Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 15
Ninjin’yoeito Ameliorates Skeletal Muscle Complications in COPD Model Mice by Upregulating Peroxisome Proliferator-Activated Receptor γ Coactivator-1α Expression
Authors Miyamoto A , Asai K , Kadotani H, Maruyama N, Kubo H , Okamoto A, Sato K , Yamada K, Ijiri N, Watanabe T, Kawaguchi T
Received 10 September 2020
Accepted for publication 9 November 2020
Published 27 November 2020 Volume 2020:15 Pages 3063—3077
DOI https://doi.org/10.2147/COPD.S280401
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Atsushi Miyamoto, Kazuhisa Asai, Hideaki Kadotani, Naomi Maruyama, Hiroaki Kubo, Atsuko Okamoto, Kanako Sato, Kazuhiro Yamada, Naoki Ijiri, Tetsuya Watanabe, Tomoya Kawaguchi
Department of Respiratory Medicine, Graduate School of Medicine, Osaka City University, Osaka-City, Osaka, Japan
Correspondence: Kazuhisa Asai
Department of Respiratory Medicine, Graduate School of Medicine, Osaka City University, 1-4-3 Asahi-Machi, Abeno-ku, Osaka-City, Osaka 545-8585, Japan
Tel +81-6-6645-3916
Fax +81-6-6646-6160
Email [email protected]
Purpose: Sarcopenia, the loss of skeletal muscle mass and strength, is a common systemic consequence of chronic obstructive pulmonary disease (COPD) and is correlated with higher mortality. Ninjin’yoeito (NYT) is a Japanese herbal medicine used to treat athrepsia and anorexia and is reported to ameliorate weight loss and muscular dysfunction. Recent studies have shown that its crude components upregulate the peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α)-related pathway, which is involved in skeletal muscle functions. Here, we examined whether NYT improves skeletal muscle complications by upregulating PGC-1α in COPD model mice.
Materials and Methods: Mice were divided into four groups: control, NYT, smoking, and smoking + NYT. The smoking and smoking + NYT groups were exposed to cigarette smoke for 60 min once daily. The mice in the NYT and smoking + NYT groups were fed an NYT-containing diet (3% w/w). We performed cellular analysis of bronchoalveolar lavage fluid, assessed pulmonary morphological changes, examined the expression of PGC-1α mRNA and protein in the gastrocnemius and soleus muscle, measured the hindlimb muscle volume with micro-computed tomography, and determined the myofiber proportion in soleus muscle after 12 weeks.
Results: Cigarette smoke exposure resulted in reduced skeletal muscle volume and slow-twitch muscle fibers and development of pulmonary emphysema. NYT feeding induced partial recovery of the damaged alveolar wall; however, NYT did not ameliorate smoke-induced alveolar enlargement. These findings revealed that NYT did not have sufficient efficacy in suppressing pulmonary emphysema. On the other hand, PGC-1α expression in muscle tissue of the NYT-fed mice increased significantly, resulting in suppression of smoke-induced loss of muscle mass and alteration in the muscle fiber distribution.
Conclusion: NYT increases PGC-1α expression in the muscle of COPD model mice and is involved in suppressing cigarette smoke-induced muscle complications. NYT may be a novel preventive and therapeutic medication for muscular dysfunctions in COPD.
Keywords: chronic obstructive pulmonary disease, sarcopenia, peroxisome proliferator-activated receptor γ coactivator-1α, Ninjin’yoeito, muscle dysfunction
Introduction
Chronic obstructive pulmonary disease (COPD) is a life-threatening lung disease caused by prolonged inhalation of noxious particles or gases, primarily cigarette smoke.1 It is characterized by persistent, progressive airflow limitations due to small airway damage and pulmonary emphysema with parenchymal destruction.2 COPD is often associated with other chronic comorbidities that contribute to patients’ clinical manifestations and prognoses, including skeletal muscle wasting and a lower fat-free mass index.3,4
Sarcopenia, which is age-related loss of skeletal muscle mass and strength, can be enhanced by catabolic processes in chronic illnesses and is a common systemic consequence of COPD.5 Systemic inflammation,3,6 oxidative stress,7 and hypoxemia,8 as well as insufficient physical activity due to respiratory dysfunction and symptoms,9 may cause skeletal muscle dysfunction. Muscle weakening can subsequently further decrease physical activity, exercise tolerance, and quality of life.10,11 Physical inactivity is an important predictor of the outcome among various factors regarding the physical status in COPD. A lower physical activity level is associated with the risk of exacerbation and all-cause mortality in patients with COPD.12,13 Therefore, strategies to get patients out of the vicious circle of muscle complications are necessary to improve the prognosis of COPD. Attempts have been made to improve skeletal muscle volume and function with nutrition or hormones in COPD patients; however, the effects remain unclear.14
Skeletal muscle features in COPD are manifested as structural and functional changes in muscle fibers. Mitochondrial dysfunction in skeletal muscles of COPD patients has been reported, including reduced enzyme activity and oxidative phosphorylation.15 Furthermore, a shift in the distribution of the muscle fiber type from type I to type II is a typical feature of COPD16,17 and is inconsistent with simple aging. These changes can be partially explained by decreased expression of peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α), which is involved in the regulation of muscular mitochondrial biogenesis.18 A case–control study showed a reduction in PGC-1α in lower limb muscles of cachectic COPD patients.19 PGC-1α may be a crucial factor for the maintenance of muscular function in COPD; therefore, we have focused on PGC-1α and its related proteins. Irisin is a myokine and is an example of a downstream peptide of PGC-1α.20 We reported that serum irisin levels are decreased significantly in patients with COPD compared to their healthy counterparts and that the irisin levels are correlated with the physical activity level.21 We also found that serum irisin levels are significantly correlated with the diffusion capacity of the lung and the low-attenuation area of chest computed tomography (CT) images in patients with COPD.22 Furthermore, we showed that an exercise-based intervention increases serum irisin levels and consequently ameliorates cigarette smoke-induced emphysema in COPD model mice through the irisin-accelerated antioxidation pathway.23 Considering these data, the PGC-1α-related pathway is potent and has a favorable effect not only on muscle fibers but also on lung tissue damaged by smoking.
