Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 14
Niacinamide Down-Regulates the Expression of DKK-1 and Protects Cells from Oxidative Stress in Cultured Human Dermal Papilla Cells
Authors Choi YH , Shin JY, Kim J , Kang NG , Lee S
Received 19 August 2021
Accepted for publication 1 October 2021
Published 18 October 2021 Volume 2021:14 Pages 1519—1528
DOI https://doi.org/10.2147/CCID.S334145
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Yun-Ho Choi, Jae Young Shin, Jaeyoon Kim, Nae-Gyu Kang, Sanghwa Lee
LG Household & Health Care (LG H&H) R&D Center, Seoul, 07795, Korea
Correspondence: Sanghwa Lee
LG Household & Health Care (LG H&H) R&D Center, 70, Magokjoongang 10-ro, Gangseo-gu, Seoul, 07795, Korea
Tel +82-2-6980-1210
Fax +82-2-6980-1615
Email [email protected]
Purpose: An increasing number of people are suffering from hair loss disorders. Niacinamide has long been used as an active ingredient for anti-hair loss preparations but the exact mechanism has not been clearly elucidated yet. The effects of niacinamide were investigated in cultured human dermal papilla cells (hDPCs).
Methods: To investigate the anti-hair loss effect of niacinamide and its molecular mechanisms, Western blot analysis, ELISA, quantitative RT-PCR and immunocytochemistry were performed. To study the protective effects of niacinamide against H2O2-induced oxidative stress, ROS generation and cytotoxicity were evaluated by DCF-DA assay and LDH release assay, respectively. Minoxidil was used as a positive control.
Results: Niacinamide decreased the protein expression level of DKK-1 which promotes regression of hair follicles by inducing catagen. The protein expression levels of cell senescence markers, p21 (CDKN1A) and p16 (CDKN2A) which are related to cell cycle arrest, were decreased. The expression of versican was increased by niacinamide treatment in cultured hDPCs. We have found that niacinamide decreased the H2O2-induced intracellular ROS production in cultured hDPCs. Moreover, niacinamide decreased the protein expression levels of H2O2-induced p21 and p16 and diminished the secretion of H2O2-induced DKK-1.
Conclusion: Our data demonstrate that niacinamide could enhance hair growth by preventing oxidative stress-induced cell senescence and premature catagen entry of hair follicles.
Keywords: anti-hair loss, hair follicle, ROS, cell cycle
Introduction
Hair loss or alopecia is a common hair disorder and an increasing number of people are suffering from hair loss.1 The causes of hair loss include aging, nutritional deficiency, hormone imbalances, diseases and stress,2–7 but the pathogenesis and underlying molecular mechanisms are not fully elucidated yet. The postnatal hair follicles undergo a cycle of growth (anagen), apoptosis driven organ involution (catagen) and resting (telogen).8 Hair loss is characterized by progressive miniaturization of hair follicles and decrease in the number of anagen hair follicles.9 The hair follicle cycle is regulated by many signaling pathways, including growth factors,10 bone morphogenetic proteins,11 hormones, and Wnt (wingless-type mouse mammary tumor virus integration site).12
A hair follicle is an organ composed of epithelial and mesenchymal tissues. The dermal compartment of hair follicle consists of dermal papilla and dermal sheath.13 The dermal papilla, a mesenchymal component in hair follicle, is a cluster of specialized fibroblasts enveloped by hair matrix keratinocytes in the bulb of anagen hair. The dermal papilla cells (DPCs) reside in the base of the hair follicle and activate follicular keratinocytes to maintain and regenerate hair follicle cycle.14
