Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
New Mechanistic Insights of Melasma
Received 14 November 2022
Accepted for publication 10 January 2023
Published 13 February 2023 Volume 2023:16 Pages 429—442
DOI https://doi.org/10.2147/CCID.S396272
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Wei Liu, Qin Chen, Yumin Xia
Department of Dermatology, The Second Affiliated Hospital of Xi’an Jiaotong University, Xi’an, 710004, People’s Republic of China
Correspondence: Yumin Xia, Department of Dermatology, The Second Affiliated Hospital of Xi’an Jiaotong University, 157 Xiwu Road, Xi’an, 710004, People’s Republic of China, Tel +86 29 87679969, Fax +86 29 87678425, Email [email protected]
Abstract: Melasma is a common acquired disorder of pigmentation that negatively impacts quality of life. Present treatments show poor therapeutic effect with frequent recurrence. This in large part is due to the currently limited understanding of the disease’s etiology. It is urgent to elucidate the pathogenesis of melasma to further the discovery of new therapeutic strategies. Recent studies show that melasma is triggered or aggravated by a variety of factors, including genetic susceptibility, ultraviolet radiation, and sex hormone dysregulation. Ultraviolet B radiation upregulates the expression of several melanocyte-specific genes and stimulates the release of key factors that participate in the synthesis of melanin. There is a significant increase in melanin in both the epidermal and dermal layers of affected skin, possibly due to abnormalities in crosstalk between the melanocytes and other cells. Melanogenesis is regulated through various signaling networks including the Wnt/β-catenin, PI3K/Akt, cAMP/PKA, and SCF/c-kit-mediated signaling pathways. In addition, inflammatory mediators, oxidative stress, neuroactive molecules, sebocytes, etc, have also been proved to be related to the pathogenesis of melasma. This review provides a comprehensive update on the current understanding of the pathogenesis of melasma.
Keywords: genetic predisposition, sex hormone, melanocyte, melasma, ultraviolet
Introduction
Melasma, also known as chloasma which is not in use nowadays, is a common disorder that affects sun-exposed areas, particularly the face, with bilateral distribution and irregular borders. There are three predominant patterns of melasma: centrofacial, malar, and mandibular patterns.1 By Wood’s lamp examination, melasma is classified as epidermal, dermal, or mixed type according to the pigment location. Wood’s lamp can also be used to evaluate the efficacy. The classification is not reflective of histopathological results in melasma. Melasma’s prevalence within the population varies according to ethnicity, sunlight intensity, and skin phototype. Epidemiological studies have identified higher prevalence in pigmented phenotypes, primarily Fitzpatrick classification III–VI. Epidemiological studies have reported higher prevalence of melasma in Indian, Pakistani, Middle Eastern, East Asian (Japanese, Korean, and Chinese), and Mediterranean-African populations. In Southeast Asian populations, the reported prevalence rate is as high as 40%.2 Melasma often brings negative psychosocial implications to patients and lowers their quality of life. Patients frequently experience shame, low self-esteem, lack of pleasure, dissatisfaction, and lack of social motivation.3 According to the score MASI (the melasma area and severity index), several studies confirmed no correlation between MelasQoL and the severity of the disease, suggesting that the patient’s concerns are beyond the severity of clinical manifestations.4–6 Consequently, it is particularly important to clarify the pathogenesis of melasma.
The pathogenesis of melasma has not been clearly elucidated, although the potential role of several factors, such as ultraviolet radiation exposure and genetic predisposition, have been considered. The clinical emergence of melasma may depend on an interplay between hormonal and environmental factors within genetically susceptible individuals. Given the incomplete understanding of its pathogenesis, along with both its chronicity and periodicity, the management of melasma remains challenging. In this review, we summarize recent progress in defining the etiology and pathogenesis of melasma.
Genetic Predisposition
The prevalence of melasma is highly variable among various ethnic populations.2 Family history is also an important risk factor for melasma.7 In a global survey of 324 women, 48% of the individuals have a family history of melasma; among those with a positive history, 97% were in first-degree relatives.8 A positive family history was obtained in 20% of the cases in a study of India.2 In a study conducted in 855 Iranian women, melasma was identified in 39.5% of the respondents. Positive family history of melasma was present in 54.7% of 400 pregnant women.9 Family history of patients with Fitzpatrick skin type II and type III is not as pronounced as patients with darker skin (IV–VI).10 When accounting for epidemiological risk factors, facial melasma seems most likely to display a dominant inheritance pattern, with exposure factors leading to disease development in genetically susceptible individuals.10 Transcriptional profiling has identified 279 genes that are differently expressed in lesional and perilesional skin.11 Hitherto, no genome-wide studies have been conducted to examine related genes, but current research indicates that related regulated proteins are involved in pigment, response to oxidative stress, energy metabolism, etc.12 Therefore, we speculate that the abnormal expression of these genes may have important implications in the pathogenesis of melasma.
Ultraviolet and Visible Light Exposure
Ultraviolet (UV) and visible light both promote melanin synthesis, activate tyrosinase activity, contribute to skin photoaging,13 and lead to pigmentation. The role of UV light in the development of melasma has been investigated quite intensively in recent years. Persons living in the tropical regions of the Americas often develop melasma because of the greater UV radiation.14 Melasmic pigmentation often diminishes during the winter and increases during the summer or intense sun exposure. One study showed that the use of a broad-spectrum sunscreen can reduce the incidence of melasma by almost 50% during pregnancy.15 Sun exposure is the most important environmental factor implicated in melasma, and solar radiation can directly or indirectly stimulate melanocytes by secreting melanogenesis factors through keratinocytes, fibroblasts, endothelial cells, and so on.13
UV radiation can induce production of alpha-melanocyte–stimulating hormone, corticotropin, and lipid peroxidation, resulting in an increase in melanin produced by melanocytes.16 This is more pronounced in melasmic lesions than in adjacent skin. Several studies have confirmed that UV radiation induces a number of different photobiological effects. UVB radiation stimulates expression of several melanocyte-specific genes such as tyrosinase, tyrosinase-related protein 1 (Tyrp1), and dopachrome tautomerase. In contrast, UVA radiation appears to cause no similar increase in factors associated with melanogenesis.17 UVB stimulates corticotropin releasing hormone, proopiomelanocortin, melanocortin 1 receptor (MC1R), MC2R, and β-endorphin, while UVA is capable of stimulating β-endorphin and, to a lesser extent proopiomelanocortin.18 UV irradiation is central in the melanogenesis of skin, and blocking UV rays may reduce the incidence of melasma.