Ninjin’yoeito (NYT) is a Japanese herbal medicine used to treat symptoms such as fatigue, athrepsia, and anorexia in the convalescent phase after illness or surgery.24 It is composed of 12 ingredients: Panax ginseng roots, Angelica acutiloba roots, Rehmannia glutinosa var. purpurea roots, Atractylodes japonica rhizomes, Wolfiporia cocos sclerotia, Paeonia lactiflora roots, Citrus unshiu peel, Polygala tenuifolia root bark, Astragalus membranaceus roots, Cinnamomum cassia bark, Schisandra chinensis fruit, and the roots and stolons of Glycyrrhiza uralensis. NYT ameliorates the reduced muscle complications in tumor-bearing model mice25 or Klotho mice.26 These reports suggest that NYT may be a potentially useful treatment for age-related or chronic disease-related sarcopenia. Furthermore, some crude components of NYT upregulate the PGC-1α-related pathway.27,28
Based on these studies, we hypothesize that NYT ameliorates the loss of skeletal muscle function by upregulating PGC-1α and suppresses emphysema by consequently activating an antioxidative pathway in COPD. The purpose of this study was to determine whether NYT improves skeletal muscle complications and pulmonary emphysema in cigarette-smoking model mice.
Materials and Methods
Animals
Four-week-old male C57BL/6 mice (13–19 g) were purchased from Japan SLC (Shizuoka, Japan) and housed in pathogen-free conditions at a constant temperature of 23°C ± 2°C and a 12 h-light/dark cycle. The mice were divided randomly into four groups: (1) control (n = 8), (2) NYT (n = 8), (3) smoking (n = 8), and (4) smoking + NYT (n = 8). The mice in the control and smoking groups were fed a standard diet (MF diet containing 7.9% water, 23.1% protein, 5.1% fat, 5.8% minerals, 2.8% fiber, and 55.3% carbohydrates). The mice in the NYT and smoking + NYT groups were fed a diet containing 3% (w/w) NYT (Kracie Ninjin’yoeito Extract Granules; Kracie Pharma Ltd., Tokyo, Japan) that was added to the standard diet. These diets were prepared by Oriental Yeast Co. Ltd. (Tokyo, Japan). The mice were provided with each diet and water ad libitum. All experimental protocols described herein were approved by the Ethics Committee of the Institutional Animal Care and Use of Osaka City University Graduate School of Medicine (Permit Number: 19,011, 5 Aug 2019). Animal experiments were conducted in accordance with the Regulations on Animal Experiments in Osaka City University following the Guidelines for Proper Conduct of Animal Experiments in Japan.
Cigarette Smoke Exposure
Following 1 week of acclimatization, the smoking and smoking + NYT groups were exposed to cigarette smoke (18 cigarettes/day) generated from commercially marketed Peace non-filtered cigarettes (2.3 mg nicotine and 28 mg tar/cigarette; Japan Tobacco, Tokyo, Japan). We used a cigarette smoke inhalation experimental system for small animals (SG-300; Shibata Scientific Technology, Tokyo, Japan) for the exposure to cigarette smoke. The control and NYT groups were exposed to ambient air in restraint tubes for 60 min, as were the other groups during the smoke exposure. Cigarette smoke exposure or mock treatment was performed once a day, for 60 min per session, 5 times per week, for 12 weeks.
Animal Preparation for Evaluation
All mice were sacrificed under deep anesthesia 24 h after the last exposure to smoke. Bronchoalveolar lavage (BAL) was performed, and the BAL fluid (BALF) was collected as previously described.23 After BAL, the left lung was dissected and immediately soaked in 10% formalin. The gastrocnemius and soleus muscles were dissected from the right leg of each mouse for muscle analysis. The muscles were cut in half, and one specimen was shock-frozen in liquid nitrogen for the protein assay, and the other was placed in RNAlater (Thermo Fisher Scientific, Waltham, MA, USA) for mRNA expression analysis. We also resected the left leg on the proximal side of the femur, removed all skin, and fixed the specimen in 10% formalin for 48 h.
BALF Analysis
The recovered BALF was treated as previously described.23 Briefly, the BALF was centrifuged (1200 × g, 4°C, 10 min), and the cell pellet was resuspended in 1 mL phosphate-buffered saline and subjected to a cytospin procedure using a Shandon Cytospin 3 centrifuge (Shandon Scientific Co., London, England). The slides were stained with Diff-Quik (Sysmex, Kobe, Japan) for cell counts (total cells and neutrophils). Cell counting was performed in a blinded manner.
Morphological Assessment of Emphysema
The left lung was perfused and fixed with 10% formalin for 48 h at 25 cm H2O and used for routine histological staining. Three-micrometer thick sections were stained with hematoxylin and eosin. The airspace enlargement was determined by the mean linear intercept (MLI), as previously described.29 Furthermore, the destructive index (DI) was also measured to evaluate alveolar destruction, as previously described.30 These analyses were performed in a blinded manner.
Gene Expression Analysis
The specimen containing the gastrocnemius and soleus muscles from the right hindlimb was homogenized in RLT lysis buffer (Qiagen NV, Venlo, Netherlands). Total RNA from the muscle was extracted with the RNeasy Fibrous Tissue Mini Kit (Qiagen NV). cDNA was synthesized using the SuperScript VILO cDNA synthesis kit (Thermo Fisher Scientific). mRNA expression of the target gene PGC-1α (Mm01208835_m1; Thermo Fisher Scientific) in comparison with housekeeping gene 36B4 (Mm00725448_s1; Thermo Fisher Scientific) was determined with TaqMan gene expression assays on an Applied Biosystems 7500 real-time PCR system (Thermo Fisher Scientific). The relative mRNA level was normalized using the ΔΔCT method.