DPCs regulate hair growth and regeneration of hair follicles through the secretion of growth factors and cytokines.15 Vascular endothelial growth factor (VEGF), a mediator of angiogenesis, was reported to improve hair follicle vascularization. It increases the size of hair follicles and hair shafts.16 Keratinocyte growth factor (KGF or FGF-7) is an important endogenous mediator of normal hair follicle growth, development, and differentiation.17 Insulin-like growth factor-1 (IGF-1) and hepatocyte growth factor (HGF) from dermal papilla are reported to play important roles in stimulating the differentiation of keratinocytes and the growth of hair follicles.18 DPCs also control the transition of hair follicle cycle. Transforming growth factor-β and dickkopf1 (DKK-1) from dermal papilla are known to induce anagen to catagen transition.19,20
DKK-1 is of special interest since a significant increase in DKK1 immunostaining was found in lesional scalp biopsies taken from patients with androgenic alopecia and alopecia areata when compared to healthy control volunteers.21 It was reported that the administration of recombinant human DKK-1 (rhDKK-1) into mouse hypodermis resulted in premature onset of catagen, a key feature of male pattern baldness. Injection of neutralizing DKK-1 antibody, on the contrary, delayed the anagen to catagen transition in mice. It was also found that treatment of rhDKK1 suppressed Wnt/β-catenin signaling, triggering apoptosis in outer root sheath keratinocytes via induction of pro-apoptotic protein Bax.20
DPCs isolated from balding scalp were previously reported to have slower growth rates in vitro compared with non-balding DPCs.22 Balding DPCs undergo premature senescence that is associated with cyclin-dependent kinase inhibitor p16INK4a and retinoblastoma tumor suppressor protein pRb. In addition, they showed changes in the expression levels and distribution of stress-response proteins including catalase, SOD-1, HSP-27, ATM and ATR.23
An oxidative stress induced by hydrogen peroxide (100 µM) promoted the secretion of TGF-β1 in human dermal papilla cells, known as an endogenous regulator of catagen induction.24
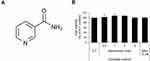
Niacinamide (Figure 1A), also known as nicotinamide, is a water-soluble amide form of vitamin B3 (niacin). It is widely used in cosmetics including skin and hair care products.25 Niacinamide has long been used as an active ingredient in anti-hair loss preparations. There are few studies, however, on the efficacy and underlying mechanisms of niacinamide for anti-hair loss, have been reported.
A pilot study was conducted on the efficacy of niacin derivatives in female pattern baldness patients. The niacin derivatives demonstrated a significant increase in hair fullness in photographic analysis.26 Additionally, in a study comparing the efficacy of adenosine and niacinamide in Japanese men with androgenic alopecia, 32% of the niacinamide treated participants showed the clear improvement in hair thickness.27 However, its effects on hair follicle cells and the underlying mechanisms are not fully understood.
In the present study, the effects of niacinamide on cellular responses were investigated in cultured hDPCs. We have found that niacinamide protected cells from the oxidative stress induced by hydrogen peroxide treatment, preventing cellular senescence in cultured hDPCs. We have also found that niacinamide decreased the protein expression levels of catagen inducer DKK-1 not only under normal state but also under oxidative stress.
Our data suggest that niacinamide could support hair growth by preventing oxidative stress-induced cell senescence and premature catagen entry of hDPCs.
Materials and Methods
Materials
Niacinamide and hydrogen peroxide were purchased from Sigma Aldrich (St. Louis, MO, USA). Niacinamide was dissolved in dimethyl sulfoxide (DMSO). Rabbit antibodies for DKK-1 (ab109416, 1:1000), p16INK4a (ab54210, 1:1000), versican (ab19345, 1:200) and goat anti-Alexa-488 conjugated rabbit IgG (ab150077, 1:1000) were from Abcam (Cambridge, UK). Mouse anti-GAPDH (sc-47724, 1:3000) was from Santa-Cruz (Santa Cruz, CA, USA). Rabbit anti-p21 (2947s), horse anti-mouse IgG (7076s) and goat anti-rabbit IgG (7074s) antibodies were from Cell Signaling Technology (Danvers, MA, USA).