One of the important pathways by which UV and visible light induces pigmentation is the secretion of stem cell factor (SCF), a ligand for the tyrosine kinase receptor c-kit, which causes downstream effects on melanocyte proliferation.19 SCF, also known as mast cell growth factor, can be secreted by human keratinocytes and fibroblasts. Skin inflammation and fibroblast activation caused by prolonged UV radiation upregulate SCF in the melasma dermis, which contributes to the increase in melanogenesis.
Studies have confirmed that, in addition to UV, visible light is also involved in the pathophysiology of melasma. Classically, opsin-3 detects blue light through the retina and has been found in melanocyte membranes. However, activation of the key sensor opsin-3 in melanocytes via blue light stimulates calcium flux, which activates mitogen-activated protein kinase pathways, resulting in incremental melanogenesis.20 Nevertheless, OPN3 receptors were not differentially expressed in keratinocytes, melanocytes, or fibroblasts between facial melasma and the healthy adjacent skin.21 Visible light-induced hyperpigmentation in darker skin types was deeper and longer-lasting over a two-week period compared to UVA1.22 In addition, compared with UVB irradiation, blue-violet light (415 nm) induced significantly evident hyperpigmentation in a dose-dependent manner. This blue-violet light-induced hyperpigmentation was observed to last for up to 3 months.23 It is reported that the blue light intensity of the device normally exposed in human daily life is different from that of the sun. In addition to the sun, other sources (such as screen smartphones, tablets, computer screens and other electronic devices) can produce low intensity blue light. However, patients with facial melasma do not show the deterioration of lesions after contacting the computer screen.24 Another systematic review also shows that BL from electronic equipments is not a hazard to human photoaging.25 Sunscreens that do not block UVA and visible radiation can also stimulate melanocytes to produce melanin. Adding iron oxide (blue blocking agent) to a wide range of UVB-UVA sunscreen prevented recurrence of melasma, confirming that visible light contributes to its pathology.26 Therefore, both UV and visible light can participate in the pathogenesis of melasma through a variety of mechanisms.
Hormonal Influence
Pregnancy, oral contraceptives, and hormone replacement therapy are the most frequently cited triggering factors of melasma, indicating that the effects of hormones may play an important role in the pathogenesis of melasma.27 The prevalence of melasma in pregnant women varies widely across different countries. A cross-sectional study on 400 pregnant women in Tehran showed the prevalence of melasma was 15.8%.9 In a multicentric cross-sectional study from India, 15.2% of the subjects reported onset of melasma during pregnancy.2 It has been reported that 8–34% of the women taking oral contraceptives or hormone replacement therapy develop melasma. A report presented spontaneous improvements in patients with melasma after switching from a combined oral contraceptive to a hormone-releasing intrauterine device.28 These studies demonstrate that abnormal hormone levels may induce or aggravate melasma.
Sexual hormones such as estrogen and, especially, 17β‐estradiol (E2) and progesterone have been identified as factors involved in the regulation of pigmentation through nonclassical membrane-bound receptors.29 The development of hyperpigmentation during pregnancy may be associated with increased melanocyte-stimulating hormone (MSH), estrogen, and progesterone, which may result in increased transcription of tyrosinase and dopachrome tautomerase.30 A study including 42 women with facial melasma demonstrated increased expression of estrogen β and progesterone receptors in the affected epithelia.31 Another study showed that progesterone stimulates the melanogenesis of melanocytes and thus participates in the pathogenesis of melasma.32 Contrary to this report, however, yet another study found that progesterone can inhibit the proliferation of human melanocytes, thereby counteracting the estrogen-stimulating effect.33 Therefore, the effect of progesterone on melanocytes remains to be further elucidated.
Hormonal regulation of pigmentation plays a key role in the switch between eumelanin and pheomelanin synthesis within melanocytes. Estrogen stimulates melanogenesis in cultured human melanocytes by inducing synthesis of melanogenic enzymes such as tyrosinase and Tyrp 1 and 2.34 Estrogen treatment in mixed cell cultures (melanocytes and keratinocytes) lacking the H19 gene promoted expression of tyrosinase, suggesting that estrogen may be involved in disease pathogenesis.35 The expression of estrogen and progesterone receptors in melasmic skin is different from that in perilesional and non‐lesional skin or cells. Immunohistochemical examination of affected and unaffected skin showed significantly enhanced expression of progesterone receptor in epidermal lesions, but no significant difference in the dermis. Estrogen receptor (ER) β expression is increased in dermal lesions compared with unaffected dermis, particularly around small blood vessels and fibroblast-like cells.31 Two different intracellular ERs (ERα and ERβ) have been identified as belonging to the superfamily of nuclear hormone receptors, with ERβ being the principal ER type in melanocytic lesions.36 In general, sexual hormones stimulate melanogenesis through multiple regulating pathways.
