sex, race, educational level, self-rated health and socioeconomic status (Table 3).
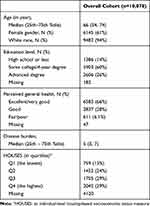 |
Table 1 Characteristics of Study Subjects Included in the Study |
 |
Table 2 Association Between Subject Characteristics and Risk of Hospitalization Status During Follow-Up Period |
 |
Table 3 Association Between Subject Characteristics and Risk of Emergency Department (ED) Status During Follow-Up Period |
We did not find any association between the phenotype of 10 PGx genes and hospitalization. We have reported all of the phenotypes for the 10 pharmacogenomics genes in Supplementary Table 1. In comparing the phenotypes of frequencies of CYP 2D6 to other world populations, they appear similar.33 There was very little variation in phenotype between those with hospitalization and those without hospitalization (Table 4). Specifically, in CYP 2D6 those with a fast phenotype had 2.6% hospitalization compared with 2.0% in those without hospitalization (p 0.40). For our second outcome, we found that there was no association between ED visits and pharmacogenomic phenotype (Table 5). Of the pharmacogenomic phenotypes, dihydropyrimidine dehydrogenase (DPYD) had a trending p value of p=0.06. We report no difference in pharmacogenomics phenotype by sex or disease burden (Supplementary Table 2), which justifies the rationale for not adjusting for these variables when testing the association between PGx phenotype and the outcomes. For the primary diagnosis for admission, we found osteoarthritis and major depression as the top two reasons for admission (Supplementary Table 1).
Discussion
In this study of 10,078 patients, we did not find a relationship between PGx phenotype and hospitalization and ED visits. In particular, we did not find a relationship between the common PGx genes of CYP 2D6, CYP 2C19 and CYP 3A4 and hospitalization and ED visits. This is a novel finding as this type of study has not been performed previously and provides some initial evidence on the utility of PGx phenotype to predict hospitalization or ED visit. These results reinforce that traditional risk factors for hospital readmission like age, sex, race, comorbid health burden,34 previous utilization,34,35 and education36 are stronger predictors of hospitalization. Our previous work also shows the importance of these traditional risk factors.5,11,37 PGx phenotype as currently defined does not help predict hospitalization or ED visit.
In our previous study, our data indicated that 8% of the cohort had ultra-rapid phenotypes for CYP 2D6 and that this phenotype was associated with hospitalization outcomes.20 In contrast, in the current study, we found only 2.1% of patients had ultra-rapid phenotype. The differences most likely reflect re-evaluation of the activity of the *2A allele that occurred in the interim period by the laboratory that conducted the phenotyping assays, suggesting that our previously reported finding was a chance finding.38 The continual improvement of pharmacogenomic assays is critically important as we work to improve the understanding of pharmacogenes, especially CYP2D6. The metabolism phenotype for CYP 2D6 has numerous clinical implications including antipsychotic medications,39 antidepressants40 and pain medication like oxycodone or codeine.41 In fact, CYP 2D6 is known to affect the metabolism of approximately 25% of the known drugs.42
Clinical medicine is just starting to understand personalized medicine and PGx. There have been some common uses of PGx for individual medications and PGx phenotypes. Clopidogrel and CYP 2C19 is one such common example as clopidogrel is a prodrug and is commonly used as an anti-platelet medication to prevent stent re-thrombosis.43 In a meta-analysis of studies of clopidogrel and CYP 2C19, there were worse health outcomes with reduced function phenotypes with hazard ratios of 1.55; 95% CI, 1.11–2.17; P = 0.01 for patients with one reduced function allele compared to normal types for cardiovascular death, stroke or myocardial infarction.44 In a study of patients starting medications for mood disorders, pre-emptive PGx testing resulted in lower costs and utilization.45 In another observational study, investigators found lower hospital length of stay in patients receiving pharmacogenomics testing compared to no testing in depressed patients.46
The strengths of this study included a comprehensive list of outcomes (hospitalization and ED visit) from the REP data system and catchment area.47 It included the pharmacogenomics information which was unique and integrated into the medical record.48 The limitations included the potential for missing outcomes or predictors if the patient sought care outside the REP catchment area; however, this is minimized because the largest health systems are included for the outcomes. In this cohort study, there may be unrecognized confounders that can influence hospitalization or ED visits. The study does not report drug-gene pairs, and we cannot assume that hospitalization or ED visits were associated with a complication of medication or of a problem that PGx can solve. We also accept that the population of the cohort is largely white1 which may generalize to the upper Midwest of the United States but not to other regions of the world.49 Additionally, we acknowledge that we may lack statistical power as some PGx phenotypes had relatively small sample sizes in abnormal categories. However, the observed effect size was in general small in the majority of the PGx phenotypes.
