Back to Journals » Cancer Management and Research » Volume 12
Nimotuzumab Combined with Induction Chemotherapy and Concurrent Chemoradiotherapy in Unresectable Locally Advanced Hypopharyngeal Carcinoma: A Single Institution Experience in China
Authors Tian X, Xuan Y, Wu R, Gao S
Received 5 February 2020
Accepted for publication 21 April 2020
Published 11 May 2020 Volume 2020:12 Pages 3323—3329
DOI https://doi.org/10.2147/CMAR.S248392
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Rudolph Navari
Xin Tian, Ying Xuan, Rong Wu, Song Gao
Department of Clinical Oncology, Shengjing Hospital of China Medical University, Shenyang, Liaoning 110004, People’s Republic of China
Correspondence: Song Gao
Department of Clinical Oncology, Shengjing Hospital of China Medical University, 36 San Hao Street, Shenyang 110004, People’s Republic of China
Email [email protected]
Objective: To investigate the curative and adverse effects (AEs) of additional use of nimotuzumab combined with induction chemotherapy and concurrent chemoradiotherapy in unresectable locoregionally advanced hypopharyngeal carcinoma.
Patients and Methods: We retrospectively evaluated 36 patients with stage III or IVA hypopharyngeal carcinoma who received induction chemotherapy followed by concurrent chemoradiotherapy with or without nimotuzumab. The induction chemotherapy included two or three cycles of TPF regimen. The intensity-modulated radiation therapy (IMRT) dose was 70 Gy to the planning target volume. Concurrent with radiotherapy, patients received chemotherapy consisting of cisplatin q3w. Adjuvant chemotherapy consisting of TPF regimen was administered 1 month later after concurrent chemoradiotherapy. Nimotuzumab (200 mg day 1, q3w) was given to patients concurrently with induction chemotherapy and was administered concurrently with IMRT at a weekly dose of 200 mg.
Results: After induction chemotherapy, the objective response rate in patients treated with nimotuzumab (group A) versus those treated without nimotuzumab (group B) was 91.7% versus 58.3% (p=0.029). After concurrent chemoradiotherapy, the objective response rate was 95.8% in group A versus 83.3% in group B (p=0.253). The median follow-up was 22.6 months (range 8.9– 39.5 months). The 2-year OS rate in group A and group B were 62.5% (95% CI 55– 70%) and 51.8% (95% CI 45– 59%), respectively, the 2-year OS rate in group A was better than group B, P< 0.05. PFS was 23 months (95% CI 19– 27) in group A versus 18 months (95% CI 12– 22) in group B, PFS was longer in group A than group B, P< 0.05. There was no significant difference in AEs between the two groups.
Conclusion: Additional use of nimotuzumab combined with induction chemotherapy and concurrent chemoradiotherapy in unresectable locoregionally advanced hypopharyngeal carcinoma yielded better short-term efficacy, also may improve overall survival and progression-free survival than patients without using nimotuzumab. The toxicity was tolerable.
Keywords: nimotuzumab, induction chemotherapy, chemoradiotherapy, unresectable, locoregionally advanced, hypopharyngeal carcinoma
Introduction
Hypopharyngeal carcinoma is rare and accounts for 4% of all head and neck cancers and 0.5% of all the human malignant tumors, and its incidence, along with aging populations is increasing.1 Because of its special anatomical position and varied clinical manifestations, most of the cases present in a locally advanced stage that is unresectable, and it often tends to recur locally or develops distant metastasis.2 This is leading to a large number of social and economic burdens.3
The patients with locally advanced unresectable hypopharyngeal cancer are often treated with concurrent chemoradiotherapy and adjuvant chemotherapy with the aim of reducing local recurrence and distant metastasis.4 Unfortunately, following concurrent chemoradiotherapy and adjuvant chemotherapy, the survival rates are not optimal.5 In recent years, increasing evidence has indicated that nimotuzumab combined with induction chemotherapy, followed by concurrent chemoradiotherapy, is feasible and results in better local control and overall survival (OS) rate.6,7 Induction chemotherapy theoretically has the advantages of reducing tumor volume, shrinking radiotherapy target volume, improving radiotherapy efficacy and reducing adverse effects (AEs).8 A few clinical trials have shown encouraging results with non-surgical management, including concurrent chemoradiotherapy, concurrent chemoradiotherapy with epidermal growth factor receptor (EGFR) inhibitor cetuximab, or induction chemotherapy followed by concurrent chemoradiotherapy with/without cetuximab.1,9,10
In the present study, we retrospectively analyzed 36 patients with stage III or IVA hypopharyngeal cancer, who received induction chemotherapy followed by concurrent chemoradiotherapy combined with or without nimotuzumab. The primary research aim of the study was to investigate whether additional use of nimotuzumab with induction chemotherapy and concurrent chemoradiotherapy could benefit patients with unresectable locoregionally advanced hypopharyngeal cancer.