Estrogen can also increase the expression of α-melanocyte-stimulating hormone (α-MSH) and PDZ domain protein kidney 1 (PDZK1) and enhance the synthesis of tyrosinase, thus promoting the production of melanin. A comparative study indicated notable expression of α-MSH and MC1R in the epidermis of lesional skin. Espósito et al confirmed higher fluorescent intensity of α-MSH and MC1R in melasmic lesions.37 MC1R is one of the main factors determining skin pigment phenotype and plays a crucial role in the switch between eumelanin and pheomelanin synthesis in melanocytes. In addition, MC1R signaling can activate antioxidant, DNA repair, and survival pathways. DNA repair ability is another major factor determining skin cancer risk. MC1R is a melanoma susceptibility gene, and its functional deletion variant impairs the DNA repair and antioxidant capacity of melanocytes.38 MC1R expression is increased by estrogens acting on nuclear receptors. When bound to an agonist, such as α-MSH and adrenocorticotropic hormone (ACTH), MC1R increases intracellular cyclic adenosine monophosphate (cAMP) levels that activate protein kinase A. This, in turn, leads to increased transcription of microphthalmia-associated transcription factor (MITF), the most critical transcription factor for melanocyte function. MITF enhances activity of tyrosinase, leading to activation of melanogenesis and increased melanin production.39
Overexpression of postsynaptic density-95, DglA, and zonula occludens-1 (PDZ) domain protein kidney 1 (PDZK1) was found to be related to estrogen in melasma. PDZK1 upregulation improved estrogen-induced melanosome transfer and tyrosinase by regulating the expression of ERα and ERβ.40
One of the primary mechanisms of pigmentation regulation is the local production of proopiomelanocortin and the processing of peptides related to α-MSH, ACTH, and β-endorphin.41 Estrogen, α-MSH, ACTH, and endorphins increase pigmentation, while androgens have a restrictive effect on melanocytes.42 The serum levels of luteinizing hormone and follicle-stimulating hormone in melasmic women were higher than those of control women. In contrast, the serum levels of follicle-stimulating hormone in melasmic men were similar to those in the control group; however, their testosterone levels were lower.43 Two studies in India also noted increased luteinizing hormone and low testosterone levels in men with melasma.43 These hormones play an important role in the pigmentation of skin. Local application of estrogen antagonists such as selective estrogen receptor modulators or aromatase inhibitors can be considered for the treatment of melasma.
Communications Between the Melanocytes and Other Cells
Melasma is driven not just by the melanocytes themselves. On the contrary, an entire network of cellular interactions among melanocytes, keratinocytes, mast cells, fibroblasts, the dermal vasculature, and endothelial cells lead to the formation of melasma.13,44 As an illustration of how pivotal this network can be, one study found no quantitative increase in melanocytes in the hyperpigmented areas of melasmic skin. However, the melanocytes were hypertrophied, more dendritic, and intensely stained. Morphological observation revealed more melanosomes in keratinocytes, melanocytes, and dendrites in lesional skin of melasma patients in comparison to adjacent normal skin.45 Electron microscopy in melasmic skin showed more cytoplasmic organelles and increased mature melanosomes in keratinocytes and melanocytes.46 The function of melanocytes is regulated by autocrine and paracrine factors produced by different types of cells, including melanocytes, keratinocytes, and fibroblasts.16 It has been confirmed that paracrine linkages among keratinocytes, fibroblasts, and melanocytes in the skin play an important part in regulating epidermal melanization under UV radiation.
One of the most pivotal paracrine interactions between keratinocytes and melanocytes is the interaction of endothelin (EDN) with its receptor. Several physiological factors induced by keratinocytes, fibroblasts, and other sources regulate the expression functions and levels of MITF. Keratinocyte-derived factors that activate melanocytes include SCF, α-MSH, ACTH, hepatocyte growth factor, nerve growth factor, granulocyte-macrophage colony-stimulating factor, endorphin, EDN1, and prostaglandin E2.47 Fibroblasts interact with melanocytes, both directly and indirectly via adjacent cells, by the synthesis of cytokines, growth factors, and proteins. Fibroblast-derived factors bind to melanocyte receptors and modulate intracellular signaling pathways involved in melanogenesis.48 Among these, dickkopf 1 and transforming growth factor-β1 have been demonstrated to contribute to the downregulation of melanin synthesis.42 Tranexamic acid partially inhibits melanin production and acts in the treatment of melasma by stimulating the expression of TGF-β1 in human epidermal keratinocytes.49 Considering that melanogenesis is dependent on crosstalk among different skin cells, these accessory cells can be considered as therapeutic targets in melasma treatment.
Dermal and Vascular Components
Histologically, melasma has been characterized by increased solar elastosis, basement membrane disruption, increased dermal blood vessels, infiltration of mast cells, and subclinical inflammation, with occasionally perivascular lymphohistiocytic infiltration.50 Basement membrane zone is important in maintaining epidermal and dermal homeostasis. Many pigmented basal cells and increased melanin were observed to protrude into the dermis of melasma patients, implying a role of basal membrane damage in melasma. There is structural damage in basement membrane zone in melasma, which is characterized by gaps, disruptions, thinning and lower density of lamina densa and loss of anchoring fibers of lamina lucida.51 Reflectance confocal microscopy displayed an increase in solar elastosis and blood vessels in the dermis.45 Increased elastosis and vascularity, moderate monocyte infiltration, and mast cells were present in the dermis of melasmic lesions. The increased prevalence of mast cells and infiltrating leukocytes in lesional skin indicates chronic skin inflammation.46 Significant solar elastosis compared with normal skin implies that accumulated dermal sun damage may be involved in the occurrence of melasma. Increased elastic material may indicate that accumulation of sun exposure is necessary for the development of melasma.52
Increased vascularization has been observed in melasma by using dermoscopy and reflectance confocal microscopy. Additionally, the expression of vascular endothelial growth factor (VEGF) was significantly increased in melasma lesions.53 VEGF is produced by keratinocytes following UV damage and can maintain human melanocytes in tissue culture. This mechanism is considered to be one of the drivers of increased melanocyte activity in melasma.54 Moreover, angiogenesis can be induced by UV radiation, so vascular changes in melasma may be the result of chronic UV exposure. A combination of pulsed dye laser and a topical formulation consisting of hydroquinone, retinoid, and corticosteroid was demonstrated to be more effective than topical treatment alone.55 These findings demonstrate that vascularization is related to melanocyte activity in melasma.