Cell Culture
Human dermal papilla cells (hDPCs) were kindly provided by Prof. Byung Cheol Park (Department of Dermatology, Dankook Medical College, Korea). hDPCs were obtained from 57-year-old male patient underwent hair transplantation surgery with a written consent and approved by the Institutional Review Board of Dankook University Hospital (IRB no. DKUH. 2017-07-003). They were cultured in Follicle Dermal Papilla Cell Growth Medium (Promocell, Heidelberg, Germany) supplemented with 4% fetal calf serum, 0.4% bovine pituitary extract, 1 ng/mL basic fibroblast growth factor (bFGF), and 5 μg/mL insulin (Supplement Mix, Promocell). hDPCs of 3–10 passages were used for experiments. Cells were maintained at 37 °C in a humidified atmosphere with 5% CO2. For H2O2-induced oxidative stress condition, serum limitation was carried out by replacing the medium with fresh DMEM (Gibco, MA, USA) supplemented with 1% fetal bovine serum (FBS; Gibco) and 1 ng/mL bFGF (Merck, Darmstadt, Germany).28
Cell Viability and Lactate Dehydrogenase (LDH) Assay
hDPCs (3.0 × 103 cells/well) were seeded in 96-well plates and cultured overnight. Then the medium was replaced with fresh DMEM with 1% FBS and 1 ng/mL bFGF. After 24 h, cells were treated with various concentrations (250 ~ 2000 μM) of H2O2 for 24 h to determine the non-toxic H2O2 concentration. After treatment, the supernatant was collected and transferred to empty 96-well transparent plate for a lactate dehydrogenase (LDH) assay. The cell viability and the leakage of LDH were measured using CCK-8 assay Kit and Cytotoxicity LDH Assay Kit-WST (Dojindo, Korea), respectively, following the manufacturer’s protocols. The absorbance was measured using Epoch micro plate spectrophotometer (BioTek, VT, USA).
Intracellular ROS Assay
Intracellular reactive oxygen species (ROS) level was evaluated using 2ʹ, 7ʹ- dichlorofluorescin diacetate (DCF-DA) method. hDPCs (2 × 104 cells/well) were seeded in 24-well plates and cultured for 24 h. The medium was replaced with fresh serum limitation medium and cultured for 24 h. Cells were then treated with niacinamide in the presence of 1 mM H2O2 for 30 min. After treatment, cells were washed with phosphate buffered saline (PBS) and stained with DCF-DA probe for 30 min. Fluorescent images were obtained using EVOSTM FLAuto 2 Imaging System (Thermo Fisher Scientific, Waltham, MA, USA). Quantification of green fluorescence intensity was performed using Celleste™ Image Analysis Software (Thermo Fisher Scientific, Waltham, MA, USA).
Western Blotting
hDPCs (5.0 × 105 cells/dish) were seeded in 60 mm dishes and cultured for 24 h. After treatment, cells were washed with 1 × PBS and lysed on ice in M-PER buffer (Thermo Fisher Scientific) supplemented with Complete™ protease inhibitor cocktail and phosphatase inhibitor (Roche, IN, USA). Forty micrograms of protein was analyzed by Western blotting using appropriate antibodies to assess protein expression. The resulting blot was quantitated by chemiluminescence detector Fusion FX5 (Vilber Lourmat, France) and iBright FL1500 (Thermo Fisher scientific).
DKK-1 ELISA
hDPCs (2 × 104 cells/well) were seeded in 24-well plates, cultured for 24 h and were treated with niacinamide for 24 h. After treatment, culture supernatants were collected and the amounts of DKK-1 were measured using Human DKK-1 DuoSet ELISA Kit (R&D Systems, MN, USA) according to the manufacturer’s instruction. Briefly, anti-human DKK-1 capture antibody was plated to 96-well plate. The plate was sealed and incubated overnight at room temperature. Then, the wells were blocked with blocking buffer (1% BSA in PBS, pH 7.2–7.4), and 100 μL of supernatant samples were added to wells in triplicate and incubated at room temperature for 2 h. After anti-human DKK-1 antibody was treated at room temperature for 2 h, 100 μL of working dilution of Streptavidin-HRP was added to each well and incubated at room temperature for 20 min. Wash steps were included between each step. After incubation with 100 μL of substrate solution for 20 min, absorbance at 450 nm was measured using Epoch microplate spectrophotometer (BioTek, VT, USA). Background wavelength correction was performed at 570 nm.