Methods
Patient Eligibility
We retrospectively evaluated 36 patients with stage III or IVA hypopharyngeal cancer, who received induction chemotherapy followed by concurrent chemoradiotherapy combined with or without nimotuzumab between January 2015 and September 2016 in the Department of Clinical Oncology, Shengjing Hospital of China Medical University. All patients had histologically proven hypopharyngeal squamous cell carcinoma and the tumor was unresectable. The inclusion criteria were: 18–70 years age; squamous cell carcinoma; stage III/IVA hypopharyngeal cancer [according to the 2010 American Joint Committee on Cancer (AJCC) staging system for hypopharyngeal cancer]; availability of complete medical data; adequate hematological, renal and hepatic function; Karnofsky score ≥70. The exclusion criteria were: history of other malignant diseases; serious concomitant illness (eg, liver cirrhosis, angina, or myocardial disease); pre-existing treatment with radiotherapy, chemotherapy or EGFR inhibitors; hypopharyngeal cancer-unrelated death. All the 36 patients with stage III or IVA hypopharyngeal cancer were suggested by us to accept nimotuzumab combined with induction chemotherapy and concurrent chemoradiotherapy, but the cost of nimotuzumab is higher than ordinary cancer medicines, and for hypopharyngeal cancer, it is not covered by national basic medical insurance; therefore, some patients cannot afford by themselves. Under such condition, we had to consider the patients’ will when we chose the therapeutic schedule. Patients in group A (n=24) received induction chemotherapy (TPF) followed by concurrent chemoradiotherapy with nimotuzumab, while patients in group B (n=12) received induction chemotherapy (TPF) followed by concurrent chemoradiotherapy without nimotuzumab. All patients signed written informed consent that their clinical data might be used for scientific research and the study was approved by the ethics committee of Shengjing Hospital of China Medical University.
Radiotherapy
All patients underwent radical IMRT 2–3 weeks after induction chemotherapy. The total dose was 70 Gy/35 fractions to the planning target volume of the primary tumor and involved cervical lymph nodes, 60 Gy to the high-risk lymph node regions, and 50 Gy to the low-risk lymph node regions. According to the Radiation Therapy Oncology Group (RTOG), 0225 protocol, the gross tumor volume (GTV) included all of the clinically evident primary tumor and lymph nodes based on clinical, endoscopic and all imaging findings. The clinical target volume (CTV) included GTV with a 0.5cm margin and adjacent soft tissue or lymphatics at risk for subclinical micrometastasis. The planning target volume (PTV) was CTV plus 0.5–1 cm margin. Dose constraints to critical normal structures including the brainstem, spinal cord, parotid glands, optic nerves, lens, eyeballs, temporomandibular joints, mandible, and hypophysis were also contoured and set according to the RTOG 0225 protocol. Radiation was delivered once daily, five fractions per week.