EDNs released by microvascular endothelial cells induce melanogenesis through the activation of endothelin receptor type B (EDNRB) and activate mitogen-activated protein kinase, extracellular signal-regulated kinase 1/2, and p38.56 EDN1, prostaglandin, and nitric oxide are derived from endothelial cells. Studies have confirmed that EDN1-induced melanogenesis occurs via the MITF-mediated glycoprotein (transmembrane) non-metastatic melanoma protein B pathway.57 In addition to its role in smooth muscle relaxation, nitric oxide triggers melanogenesis in response to UV radiation.42 Nitric oxide interacts with and activates heme-containing proteins such as guanylate cyclase, inducing the production of cyclic guanosine monophosphate and leading to MITF expression and melanogenesis.58 Studies have confirmed that inducible nitric oxide synthase (iNOS) expression is elevated in melasmic lesions, possibly through activation of the Akt/nuclear factor-κB pathway, suggesting that nitric oxide production plays an important role in the hyperpigmentation mechanism of melasma.59
A study showed a significant increase in the expression of SCF in the dermis and c-kit in the epidermis of lesional skin.60 Another study found that the expression of c-kit in the dermis was increased compared with perilesional skin, with c-kit-positive basal cells protruding into the dermis at up to 70%, compared to only 29% of the perilesional skin. SCF plays a large role in skin pigmentation disorders, and therefore represents a significant therapeutic target.
Relevant Signaling Pathways
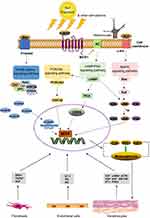
Melanogenesis is a complex process regulated by paracrine, autocrine, and environmental stimuli, and can be mediated by a series of intracellular signal pathways, including the Wnt/β-catenin, PI3K/Akt, cAMP/PKA, and SCF/c-kit mediated signaling pathways (Figure 1).
 |
Table 1 Studies Involved in This Paper |
Studies have shown an increase in the level of Wnt signaling-related genes, which is noteworthy because Wnt is involved in the proliferation of melanocyte stem cells.11 Activation of the Wnt receptor complexes triggers the replacement of the multifunctional glycogen synthase kinase-3β (GSK3β) and leads to the accumulation of β-catenin. Stable β-catenin is transferred to the nucleus, where it increases the expression of MITF, thereby stimulating melanogenesis. The expression of Wnts, either in the canonical or noncanonical signaling pathway, is occurring by increments in the hyperpigmented skin of patients with melasma.
Wnt inhibitory factor-1 (WIF-1) was identified to be expressed in cultured skin keratinocytes and fibroblasts, but not in melanocytes. Despite UV exposure, the expression of WIF-1 was notably decreased in melasmic skin. The downregulation of WIF-1 promotes melanosome transfer, along with MITF and tyrosinase expression via upregulation of the Wnt signaling pathway. This was found to be reversible by WIF-1 overexpression.61 MITF expression was increased, whereas GSK3β, β-catenin, and nuclear factor of activated T cells 2 (NFAT2) phosphorylation was reduced following WIF-1 knockdown. Bioinformatics analysis confirmed that a subset of Wnt signaling modulators, including WIF-1, Wnt5a, and secreted frizzled-related protein (SFRP)-2, were upregulated in lesional skin.11 The SFRP family includes a cysteine-rich domain, which is homologous to the supposed Wnt-binding site of Frizzled proteins, and SFRPs act as soluble modulators of Wnt signaling.74 It was found that SFRP2 is overexpressed in the hyperpigmented skin of melasma patients. SFRP2 plays a role as a melanogenic stimulant by MITF and tyrosinase upregulation via β-catenin signaling. This discovery was corroborated in melanocytes co-cultured with fibroblasts and in ex vivo cultured skin.62 Hence, inhibition of WIF-1 expression leads to tyrosinase upregulation and subsequent melanosome transfer.
PI3K/Akt signaling is another important pathway involved in regulating melanin production. Recent studies have shown that the activation of the Akt signaling pathway contributes to the inhibition of melanogenesis.63 Intracellular cAMP inhibits phosphatidylinositol 3-kinase (PI3K) and stimulates GSK3β activity, subsequently controlling MITF expression. Activation of GSK3β phosphorylates MITF, thereby enhancing transcriptional activation of tyrosinase-related genes and melanogenesis.39,64 The activation of the PI3K/Akt signaling pathway connects intracellular cAMP to GSK3β activity.
When EDN1 binds to EDNRB, the resulting diglyceride (DAG) mobilizes intracellular Ca2+ and activates protein kinase C (PKC). Activated PKC phosphorylates Raf or Raf-1, which activates the mitogen-activated protein kinase (MAPK) cascade through phosphorylation. Intracellular signaling interactions between EDN1 and SCF synergistically stimulate melanin synthesis through crosstalk between EDN1-induced PKC activation and SCF-induced c-kit activation.75 Interrupting the EDN1- or SCF-specific intracellular signaling pathways would, therefore, likely eliminate these synergistic signals, resulting in a significant anti-pigmentation effect.
ERK, JNK, and p38 also play important roles in the regulation of melanogenesis by the MAPK cascade. In this pathway, ligand–receptor interactions activate the Ras proteins, which activate B-Raf kinase, which, in turn, activate ERK, JNK, or p38. Phosphorylation of ERK or JNK leads to the degradation of MITF, which subsequently downregulates melanogenic related proteins and inhibits melanin synthesis.76 By contrast, phosphorylation of p38 promotes MITF activation and stimulates melanogenesis.65 These findings further underscore the important role of MITF as a key transcription factor in melanin synthesis, one controlled by several regulators.
Other Potential Factors
In addition, other factors may also be involved in the occurrence or development of melasma. The high expression of various inflammatory mediators in melasma such as iNOS, EDN1, and VEGF, indicates a general state of inflammation in melasmic skin. Arachidonate-derived chemical mediators, prostaglandins, thromboxanes, and leukotrienes can all enhance the activity of tyrosinase, thereby promoting pigmentation during inflammatory process.77 Lesional skin of patients with malar melasma shows increased infiltration by CD4+ T cells, mast cells, and macrophages. The levels of interleukin-17 and cyclooxygenase-2 were both significantly elevated compared with healthy skin.77 Increased expression of advanced glycation end products, NOD-like receptor thermal protein domain associated protein 3, and interleukin-18 was observed in melasma lesions.78 Although the relationship between inflammation and pigmentation is not clear, we believe that inflammatory mediators participate in the skin cell crosstalk relevant to physiological homeostasis and pathological processes. Elucidating this effect can provide a new perspective for the treatment of pigmentary disorders.
