Back to Journals » Drug Design, Development and Therapy » Volume 14
Nimodipine Improves Cognitive Impairment After Subarachnoid Hemorrhage in Rats Through IncRNA NEAT1/miR-27a/MAPT Axis
Authors Li JW, Ren SH, Ren JR, Zhen ZG, Li LR, Hao XD, Ji HM
Received 2 February 2020
Accepted for publication 27 April 2020
Published 10 June 2020 Volume 2020:14 Pages 2295—2306
DOI https://doi.org/10.2147/DDDT.S248115
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qiongyu Guo
Jun-Wei Li, Shao-Hua Ren, Jin-Rui Ren, Zi-Gang Zhen, Li-Rong Li, Xu-Dong Hao, Hong-Ming Ji
Department of Neurosurgery, The People’s Hospital of Shanxi Province, Taiyuan, Shanxi Province, People’s Republic of China
Correspondence: Hong-Ming Ji Tel +86 0351-4960650
Email [email protected]
Background: Subarachnoid hemorrhage (SAH) is a cerebral hemorrhage disease that severely damages the brain and causes cognitive impairment (CI). Therefore, accurate and appropriate treatment strategies are urgently needed. The application of nimodipine can not only improve blood circulation in patients with SAH but also repair ischemic neuron injury.
Purpose: To investigate the effects of nimodipine and lncRNA nuclear paraspeckle assembly transcript 1 (NEAT1)/miR-27a/microtubule-associated protein tau (MAPT) axis on CI after SAH.
Methods: One hundred and twenty healthy male rats were selected and equally divided into control group, sham operation group, model group, PBS group, nimodipine group (drug group), NC siRNA group, NC mimics group, NEAT1 siRNA, miR-27a mimics, MAPT siRNA, drug + NEAT1-ad, and drug + NC-ad groups by random number table. Rats in the model group were constructed by double-hemorrhage model, and expression vectors were injected into the tail to regulate the expression of lncRNA NEAT1, miR-27a and MAPT. In addition, Western blot was employed to detect brain tissue protein, flow cytometry was applied to measure brain tissue apoptosis, and MTT was utilized to determine cell activity, so as to evaluate brain damage and cognitive function in each group.
Results: Nimodipine, down-regulated lncRNA NEAT1, up-regulated miR-27a and down-regulated MAPT all improved brain damage and CI, inhibited brain tissue cell apoptosis, and enhanced brain cell activity. The common binding sites of lncRNA NEAT1 and MAPT were found on the miR-27a sequence fragment, and miR-27a could be paired with the former two. Nimodipine was found to cause the down-regulation of lncRNA NEAT1 and MAPT, as well as the up-regulation of miR-27a.
Conclusion: Nimodipine can improve CI after SAH in rats through the lncRNA NEAT1/miR-27a/MAPT axis.
Keywords: subarachnoid hemorrhage, cognitive impairment, lncRNA NEAT1/miR-27a/MAPT axis, nimodipine
Introduction
Subarachnoid hemorrhage (SAH) is a type of cerebral hemorrhage that causes devastating damage to the brain with extremely high morbidity and mortality.1–3 Early brain injury induced by SAH is the main cause of neurological impairment in patients.4 Apoptosis of cerebral microvascular endothelial cells caused by arachnoid hemorrhage may damage the microenvironment of peripheral cerebral nerves, and apoptosis of cerebral microvascular endothelial cells may also lead to hypoxia of cerebral nerves. Since the nerve function is affected by SAH, cognitive impairment (CI) has also become a common complication of SAH.5–7 Cognitive impairment caused by arachnoid hemorrhage seriously affects the patient’s quality of life and social relationships, so accurate and appropriate treatment strategies are urgently needed. Understanding the pathogenesis of CI after SAH is beneficial to select appropriate drugs for targeted treatment.