Versican Immunocytochemistry
hDPCs (2 × 104 cells/well) were seeded in 24-well plates and cultured for 24 h. Then, cells were treated with 1 and 2 mM of niacinamide for 24 h. After treatment, the cells were fixed with 4% paraformaldehyde at room temperature for 10 min. The cells were then permeabilized with phosphate buffered saline containing 0.1% triton X-100 and blocked with phosphate buffered saline containing 5% FBS and 1% BSA. After a consecutive incubation with anti-versican antibody (1:200 dilution) at 4 °C for 12 h and Alexa 488 nm conjugated secondary antibody (1:1000 dilution; Abcam, Cambridge, MA) at room temperature for 1 h, nuclei were stained with DAPI (1:2000 dilution, Thermo Fisher Scientific) in dark for 10 min. High-resolution fluorescence images were taken using EVOSTM FL Auto 2 Imaging System.
Quantitative Real-Time PCR
Real-time Polymerase Chain Reaction (RT-PCR) was used to study the effects of niacinamide on cell cycle and apoptosis. Cells were seeded in 6-well plates (1.5 × 105 cells/well) and cultured for 24 h. After serum limitation for 24 h, niacinamide was treated at concentrations of 1, 2, and 4 mM for 24 h in the presence of H2O2. Total RNA was extracted using RNeasy RNA extraction kits (Qiagen Inc., CA, USA). cDNA synthesis was performed using cDNA synthesis kit (Philekorea, Seoul, Korea) with ThermoCycler (R&D systems) according to the manufacturer’s protocol. cDNA samples obtained from control and treated cells were subjected to RT-PCR analysis. TaqMan probes for RT-PCR used in this study are as follows: GAPDH assay id 4352934E; CDKN1A assay id Hs00355782_m1; CDKN2A assay id Hs00923894_m1. For PCR reaction, TaqMan One-Step RT-PCR Master Mix Reagent (Life Technologies, CA, USA) was used. The PCR reactions were performed on ABI7500 Real-Time PCR system following the manufacturer’s protocol. The resulting data were analyzed with ABI software.
Statistical Analysis
The data were expressed as mean ± standard deviation (S.D.) from at least three independent experiments, otherwise indicated. The data were analyzed using two-tailed unpaired Student’s t-test. The value of p < 0.05 was considered statistically significant.
Results
Niacinamide Enhanced the Cell Viability in Cultured hDPCs
As shown in Figure 1B, niacinamide (0.5, 1, 2 and 4 mM) increased the viability of cultured hDPCs when treated only in complete medium. The increased viability (proliferation) ranged about 10% but with significance. When treated in serum limitation medium, however, niacinamide did not induce any changes in cell viability (data not shown).
Niacinamide Decreased the Expression of DKK-1 and Increased the Expression of Versican in Cultured hDPCs
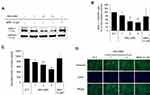
When anagen to catagen transition occurs in hair follicle, the hair follicle cells alter the expression pattern of hair-growth related factors.29 Dickkopf-1 (DKK-1), known as a Wnt antagonist and a catagen inducer, promotes the regression of hair follicle by blocking Wnt/β-catenin signaling.30
As shown in Figure 2, niacinamide significantly down-regulated the protein expression level of DKK-1 in a concentration-dependent manner. 4 mM niacinamide decreased the DKK-1 expression level in whole cell lysate to 55.3% of non-treated control in cultured hDPCs (Figure 2A and B). Treatment of niacinamide also significantly decreased the secretion of DKK-1 protein in a concentration-dependent manner (Figure 2C). Our data suggest that niacinamide could inhibit the catagen progression via down-regulating the expression of DKK-1.
Versican, a large chondroitin sulfate proteoglycan, is reported to be highly expressed during anagen phase, so is regarded as one of the hair follicle cycle-related markers.31 Immunocytochemical staining data with anti-versican antibody have revealed that niacinamide increased the expression of versican in cultured hDPCs, suggesting that niacinamide could prolong the anagen phase (Figure 2D).