H19 is a non-coding RNA gene that has been observed to be downregulated in patients with melasma. Downregulation of H19 in mixed cultures induces overexpression of tyrosinase and melanosome transfer to keratinocytes.35 H19 stimulates the expression of microRNA 675 (miR-675), which targets MITF.66 Notably, downregulation of miR-675 was observed in melasma patients. Another study demonstrated effective inhibition of melanogenesis via upregulation of the H19/miR-675 axis.67
Microarrays revealed that guanine deaminase (GDA) gene expression is highly increased in melasma, and GDA in epidermal keratinocytes can promote melanin production by upregulating SCF and ET-1.68 GDA may therefore serve as a therapeutic target for inhibiting melanogenesis.
Higher superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) activities have been found in patients with melasma, implying an imbalance between oxidant and antioxidant levels in melasma. Compared with the control group, the serum levels of malondialdehyde, SOD, and GSH were remarkably elevated in patients with melasma, with no statistical difference between male and female patients.69 Bilirubin, as an antioxidant, may participate in the process of oxidative stress. Compared with healthy controls, the serum concentration of bilirubin was significantly higher in melasma patients.70 These results confirmed that oxidative stress may be involved in the pathogenesis of melasma. Antioxidants have been applied in the treatment of melasma for inhibition of free radicals.
A cross-sectional study performed in Brazil found that patients with melasma displayed higher recurrence rates of post-inflammatory hyperpigmentation.71 A study in Iran showed that patients with melasma displayed almost six times more development of post-acne pigmentation.72 Downregulation of lipid metabolism-related genes such as peroxisome proliferator-activated receptor-alpha (PPAR-α) and arachidonate 15-lipoxygenase type B (ALOX15B) in lesions may indicate that impaired barrier function contributes to the pathogenesis of melasma.11 A comparative study indicated markedly increased expression of nerve growth factor receptor and neural endopeptidase compared to nonlesional skin, along with increased numbers of keratinocytes expressing nerve growth factor receptor, neural endopeptidase, and nerve fibers in the dermis of lesional skin.79 We suggest that oxidative stress and neuroactive molecules are critical factors for the pathogenesis of melasma, which may directly affect the microenvironment and damage barrier function around melanocytes and lead to hyperpigmentation.
In addition, melasma’s predominance in the region of the body with the highest density of sebaceous glands suggesting that the function of sebaceous glands may be related to the pathogenesis of melasma. Sebum is a source of a variety of antioxidants, including vitamin E and coenzyme Q10. In addition to producing lipids, sebocytes also have inflammatory roles. Under normal and pathological conditions, leptin and its receptor were strongly expressed in sebaceous glands. In order to respond to leptin, sebum cells can perform inflammatory response by activating NF-κB and STAT3 pathways in vitro, such as enhanced inflammatory enzymes and cytokines.80 Moreover, sebaceous gland cells are regulated by α-MSH, and thus overexpression of the hormone can affect sebocytes and melanocytes. The sebaceous glands can synthesize several cytokines and growth factors, such as angiopoietin and adipokines, which can regulate melanocyte function. Lipids found in sebum, like linoleic acid, have been shown to inhibit melanogenesis.81 This implicates sebocytes as one of the actors in melasma pathogenesis, contributing to the generation growth factors and other mediators participating in photoaging and melasma appearance.
The gut microbiota structure of patients with melasma is different from that of normal control group. The abundance of Collinsella spp., Parabacteroides spp., Bacteroides spp., Actinomyces spp., Paraprevotella spp., Roseburia spp., and Blautia spp. in melasma patients was significantly different from that in healthy controls. Among them, Collinsella spp. is a distinctive member of the microbiota in patients with melasma. Their biological effects may play an important role in the development of chloasma by influencing estrogen metabolism.73 This suggests that the regulation of the gut-skin axis is also related to the pathogenesis of melasma (Table 1).
Conclusions
The development of melasma involves complex interactions among environmental, hormonal, and cellular factors. Studies have indicated that melasma may be a multifactorial pigmentation skin disease affecting genetically susceptible individuals. The short- and long-term effects of visible light on human skin pigmentation should be further investigated in order to elucidate its role in the development of melasma. In the future, it is necessary to further explore the interaction among the signaling pathways of keratinocytes, fibroblasts, sebocytes, and endothelial cells in the regulation of melanogenesis. A better understanding of the biological basis of melasma will help more effective and safe treatments for this common but challenging disease.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No.82173445).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Sarkar R, Gokhale N, Godse K, et al. Medical management of melasma: a review with consensus recommendations by Indian pigmentary expert group. Indian J Dermatol. 2017;62(6):558–577. doi:10.4103/ijd.IJD_489_17
2. Sarkar R, Jagadeesan S, Basavapura Madegowda S, et al. Clinical and epidemiologic features of melasma: a multicentric cross-sectional study from India. Int J Dermatol. 2019;58(11):1305–1310. doi:10.1111/ijd.14541
3. Espósito MCC, Espósito ACC, Jorge MFS, et al. Depression, anxiety, and self‐esteem in women with facial melasma: an Internet‐based survey in Brazil. Int J Dermatol. 2021;60(9):e346–e347. doi:10.1111/ijd.15490
4. Harumi O, Goh CL. The effect of melasma on the quality of life in a sample of women living in Singapore. J Clin Aesthet Dermatol. 2016;9(1):21–24.