Conclusion
We found no association between pharmacogenomics phenotype and hospitalization or ED visit in patients who had pharmacogenomics testing. This lack of association adds to our knowledge about the effect of individualized medicine and risk stratification for hospitalization and ED visit. Traditional risk factors for hospitalization including age, comorbid health concerns and previous hospitalization4,5 will still be standard methods to help predict future hospitalization. Pharmacogenomics will continue to play instrumental roles in preventing adverse drug reactions in high-risk patient populations.17
Acknowledgments
We want to acknowledge Kari Takahashi MEd for assistance with the manuscript preparation. This publication was made possible by CTSA Grant Number UL1 TR002377 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH. We also acknowledge the Mayo Clinic Center for Individualized Medicine for support of the Mayo Clinic Biobank and the RIGHT Protocol.
Disclosure
Dr James R Cerhan reports grants from NanoString, personal fees from Jannsen, nothing from Celgene, nothing from Genentech, outside the submitted work. The authors report no other potential conflicts of interest in this manuscript or work.
References
1. Bielinski SJ, St Sauver JL, Olson JE, et al. Cohort Profile: the right drug, right dose, right time: using genomic data to individualize treatment protocol (RIGHT protocol). Int J Epidemiol. 2020;49(1):23–24k. doi:10.1093/ije/dyz123
2. Porter ME, Pabo EA, Lee TH. Redesigning primary care: a strategic vision to improve value by organizing around patients’ needs. Health Aff (Millwood). 2013;32(3):516–525. doi:10.1377/hlthaff.2012.0961
3. Clough JD, Riley GF, Cohen M, et al. Patterns of care for clinically distinct segments of high cost Medicare beneficiaries. Healthc (Amst). 2016;4(3):160–165. doi:10.1016/j.hjdsi.2015.09.005
4. Boult C, Dowd B, McCaffrey D, Boult L, Hernandez R, Krulewitch H. Screening elders for risk of hospital admission. J Am Geriatr Soc. 1993;41(8):811–817. doi:10.1111/j.1532-5415.1993.tb06175.x
5. Crane SJ, Tung EE, Hanson GJ, Cha S, Chaudhry R, Takahashi PY. Use of an electronic administrative database to identify older community dwelling adults at high-risk for hospitalization or emergency department visits: the elders risk assessment index. BMC Health Serv Res. 2010;10:338. doi:10.1186/1472-6963-10-338
6. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi:10.1016/0021-9681(87)90171-8
7. Abdel-Qadir H, Thavendiranathan P, Austin PC, et al. Development and validation of a multivariable prediction model for major adverse cardiovascular events after early stage breast cancer: a population-based cohort study. Eur Heart J. 2019. doi:10.1093/eurheartj/ehz460
8. Takahashi PY, Ryu E, Olson JE, et al. Health behaviors and quality of life predictors for risk of hospitalization in an electronic health record-linked biobank. Int J Gen Med. 2015;8:247–254. doi:10.2147/IJGM.S85473
9. Takahashi PY, Ryu E, Hathcock MA, et al. A novel housing-based socioeconomic measure predicts hospitalisation and multiple chronic conditions in a community population. J Epidemiol Community Health. 2016;70(3):286–291. doi:10.1136/jech-2015-205925
10. Porter ME. What is value in health care? N Engl J Med. 2010;363(26):2477–2481. doi:10.1056/NEJMp1011024
11. Haas LR, Takahashi PY, Shah ND, et al. Risk-stratification methods for identifying patients for care coordination. Am J Manag Care. 2013;19(9):725–732.
12. Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365(21):2002–2012. doi:10.1056/NEJMsa1103053
13. Thorsteinsdottir B, Peterson SM, Naessens JM, et al. Care transitions program for high-risk frail older adults is most beneficial for patients with cognitive impairment. J Hosp Med. 2019;14:E1–E8.