Niacinamide Protected Cultured hDPCs from H2O2-Induced Oxidative Stress
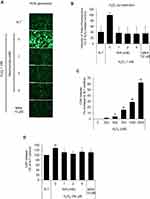
Dermal papilla cells were reported to become prematurely senescent under oxidative stress.24,32 In this study, hydrogen peroxide was chosen for an oxidative stressor, decreased the cell viability of cultured hDPCs in a concentration-dependent manner (data not shown). To determine whether niacinamide treatment protect hDPCs from oxidative stress, we performed DCF-DA assay to detect intracellular ROS generation. As shown Figure 3A and B, the treatment of niacinamide decreased the H2O2-induced ROS generation in cultured hDPCs. The treatment of H2O2 resulted in increased LDH activity which indicates the loss of cell membrane integrity and chemical-induced cytotoxicity (Figure 3C). We have found that niacinamide decreased the LDH activities induced by 750 µM H2O2 in a concentration-dependent manner (Figure 3D).
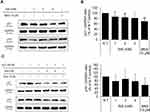 |
Figure 4 Continued. |
Niacinamide Abolished the H2O2-Induced p21 and p16 Expression in Cultured hDPCs
The expression of p21, a cell-cycle regulatory protein, is often elevated in senescent cells, blocking the cell cycle progression.33,34 It was found that the protein expression level of p21 was slightly decreased by niacinamide treatment with statistical significance. The expression of p16, which was reported to be associated with premature senescence in balding DPCs, was also decreased by niacinamide, but was not significant (Figure 4A and B). We have found that both mRNA and protein expression levels of p21 were increased by H2O2 treatment (Figure 4C–E), which were abrogated by niacinamide treatment. Western blot analysis revealed that niacinamide at concentrations of 1 and 2 mM significantly decreased the H2O2-induced p21 protein expression by 46.8% and 54.1%, respectively.
The protein expression level of p16 was increased by H2O2 without significance but significantly decreased by niacinamide treatment. The mRNA expression level of p16 was not significantly changed by H2O2, decreased significantly by niacinamide only at the lowest concentration (Figure 4C–E).
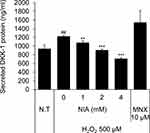
Niacinamide Decreased H2O2-Induced Secretion of DKK-1 Protein in Cultured hDPCs
We have found that treatment of H2O2 at concentration of 500 µM significantly increased the secretion of DKK-1 protein in cultured hDPCs. As shown Figure 5, niacinamide significantly decreased the secretion of DKK-1 in a concentration-dependent manner. DKK-1 secretion was diminished by 26.0% and 42.0%, when treated with niacinamide at concentrations of 2 and 4 mM, respectively.
Discussion
Niacinamide, a member of vitamin B family, has long been used in hair care products, especially for the purpose of anti-hair loss. Neither its effects on hair follicle cells nor the underlying mechanisms concerning the efficacy for anti-hair loss, however, have been clarified.
In the present study, we first provide in vitro evidences that niacinamide could support hair growth in cultured hDPCs. We have found that the expression of DKK-1 in cultured hDPCs was decreased by niacinamide treatment in a concentration-dependent manner (Figure 2A and C). DKK-1, a representative inhibitor of Wnt/β-catenin signaling pathway, was reported to promote the catagen entry of anagen hair follicles in murine and human models, inducing the apoptosis in hair follicle cells and reducing the hair follicle enlargement in the course of hair follicle regeneration.35 Injection of recombinant human DKK-1 (rhDKK-1) promoted catagen progression in mice by blocking the Wnt/β-catenin signaling and by inducing pro-apoptotic protein BAX, leading to apoptosis in outer root sheath (ORS) keratinocytes.20 Furthermore, the expression level of DKK-1 in balding scalp was up-regulated compared with non-balding scalp.36 In this context, our data suggest that niacinamide could support hair growth by preventing catagen entry through the down-regulation of DKK-1 protein level.
On the other hand, niacinamide increased the expression of versican which is involved in matrix assembly and cell adhesion.37 In hair follicles, accumulation of versican protein was observed in the DP during anagen phase.31 Furthermore, the expression level of versican was lower in the DP of vellus-like hair follicles in anagen phase. Based on these results, niacinamide possibly prolongs the anagen duration (Figure 2D).