5. Jusuf NK, Putra IB, Mahdalena M. Is there a correlation between severity of melasma and quality of life? Open Access Maced J Med Sci. 2019;7(16):2615–2618. doi:10.3889/oamjms.2019.407
6. Anderson L, Rodrigues M. Quality of life in a cohort of melasma patients in Australia. Australas J Dermatol. 2019;60(2):160–162. doi:10.1111/ajd.12969
7. D’Elia MP, Brandão MC, de Andrade Ramos BR, et al. African ancestry is associated with facial melasma in women: a cross-sectional study. BMC Med Genet. 2017;18(1):17. doi:10.1186/s12881-017-0378-7
8. Ogbechie-Godec OA, Elbuluk N. Melasma: an up-to-date comprehensive review. Dermatol Ther (Heidelb). 2017;7(3):305–318. doi:10.1007/s13555-017-0194-1
9. Espósito ACC, Cassiano DP, da Silva CN, et al. Update on melasma-part I: pathogenesis. Dermatol Ther (Heidelb). 2022;12(9):1967–1988. doi:10.1007/s13555-022-00779-x
10. Holmo NF, Ramos GB, Salomão H, et al. Complex segregation analysis of facial melasma in Brazil: evidence for a genetic susceptibility with a dominant pattern of segregation. Arch Dermatol Res. 2018;310(10):827–831. doi:10.1007/s00403-018-1861-5
11. Kang HY, Suzuki I, Lee DJ, et al. Transcriptional profiling shows altered expression of Wnt Pathway- and lipid metabolism-related genes as well as melanogenesis-related genes in melasma. J Invest Dermatol. 2011;131(8):1692–1700. doi:10.1038/jid.2011.109
12. Schaefer LV, Pontes LG, Cavassan NRV, Santos LDD, Miot HA. Proteomic study of facial melasma. An Bras Dermatol. 2022;97(6):808–814. doi:10.1016/j.abd.2021.06.010
13. Passeron T, Picardo M. Melasma, a photoaging disorder. Pigment Cell Melanoma Res. 2018;31(4):461–465. doi:10.1111/pcmr.12684
14. Yang J, Zeng J, Lu J. Mechanisms of ultraviolet-induced melasma formation: a review. J Dermatol. 2022;49(12):1201–1210. doi:10.1111/1346-8138.16542
15. Fatima S, Braunberger T, Mohammad TF, Kohli I, Hamzavi IH. The role of sunscreen in melasma and postinflammatory hyperpigmentation. Indian J Dermatol. 2020;65(1):5–10. doi:10.4103/ijd.IJD_295_18
16. Serre C, Busuttil V, Botto JM. Intrinsic and extrinsic regulation of human skin melanogenesis and pigmentation. Int J Cosmet Sci. 2018;40(4):328–347. doi:10.1111/ics.12466
17. Bernerd F, Marionnet C, Duval C. Solar ultraviolet radiation induces biological alterations in human skin in vitro: relevance of a well-balanced Uva/Uvb protection. Indian J Dermatol Venereol Leprol. 2012;78(Suppl 1):S15–S23. doi:10.4103/0378-6323.97351
18. Han M, Ban JJ, Bae JS, Shin CY, Lee DH, Chung JH. UV irradiation to mouse skin decreases hippocampal neurogenesis and synaptic protein expression via Hpa axis activation. Sci Rep. 2017;7(1):15574. doi:10.1038/s41598-017-15773-z
19. Kapoor R, Dhatwalia SK, Kumar R, Rani S, Parsad D. Emerging role of dermal compartment in skin pigmentation: comprehensive review. J Eur Acad Dermatol Venereol. 2020;34(12):2757–2765. doi:10.1111/jdv.16404
20. Regazzetti C, Sormani L, Debayle D, et al. Melanocytes sense blue light and regulate pigmentation through opsin-3. J Invest Dermatol. 2018;138(1):171–178. doi:10.1016/j.jid.2017.07.833
21. Espósito ACC, de Souza NP, Miot LDB, Miot HA. Expression of opn3 in fibroblasts, melanocytes, and keratinocytes of skin with facial melasma in comparison with unaffected adjacent skin. An Bras Dermatol. 2021;96(3):367–369. doi:10.1016/j.abd.2020.05.016
22. Narla S, Kohli I, Hamzavi IH, Lim HW. Visible light in photodermatology. Photochem Photobiol Sci. 2020;19(1):99–104. doi:10.1039/c9pp00425d
23. Duteil L, Cardot-Leccia N, Queille-Roussel C, et al. Differences in visible light-induced pigmentation according to wavelengths: a clinical and histological study in comparison with uvb exposure. Pigment Cell Melanoma Res. 2014;27(5):822–826. doi:10.1111/pcmr.12273
24. Duteil L, Queille-Roussel C, Lacour JP, Montaudié H, Passeron T. Short-term exposure to blue light emitted by electronic devices does not worsen melasma. J Am Acad Dermatol. 2020;83(3):913–914. doi:10.1016/j.jaad.2019.12.047
25. Ceresnie MS, Patel J, Lim HW, Kohli I. The cutaneous effects of blue light from electronic devices: a systematic review with health hazard identification. Photochem Photobiol Sci. 2022. doi:10.1007/s43630-022-00318-9
26. Boukari F, Jourdan E, Fontas E, et al. Prevention of melasma relapses with sunscreen combining protection against UV and short wavelengths of visible light: a prospective randomized comparative trial. J Am Acad Dermatol. 2015;72(1):189–190.e1. doi:10.1016/j.jaad.2014.08.023
27. Handel AC, Lima PB, Tonolli VM, Miot LD, Miot HA. Risk factors for facial melasma in women: a case-control study. Br J Dermatol. 2014;171(3):588–594. doi:10.1111/bjd.13059
28. Locci-Molina N, Wang A, Kroumpouzos G. Melasma improving spontaneously upon switching from a combined oral contraceptive to a hormone-releasing intrauterine device: a report of four cases. Acta Derm Venereol. 2015;95(5):624–625. doi:10.2340/00015555-2013
29. Natale CA, Duperret EK, Zhang J, et al. Sex steroids regulate skin pigmentation through nonclassical membrane-bound receptors. Elife. 2016;5:e15104. doi:10.7554/eLife.15104
30. Goandal NF, Rungby J, Karmisholt KE. The role of sex hormones in the pathogenesis of melasma. Ugeskr Laeger. 2022;184(6):V10210769.