Western Blot Analysis
Frozen muscles containing the gastrocnemius and soleus from the right leg were subjected to Western blot analysis. About 30 mg of the muscle sample was homogenized in 300 µL radioimmunoprecipitation assay buffer (Beyotime Biotechnology, Shanghai, China) supplemented with the Protease Inhibitor Cocktail (Cell Signaling Technology) and phenylmethanesulfonyl fluoride (Cell Signaling Technology, Danvers, MA, USA). Subsequently, the samples were placed on ice for 5 min and then centrifuged at 11,800 × g and 4°C for 4 min. The supernatant was collected, and the proteins were separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis with Mini-PROTEAN TGX Precast Protein Gels (#4,561,083; Bio-Rad, Hercules, CA, USA) and transferred onto polyvinylidene fluoride membranes (IB24001; Thermo Fisher Scientific). Four 10-well gels were electrophoresed simultaneously and transferred to a single membrane to avoid inter-membrane variation in quantitative analysis. The membrane was incubated with primary anti-PGC-1α antibody (1:1000, ab54481; Abcam, Cambridge, UK) or anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody (1:10,000, ab8245; Abcam) at 4°C overnight. The membrane was then incubated with the appropriate horseradish peroxidase-conjugated secondary antibodies (goat anti-rabbit IgG; #4030-05 and goat anti-mouse IgG; #1030-05, SouthernBiotech, Birmingham, AL, USA) for 2 h at room temperature. Western blot signals were acquired using a Fuji LAS-4000 fluorescence imager (Fujifilm Corporation, Tokyo, Japan) with SuperSignal West Dura Extended Duration Substrate (Thermo Fisher Scientific). The level of target protein was normalized to the level of GAPDH.
Volumetric Evaluation of Lower Leg Muscle
We performed CT of mice with the third generation CT scanner, LaTheta LCT-200 (Hitachi-Aloka, Tokyo, Japan) for muscle volume analysis. The tube voltage was set at 50 kV, and the current was constant at 0.5 mA. Scanning was carried out with a resolution of 96-µm pixels over 360° in a 48-mm wide holder. Just before sacrifice and under deep anesthesia, the lower body of each mouse was scanned to detect the length of the tibia. We used the formalin-fixed leg to measure the volume of muscles located below the knee, the principal components of which are the extensor digitorum longus, tibialis anterior, gastrocnemius, and soleus muscles. The lower portion was cut out by dissecting at the patella joint and the ankle joint, and upper hindlimb muscles left over were removed. The resected specimen was scanned using the same procedure above with the LCT-200. The scanned images were reconstructed in 3D and analyzed using the free, open source software, 3D slicer (version 4.10.2, https://www.slicer.org/). The length of the tibia (LT µm) was defined as the distance from the upper extremity of the left tibia to the medial malleolus. The volume of the left lower hindlimb muscle (V µm3) was measured as the quantity of voxels with densities within the range of (−140) – (+350) Hounsfield Units according to the definition in the LCT-200 software. We used a definition of corrected muscle volume (VC µm) for analysis, considering the difference in body length, as appendicular skeletal muscle mass adjusted by height squared (appendicular skeletal muscle mass index) for evaluating sarcopenia in humans.31,32 The corrected muscle volume was calculated with the following formula: VC = V/(LT)2.
Analysis of Muscle Fiber Characteristics
The posterior muscles of the lower hindlimbs were dissected after CT scanning, embedded in paraffin, and sectioned at a thickness of 3 μm. After deparaffinization, the sections were heated in citrate buffer (10 mM sodium citrate, pH 6.0) for antigen retrieval. Subsequently, they were preincubated for 10 min in Blocking One Histo (Nacalai Tesque, Kyoto, Japan) at room temperature and incubated overnight at 4°C with primary antibodies for slow-type myosin heavy chain (sMyHC) (1:1000, ab11083; Abcam) and dystrophin (1:100, ab15277; Abcam). Secondary antibody incubation was performed with Alexa Fluor 488- (1:500, ab150113; Abcam) and Alexa Fluor 594- (1:500, ab150080; Abcam) conjugated antibodies for 2 h at room temperature in the dark to detect sMyHC and dystrophin, respectively. Specimens were mounted in ProLong Gold Antifade Mountant (Thermo Fisher Scientific). Digitized images of immunolabeled sections were obtained using a camera housing attached to a fluorescence microscope, BX53/BX3-URA/DP70 (Olympus Co., Tokyo, Japan) for analysis of the distribution of myofiber type. All fibers composing the soleus on each section were evaluated. The type I fiber mass was quantified as the ratio of the number of sMyHC-positive fibers to that of all myofibers.
Statistical Analysis
Data are expressed as the mean ± standard error or in scatter plots with the mean. Differences were evaluated using two-way analysis of variance (ANOVA) with two factors (exposure: air or smoke; diet: vehicle or NYT) for multiple-group comparisons. Post-hoc comparison was assessed with the Tukey–Kramer test when a significant interaction between exposure and diet was detected. Statistical significance was accepted at p < 0.05. All statistical analyses were performed using GraphPad Prism 7.04 (GraphPad Software, San Diego, CA, USA).
Results
Daily Food Consumption and Changes in Body Weight
One mouse from the smoking group and another from the smoking + NYT group were excluded from the analysis due to death from unknown causes after cigarette smoke exposure. The average daily consumption of food per mouse was calculated by weekly measurement of food remaining in the cage. Food consumption during the entire experimental period was 3.39 ± 0.040, 3.39 ± 0.027, 2.92 ± 0.024, and 3.09 ± 0.035 g/mouse/day for the control, NYT, smoking, and smoking + NYT groups, respectively. Although the cigarette smoke-exposed groups appeared to have lower consumption, more accurate measurement will be necessary to confirm this observation. Weekly body weight measurements for each group are shown in Figure 1A. Body weights steadily increased during the 12 weeks. Two-way ANOVA for body weight at the 12th week showed a significant difference in exposure (F (1, 26) = 6.126, p = 0.020), but not in diet (F (1, 26) = 1.833, p = 0.19) (Figure 1B). No interaction was detected between exposure and diet (F (1, 26) = 0.947, p = 0.34).