In addition, we also have found that niacinamide diminished the H2O2 induced DKK-1 secretion in cultured hDPCs (Figure 5). Exposure to H2O2 is a widely used procedure to cause oxidative damage/stress in cellular models.38 H2O2 treatment caused premature senescence in rat dermal papilla cells, resulting in inhibition of cell proliferation in follicular keratinocytes.32
We have found that niacinamide protected hDPCs from H2O2 induced oxidative stress (Figure 3), eliminating the H2O2 induced ROS production and decreasing the cytotoxic effect of H2O2, revealed by DCF-DA and LDH assays, respectively. Our data coincide with previously reported studies where niacinamide functioned as an antioxidant that effectively inhibited oxidative stress.39,40
Cell senescence is associated with cell cycle arrest, where cell cycle regulatory proteins such as p21CIP/WAF1 and p16 INK4a play pivotal roles. The cyclin-dependent kinase inhibitors p21 and p16 control G1/S cell cycle check point.34,41 We have found that in the presence of H2O2 both mRNA and protein expression levels of p21 were significantly up-regulated, and niacinamide decreased the H2O2-induced p21 expression (Figure 4). The protein expression level of p16, another cell senescence marker, was also significantly decreased by niacinamide.
It has been reported that niacinamide inhibited human hair follicle growth ex vivo,42 where hair follicle organ culture system was adopted. Although the hair follicle organ culture system is superior to cell culture system, the experimental results could vary according to the culture conditions. Another report demonstrated that topical niacinamide did not stimulate hair growth,43 whose conclusion was derived only by VEGF synthesis assay. Although VEGF plays important role in hair growth, it could not be concluded that some chemicals without VEGF stimulatory effect do not promote hair growth. The cellular and molecular mechanisms of niacinamide were not investigated in hDPCs. Our findings provide some cellular and molecular clues of niacinamide for its widely used hair growth promoting efficacy in cultured hDPCs. Taken together, our data demonstrate that niacinamide could prevent premature catagen entry by inhibiting the expression of DKK-1 and protecting hDPCs against oxidative stress.
Conclusion
In conclusion, our data demonstrate that niacinamide could promote hair growth possibly by preventing premature catagen entry and cell senescence of hDPCs via down-regulation of DKK-1, p16, and p21, leading to elongation of anagen phase with increased versican expression.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, agreed to the submitted journal, and agreed to be accountable for all aspects of the work.
Disclosure
The authors declare no conflict of interest.
References
1. Hunt N, McHale S. The psychological impact of alopecia. BMJ. 2005;331(7522):951–953. doi:10.1136/bmj.331.7522.951
2. Guo EL, Katta R. Diet and hair loss: effects of nutrient deficiency and supplement use. Dermatol Pract Concept. 2017;7(1):1–10. doi:10.5826/dpc.0701a01
3. Schmidt JB, Lindmaier A, Trenz A, Schurz B, Spona J. Hormone studies in females with androgenic hairloss. Gynecol Obstet Invest. 1991;31(4):235–239. doi:10.1159/000293166
4. Vidali S, Knuever J, Lerchner J, et al. Hypothalamic-pituitary-thyroid axis hormones stimulate mitochondrial function and biogenesis in human hair follicles. J Invest Dermatol. 2014;134(1):33–42. doi:10.1038/jid.2013.286
5. Rushton DH. Nutritional factors and hair loss. Clin Exp Dermatol. 2002;27(5):396–404. doi:10.1046/j.1365-2230.2002.01076.x
6. Olsen EA. Female pattern hair loss. J Am Acad Dermatol. 2001;45(3, Supplement):S70–S80. doi:10.1067/mjd.2001.117426
7. Thom E. Stress and the hair growth cycle: cortisol-induced hair growth disruption. J Drugs Dermatol. 2016;15(8):1001–1004.