Chemotherapy and Targeted Drug Therapy
The induction chemotherapy included 2 or 3 cycles of TPF regimen, docetaxel (75 mg/m2 on day 1), cisplatin (25 mg/m2 on days 1–3) plus raltitrexed (2.5 mg/m2 on day 1), every 3 weeks (q3w). Concurrent with radiotherapy, patients received chemotherapy consisting of cisplatin (25 mg/m2 on days 1–3), q3w. Adjuvant chemotherapy consisting of TPF regimen was administered 1 month later after concurrent chemoradiotherapy. Nimotuzumab (200 mg on day 1, q3w) was given to the patients concurrently with induction chemotherapy. Nimotuzumab was administered concurrently with IMRT at a weekly dose of 200 mg in all patients.
Evaluation
Prior to enrollment, each patient provided a detailed medical history and underwent a full assessment comprising complete physical examination, complete blood cell count and biochemical testing, electrocardiography, and staging with magnetic resonance imaging (MRI) of the hypopharynx and neck, computed tomography (CT) of the thorax, abdomen, and pelvis, and skeletal scintigraphy. Assessment of tumor response was performed three times: after completion of induction chemotherapy, at the end of IMRT, and 3 months after radiation. It was based on MRI and fibroscopy according to the Response Evaluation Criteria for Solid Tumors (RECIST version 1.1). The responses included complete response (CR), partial response (PR), progression of disease (PD), and stable disease (SD). All AEs were documented according to the National Cancer Institute Common Toxicity Criteria for Adverse Events version 3.0 (NCI-CTCAE v3.0). Patients were followed up every 3 months in the first 2 years, every 6 months from the third to the fifth year, and then annually. Each follow-up included careful examination of the hypopharyngeal and neck nodes, thorax, abdomen, and pelvis, and skeletal scintigraphy by an experienced doctor.
Statistical Analysis
Data were analyzed using SPSS version 21.0 (SPSS, Inc., Chicago, IL, USA) and GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA). The following endpoints were assessed: overall survival (OS) and progression-free survival (PFS). All the endpoints were defined as the interval from the date of treatment initiation to the date of failure or last follow-up. Survival curves were estimated using the Kaplan–Meier method, and the differences between the survival curves were evaluated using the Log-rank test. Patient’s clinical characteristics, response rates and adverse effect were compared between the two groups using Fisher’s Exact test. All results were shown as means ± SD and p<0.05 was considered statistically significant.
Results
Baseline Patient Characteristics
These patients included 32 men and 4 women, ranging in age from 34 to 69 years, with a median age of 60 years. No patients had a history of malignant disease. Cancer stage was classified according to the 2010 Edition of the American Joint Committee on Cancer (AJCC) TNM classification. Baseline characteristics of patients are summarized in Table 1. All patients had a good performance status with Karnofsky score ≥70 and completed at least two cycles of induction chemotherapy and adjuvant chemotherapy, and all of them completed concurrent chemoradiotherapy.
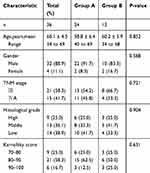 |
Table 1 Clinical Characteristic of the Patients in the Two Groups |
Short-Term Efficacy After Induction Chemotherapy and Chemoradiotherapy
After completion of induction chemotherapy, no patients achieved CR and 22 achieved PR in group A, and no patients achieved CR and 7 achieved PR in group B. The objective response rate (CR+PR) in group A was 91.7% versus 58.3% in group B (p=0.029). Three months after chemoradiotherapy, 5 patients achieved CR and 18 achieved PR in group A, and 1 patient achieved CR and 9 achieved PR in group B. The objective response rate in group A was 95.8% versus 83.3% in group B (p=0.253), although the difference was not significant (Table 2).
 |
Table 2 Short-Term Effect of the Patients After Induction Chemotherapy and Concurrent Chemoradiotherapy |
Treatment‑Related Toxicity
All 36 patients completed at least two cycles of induction chemotherapy and adjuvant chemotherapy, and all of them completed concurrent chemoradiotherapy. Eight patients in group A and 6 in group B were unable to undergo chemoradiotherapy for 25 days due to toxic AEs, thus prolonging the time required, or had an interruption of chemoradiotherapy. The main AEs were mucositis and hematological toxicity, particularly neutropenia, anemia and thrombocytopenia after induction chemotherapy and concurrent chemoradiotherapy. Patients also developed low-grade nausea and vomiting. Chronic toxicity was mainly grade I/II radiation-induced xerostomia after concurrent chemoradiotherapy. Toxicity is summarized in Tables 3 and 4. There was no significant difference in toxicity between the two groups.