Previous studies8–11 have confirmed that non-coding RNA is an important regulatory element involved in the pathogenesis of SAH in living organisms. Of these, LncRNA nuclear paraspeckle assembly transcript 1 (NEAT1) is a long non-coding RNA located on human chromosome 11 that binds to a variety of downstream sequences through the 3’ non-coding region of its nucleic acid sequence, thus participating in disease progression.12–14 Memory is one of the cognitive functions. LncRNA NEAT1 down-regulates the formation and maintenance of memory by interfering with histone methylation, resulting in memory decline.15 CI after SAH is a disease involving cerebral neuropathy, and lncRNA NEAT1 may participate in CI after SAH through interference with memory, but unfortunately, no relevant data have been found in current studies. While miR-27a is a small non-coding RNA of about 78 bp that is capable of up-regulating or down-regulating the mRNA level of the gene by binding to the target gene. Many a study16–20 has shown that the abnormal expression of miR-27a is related to the pathogenesis of many diseases. In cerebral hemorrhage, down-regulation of miR-27a gives rise to increased intracranial hematoma volume and vascular barrier permeability, and inhibits the survival of cerebral vascular endothelial cells.21,22 Therefore, miR-27a is inextricably linked to cerebral hemorrhage-related diseases. While the case in SAH is that, the tau protein encoded by microtubule-associated protein tau (MAPT) gene is synthesized in large quantities and causes brain damage and cognitive dysfunction by inducing neural inflammation and brain metabolic disorders.23–25
Nimodipine enjoys the advantages of improving blood circulation in patients with SAH while repairing ischemic neuron damage at the same time. In this paper, an SAH model was established in rats where nimodipine was applied for treatment. The lncRNA NEAT1, miR-27a, and MAPT genes were observed to be changed before and after treatment, while these three were inextricably related to cerebral hemorrhage disease and CI. Therefore, it was speculated that nimodipine might be able to improve SAH through the above three factors. In this regard, the relationship between nimodipine and the three factors was discussed, so as to explore the possibility of nimodipine as a new approach in the treatment of CI after SAH.
Materials and Methods
SAH Model
A model of SAH was constructed with reference to Zhao et al.10 Haven confirmed that there was no obvious abnormal behavior, 120 healthy SD male rats (Hunan SJA Laboratory Animal Co., Ltd.) were divided into control group, sham operation group, model group, PBS group, nimodipine group (drug group), NC siRNA group, NC mimics group, NEAT1 siRNA, miR-27a mimics, MAPT siRNA, drug + NEAT1-ad, and drug + NC-ad groups by random number table. The control group was left untreated. While the remaining groups were anesthetized with 1% pentobarbital sodium (35mg/kg), and a syringe was inserted into the femoral artery in a sterile environment to collect autologous blood. Apart from that, 0.2mL of normal saline was injected into the cisterna magna of rats in sham operation group, while the rest groups were injected with 0.2mL of fresh autologous blood at the cisterna magna, and 48 hours later, 0.2 mL fresh autogenous blood was injected again. After that, the PBS group, nimodipine group (drug group), NC siRNA group, NC mimics group, NEAT1 siRNA group, miR-27a mimics group, MAPT siRNA group, drug + NEAT1-ad group, drug + NC-ad group were treated with PBS, while nimodipine, NC siRNA, NC mimics, NEAT1 siRNA, miR-27a mimics, MAPT siRNA, nimodipine + NEAT1-ad, nimodipine + NC-ad groups were administered with nimodipine. After the operation, the rats were kept in a culture cage where constant temperature and humidity were maintained, and they were free to move and eat. The study was approved by the Medical Ethics Committee of the People’s Hospital of Shanxi Province and the procedure was conducted in strict accordance with the guidelines for the Care and Use of Experimental Animals (NIH publication, 1996 revision, No. 85–23). NC siRNA, NC mimics, NEAT1 siRNA, miR-27a mimics, MAPT siRNA, NEAT1-ad, and NC-ad vectors were all purchased from Shanghai Sangon Biotechnology Co., Ltd. Nimodipine was injected intraperitoneally with 2 mg/(kg d−1) 30 min after the second injection of autologous blood.