31. Tamega Ade A, Miot HA, Moço NP, Silva MG, Marques ME, Miot LD. Gene and protein expression of oestrogen-β and progesterone receptors in facial melasma and adjacent healthy skin in women. Int J Cosmet Sci. 2015;37(2):222–228. doi:10.1111/ics.12186
32. Lee AY. Recent progress in melasma pathogenesis. Pigment Cell Melanoma Res. 2015;28(6):648–660. doi:10.1111/pcmr.12404
33. Cario M. How hormones may modulate human skin pigmentation in melasma: an in vitro perspective. Exp Dermatol. 2019;28(6):709–718. doi:10.1111/exd.13915
34. Filoni A, Mariano M, Cameli N. Melasma: how hormones can modulate skin pigmentation. J Cosmet Dermatol. 2019;18(2):458–463. doi:10.1111/jocd.12877
35. Kim NH, Lee CH, Lee AY. H19 RNA downregulation stimulated melanogenesis in melasma. Pigment Cell Melanoma Res. 2010;23(1):84–92. doi:10.1111/j.1755-148X.2009.00659.x
36. Spałkowska M, Dyduch G, Broniatowska E, Damiani G, Wojas-Pelc A. Molecular proof of a clinical concept: expression of estrogen alpha-, beta-receptors and G protein-coupled estrogen receptor 1 (Gper) in histologically assessed common nevi, dysplastic nevi and melanomas. Medicina. 2021;57(11):1228. doi:10.3390/medicina57111228
37. Esposito ACC, Brianezi G, de Souza NP, Miot LDB, Marques MEA, Miot HA. Exploring pathways for sustained melanogenesis in facial melasma: an immunofluorescence study. Int J Cosmet Sci. 2018;40(4):420–424. doi:10.1111/ics.12468
38. Swope VB, Abdel-Malek ZA. Mc1r: front and center in the bright side of dark eumelanin and DNA repair. Int J Mol Sci. 2018;19(9):2667. doi:10.3390/ijms19092667
39. Gelmi MC, Houtzagers LE, Strub T, Krossa I, Jager MJ. Mitf in normal melanocytes, cutaneous and uveal melanoma: a delicate balance. Int J Mol Sci. 2022;23(11):6001. doi:10.3390/ijms23116001
40. Kim NH, Cheong KA, Lee TR, Lee AY. Pdzk1 upregulation in estrogen-related hyperpigmentation in melasma. J Invest Dermatol. 2012;132(11):2622–2631. doi:10.1038/jid.2012.175
41. Harno E, Gali Ramamoorthy T, Coll AP, White A. Pomc: the physiological power of hormone processing. Physiol Rev. 2018;98(4):2381–2430. doi:10.1152/physrev.00024.2017
42. Yardman-Frank JM, Fisher DE. Skin pigmentation and its control: from ultraviolet radiation to stem cells. Exp Dermatol. 2021;30(4):560–571. doi:10.1111/exd.14260
43. Sarkar R, Ailawadi P, Garg S. Melasma in men: a review of clinical, etiological, and management issues. J Clin Aesthet Dermatol. 2018;11(2):53–59.
44. Brianezi G, Handel AC, Schmitt JV, Miot LD, Miot HA. Changes in nuclear morphology and chromatin texture of basal keratinocytes in melasma. J Eur Acad Dermatol Venereol. 2015;29(4):809–812. doi:10.1111/jdv.12453
45. Lentsch G, Balu M, Williams J, et al. In vivo multiphoton microscopy of melasma. Pigment Cell Melanoma Res. 2019;32(3):403–411. doi:10.1111/pcmr.12756
46. Espósito ACC, Brianezi G, de Souza NP, Miot LDB, Miot HA. Exploratory study of epidermis, basement membrane zone, upper dermis alterations and Wnt pathway activation in melasma compared to adjacent and retroauricular skin. Ann Dermatol. 2020;32(2):101–108. doi:10.5021/ad.2020.32.2.101
47. Yuan XH, Jin ZH. Paracrine regulation of melanogenesis. Br J Dermatol. 2018;178(3):632–639. doi:10.1111/bjd.15651
48. Wang Y, Viennet C, Robin S, Berthon JY, He L, Humbert P. Precise role of dermal fibroblasts on melanocyte pigmentation. J Dermatol Sci. 2017;88(2):159–166. doi:10.1016/j.jdermsci.2017.06.018
49. Xing X, Xu Z, Chen L, Jin S, Zhang C, Xiang L. Tranexamic acid inhibits melanogenesis partially via stimulation of TGF-beta1 expression in human epidermal keratinocytes. Exp Dermatol. 2022;31(4):633–640. doi:10.1111/exd.14509
50. Kwon SH, Hwang YJ, Lee SK, Park KC. Heterogeneous pathology of melasma and its clinical implications. Int J Mol Sci. 2016;17(6):824. doi:10.3390/ijms17060824
51. Esposito ACC, Brianezi G, de Souza NP, Santos DC, Miot LDB, Miot HA. Ultrastructural characterization of damage in the basement membrane of facial melasma. Arch Dermatol Res. 2020;312(3):223–227. doi:10.1007/s00403-019-01979-w
52. Gautam M, Patil S, Nadkarni N, Sandhu M, Godse K, Setia M. Histopathological comparison of lesional and perilesional skin in melasma: a cross-sectional analysis. Indian J Dermatol Venereol Leprol. 2019;85(4):367–373. doi:10.4103/ijdvl.IJDVL_866_17
53. Byun JW, Park IS, Choi GS, Shin J. Role of fibroblast-derived factors in the pathogenesis of melasma. Clin Exp Dermatol. 2016;41(6):601–609. doi:10.1111/ced.12874
54. Zhu JW, Ni YJ, Tong XY, Guo X, Wu XP. Activation of VEGF receptors in response to uvb promotes cell proliferation and melanogenesis of normal human melanocytes. Exp Cell Res. 2020;387(2):111798. doi:10.1016/j.yexcr.2019.111798
55. Trivedi MK, Yang FC, Cho BK. A review of laser and light therapy in melasma. Int J Womens Dermatol. 2017;3(1):11–20. doi:10.1016/j.ijwd.2017.01.004
56. Regazzetti C, De Donatis GM, Ghorbel HH, et al. Endothelial cells promote pigmentation through endothelin receptor B activation. J Invest Dermatol. 2015;135(12):3096–3104. doi:10.1038/jid.2015.332
57. Niwano T, Terazawa S, Sato Y, Kato T, Nakajima H, Imokawa G. Glucosamine abrogates the stem cell factor + endothelin-1-induced stimulation of melanogenesis via a deficiency in mitf expression due to the proteolytic degradation of creb in human melanocytes. Arch Dermatol Res. 2018;310(8):625–637. doi:10.1007/s00403-018-1850-8
58. Lajis AFB, Ariff AB. Discovery of new depigmenting compounds and their efficacy to treat hyperpigmentation: evidence from in vitro study. J Cosmet Dermatol. 2019;18(3):703–727. doi:10.1111/jocd.12900
59. Samaka RM, Bakry OA, Shoeib MA, Zaaza MM. Expression of iNOS and NF-Kappab in melasma: an immunohistochemical study. Anal Quant Cytopathol Histpathol. 2014;36(5):245–257.