 |
Figure 1 Changes in body weight over time and at week 12. |
Smoke Exposure Induced Alveolar Inflammatory Cell Infiltration and Emphysema
Representative images of cells in BALF are shown in Figure 2A. The cell population analysis revealed a significant effect of exposure on increasing the number of inflammatory cells in BALF (total cell count: F (1, 26) = 71.121, p < 0.001; number of neutrophils: F (1, 26) = 124.265, p < 0.001) (Figure 2B and C). On the other hand, neither an effect of diet nor an interaction between exposure and diet was detected (total cell count: F (1, 26) = 1.971, p = 0.17 and F (1, 26) = 0.014, p = 0.91; number of neutrophils: F (1, 26) = 1.165, p = 0.29 and F (1, 26) = 1.064, p = 0.31). Smoke exposure also resulted in air space enlargement and alveolar destruction (Figure 3A). Two-way ANOVA showed a significant effect of exposure (MLI: F (1, 26) = 5.369, p = 0.029; DI: F (1, 26) = 12.437, p < 0.001) (Figure 3B and C). Neither morphological indication was affected by diet (MLI: F (1, 26) = 0.255, p = 0.62; DI: F (1, 26) = 3.257, p = 0.083). However, an interaction of exposure and diet was detected only for the DI (MLI: F (1, 26) = 0.050, p = 0.83; DI: F (1, 26) = 9.679, p = 0.0045). The post-hoc test revealed that exposure to cigarette smoke for 12 weeks caused a significant increase in DI in the lungs of mice in the smoking group compared to the control group (p < 0.001), and the lung tissues from the smoking + NYT group showed a lower DI than tissues from the smoking group (p = 0.012).
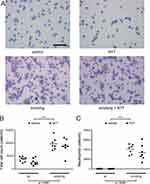 |
Figure 2 Total cell count and proportion of neutrophils in BALF. |
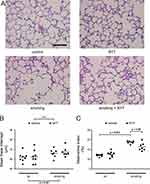 |
Figure 3 MLI and DI for the mice in each group. |
PGC-1α Expression Levels Were Increased in the Lower Leg Muscle of NYT-Fed Mice
We performed qPCR with homogenates of the lower leg muscle to evaluate the mRNA expression level of PGC-1α. Two-way ANOVA showed a significant difference in diet (F (1, 26) = 13.834, p = 0.0010), but not in exposure (F (1, 26) = 1.225, p = 0.28) (Figure 4A). No interaction was detected between exposure and diet (F (1, 26) = 1.436, p = 0.24). Representative images of Western blot signals from the same gel are shown in Figure 4B. The result showed a similar tendency as that of mRNA (exposure: F (1, 24) = 1.098, p = 0.31; diet: F (1, 24) = 5.714, p = 0.025; exposure–diet interaction: F (1, 24) = 1.416, p = 0.25) (Figure 4C), which revealed upregulation of PGC-1α following NYT intake.
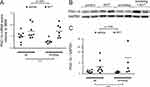 |
Figure 4 PGC-1α expression in the muscle homogenate. |
NYT Ameliorated the Loss of Muscle Mass in Lower Hindlimbs Induced by Cigarette Smoke
Representative reconstructed CT images of lower hindlimb muscles are shown in Figure 5A. The results of two-way ANOVA for VC were as follows: exposure: F (1, 26) = 4.105, p = 0.053; diet: F (1, 26) = 3.578, p = 0.070; exposure–diet interaction: F (1, 26) = 8.994, p = 0.0059 (Figure 5B). Post-hoc analysis was performed after detection of the significant interaction. Cigarette smoke significantly decreased muscle mass in the vehicle-fed groups (the control and smoking groups) (p = 0.0076). In contrast, no significant change was detected between the NYT-fed groups (the NYT and smoking + NYT groups). In addition, NYT feeding ameliorated the loss of muscle volume in the smoking groups (the smoking and smoking + NYT groups) (p = 0.013).
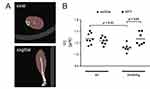 |
Figure 5 Volumetric evaluation of lower hindlimb muscle. |
Muscle Fiber Shift Toward Type II Fibers Was Suppressed by NYT
Representative immunofluorescence staining images of the soleus muscle from each group are shown in Figure 6A. Distribution analysis of soleus muscle fibers revealed a significant effect of diet (F (1, 26) = 4.664, p = 0.040) and an interaction between exposure and diet (F (1, 26) = 4.873, p = 0.036), but did not show a significant effect of exposure (F (1, 26) = 1.946, p = 0.17) (Figure 6B). According to post-hoc tests, sMyHC-positive fibers increased significantly as a simple effect of the NYT diet in the cigarette smoke-exposed groups (the smoking and smoking + NYT groups) (p = 0.029), but no change was detected between the control and NYT groups. Furthermore, the reduction in sMyHC-positive fibers in the control compared to the smoking groups was close to significant (p = 0.076), suggesting that cigarette smoke caused a muscle fiber shift toward type II fibers in smoking model mice.
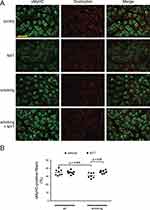 |
Figure 6 Determination of myofiber type distribution in soleus muscle. |
Discussion
Our present study provides three key findings. First, cigarette smoke exposure results in reduced skeletal muscle volume and slow-twitch muscle fibers in addition to development of pulmonary emphysema. Second, treatment with NYT increases PGC-1α expression in muscle tissue. Third, NYT ingestion suppresses smoke-induced loss of muscle mass and alteration in the distribution of muscle fibers, although NYT does not have a strong effect on ameliorating pulmonary emphysema.