Water Maze
The rats in each group were cognitively evaluated in the Morris water maze system. Morris water maze was a cylindrical pool, which was divided into four quadrants, all containing an equal amount of water, and a quadrant platform was selected randomly. Place navigation test: One day before the experiment, the rats were made familiar with the environment in Morris system and to learn to swim within 120 s to find a platform. The rats who failed to completed within 120s were guided to climb onto the stage and stop on the platform for 30 s. The rats that could not swim were excluded. One day after the experiment, Morris system experiment was carried out. The rat in each group were randomly put into the water with its back to the pool wall, and the time it took to find a fixed platform was called escape latency. The escape latency and swimming distance were recorded. If the platform was not found within 2 min, it would be recorded as 120s. Spatial probe test: The platform was removed from the water, and the rats was placed in the water from any point in the corresponding quadrant of the platform area. The time of the rats staying in platform quadrant and the the number of times of accurately crossing the platform within 2 minutes were recorded. The worse the cognitive function of the experimental rats, the longer the escape latency and the swimming distance, the less target quadrant residence time, and the fewer times they crossed the platform.
Neurological Scores and Brain Water Content
Twenty-four hours after modelling, the rats in each group were scored with the following criteria: Spontaneous motor activity, 0–3 points; Symmetry in the movement of four limbs, 0–3 points; Forepaw outstretching, 0–3 points; Climbing, 0–3 points; Body proprioception, 1–3 points; Response to vibrissae touch, 1–3 points.
After cervical vertebra dislocation, rats in each group were intraperitoneally injected with pentobarbital sodium (150 mg/kg) until respiration and cardiac arrest. The brains of 3 rats in each group were randomly isolated and weighed. After that, the brain was dried at 105 °C for 72 hours to weigh the dry weight. Brain water content = (wet weight-dry weight)/wet weight ×100%.
Extraction and Culture of Brain Microvascular Endothelial Cells
In this section, the experimental brain was taken from a portion of the brain extracted in the previous paragraph. After careful separation of cerebellum, interstitial, white matter, brain stem and superficial blood vessels, the cerebral cortex was washed three times with PBS, minced with a scalpel, and digested with 0.1% collagenase II at 37 °C for 30 min. The hydrolysates were then filtered through a sterile cell sieve and centrifuged at 160 xg for 8 min at 4 °C. Then, the supernatant was discarded, and the substrate pellet was resuspended using 20% fetal bovine serum (FBS, Gibco) before it was centrifuged at 1*103 xg for 20 min at 4 °C. After that, the lower microvascular cell precipitate was mixed with PBS solution and centrifuged at 0.5*103 xg at 4 °C for 5 min. Followed by its re-suspension in fresh DMEM medium containing 10%FBS and 1% penicillin/streptomycin solution (Hyclone company) and then cultured in 6-well plates at 37 °C/5%CO2.
qPCR
Part of the brain tissue was ground and prepared into homogenate, and the total RNA of homogenate was extracted by Trizol method. The OD value of total RNA at 260–280nm was obtained by ultraviolet spectrophotometer, and those with OD260/OD280>1.8 was used for subsequent RT-PCR quantification. RNA was amplified and quantified by reverse transcription and PCR using FastKing one-step reverse transcription-fluorescence quantitative kit (Tiangen Biotechnology Co., Ltd., Beijing, China), and ABI PRISM 7000 (Applied Biosystems, USA) were applied for quantitative analysis of the RNA to be tested. Shanghai Sangon Biotechnology Co., Ltd. was in charge of the design and synthesis of the primers of NEAT1, miR-27a and MAPT. Detailed primer sequences can be found in Table 1. The qPCR reaction system (50 uL) was as follows: upstream primer 1.25 μL, downstream primer: 1.25 μL, probe: 1.0 μL, RNA template: 10 pg/μg, 50 *ROX Reference Dye ROX: 5 μL, and RNase-Free ddH2O was added to reach the overall reaction system of 50 μL. Reaction process: reverse transcription at 50°C for 30 min, cycled once; pre-denaturation at 95°C for 3 min, cycled once; denaturation at 95°C for 15 seconds, and annealing at 60 for 30 seconds, cycled 40 times. The results were analyzed by ABI PRISM 7000 instrument. The internal reference genes were U6 and GAPDH, and the data were standardized by 2–ΔΔCt.