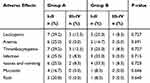 |
Table 3 Treatment‑Related Toxicities After Induction Chemotherapy Between the Two Groups |
 |
Table 4 Treatment‑related Toxicities After Concurrent Chemoradiotherapy Between the Two Groups |
Survival Analysis
Up to the last visit (30 September 2019), the median follow-up was 22.6 months (range 8.9–39.5 months). The 2-year OS rate in group A and group B were 62.5% (95% CI 55–70%) and 51.8% (95% CI 45–59%), respectively (Figure 1A), the 2-year OS rate in group A was better than group B, P<0.05. PFS was 23 months (95% CI 19–27) in group A versus 18 months (95% CI 12–22) in group B (Figure 1B), PFS was longer in group A than group B, P<0.05.
Discussion
Locoregionally advanced hypopharyngeal cancer has always been difficult because the prognosis for this subgroup is poor. Surgical treatment is not suitable for these patients. Concurrent chemoradiotherapy along with adjuvant chemotherapy has become the standard treatment for local advanced hypopharyngeal cancer, as stated in the National Comprehensive Cancer Network guidelines.11 Although a variety of therapeutic methods have been tried, the effect is not satisfactory. Many studies have shown that patients with squamous cell carcinoma of the head and neck have good tolerance to induction chemotherapy.7,12,13 Induction chemotherapy can kill small metastatic lesions at an early stage and reduce the incidence of distant metastasis.14 It can also reduce tumor volume, shrinking radiotherapy target volume, improving radiotherapy efficacy and reducing AEs, and potentially improve the success rate of organ preservation, and improve quality of life.15–17
Targeted drug therapy is a new therapeutic approach for head and neck carcinoma, mainly including EGFR and vascular endothelial growth factor receptor; two intervention targets.18 Overexpression of EGFR is a feature of nearly 90% of head and neck carcinomas, and 68–89% of high EGFR expression is associated with targeted therapy.19 Nimotuzumab is the first monoclonal antibody to target EGFR, and it has been used in head and neck carcinoma, as well as in esophageal cancer, glioma and many other malignant tumors.20–22 Nimotuzumab with induction chemotherapy and concurrent chemoradiotherapy in locoregionally advanced head and neck carcinoma has achieved encouraging results. This is supported by results from several studies. For example, Kumar et al reported that the addition of nimotuzumab to standard concurrent chemoradiotherapy showed improved survival rate in unresectable squamous cell carcinoma of the head and neck, without producing additional toxicity.23 In 2018, Subramanian et al published a retrospective study of 14 patients, which demonstrated that the addition of nimotuzumab to standard treatment yielded a promising response rate as well as survival outcomes in recurrent and/or metastatic head and neck squamous cell carcinoma, without producing additional toxicity.24 Despite our knowledge of its anticancer effects, little is known concerning the efficacy and safety of nimotuzumab combined with induction chemotherapy and concurrent chemoradiotherapy in locoregionally advanced hypopharyngeal carcinoma. To the best of our knowledge, our study is the first to evaluate the efficacy and safety of nimotuzumab combined with induction chemotherapy and concurrent chemoradiotherapy in locoregionally advanced hypopharyngeal cancer in a Chinese population, and the preliminary outcome data appear encouraging.