8. Paus R, Muller-Rover S, Botchkarev VA. Chronobiology of the hair follicle: hunting the “hair cycle clock”. J Investig Dermatol Symp Proc. 1999;4(3):338–345. doi:10.1038/sj.jidsp.5640241
9. Messenger AG, Sinclair R. Follicular miniaturization in female pattern hair loss: clinicopathological correlations. Br J Dermatol. 2006;155(5):926–930. doi:10.1111/j.1365-2133.2006.07409.x
10. Yue Z, Jiang TX, Wu P, Widelitz RB, Chuong CM. Sprouty/FGF signaling regulates the proximal-distal feather morphology and the size of dermal papillae. Dev Biol. 2012;372(1):45–54. doi:10.1016/j.ydbio.2012.09.004
11. Rendl M, Polak L, Fuchs E. BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes Dev. 2008;22(4):543–557. doi:10.1101/gad.1614408
12. Shimizu H, Morgan BA. Wnt signaling through the beta-catenin pathway is sufficient to maintain, but not restore, anagen-phase characteristics of dermal papilla cells. J Invest Dermatol. 2004;122(2):239–245. doi:10.1046/j.0022-202X.2004.22224.x
13. Yang CC, Cotsarelis G. Review of hair follicle dermal cells. J Dermatol Sci. 2010;57(1):2–11. doi:10.1016/j.jdermsci.2009.11.005
14. Stenn KS, Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;81(1):449–494. doi:10.1152/physrev.2001.81.1.449
15. Peus D, Pittelkow MR. Growth factors in hair organ development and the hair growth cycle. Dermatol Clin. 1996;14(4):559–572. doi:10.1016/S0733-8635(05)70384-3
16. Yano K, Brown LF, Detmar M. Control of hair growth and follicle size by VEGF-mediated angiogenesis. J Clin Invest. 2001;107(4):409–417. doi:10.1172/JCI11317
17. Danilenko DM, Ring BD, Yanagihara D, et al. Keratinocyte growth factor is an important endogenous mediator of hair follicle growth, development, and differentiation. Normalization of the nu/nu follicular differentiation defect and amelioration of chemotherapy-induced alopecia. Am J Pathol. 1995;147(1):145–154.
18. Madaan A, Verma R, Singh AT, Jaggi M. Review of hair follicle dermal papilla cells as in vitro screening model for hair growth. Int J Cosmet Sci. 2018;40(5):429–450. doi:10.1111/ics.12489
19. Foitzik K, Lindner G, Mueller-Roever S, et al. Control of murine hair follicle regression (catagen) by TGF-β1 in vivo. FASEB J. 2000;14(5):752–760. doi:10.1096/fasebj.14.5.752
20. Kwack MH, Kim MK, Kim JC, Sung YK. Dickkopf 1 promotes regression of hair follicles. J Invest Dermatol. 2012;132(6):1554–1560. doi:10.1038/jid.2012.24
21. Mahmoud EA, Elgarhy LH, Hasby EA, Mohammad L. Dickkopf-1 expression in androgenetic alopecia and alopecia areata in male patients. Am J Dermatopathol. 2019;41(2):122–127. doi:10.1097/DAD.0000000000001266
22. Randall VA, Hibberts NA, Hamada K. A comparison of the culture and growth of dermal papilla cells from hair follicles from non-balding and balding (androgenetic alopecia) scalp. Br J Dermatol. 1996;134(3):437–444. doi:10.1111/j.1365-2133.1996.tb16227.x
23. Bahta AW, Farjo N, Farjo B, Philpott MP. Premature senescence of balding dermal papilla cells in vitro is associated with p16INK4a expression. J Investig Dermatol. 2008;128(5):1088–1094. doi:10.1038/sj.jid.5701147
24. Upton JH, Hannen RF, Bahta AW, Farjo N, Farjo B, Philpott MP. Oxidative stress–associated senescence in dermal papilla cells of men with androgenetic alopecia. J Investig Dermatol. 2015;135(5):1244–1252. doi:10.1038/jid.2015.28
25. Bissett DL, Oblong JE, Berge CA. Niacinamide: a B vitamin that improves aging facial skin appearance. Dermatol Surg. 2005;31(s1):860–866. doi:10.1111/j.1524-4725.2005.31732