 |
Table 1 Primer Sequences |
Western Blot
Protein extract, which was 20 mmol/L Tris-HCl solution (pH7.5, Solarbio company), was mixed with protein inhibitor (Solarbio company). A portion of the hippocampal tissue was milled and prepared as a homogenate. Then 1 mL cell protein extract was added to the above homogenate and the solution was pipetted repeatedly to facilitate full cell lysis before centrifuging at 4 °C and 1.6104 g for 20 min. Next, the supernatant was taken, and the protein concentration was determined by BCA kit (Thermo Fisher). Having separated by SDS-PAGE, the isolated protein was transferred to polyvinylidene fluoride membrane (EMD millipore Co., Ltd.) and blocked by 5% skim milk-PBS solution at room temperature for 1 h. The protein to be tested and β-actin primary antibody were then added and left to stand overnight at 4 °C. After that, the NC membrane was washed with PBS solution for three times, followed by the addition of goat anti-rabbit secondary antibody (HRP cross-linking), then left at room temperature for 1h. Finally, the polyvinylidene fluoride (PVDF) membrane was washed with PBS solution and visualized using ECL luminescent solution. With β-actin as the internal reference protein, the relative expression level of the protein to be measured=the gray value of the band to be measured/the gray value of the β-actin band. Tau, apoptosis-related proteins (Caspase 3/Caspase 9/Bax/Bcl2), β-actin primary antibody and goat anti-rabbit second antibody (HRP cross-linking) were all obtained from Abcam company.
Flow Cytometry
Part of the hippocampal tissue was milled and prepared into suspension, and the total number of cells was controlled to 1*106. At 4°C, the cells were fixed in 70% ethanol ice-cold solution for 30 min before the ethanol solution was removed. Then, the cells were incubated at room temperature for 30 min with Annexin V-FITC/PI-A solution. Cell apoptosis was analyzed by FACScan flow cytometry (Becton Dickinson Company, USA).
MTT
Four 96-well plates were gathered, and three wells were randomly selected from each well plate to inoculate cells, ensuring a cell density of 5*103/100 μL per well. After that, one well plate was taken out at 24 h, 48 h, 72 h and 96 h respectively after inoculation. Followed by the addition of MTT solution according to 10 μL/well. After 4 h of culture at 37 °C/5% CO2, the culture medium was removed, and the Violet-blue Formazan crystalline was dissolved by DMSO. The OD value at 570 nm was measured by a microplate reader.
DLR Gene Assay
The extracted primary cells were seeded in a 24-well plate (5*103/well) in advance and cultured until they grew well. Starbase2.0 (http://starbase.sysu.edu.cn/starbase2/index.php) was adopted to predict the binding sites of lncRNA NEAT1 and miR-27a, while the binding sites of miR-27a and MAPT was tested by Targetscan7.2 (http://www.targetscan.org/vert_72/). According to the binding site, GLO-NEAT1- wt (containing miR-27a binding site), GLO-NEAT1-mut (without miR-27a binding site), GLO-MAPT-wt (containing miR-27a binding site) and GLO-MAPT-mut (without miR-27a binding site) were constructed and cotransfected with miR-27a mimics and NC mimics, respectively. Forty-eight hours after transfection, luciferase intensity was detected by DLR gene detection system (Promega).
Statistics and Analysis
The data were collected and analyzed by SPSS 20.0 (Asia analytics formally SPSS China), and the pictures were drawn by GraphPad 8.0. The measurement data were expressed as Mean±SD, and the inter-group difference was compared by the independent sample t-test, while the difference among groups was determined by one-way ANOVA. LSD-t test was applied for post hoc pairwise comparison. All data were double-tailed, with 95% as its confidence interval, a statistically significant difference was assumed at P<0.05.
Results
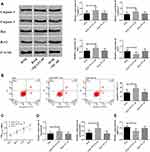
Nimodipine Improved CI and Brain Damage Caused by SAH
SAH is a cerebral hemorrhage disorder that can cause brain damage and subsequent CI. In this paper, brain water content and neurological scores were used to evaluate the degree of brain injury. The higher the brain water content or the lower the neurological scores, the more serious the brain injury. As can be seen from Figure 1A and B, compared with the control group, the neurological scores decreased while the brain water content increased in the model group and PBS group. Compared with the model group, the drug group had higher neurological scores and lower brain water content. The cognitive function of rats was tested by water maze. When the rats had obvious CI, the escape latency and swimming distance increased, while the number of times of crossing the platform and the time of staying in platform quadrant decreased. In addition, it was observed that, compared with the control group, the escape latency and swimming distance of the model group and the PBS group increased, while the number of times of crossing the platform and the time of staying in platform quadrant decreased (Figure 1C–F). Compared with the model group, the escape latency and swimming distance of the drug group decreased, while the number of times of crossing the platform and the time of staying in platform quadrant increased. The above results indicated that nimodipine improved CI and brain damage caused by SAH. In addition, down-regulation of NEAT1, up-regulation of miR-27a, and down-regulation of MAPT could achieve similar results as those of the drug group.