In the present study, the short-term effect in patients with nimotuzumab after induction chemotherapy was 91.7%, in which 22 cases reached PR. The short-term effect in patients with nimotuzumab after concurrent chemoradiotherapy was 95.8%, in which 5 cases reached CR and 18 reached PR. The short-term effect was better in the nimotuzumab group. We also showed encouraging clinical activity in the 2-year OS and PFS rates. The 2-year OS in patients treated with nimotuzumab was 62.5% (95% CI 55–70%) versus 51.8% (95% CI 45–59%) in those without nimotuzumab. PFS in patients treated with nimotuzumab was 23 months (95% CI 19–27) versus 18 months (95% CI 12–22) in those without nimotuzumab. The 2-year OS and PFS rates were better in patients treated with nimotuzumab, both p-values<0.05. But because of the small sample size of our study, the power of Log-rank test was low, although both p-values<0.05 between the 2-year OS and PFS rates, the results showed that additional use of nimotuzumab with induction chemotherapy and concurrent chemoradiotherapy may prolong the OS and PFS in locoregionally advanced hypopharyngeal cancer. A larger number of multicenter randomized controlled trials with a larger sample size should be conducted to increase the power of Log-rank test in order to confirm the OS and PFS rates. The short-term efficiency and survival observed in our study were satisfactory compare to other studies. In a randomized Phase 3 trial conducted in India in 2012, 536 patients with locoregionally advanced head and neck cancer were evaluated after administration of nimotuzumab or cisplatin in combination with radiotherapy as the first line of therapy.25 In 2014, Reddy BK et al published a randomized, open-label, phase IIb study, which demonstrated that addition of nimotuzumab is safe and provides long-term survival benefit in patients with squamous cell carcinoma of the head and neck. Consistent with our results, there was an increase in OS and PFS with the use of nimotuzumab.9
The addition of nimotuzumab was safe without serious AEs in our study. The common AEs observed were grade I/II and included mucositis, anemia, hematological toxicity, nausea and vomiting, which were similar to the AEs in patients without nimotuzumab. After induction chemotherapy, patients in group A showed twice more grade I–II mucositis and rash than those in group B. After concurrent chemoradiotherapy, patients in group A showed twice more grade III–IV mucositis and xerostomia than those in group B. But the incidence rate of grade III–IV AEs was very low and both p-values were not significant, so we considered these toxicities may be caused by chemotherapy or radiotherapy rather than nimotuzumab. The toxicity normally happened during chemotherapy or radiotherapy for the patients with stage III or IVA hypopharyngeal cancer. However, we still need to observe these AEs in future study with a larger sample size. Although more than one-third of the patients had RT delays, but the total delays were 25 days for these patients which is short for every patient and not affect the therapeutic effect. No patient had RT delays more than 2 days, and these delays are normal toxicity during radiotherapy for the patients with stage III or IVA hypopharyngeal cancer because of the large tumor. Also, these patients don’ t need to accept additional treatment and get well soon. No typical anti-EGFR-related toxicity such as severe rash or infusion reaction was observed. The benign AEs of nimotuzumab can be attributed to the fact that the drug requires bivalent binding for stable attachment, leading to selective binding to tumor cells that overexpress moderate-to-high EGFR levels. When EGFR expression is low, such as in normal tissues, monovalent interaction of nimotuzumab is transient, thus sparing normal healthy tissues and avoiding severe toxicity.26
There were limitations to this study because of its retrospective nature. As we have known, the hypopharyngeal carcinoma is rare with very low incidence and the prognosis of unresectable locally advanced hypopharyngeal carcinoma is very poor, so it is difficult to collect large amounts of data in a short time, the sample size was small, and the study was conducted at a single hospital. A large number of multicenter randomized controlled trials with a larger sample size should be conducted to confirm the efficacy of this therapeutic approach.
Conclusion
Our findings show that additional use of nimotuzumab combined with induction chemotherapy and concurrent chemoradiotherapy in locoregionally advanced hypopharyngeal cancer yielded a better short-term efficacy, also may improve overall survival and progression-free survival than patients without using nimotuzumab. The toxicity was tolerable. Our results confirmed the feasibility of the combined induction chemotherapy and target drug with chemoradiotherapy. Our results may provide novel evidence for the treatment of unresectable locally advanced hypopharyngeal cancer which needs a larger number of randomized controlled trials to confirm the efficacy of this therapeutic approach in the future.