14. Flaherty JH, Perry HM
15. Budnitz DS, Lovegrove MC, Crosby AE. Emergency department visits for overdoses of acetaminophen-containing products. Am J Prev Med. 2011;40(6):585–592. doi:10.1016/j.amepre.2011.02.026
16. By the American Geriatrics Society Beers Criteria Update Expert P. American Geriatrics Society 2019 updated AGS beers criteria(R) for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674–694. doi:10.1111/jgs.15767
17. Weinshilboum RM, Wang L. Pharmacogenomics: precision medicine and drug response. Mayo Clin Proc. 2017;92(11):1711–1722. doi:10.1016/j.mayocp.2017.09.001
18. Ji Y, Skierka JM, Blommel JH, et al. Preemptive pharmacogenomic testing for precision medicine: a comprehensive analysis of five actionable pharmacogenomic genes using next-generation DNA sequencing and a customized CYP2D6 genotyping cascade. J Mol Diagn. 2016;18(3):438–445. doi:10.1016/j.jmoldx.2016.01.003
19. Bielinski SJ, Olson JE, Pathak J, et al. Preemptive genotyping for personalized medicine: design of the right drug, right dose, right time-using genomic data to individualize treatment protocol. Mayo Clin Proc. 2014;89(1):25–33. doi:10.1016/j.mayocp.2013.10.021
20. Takahashi PY, Ryu E, Pathak J, et al. Increased risk of hospitalization for ultrarapid metabolizers of cytochrome P450 2D6. Pharmgenomics Pers Med. 2017;10:39–47. doi:10.2147/PGPM.S114211
21. Leary E, Brilliant M, Peissig P, Griesbach S. Preliminary outcomes of preemptive warfarin pharmacogenetic testing at a large rural healthcare center. Am J Health Syst Pharm. 2019;76(6):387–397. doi:10.1093/ajhp/zxy072
22. Nofziger C, Turner AJ, Sangkuhl K, et al. PharmVar GeneFocus: CYP2D6. Clin Pharmacol Ther. 2020;107(1):154–170. doi:10.1002/cpt.1643
23. Devarajan S, Moon I, Ho MF, et al. Pharmacogenomic next-generation DNA sequencing: lessons from the identification and functional characterization of variants of unknown significance in CYP2C9 and CYP2C19. Drug Metab Dispos. 2019;47(4):425–435. doi:10.1124/dmd.118.084269
24. Lauschke VM, Ingelman-Sundberg M. The importance of patient-specific factors for hepatic drug response and toxicity. Int J Mol Sci. 2016;17(10):1714. doi:10.3390/ijms17101714
25. Olson JE, Ryu E, Hathcock MA, et al. Characteristics and utilisation of the Mayo Clinic Biobank, a clinic-based prospective collection in the USA: cohort profile. BMJ Open. 2019;9(11):e032707. doi:10.1136/bmjopen-2019-032707
26. Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ
27. Wu P, Gifford A, Meng X, et al. Developing and evaluating mappings of ICD-10 and ICD-10-CM codes to PheCodes. JMIR Med Inform. 2019;7(4):e14325. doi:10.2196/14325
28. Wei WQ, Bastarache L, Carroll RJ, et al. Evaluating phecodes, clinical classification software, and ICD-9-CM codes for phenome-wide association studies in the electronic health record. PLoS One. 2017;12(7):e0175508. doi:10.1371/journal.pone.0175508
29. Goodman RA, Boyd C, Tinetti ME, Von Kohorn I, Parekh AK, McGinnis JM. IOM and DHHS meeting on making clinical practice guidelines appropriate for patients with multiple chronic conditions. Ann Fam Med. 2014;12(3):256–259. doi:10.1370/afm.1646
30. Rocca WA, Boyd CM, Grossardt BR, et al. Prevalence of multimorbidity in a geographically defined American population: patterns by age, sex, and race/ethnicity. Mayo Clin Proc. 2014;89(10):1336–1349. doi:10.1016/j.mayocp.2014.07.010