Nimodipine Affected CI After SAH Through the lncRNA NEAT1/miR-27a/MAPT Axis
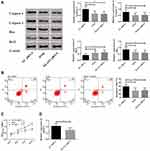
When nimodipine was used to treat SAH, it was found that lncRNA NEAT1 decreased, miR-27a increased and MAPT declined in rats with SAH (Figure 2C–E). From Results 2.1, it can be seen that down-regulation of NEAT1, up-regulation of miR-27a and down-regulation of MAPT can all improve CI and brain damage caused by SAH, which was suggestive that nimodipine may improve CI after SAH through lncRNA NEAT1, miR-27a, and MAPT. In order to verify this inference, Targetscan7.2 and Starbase2.0 were utilized to predict the binding sites of the three. It turned out that there were common binding sites of lncRNA NEAT1 and MAPT on the miR-27a sequence (Figure 2A). Then, DLR gene assay was employed to test whether miR-27a could bind to lncRNA NEAT1 or MAPT. As indicated by Figure 2B, miR-27a could bind to lncRNA NEAT1 or MAPT, respectively. These results exhibited that nimodipine might affect CI after SAH through the lncRNA NEAT1/miR-27a/MAPT axis.
Nimodipine Inhibited Brain Apoptosis and Increased Brain Cell Activity by Down-Regulating lncRNA NEAT1
Increased apoptosis and decreased cell activity are likely to cause brain damage and subsequent CI, so the mechanism of nimodipine in the treatment of CI can be evaluated through the two. As nimodipine caused the down-regulation of lncRNA NEAT1, lncRNA NEAT1 siRNA was used to simulate the effect of nimodipine on NEAT1 (Figure 3D), and the changes of apoptosis and cell activity in brain tissues of rats were observed. Caspase 3, Caspase 9, Bax, Bcl2 are apoptosis-related proteins, and their level changes are closely related to the initiation and development of apoptosis. It was noticed that nimodipine and NEAT1 siRNA induced decreased apoptosis, down-regulation of Caspase 3, Caspase 9, Bax, and up-regulation of Bcl2 (Figure 3A and B), as well as elevated cell activity (Figure 3C). All these demonstrated that nimodipine inhibited brain apoptosis and increased brain cell activity by down-regulating lncRNA NEAT1.
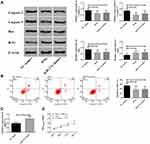
Up-Regulation of miR-27a Induced by Nimodipine Inhibited the Apoptosis of Brain Tissue Cells and Enhanced the Activity of Brain Tissue Cells
As we had verified that nimodipine might influence CI after SAH through the lncRNA NEAT1/miR-27a/MAPT axis and up-regulated miR-27a, here miR-27a mimics was used to simulate nimodipine on miR-27a (Figure 4C), and to observe the changes in apoptosis and cell activity in brain tissues of rats. It was noticed that nimodipine and miR-27a mimics reduced apoptosis, down-regulated Caspase-3, Caspase-9, Bax, up-regulated Bcl2 (Figure 4A and B), and enhanced cell activity (Figure 4D). The above results exhibited that the up-regulation of miR-27a caused by nimodipine could inhibit apoptosis and increase cell activity of brain tissues.
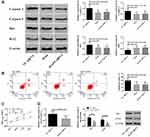
Up-Regulation of MAPT Induced by Nimodipine Inhibited the Apoptosis of Brain Tissue Cells and Enhanced the Activity of Brain Tissue Cells
As nimodipine also caused down-regulation of MAPT, in this part, we utilized MAPT siRNA to simulate the effects of nimodipine on MAPT (Figure 5D), and to observe the changes of rat brain tissue cell apoptosis and cell activity. It was found that nimodipine and MAPT siRNA reduced apoptosis, down-regulated Caspase-3, Caspase-9, Bax and up-regulated Bcl2, (Figure 5A and B), and meanwhile improved cell activity (Figure 5C). The above results revealed that the up-regulation of MAPT induced by nimodipine could inhibit the apoptosis and improve the activity of brain cells.