We exposed the mice to cigarette smoke by using a well-characterized nose-only smoke exposure system. We began with BALF analysis and morphological analysis of the lung to validate our mice as a COPD model. The cigarette smoke-exposed mice showed less weight gain, increased inflammatory cell infiltration in BALF, and development of emphysema characterized by alveolar enlargement and greater destruction of alveoli, similar to mice in a previous report.23,33,34 Our results showed a discrepancy regarding the effect of NYT on smoking-induced change in pulmonary morphology: NYT induced partial recovery of alveolar damage as indicated by the DI; however, NYT did not reduce air space enlargement as indicated by the MLI. Some reports showed that the DI is a more sensitive parameter of pulmonary destruction than the MLI,30,35 whereas others revealed a correlation between the MLI and loss of elastic recoil.36,37 Considering this difference in morphological parameters assessed with the DI and MLI, our results suggest that NYT has some favorable effects on localized damage to the alveolar wall including epitheliums; however, NYT had no effects on other connective or supportive structures such as elastic fibers, although the specific effect of NYT on each type of cells remains to be confirmed. We finally concluded that NYT did not have sufficient efficacy in suppressing pulmonary emphysema, because emphysema is defined as coexistence of destroyed alveolar wall and air space enlargement.2 Skeletal muscles in COPD patients exhibit poor contractile performance, reduced endurance, low capacity for muscle aerobic metabolism, and atrophy, resulting in exercise intolerance and limited physical activity.38 According to the International European Working Group on Sarcopenia in Older People (EWGSOP) criteria revised in 2018 (EWGSOP2), COPD patients with low muscle strength and low muscle quantity or quality are susceptible to secondary sarcopenia.31,39 Additionally, EWGSOP2 newly defined “severe” sarcopenia as poor physical performance, which predicts higher mortality. In contrast to primary sarcopenia,40 which occurs with only aging and no other specific cause, secondary sarcopenia with COPD includes a shift in the distribution of skeletal muscle fiber type from type I to type II fibers.16,17 Furthermore, patients with sarcopenia, which is defined by a low appendicular skeletal muscle mass index, show a decreased proportion and lower size of type I fibers than non-sarcopenic COPD patients.41 The muscular characteristics similar to those observed in human COPD patients as mentioned above have been reported in cigarette smoke-exposed rodent models. The muscle weight and oxidative fiber mass of the tibialis anterior are lower in male BALB/c mice exposed to cigarette smoke for 8 weeks.42 Another group reported that 16-week exposure of male Sprague-Dawley rats to cigarette smoke leads to less contractility of tibialis anterior muscles and a reduced cross-sectional area of gastrocnemius muscles, as well as emphysematous changes in the lung, elevated inflammatory factors in the serum, and ubiquitin protease induction in muscle tissue.43 Additionally, in the present study, we showed a reduction in muscle mass and sMyHC-positive fibers in the smoke-exposed mice. Considering these data, rodents exposed to cigarette smoke for several months represent a well-established model for sarcopenia with COPD.
Although the underlying pathophysiology of muscular impairment in COPD remains to be completely elucidated, some mechanisms have been described. For example, the loss of muscle endurance is explained by decreased oxidative capacity of the muscle, reflecting pathological alterations in mitochondrial function.44 A fiber-type shift toward type II is also associated with mitochondrial impairment in COPD, because type I fibers contain mitochondria at higher density.45 PGC-1α is a key regulating factor of mitochondrial biogenesis.18 PGC-1α has the ability to redirect fiber-type distribution in skeletal muscle to a more oxidative phenotype or a type I-rich phenotype.46 Transgenic expression of PGC-1α in mice protects mice against muscle atrophy induced by denervation47 or hindlimb unloading.48 Furthermore, PGC-1α expression is reduced in the quadriceps of COPD patients with low body mass indices and low fat-free mass indices.19
NYT is a Japanese herbal medicine used to facilitate recovery from diseases or postoperative fatigue and is indicated in patients with anemia or symptoms such as anorexia and fatigue.49 Because of its efficacy, NYT is often used in the elderly. According to a post-marketing surveillance study,50 NYT is well tolerated with an incidence of adverse reactions in 3.1% of patients; only one patient experienced non-mild constipation causally related to the administration of NYT. The significant effectiveness of NYT in fatigue, malaise, and anorexia was revealed based on visual analog scale scores. In addition, NYT ameliorates skeletal muscle reduction and symptoms of frailty. NYT-fed melanoma tumor-bearing model mice show improved protein degradation in the quadriceps muscle via recovery of myogenetic signals.25 NYT feeding restores the loss of gastrocnemius and soleus muscle volume in Klotho-deficient senescence-accelerated mice.26 A recent clinical trial revealed that that NYT improves symptoms and quality of life in COPD patients with frailty.51 Additionally, previous reports have revealed that some of the crude components of NYT, namely the fruit of Schisandra chinensis and roots of Panax ginseng, potently induce muscle energy metabolism. Schisandra fruit upregulates PGC-1α expression in the rat gastrocnemius and soleus muscles, extending the running endurance time and activating energy metabolism.27 Ginsenoside from ginseng activates adenosine monophosphate-activated protein kinase, which phosphorylates PGC-1α.28
Based on these data, upregulating PGC-1α is likely a potent strategy for attenuating the muscle complications in COPD, and NYT may improve muscular function via PGC-1α expression. Hence, we hypothesized that NYT ameliorated the loss of muscle volume and function in COPD mice via PGC-1α upregulation. The results in the present study support this hypothesis; that is, an NYT-containing diet facilitated the expression level of PGC-1α in lower hindlimb muscles and salvaged the reduction in muscle volume and the number of sMyHC-positive fibers induced by smoke exposure.
COPD is the third leading cause of death worldwide.52 Muscle dysfunction is an important systemic consequence of COPD because it has a large impact on mortality in patients with this disease.53 Muscle dysfunction is also likely to induce a lower physical activity level, which is the factor that is most associated with COPD mortality.13 Moreover, a recent study using data from the Nationwide Inpatient Sample in the United States reported that COPD patients with a muscle loss phenotype show higher in-hospital mortality, longer length of stay, and greater healthcare cost when they are admitted for a COPD exacerbation.54 These data emphasize the importance of developing targeted interventions to improve muscle dysfunction in COPD. Whether muscle dysfunction in COPD is a simple result of disuse due to physical inactivity or a disease-specific myopathy remains controversial.17 Skeletal muscle dysfunction in COPD has been considered to be the result of a sedentary lifestyle that is commonly observed in these patients.55 Although disuse in COPD patients plays a significant role in inducing muscle dysfunction, at least to some extent, several observations argue against the perception that disuse is the only mechanism. One example is structural differences in muscle, including the proportion of the types of fibers in patients with COPD; COPD patients have a lower number of type I fibers compared with healthy subjects with comparable low physical activity.17,56 In this context, we showed in the present study that NYT improves “myopathic” muscle dysfunction in COPD. Exercise-based intervention is promising from the aspect of disuse, but it is challenging for COPD patients. In a randomized controlled trial, the effectiveness of an exercise intervention to increase physical activity measured by daily steps in patients with COPD was significant in per-protocol analysis, whereas the efficacy disappeared in intention-to-treatment analysis. The authors speculated that patients who are unwilling and self-reported non-adherent do not have efficacy for exercise training.57 The reason why COPD patients have difficulty beginning or completing an exercise-based intervention is partially explained by symptoms and frailty.58 These studies suggest that medications that affect myopathy in COPD may remove patients from the vicious circle of inactivity and facilitate the efficacy of exercise by ameliorating symptoms or improving exercise tolerance. Several interventions are oriented toward improving myopathic dysfunction in patients with COPD by supplementation with potential therapeutic substances such as nutrition support,59 growth hormone,60 and creatine.61 However, these strategies per se have a very limited beneficial effect, especially without concurrent physical exercise.62 On the other hand, our results suggest that NYT may improve muscle dysfunction even without interventional exercise.