31. Schultz R, Eden J. Families Caring for an Aging America. Washington, DC: National Academies Press; 2016.
32. Juhn YJ, Qin R, Urm S, Katusic S, Vargas-Chanes D. The influence of neighborhood environment on the incidence of childhood asthma: a propensity score approach. J Allergy Clin Immunol. 2010;125(4):838–843 e832. doi:10.1016/j.jaci.2009.12.998
33. Gaedigk A, Sangkuhl K, Whirl-Carrillo M, Klein T, Leeder JS. Prediction of CYP2D6 phenotype from genotype across world populations. Genet Med. 2017;19(1):69–76. doi:10.1038/gim.2016.80
34. Shelton P, Sager MA, Schraeder C. The community assessment risk screen (CARS): identifying elderly persons at risk for hospitalization or emergency department visit. Am J Manag Care. 2000;6(8):925–933.
35. Reuben DB, Keeler E, Seeman TE, Sewall A, Hirsch SH, Guralnik JM. Development of a method to identify seniors at high risk for high hospital utilization. Med Care. 2002;40(9):782–793. doi:10.1097/00005650-200209000-00008
36. Calvillo-King L, Arnold D, Eubank KJ, et al. Impact of social factors on risk of readmission or mortality in pneumonia and heart failure: systematic review. J Gen Intern Med. 2013;28(2):269–282. doi:10.1007/s11606-012-2235-x
37. Takahashi PY, Ryu E, Olson JE, et al. Hospitalizations and emergency department use in Mayo Clinic Biobank participants within the employee and community health medical home. Mayo Clin Proc. 2013;88(9):963–969. doi:10.1016/j.mayocp.2013.06.015
38. Ann Moyer MDP. CYP 2d6. In: phD JO, ed; 2020.
39. Kneller LA, Abad-Santos F, Hempel G. Physiologically based pharmacokinetic modelling to describe the pharmacokinetics of risperidone and 9-hydroxyrisperidone according to cytochrome P450 2D6 phenotypes. Clin Pharmacokinet. 2020;59(1):51–65. doi:10.1007/s40262-019-00793-x
40. Chen R, Wang H, Shi J, Shen K, Hu P. Cytochrome P450 2D6 genotype affects the pharmacokinetics of controlled-release paroxetine in healthy Chinese subjects: comparison of traditional phenotype and activity score systems. Eur J Clin Pharmacol. 2015;71(7):835–841. doi:10.1007/s00228-015-1855-6
41. Dagostino C, Allegri M, Napolioni V, et al. CYP2D6 genotype can help to predict effectiveness and safety during opioid treatment for chronic low back pain: results from a retrospective study in an Italian cohort. Pharmgenomics Pers Med. 2018;11:179–191. doi:10.2147/PGPM.S181334
42. Zhou SF. Polymorphism of human cytochrome P450 2D6 and its clinical significance: part I. Clin Pharmacokinet. 2009;48(11):689–723.
43. Siller-Matula JM, Delle-Karth G, Lang IM, et al. Phenotyping vs. genotyping for prediction of clopidogrel efficacy and safety: the PEGASUS-PCI study. J Thromb Haemost. 2012;10(4):529–542. doi:10.1111/j.1538-7836.2012.04639.x
44. Mega JL, Simon T, Collet JP, et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA. 2010;304(16):1821–1830. doi:10.1001/jama.2010.1543
45. Perlis RH, Mehta R, Edwards AM, Tiwari A, Imbens GW. Pharmacogenetic testing among patients with mood and anxiety disorders is associated with decreased utilization and cost: a propensity-score matched study. Depress Anxiety. 2018;35(10):946–952. doi:10.1002/da.22742
46. Battig VAD, Roll SC, Hahn M. Pharmacogenetic testing in depressed patients and interdisciplinary exchange between a pharmacist and psychiatrists results in reduced hospitalization times. Pharmacopsychiatry. 2020;53(4):185–192. doi:10.1055/a-1096-1171
47. Rocca WA, Grossardt BR, Brue SM, et al. Data resource profile: expansion of the Rochester epidemiology project medical records-linkage system (E-REP). Int J Epidemiol. 2018;47(2):368–368j. doi:10.1093/ije/dyx268
48. Caraballo PJ, Sutton JA, Giri J, et al. Integrating pharmacogenomics into the electronic health record by implementing genomic indicators. J Am Med Inform Assoc. 2019. doi:10.1093/jamia/ocy156
49. St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