26. Draelos ZD, Jacobson EL, Kim H, Kim M, Jacobson MK. A pilot study evaluating the efficacy of topically applied niacin derivatives for treatment of female pattern alopecia. J Cosmet Dermatol. 2005;4(4):258–261. doi:10.1111/j.1473-2165.2005.00201.x
27. Watanabe Y, Nagashima T, Hanzawa N, et al. Topical adenosine increases thick hair ratio in Japanese men with androgenetic alopecia. Int J Cosmet Sci. 2015;37(6):579–587. doi:10.1111/ics.12235
28. Kim J, Shin JY, Choi YH, et al. Hair growth promoting effect of Hottuynia cordata extract in cultured human hair follicle dermal papilla cells. Biol Pharm Bull. 2019;42(10):1665–1673. doi:10.1248/bpb.b19-00254
29. Danilenko DM, Ring BD, Pierce GF. Growth factors and cytokines in hair follicle development and cycling: recent insights from animal models and the potentials for clinical therapy. Mol Med Today. 1996;2(11):460–467. doi:10.1016/1357-4310(96)10045-9
30. Hall CL, Zhang H, Baile S, Ljungman M, Kuhstoss S, Keller ET. p21CIP-1/WAF-1 induction is required to inhibit prostate cancer growth elicited by deficient expression of the Wnt inhibitor Dickkopf-1. Cancer Res. 2010;70(23):9916–9926. doi:10.1158/0008-5472.CAN-10-0440
31. Soma T, Tajima M, Kishimoto J. Hair cycle-specific expression of versican in human hair follicles. J Dermatol Sci. 2005;39(3):147–154. doi:10.1016/j.jdermsci.2005.03.010
32. Huang W-Y, Huang Y-C, Huang K-S, et al. Stress-induced premature senescence of dermal papilla cells compromises hair follicle epithelial-mesenchymal interaction. J Dermatol Sci. 2017;86(2):114–122. doi:10.1016/j.jdermsci.2017.01.003
33. Cho A-R, Kim JY, Munkhbayer S, Shin C-Y, Kwon O. p21 upregulation in hair follicle stem cells is associated with telogen retention in aged mice. Exp Dermatol. 2016;25(1):76–78. doi:10.1111/exd.12862
34. Waga S, Hannon GJ, Beach D, Stillman B. The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA. Nature. 1994;369(6481):574–578. doi:10.1038/369574a0
35. Lei M, Guo H, Qiu W, et al. Modulating hair follicle size with Wnt10b/DKK1 during hair regeneration. Exp Dermatol. 2014;23(6):407–413. doi:10.1111/exd.12416
36. Kwack MH, Sung YK, Chung EJ, et al. Dihydrotestosterone-inducible dickkopf 1 from balding dermal papilla cells causes apoptosis in follicular keratinocytes. J Investig Dermatol. 2008;128(2):262–269. doi:10.1038/sj.jid.5700999
37. Shinomura T, Nishida Y, Ito K, Kimata K. cDNA cloning of PG-M, a large chondroitin sulfate proteoglycan expressed during chondrogenesis in chick limb buds. Alternative spliced multiforms of PG-M and their relationships to versican. J Biol Chem. 1993;268(19):14461–14469. doi:10.1016/S0021-9258(19)85261-4
38. Liu W, Mao L, Ji F, Chen F, Hao Y, Liu G. Targeted activation of AMPK by GSK621 ameliorates H2O2-induced damages in osteoblasts. Oncotarget. 2017;8(6):10543–10552. doi:10.18632/oncotarget.14454
39. Zhen AX, Piao MJ, Kang KA, et al. Niacinamide protects skin cells from oxidative stress induced by particulate matter. Biomol Ther. 2019;27(6):562–569. doi:10.4062/biomolther.2019.061
40. Burgess C. Topical vitamins. J Drugs Dermatol. 2008;7(7 Suppl):s2–6.
41. Shapiro GI, Edwards CD, Ewen ME, Rollins BJ. p16INK4A participates in a G1 arrest checkpoint in response to DNA damage. Mol Cell Biol. 1998;18(1):378–387. doi:10.1128/MCB.18.1.378
42. Haslam IS, Hardman JA, Paus R. Topically applied nicotinamide inhibits human hair follicle growth ex vivo. J Investig Dermatol. 2018;138(6):1420–1422. doi:10.1016/j.jid.2017.12.019
43. Oblong JE, Peplow AW, Hartman SM, Davis MG. Topical niacinamide does not stimulate hair growth based on the existing body of evidence. Int J Cosmet Sci. 2020;42(2):217–219. doi:10.1111/ics.12599
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.