Acknowledgments
The study was funded by the PhD research startup foundation of Liaoning Province (No.201805400242.345), and Talent Project, Shengjing Hospital of China Medical University.
Disclosure
The authors of this study declare no conflicts of interest.
References
1. Zhang X, Wang J, Wu W, et al. Efficacy and safety of combined radiotherapy with EGFR inhibitors and chemotherapy for laryngeal organ preservation in patients with locally advanced hypopharyngeal carcinomas. Curr Cancer Drug Targets. 2014;14(6):589–598. doi:10.2174/1568009614666140716115349
2. Schultz JD, Bran G, Anders C, et al. Induction chemotherapy with TPF (Docetaxel, Carboplatin and Fluorouracil) in the treatment of locally advanced squamous cell carcinoma of the head and neck. Oncol Rep. 2010;24(5):1213–1216. doi:10.3892/or_00000974
3. Eun-Jae C, Woo-Jin J, Ho JY, et al. Long-term oncological and functional outcomes of induction chemotherapy followed by (chemo)radiotherapy vs definitive chemoradiotherapy vs surgery-based therapy in locally advanced stage III/IV hypopharyngeal cancer: multicenter review of 266 cases. Oral Oncol. 2019;89:84–94. doi:10.1016/j.oraloncology.2018.12.015
4. Yi-Jun K, Rena L. Surgery vs. radiotherapy for locally advanced hypopharyngeal cancer in the contemporary era: a population-based study. Cancer Med. 2018;7(12):5889–5900. doi:10.1002/cam4.1811
5. Bozec A, Benezery K, Ettaiche M, et al. Induction chemotherapy-based larynx preservation program for locally advanced hypopharyngeal cancer: oncologic and functional outcomes and prognostic factors. Eur Arch Otorhinolaryngol. 2016;273(10):3299–3306. doi:10.1007/s00405-016-3919-3
6. Athanassios A, Jean Louis L. Laryngeal preservation strategies in locally advanced laryngeal and hypopharyngeal cancers. Front Oncol. 2019;9:419. doi:10.3389/fonc.2019.00419
7. Ghi MG, Paccagnella A, Ferrari D, et al. Induction TPF followed by concomitant treatment versus concomitant treatment alone in locally advanced head and neck cancer. A Phase II-III trial. Ann Oncol. 2017;28(9):2206–2212. doi:10.1093/annonc/mdx299
8. Subramanium S, Balasundaram V, Nithya S, et al. Nimotuzumab with induction chemotherapy and chemo-radiation in patients with advanced head and neck cancer. J Cancer Ther. 2015;6:146–152. doi:10.4236/jct.2015.62016
9. Reddy BK, Lokesh V, Vidyasagar MS, et al. Nimotuzumab provides survival benefit to patients with inoperable advanced squamous cell carcinoma of the head and neck: a randomized, open-label, phase IIb, 5-year study in Indian patients. Oral Oncol. 2014;50:498–505. doi:10.1016/j.oraloncology.2013.11.008
10. Vermorken JB, Trigo J, Hitt R, et al. Open-label,uncontrolled,multicenter Phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J Clin Oncol. 2007;25(16):2171–2177. doi:10.1200/JCO.2006.06.7447
11. Agulnik M. New approaches to EGFR inhibition for locally advanced or metastatic squamous cell carcinoma of the head and neck (SCCHN). Med Oncol. 2012;29(4):2481–2491. doi:10.1007/s12032-012-0159-2
12. Posner MR, Hershock DM, Blajman CR, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357(17):1705–1715. doi:10.1056/NEJMoa070956
13. Kamnerdsupaphon P, Chitapanarux I, Lorvidhaya V, et al. cisplatin, fluorouracil, and leucovorin (TPFL)as induction chemotherapy for locally advanced squamous cell carcinoma of the head and neck. Gan to Kagaku Ryoho. 2008;35(11):1869–1873.