60. Atef A, El-Rashidy MA, Abdel Azeem A, Kabel AM. The role of stem cell factor in hyperpigmented skin lesions. Asian Pac J Cancer Prev. 2019;20(12):3723–3728. doi:10.31557/APJCP.2019.20.12.3723
61. Kim JY, Lee TR, Lee AY. Reduced Wif-1 expression stimulates skin hyperpigmentation in patients with melasma. J Invest Dermatol. 2013;133(1):191–200. doi:10.1038/jid.2012.270
62. Kim M, Han JH, Kim JH, Park TJ, Kang HY. Secreted frizzled-related protein 2 (Sfrp2) functions as a melanogenic stimulator; the role of sfrp2 in UV-induced hyperpigmentary disorders. J Invest Dermatol. 2016;136(1):236–244. doi:10.1038/JID.2015.365
63. Eom YS, Jeong D, Ryu AR, Song KH, Im DS, Lee MY. Daphne odora exerts depigmenting effects via inhibiting Creb/Mitf and activating Akt/Erk-signaling pathways. Curr Issues Mol Biol. 2022;44(8):3312–3323. doi:10.3390/cimb44080228
64. Kim T, Hyun CG. Imperatorin positively regulates melanogenesis through signaling pathways involving Pka/ Creb, Erk, Akt, and Gsk3β/Β-catenin. Molecules. 2022;27(19):6512. doi:10.3390/molecules27196512
65. Yoon JH, Youn K, Jun M. Discovery of pinostrobin as a melanogenic agent in cAMP/PKA and p38 MAPK signaling pathway. Nutrients. 2022;14(18):3713. doi:10.3390/nu14183713
66. Kim NH, Choi SH, Kim CH, Lee CH, Lee TR, Lee AY. Reduced Mir-675 in exosome in H19 RNA-related melanogenesis via mitf as a direct target. J Invest Dermatol. 2014;134(4):1075–1082. doi:10.1038/jid.2013.478
67. Jin S, Chen L, Xu Z, Xing X, Zhang C, Xiang L. 585 Nm light-emitting diodes inhibit melanogenesis through upregulating H19/Mir-675 axis in LEDs-irradiated keratinocytes by paracrine effect. J Dermatol Sci. 2020;98(2):102–108. doi:10.1016/j.jdermsci.2020.03.002
68. Jung JM, Noh TK, Jo SY, et al. Guanine deaminase in human epidermal keratinocytes contributes to skin pigmentation. Molecules. 2020;25(11):2637. doi:10.3390/molecules25112637
69. Choubey V, Sarkar R, Garg V, Kaushik S, Ghunawat S, Sonthalia S. Role of oxidative stress in melasma: a prospective study on serum and blood markers of oxidative stress in melasma patients. Int J Dermatol. 2017;56(9):939–943. doi:10.1111/ijd.13695
70. Rahimi H, Mirnezami M, Yazdabadi A. Bilirubin as a new antioxidant in melasma. J Cosmet Dermatol. 2022;21(11):5800–5803. doi:10.1111/jocd.15240
71. Hexsel D, Lacerda DA, Cavalcante AS, et al. Epidemiology of melasma in Brazilian patients: a multicenter study. Int J Dermatol. 2014;53(4):440–444. doi:10.1111/j.1365-4632.2012.05748.x
72. Adalatkhah H, Sadeghi Bazargani H. The Association between melasma and postin flammatory hyperpigmentation in acne patients. Iran Red Crescent ME. 2013;15(5):400–403. doi:10.5812/ircmj.5358
73. Liu C, He D, Yu A, Deng Y, Wang L, Song Z. Correlation analysis between gut microbiota characteristics and melasma. Front Microbiol. 2022;13:1051653. doi:10.3389/fmicb.2022.1051653
74. Katoh M, Katoh M. Molecular genetics and targeted therapy of wnt-related human diseases (review). Int J Mol Med. 2017;40(3):587–606. doi:10.3892/ijmm.2017.3071
75. Imokawa G, Ishida K. Inhibitors of intracellular signaling pathways that lead to stimulated epidermal pigmentation: perspective of anti-pigmenting agents. Int J Mol Sci. 2014;15(5):8293–8315. doi:10.3390/ijms15058293
76. Zhou S, Sakamoto K. Pyruvic acid/ethyl pyruvate inhibits melanogenesis in b16f10 melanoma cells through Pi3k/Akt, Gsk3beta, and Ros-Erk signaling pathways. Genes Cells. 2019;24(1):60–69. doi:10.1111/gtc.12654
77. Hossain MR, Ansary TM, Komine M, Ohtsuki M. Diversified stimuli-induced inflammatory pathways cause skin pigmentation. Int J Mol Sci. 2021;22(8):3970. doi:10.3390/ijms22083970
78. Fang J, Ouyang M, Qu Y, et al. Advanced glycation end products promote melanogenesis by activating NLRP3 inflammasome in human dermal fibroblasts. J Invest Dermatol. 2022;142(10):2591–2602.e8. doi:10.1016/j.jid.2022.03.025
79. Khunger N, Kandhari R, Singh A, Ramesh V. A clinical, dermoscopic, histopathological and immunohistochemical study of melasma and facial pigmentary demarcation lines in the skin of color. Dermatol Ther. 2020;33(6):e14515. doi:10.1111/dth.14515
80. Kovács D, Fazekas F, Oláh A, Törőcsik D. Adipokines in the Skin and in Dermatological Diseases. Int J Mol Sci. 2020;21(23):9048. doi:10.3390/ijms21239048
81. Flori E, Mastrofrancesco A, Mosca S, et al. Sebocytes contribute to melasma onset. iScience. 2022;25(3):103871. doi:10.1016/j.isci.2022.103871
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.