Rescue Experiment
Based on the above results, it was speculated that nimodipine could improve CI after SAH through lncRNA NEAT1/miR-27a/MAPT axis. In this section, this hypothesis was tested with drug+NEAT1-ad (with up-regulation of NEAT1 and nimodipine at the same time). The up-regulation of NEAT1 could offset the decrease of apoptosis and increase of cell activity induced by nimodipine (Figure 6). Meanwhile, the up-regulation of NEAT1 could counteract the up-regulation of miR-27a, and the down-regulation of MAPT and tau caused by nimodipine. These results suggested that nimodipine improved CI after SAH via lncRNA NEAT1/miR-27a/MAPT axis.
Discussion
Cerebral hemorrhage, such as subarachnoid hemorrhage (SAH), can easily lead to brain damage and neurological dysfunction. Therefore, not only should we consider how to restore brain blood circulation, but should take brain protection into consideration when formulating treatment strategies. This article constructed a SAH model in rats and observed the effect of SAH on cognitive function in rats. At the same time, nimodipine was given to rats with SAH to observe the protective effect of nimodipine on rats’ cognitive function.
Water mazes are commonly applied to assess cognitive function in rats. The results of the water maze in this paper (Figure 1) showed that, compared with the control group, the neurological score, number of times of crossing the platform and time of staying in platform quadrant of the model group decreased statistically, and the brain water content, escape latency and swimming distance increased dramatically. Compared with the model group, the neurological score, number of times of crossing the platform and time of staying in platform quadrant of nimodipine group increased remarkably, and the brain water content, escape latency and swimming distance reduced statistically. The above results indicated that SAH led to significant CI, while nimodipine could greatly promote the recovery of cognitive function in rats with SAH. It is worth mentioning that compared to the control group, miR-27a decreased while lncRNA NEAT1 and tau protein increased in the model group, while compared with the model group, miR-27a increased while lncRNA NEAT1 and tau protein dropped in the nimodipine group (Figure 2), which is suggestive that elevated lncRNA NEAT1, tau protein and reduced miR-27a are involved in CI after SAH, and nimodipine may improve CI after SAH by regulating these three factors.
In order to verify the above conjectures, we constructed lncRNA NEAT1 siRNA, miR-27a mimics and MAPT siRNA to simulate the effects of nimodipine on the three. The formation of CI after SAH is closely related to the survival of brain cells. Active brain apoptosis and weak cell activity will obviously disrupt brain function. In the present study, the degree of apoptosis was evaluated by flow cytometry and apoptosis-related proteins Caspase 3, Caspase 9, Bax, Bcl2, and the cell activity was detected by MTT. The results (Figure 3–5) told that nimodipine inhibited cell apoptosis and promoted cell activity recovery by down-regulating Caspase 3, Caspase 9, Bax and up-regulation of Bcl2, while the down-regulation of lncRNA NEAT1 and MAPT and the up-regulation of miR-27a all produced the same effects as those of nimodipine, indicating that nimodipine could regulate brain cognitive function through lncRNA NEAT1, miR-27a and MAPT.
But how lncRNA NEAT1, miR-27a, and MAPT achieve this regulation? Based on the characteristics of non-coding RNA regulation, it is hypothesized that lncRNA NEAT1 may regulate MAPT by binding to miR-27a, thus causing tau protein of MAPT encoding protein to change brain metabolic disorders and inflammatory responses. To verify this hypothesis, starbase2.0 and Targetscan were used to predict binding sites first. The results (Figure 2) indicated that the nucleic acid fragment of miR-27a had sequence sites shared by the 3’ non-coding region of lncRNA NEAT1 and the 3ʹ non-coding region of MAPT, while later the DLR gene assay confirmed that lncRNA NEAT1 and MAPT could bind to miR-27a, suggesting that nimodipine promoted the recovery of cognitive function after SAH through lncRNA NEAT1/miR-27a/MAPT axis. Among the above regulatory axes, tau protein encoded by MAPT is the terminal regulatory object, and nimodipine affects SAH and CI by regulating this protein. The high enrichment and phosphorylation of Tau protein seriously affect the long-term function and quality of life of patients with SAH, and also give rise to the increase of cerebrospinal fluid, disturbance of brain inflammatory microenvironment and metabolic abnormalities.23–26 The down-regulation effect of nimodipine on tau protein slows down the damage of the latter to neurons and other brain cells,27 thus mitigating CI after SAH.