Beyond the impact on muscle function, exercise-based intervention will improve COPD itself. We previously reported that irisin, a myokine mainly released by skeletal muscles in response to exercise via stimulation of PGC-1α, suppresses features of COPD in vitro and in vivo. We showed that irisin significantly reduces apoptosis induced by cigarette smoke extract in A549 cells.22 We also found that treadmill exercise suppresses cigarette smoke-induced alveolar enlargement and destruction in COPD model mice via the irisin-accelerated antioxidative pathway.23 Other groups have also reported the effect of exercise on suppressing COPD and have focused on different mechanisms other than irisin.63,64 NYT may be considered an ergogenic medicine to enhance these favorable effects of exercise.
This study has some limitations. First, we did not measure plasma levels of metabolites of NYT in the mice. Therefore, we could not examine the bioavailability of NYT or the dose-response of NYT. Second, whether the NYT dose used in this study is relevant in humans is unclear. The concentration of NYT (3% w/w) in the diet was taken from a previous study.26 Further research is needed to clarify the optimal effective concentration of NYT. Third, we did not examine how cigarette smoke affects muscle volume loss other than via PGC-1α. From the results showing a significant simple main effect of smoke exposure on decreasing muscle volume, other mechanisms are assumed, although no significant main effect of smoke on decreased PGC-1α expression was seen. Nevertheless, we confirmed that NYT suppressed muscle complications in COPD model mice regardless of the direction of the causal relationship between the muscle condition and PGC-1α expression.
In summary, NYT improved muscular complications in COPD model mice in the present study. NYT may play a role as a complementary medicine, especially in combination with physical exercise, in treating muscle dysfunction in COPD and further by ameliorating pulmonary damage caused by smoking.
Conclusion
COPD is associated with muscular complications, which lead to less physical activity and increased mortality. NYT increases PGC-1α expression in the muscle of COPD model mice and is involved in suppressing cigarette smoke-induced muscle volume loss and slow-twitch oxidative fiber reduction. NYT ingestion may be a novel prophylactic and therapeutic strategy for muscular complications in COPD.
Abbreviations
ANOVA, analysis of variance; BAL, bronchoalveolar lavage; BALF, bronchoalveolar lavage fluid; COPD, chronic obstructive pulmonary disease; CT, computed tomography; DI, destructive index; EWGSOP, European Working Group on Sarcopenia in Older People; EWGSOP2, EWGSOP criteria revised in 2018; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; MLI, mean linear intercept; NYT, ninjin’yoeito; PGC-1α, peroxisome proliferator-activated receptor γ coactivator-1α; sMyHC, slow-type myosin heavy chain.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Consent for Publication
Not applicable.
Acknowledgments
Real-time PCR analysis, Western blot analysis and histological analysis with immunofluorescence staining were performed at the Research Support Platform of Osaka City University Graduate School of Medicine. The authors would like to thank Kracie Pharma Ltd for providing NYT.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This work was partially supported by JSPS KAKENHI (Grant-in-Aid for Scientific Research [C]) Grant Number 19K08660 to K. A.
Disclosure
The authors report no competing of interest in this work.
References
1. Eisner MD, Anthonisen N, Coultas D, et al. An official American Thoracic Society public policy statement: novel risk factors and the global burden of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;182(5):693–718. doi:10.1164/rccm.200811-1757ST
2. Hogg JC, Timens W. The pathology of chronic obstructive pulmonary disease. Annu Rev Pathol. 2009;4:435–459. doi:10.1146/annurev.pathol.4.110807.092145
3. Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009;33(5):1165–1185. doi:10.1183/09031936.00128008
4. Miller J, Edwards LD, Agustí A, et al. Comorbidity, systemic inflammation and outcomes in the ECLIPSE cohort. Respir Med. 2013;107(9):1376–1384. doi:10.1016/j.rmed.2013.05.001
5. Jones SE, Maddocks M, Kon SS, et al. Sarcopenia in COPD: prevalence, clinical correlates and response to pulmonary rehabilitation. Thorax. 2015;70(3):213–218. doi:10.1136/thoraxjnl-2014-206440
6. Montes de Oca M, Torres SH, De Sanctis J, Mata A, Hernández N, Tálamo C. Skeletal muscle inflammation and nitric oxide in patients with COPD. Eur Respir J. 2005;26(3):390–397. doi:10.1183/09031936.05.00107404
7. Barreiro E, Gea J, Corominas JM, Hussain SN. Nitric oxide synthases and protein oxidation in the quadriceps femoris of patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2003;29(6):771–778. doi:10.1165/rcmb.2003-0138OC
8. de Theije C, Costes F, Langen RC, Pison C, Gosker HR. Hypoxia and muscle maintenance regulation: implications for chronic respiratory disease. Curr Opin Clin Nutr Metab Care. 2011;14(6):548–553. doi:10.1097/MCO.0b013e32834b6e79
9. O’Donnell DE, James MD, Milne KM, Neder JA. The pathophysiology of dyspnea and exercise intolerance in Chronic Obstructive Pulmonary Disease. Clin Chest Med. 2019;40(2):343–366. doi:10.1016/j.ccm.2019.02.007
10. Bossenbroek L, de Greef MHG, Wempe JB, Krijnen WP, Ten Hacken NHT. Daily physical activity in patients with chronic obstructive pulmonary disease: a systematic review. COPD. 2011;8(4):306–319. doi:10.3109/15412555.2011.578601
11. Mostert R, Goris A, Weling-Scheepers C, Wouters EF, Schols AM. Tissue depletion and health related quality of life in patients with chronic obstructive pulmonary disease. Respir Med. 2000;94(9):859–867. doi:10.1053/rmed.2000.0829
12. Gimeno-Santos E, Frei A, Steurer-Stey C, et al. Determinants and outcomes of physical activity in patients with COPD: a systematic review. Thorax. 2014;69(8):731–739. doi:10.1136/thoraxjnl-2013-204763
13. Waschki B, Kirsten A, Holz O, et al. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest. 2011;140(2):331–342. doi:10.1378/chest.10-2521
14. Jaitovich A, Barreiro E. Skeletal muscle dysfunction in Chronic Obstructive Pulmonary Disease. What we know and can do for our patients. Am J Respir Crit Care Med. 2018;198(2):175–186. doi:10.1164/rccm.201710-2140CI
15. Puente-Maestu L, Pérez-Parra J, Godoy R, et al. Abnormal mitochondrial function in locomotor and respiratory muscles of COPD patients. Eur Respir J. 2009;33(5):1045–1052. doi:10.1183/09031936.00112408
16. Jobin J, Maltais F, Doyon JF, et al. Chronic obstructive pulmonary disease: capillarity and fiber-type characteristics of skeletal muscle. J Cardiopulm Rehabil. 1998;18(6):432–437. doi:10.1097/00008483-199811000-00005
17. Couillard A, Prefaut C. From muscle disuse to myopathy in COPD: potential contribution of oxidative stress. Eur Respir J. 2005;26(4):703–719.