14. Ma J, Liu Y, Huang X-L, et al. Induction chemotherapy decreases the rate of distant metastasis in patients with head and neck squamous cell carcinoma but does not improve survival or locoregional control: a meta-analysis. Oral Oncol. 2012;48(11):1076–1084. doi:10.1016/j.oraloncology.2012.06.014
15. Susumu O, Tomohiro E, Takuma O, et al. Induction TPF chemotherapy followed by CRT with fractionated administration of cisplatin in patients with unresectable locally advanced head and neck cancer. Int J Clin Oncol. 2019;24(7):789–797. doi:10.1007/s10147-019-01418-w
16. Zorat PL, Paccagnella A, Cavaniglia G, et al. Randomized Phase III trial of neoadjuvant chemotherapy in head and neck cancer: 10-year follow-up. J Natl Cancer Inst. 2004;96(22):1714–1717. doi:10.1093/jnci/djh306
17. Fayettea J, Bonnina N, Ferlay C, et al. Neoadjuvant TPF in locally advanced head and neck cancer can be followed by radiotherapy combined with cisplatin or cetuximab: a study of 157 patients. Anticancer Drugs. 2013;24(6):623–629. doi:10.1097/CAD.0b013e328360b9d6
18. Mendelsohn J. The epidermal growth factor receptor as a target for cancer therapy. Endocr Relat Cancer. 2001;8:3–9. doi:10.1677/erc.0.0080003
19. Bhatnagar AR, Singh DP. A comparative study of a monoclonal antibody against EGFR (nimotuzumab) used in combination with chemoradiation versus chemoradiation alone in the treatment of locally advanced inoperable squamous cell carcinoma of the head and neck. J Clin Oncol. 2012;30(30_suppl):51. doi:10.1200/jco.2012.30.30_suppl.51
20. Strumberg D, Scheulen ME, Hilger RA, et al. Safety, efficacy and pharmacokinetics of nimotuzumab, a humanized monoclonal anti-epidermal growth factor receptor (EGFR) antibody, as monotherapy in patients with locally advanced or metastatic pancreatic cancer (PC). J Clin Oncol. 2006;24:18S. doi:10.1200/jco.2006.24.18_suppl.12504
21. Wang F, Sun Q, Jiang C, et al. Additional induction chemotherapy to concurrent chemotherapy and intensity- modulated radiotherapy with or without nimotuzumab in first- line treatment for locoregionally advanced nasopharyngeal carcinoma: a propen- sity score matched analysis. J Cancer. 2018;9(3):594–603. doi:10.7150/jca.20461
22. Liang J, Mingyan E, Wu G, et al. Nimotuzumab combined with radiotherapy for esophageal cancer: preliminary study of a Phase II clinical trial. Onco Targets Ther. 2013;6:1589–1596. doi:10.2147/OTT.S50945
23. Kumar A, Chakravarty N, Bhatnagar S, et al. Efficacy and safety of concurrent chemoradiotherapy with or without Nimotuzumab in unresectable locally advanced squamous cell carcinoma of head and neck: prospective comparative study -ESCORT-N study. South Asian J Cancer. 2019;8(2):108–111. doi:10.4103/sajc.sajc_38_18
24. Subramanian S, Sridharan N, Balasundaram V, et al. Efficacy and safety of Nimotuzumab in unresectable,recurrent,and/or metastatic squamous cell carcinoma of the head and neck: a hospital-based retrospective evidence. South Asian J Cancer. 2018;7(3):188–192. doi:10.4103/sajc.sajc_87_18
25. Maruti PV, Vanita N, Amit J, et al. A randomized phase 3 trial comparing nimotuzumab plus cisplatin chemoradiotherapy versus cisplatin chemoradiotherapy alone in locally advanced head and neck cancer. Cancer. 2019;125(18):3184–3197. doi:10.1002/cncr.32179
26. Rodríguez MO, Rivero TC, Del Castillo Bahi R, et al. Nimotuzumab plus radiotherapy for unresectable squamous-cell carcinoma of the head and neck. Cancer Biol Ther. 2010;9:343–349. doi:10.4161/cbt.9.5.10981
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