In this paper, the effects of nimodipine on brain cell apoptosis and cerebral microvascular endothelial cell activity were preliminarily investigated. Whereas, the survival of neurons also play an important role in the formation and development of cognitive functions, so the effect of nimodipine on brain neurons will be a topic in our future research.
To sum up, nimodipine can improve CI after SAH through lncRNA NEAT1/miR-27a/MAPT axis. Therefore, in related treatment, the efficacy of nimodipine can be improved through lncRNA NEAT1/miR-27a/MAPT axis, so as to promote the recovery of patients’ cognitive function after SAH.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lublinsky S, Major S, Kola V, et al. Early blood-brain barrier dysfunction predicts neurological outcome following aneurysmal subarachnoid hemorrhage. EBioMedicine. 2019;43:460–472.
2. Zheng ZV, Lam PK, Poon WS, Wong KCG. The time course of cognitive deficits in experimental subarachnoid hemorrhage. Acta Neurochir Suppl. 2020;127:121–125.
3. Geraghty JR, Davis JL, Testai FD. Neuroinflammation and microvascular dysfunction after experimental subarachnoid hemorrhage: emerging components of early brain injury related to outcome. Neurocrit Care. 2019;31(2):373–389. doi:10.1007/s12028-019-00710-x
4. Sun C, Enkhjargal B, Reis C, et al. Osteopontin-enhanced autophagy attenuates early brain injury via FAK-ERK pathway and improves long-term outcome after subarachnoid hemorrhage in rats. Cells. 2019;8(9):980. doi:10.3390/cells8090980
5. Eagles ME, Tso MK, Macdonald RL. Cognitive impairment, functional outcome, and delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. World Neurosurg. 2019;124:e558–e562. doi:10.1016/j.wneu.2018.12.152
6. Haug Nordenmark T, Karic T, Sorteberg W, Sorteberg A. Predictors of cognitive function in the acute phase after aneurysmal subarachnoid hemorrhage. Acta Neurochir (Wien). 2019;161(1):177–184. doi:10.1007/s00701-018-3760-0
7. Su J, Tongzhou E, Guo Q, Lei Y, Gu Y. Memory deficits after aneurysmal subarachnoid hemorrhage: a functional magnetic resonance imaging study. World Neurosurg. 2018;111:e500–e506. doi:10.1016/j.wneu.2017.12.102
8. Muller AH, Povlsen GK, Bang-Berthelsen CH, et al. Regulation of microRNAs miR-30a and miR-143 in cerebral vasculature after experimental subarachnoid hemorrhage in rats. BMC Genomics. 2015;16(1):119. doi:10.1186/s12864-015-1341-7
9. Stylli SS, Adamides AA, Koldej RM, et al. miRNA expression profiling of cerebrospinal fluid in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg. 2017;126(4):1131–1139. doi:10.3171/2016.1.JNS151454
10. Zhao H, Li Y, Chen L, et al. HucMSCs-derived miR-206-knockdown exosomes contribute to neuroprotection in subarachnoid hemorrhage induced early brain injury by targeting BDNF. Neuroscience. 2019;417:11–23. doi:10.1016/j.neuroscience.2019.07.051
11. Yang S, Tang W, He Y, Wen L, Sun B, Li S. Long non-coding RNA and microRNA-675/let-7a mediates the protective effect of melatonin against early brain injury after subarachnoid hemorrhage via targeting TP53 and neural growth factor. Cell Death Dis. 2018;9(2):99. doi:10.1038/s41419-017-0155-8
12. Xie SP, Zhou F, Li J, Duan SJ. NEAT1 regulates MPP(+)-induced neuronal injury by targeting miR-124 in neuroblastoma cells. Neurosci Lett. 2019;708:134340. doi:10.1016/j.neulet.2019.134340
13. Wang X. Down-regulation of lncRNA-NEAT1 alleviated the non-alcoholic fatty liver disease via mTOR/S6K1 signaling pathway. J Cell Biochem. 2018;119(2):1567–1574. doi:10.1002/jcb.26317
14. Muller V, Oliveira-Ferrer L, Steinbach B, Pantel K, Schwarzenbach H. Interplay of lncRNA H19/miR-675 and lncRNA NEAT1/miR-204 in breast cancer. Mol Oncol. 2019;13(5):1137–1149. doi:10.1002/1878-0261.12472
15. Butler AA, Johnston DR, Kaur S, Lubin FD. Long noncoding RNA NEAT1 mediates neuronal histone methylation and age-related memory impairment. Sci Signal. 2019;12(588):eaaw9277. doi:10.1126/scisignal.aaw9277
16. Luo Y, Yang J, Zhang C, et al. Up-regulation of miR-27a promotes monocyte-mediated inflammatory responses in Kawasaki disease by inhibiting function of B10 cells. J Leukoc Biol. 2019;107(1):133–44.