18. Koves TR, Li P, An J, et al. Peroxisome proliferator-activated receptor-gamma co-activator 1alpha-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J Biol Chem. 2005;280(39):33588–33598. doi:10.1074/jbc.M507621200
19. Remels AH, Schrauwen P, Broekhuizen R, et al. Peroxisome proliferator-activated receptor expression is reduced in skeletal muscle in COPD. Eur Respir J. 2007;30(2):245–252. doi:10.1183/09031936.00144106
20. Mahgoub MO, D’Souza C, Al Darmaki R, Baniyas M, Adeghate E. An update on the role of irisin in the regulation of endocrine and metabolic functions. Peptides. 2018;104:15–23. doi:10.1016/j.peptides.2018.03.018
21. Ijiri N, Kanazawa H, Asai K, Watanabe T, Hirata K. Irisin, a newly discovered myokine, is a novel biomarker associated with physical activity in patients with chronic obstructive pulmonary disease. Respirology. 2015;20(4):612–617. doi:10.1111/resp.12513
22. Sugiyama Y, Asai K, Yamada K, et al. Decreased levels of irisin, a skeletal muscle cell-derived myokine, are related to emphysema associated with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2017;12:765–772. doi:10.2147/COPD.S126233
23. Kubo H, Asai K, Kojima K, et al. Exercise ameliorates emphysema of cigarette smoke-induced COPD in mice through the exercise-Irisin-Nrf2 axis. Int J Chron Obstruct Pulmon Dis. 2019;14:2507–2516. doi:10.2147/COPD.S226623
24. Uto NS, Amitani H, Atobe Y, et al. Herbal medicine Ninjin’yoeito in the treatment of sarcopenia and frailty. Front Nutr. 2018;5:126. doi:10.3389/fnut.2018.00126
25. Ohsawa M, Maruoka J, Inami C, Iwaki A, Murakami T, Ishikura KI. Effect of Ninjin’yoeito on the loss of skeletal muscle function in cancer-bearing mice. Front Pharmacol. 2018;9:1400. doi:10.3389/fphar.2018.01400
26. Takahashi R, Chiba S, Takemoto R, Michihara S, Han LK, Hujita H. Ninjin’yoeito improves survival and aging phenotype on accelerated aging model (in Japanese). Jpn J Psychosom Int Med. 2018;22(1):16–19.
27. Kim YJ, Yoo SR, Chae CK, Jung UJ, Choi MS. Omija fruit extract improves endurance and energy metabolism by upregulating PGC-1α expression in the skeletal muscle of exercised rats. J Med Food. 2014;17(1):28–35. doi:10.1089/jmf.2013.3071
28. Park MW, Ha J, Chung SH. 20(S)-ginsenoside Rg3 enhances glucose-stimulated insulin secretion and activates AMPK. Biol Pharm Bull. 2008;31(4):748–751. doi:10.1248/bpb.31.748
29. Thurlbeck WM. The internal surface area of nonemphysematous lungs. Am Rev Respir Dis. 1967;95(5):765–773.
30. Saetta M, Shiner RJ, Angus GE, et al. Destructive index: a measurement of lung parenchymal destruction in smokers. Am Rev Respir Dis. 1985;131(5):764–769.
31. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31.
32. Kim KM, Jang HC, Lim S. Differences among skeletal muscle mass indices derived from height-, weight-, and body mass index-adjusted models in assessing sarcopenia. Korean J Intern Med. 2016;31(4):643–650. doi:10.3904/kjim.2016.015
33. Kojima K, Asai K, Kubo H, et al. Isoflavone aglycones attenuate cigarette smoke-induced emphysema via suppression of neutrophilic inflammation in a COPD murine model. Nutrients. 2019;11(9):2023. doi:10.3390/nu11092023
34. Kubo H, Asai K, Kojima K, et al. Astaxanthin suppresses cigarette smoke-induced emphysema through Nrf2 activation in mice. Mar Drugs. 2019;17(12):673. doi:10.3390/md17120673
35. Eidelman DH, Ghezzo H, Kim WD, Cosio MG. The destructive index and early lung destruction in smokers. Am Rev Respir Dis. 1991;144(1):156–159. doi:10.1164/ajrccm/144.1.156
36. Greaves IA, Colebatch HJ. Elastic behavior and structure of normal and emphysematous lungs post mortem. Am Rev Respir Dis. 1980;121(1):127–136.
37. Verbeken EK, Cauberghs M, Mertens I, Clement J, Lauweryns JM, Van de Woestijne KP. The senile lung. Comparison with normal and emphysematous lungs. 2. Functional aspects. Chest. 1992;101(3):800–809. doi:10.1378/chest.101.3.800
38. Maltais F, Decramer M, Casaburi R, et al. An official American Thoracic Society/European Respiratory Society statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189(9):e15–62. doi:10.1164/rccm.201402-0373ST
39. Benz E, Trajanoska K, Lahousse L, et al. Sarcopenia in COPD: a systematic review and meta-analysis. Eur Respir Rev. 2019;28(154):13.