17. Zhang J, Qiu W, Ma J, et al. miR-27a-5p attenuates hypoxia-induced rat cardiomyocyte injury by inhibiting Atg7. Int J Mol Sci. 2019;20(10):2418.
18. Ljepoja B, Garcia-Roman J, Sommer AK, Wagner E, Roidl A. MiRNA-27a sensitizes breast cancer cells to treatment with selective estrogen receptor modulators. Breast. 2019;43:31–38. doi:10.1016/j.breast.2018.10.007
19. Maghsudlu M, Farashahi Yazd E, Amiriani T. Increased expression of MiR-27a and MiR-24-2 in esophageal squamous cell carcinoma. J Gastrointest Cancer. 2019;51(1):227–33.
20. Toden S, Okugawa Y, Buhrmann C, et al. Novel evidence for curcumin and boswellic acid-induced chemoprevention through regulation of miR-34a and miR-27a in colorectal cancer. Cancer Prev Res (Phila). 2015;8(5):431–443. doi:10.1158/1940-6207.CAPR-14-0354
21. Fang X, Hu W, Zhou L, et al. Downregulation of miR-27a-3p may increase hematoma volume in rats with intracerebral hemorrhage. Int J Clin Exp Med. 2018;11(10):10631–10638.
22. Xi T, Jin F, Zhu Y, et al. miR-27a-3p protects against blood-brain barrier disruption and brain injury after intracerebral hemorrhage by targeting endothelial aquaporin-11. J Biol Chem. 2018;293(52):20041–20050. doi:10.1074/jbc.RA118.001858
23. Schiefecker AJ, Dietmann A, Beer R, et al. Neuroinflammation is associated with brain extracellular TAU-protein release after spontaneous subarachnoid hemorrhage. Curr Drug Targets. 2017;18(12):1408–1416. doi:10.2174/1389450117666160201111804
24. Helbok R, Schiefecker A, Delazer M, et al. Cerebral tau is elevated after aneurysmal subarachnoid haemorrhage and associated with brain metabolic distress and poor functional and cognitive long-term outcome. J Neurol Neurosurg Psychiatry. 2015;86(1):79–86. doi:10.1136/jnnp-2013-307326
25. Zanier ER, Zoerle T, Fiorini M, et al. Heart-fatty acid-binding and tau proteins relate to brain injury severity and long-term outcome in subarachnoid haemorrhage patients. Br J Anaesth. 2013;111(3):424–432. doi:10.1093/bja/aet149
26. Joswig H, Korte W, Fruh S, et al. Neurodegenerative cerebrospinal fluid biomarkers tau and amyloid beta predict functional, quality of life, and neuropsychological outcomes after aneurysmal subarachnoid hemorrhage. Neurosurg Rev. 2018;41(2):605–614. doi:10.1007/s10143-017-0900-6
27. Hatsuta H, Takao M, Nogami A, et al. Tau and TDP-43 accumulation of the basal nucleus of Meynert in individuals with cerebral lobar infarcts or hemorrhage. Acta Neuropathol Commun. 2019;7(1):49. doi:10.1186/s40478-019-0700-z
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.