40. Larsson L. Histochemical characteristics of human skeletal muscle during aging. Acta Physiol Scand. 1983;117(3):469–471. doi:10.1111/j.1748-1716.1983.tb00024.x
41. van de Bool C, Gosker HR, van den Borst B, Op den Kamp CM, Slot IGM, Schols AMWJ. Muscle quality is more impaired in sarcopenic patients with Chronic Obstructive Pulmonary Disease. J Am Med Dir Assoc. 2016;17(5):415–420. doi:10.1016/j.jamda.2015.12.094
42. Chan SMH, Cerni C, Passey S, et al. Cigarette smoking exacerbates skeletal muscle injury without compromising its regenerative capacity. Am J Respir Cell Mol Biol. 2020;62(2):217–230. doi:10.1165/rcmb.2019-0106OC
43. Su J, Li J, Lu Y, et al. The rat model of COPD skeletal muscle dysfunction induced by progressive cigarette smoke exposure: a pilot study. BMC Pulm Med. 2020;20(1):74.
44. Taivassalo T, Hussain SNA. Contribution of the mitochondria to locomotor muscle dysfunction in patients with COPD. Chest. 2016;149(5):1302–1312. doi:10.1016/j.chest.2015.11.021
45. Puente-Maestu L, Lázaro A, Humanes B. Metabolic derangements in COPD muscle dysfunction. J Appl Physiol (1985). 2013;114(9):1282–1290.
46. Lin J, Wu H, Tarr PT, et al. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418(6899):797–801. doi:10.1038/nature00904
47. Sandri M, Lin J, Handschin C, et al. PGC-1alpha protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc Natl Acad Sci U S A. 2006;103(44):16260–16265. doi:10.1073/pnas.0607795103
48. Cannavino J, Brocca L, Sandri M, Bottinelli R, Pellegrino MA. PGC1-α over-expression prevents metabolic alterations and soleus muscle atrophy in hindlimb unloaded mice. J Physiol. 2014;592(20):4575–4589. doi:10.1113/jphysiol.2014.275545
49. Morinaga A, Nakamura H, Hattanmaru K, Rokot NT, Kimura Y, Ito T. Good rehabilitation outcomes and improved nutritional status after treatment with the japanese herbal medicine Ninjin’yoeito in an elderly patient with hip fracture and sarcopenia: a case report. Front Nutr. 2020;7:85. doi:10.3389/fnut.2020.00085
50. Suzuki S, Aihara F, Shibahara M, Sakai K. Safety and effectiveness of Ninjin’yoeito: a utilization study in elderly patients. Front Nutr. 2019;6:14. doi:10.3389/fnut.2019.00014
51. Hirai K, Homma T, Matsunaga T, et al. Usefulness of Ninjin’yoeito for Chronic Obstructive Pulmonary Disease patients with frailty. J Altern Complement Med. 2020;26(8):750–757. doi:10.1089/acm.2020.0083.
52. The top 10 causes of death[homepage on the internet]. World Health Organization; 2018. Available from: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
53. Swallow EB, Reyes D, Hopkinson NS, et al. Quadriceps strength predicts mortality in patients with moderate to severe chronic obstructive pulmonary disease. Thorax. 2007;62(2):115–120. doi:10.1136/thx.2006.062026
54. Attaway AH, Welch N, Hatipoğlu U, Zein JG, Dasarathy S. Muscle loss contributes to higher morbidity and mortality in COPD: an analysis of national trends. Respirology. 2020. doi:10.1111/resp.13877.
55. Young A. Rehabilitation of patients with pulmonary disease. Ann Acad Med Singapore. 1983;12(3):410–416.
56. Gifford JR, Trinity JD, Kwon OS, et al. Altered skeletal muscle mitochondrial phenotype in COPD: disease vs. disuse. J Appl Physiol (1985). 2018;124(4):1045–1053. doi:10.1152/japplphysiol.00788.2017
57. Arbillaga-Etxarri A, Gimeno-Santos E, Barberan-Garcia A, et al. Long-term efficacy and effectiveness of a behavioural and community-based exercise intervention (Urban Training) to increase physical activity in patients with COPD: a randomised controlled trial. Eur Respir J. 2018;52(4):1800063. doi:10.1183/13993003.00063-2018
58. Robinson H, Williams V, Curtis F, Bridle C, Jones AW. Facilitators and barriers to physical activity following pulmonary rehabilitation in COPD: a systematic review of qualitative studies. NPJ Prim Care Respir Med. 2018;28(1):19. doi:10.1038/s41533-018-0085-7
59. Steiner MC, Barton RL, Singh SJ, Morgan MD. Nutritional enhancement of exercise performance in chronic obstructive pulmonary disease: a randomised controlled trial. Thorax. 2003;58(9):745–751. doi:10.1136/thorax.58.9.745
60. Burdet L, de Muralt B, Schutz Y, Pichard C, Fitting JW. Administration of growth hormone to underweight patients with chronic obstructive pulmonary disease. A prospective, randomized, controlled study. Am J Respir Crit Care Med. 1997;156(6):1800–1806. doi:10.1164/ajrccm.156.6.9704142
61. Fuld JP, Kilduff LP, Neder JA, et al. Creatine supplementation during pulmonary rehabilitation in chronic obstructive pulmonary disease. Thorax. 2005;60(7):531–537. doi:10.1136/thx.2004.030452
62. Ferreira IM, Brooks D, White J, Goldstein R. Nutritional supplementation for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;12:Cd000998.
63. Toledo-Arruda AC, Vieira RP, Guarnier FA, et al. Time-course effects of aerobic physical training in the prevention of cigarette smoke-induced COPD. J Appl Physiol (1985). 2017;123(3):674–683. doi:10.1152/japplphysiol.00819.2016
64. Rodrigues Brandao-Rangel MA, Bachi ALL, Oliveira-Junior MC, et al. Exercise inhibits the effects of smoke-induced COPD involving modulation of STAT3. Oxid Med Cell Longev. 2017;2017:1–13. doi:10.1155/2017/6572714.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
