Back to Journals » Infection and Drug Resistance » Volume 8
New developments in the treatment of drug-resistant tuberculosis: clinical utility of bedaquiline and delamanid
Authors Brigden G, Hewison C, Varaine F
Received 19 May 2015
Accepted for publication 8 July 2015
Published 30 October 2015 Volume 2015:8 Pages 367—378
DOI https://doi.org/10.2147/IDR.S68351
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Grania Brigden,1 Cathy Hewison,2 Francis Varaine2
1Access Campaign, Médecins Sans Frontières, Geneva, Switzerland; 2Medical Department, Médecins Sans Frontières, Paris, France
Abstract: The current treatment for drug-resistant tuberculosis (TB) is long, complex, and associated with severe and life-threatening side effects and poor outcomes. For the first time in nearly 50 years, there have been two new drugs registered for use in multidrug-resistant TB (MDR-TB). Bedaquiline, a diarylquinoline, and delamanid, a nitromidoxazole, have received conditional stringent regulatory approval and have World Health Organization interim policy guidance for their use. As countries improve and scale up their diagnostic services, increasing number of patients with MDR-TB and extensively drug-resistant TB are identified. These two new drugs offer a real opportunity to improve the outcomes of these patients. This article reviews the evidence for these two new drugs and discusses the clinical questions raised as they are used outside clinical trial settings. It also reviews the importance of the accompanying drugs used with these new drugs. It is important that barriers hindering the use of these two new drugs are addressed and that the existing clinical experience in using these drugs is shared, such that their routine-use programmatic conditions is scaled up, ensuring maximum benefit for patients and countries battling the MDR-TB crisis.
Keywords: MDR-TB, XDR-TB, tuberculosis drugs, group 5 drugs
Introduction
Tuberculosis (TB) is one of the leading causes of death worldwide: an estimated 9 million people developed active TB in 2013, of which approximately 1.5 million died. In 2013, the World Health Organization (WHO) estimated that there were 480,000 new multidrug-resistant TB (MDR-TB) cases (defined as resistance to isoniazid and rifampicin) and 210,000 deaths. Up to 30% of MDR-TB patients have further resistance to either fluoroquinolones (FQ) or injectable anti-TB agents; approximately 9% of MDR-TB cases have both, which is defined as extensively drug-resistant TB (XDR-TB). In 2013, cases of XDR-TB were reported in 100 countries.1 In some regions, MDR-TB appears to be an epidemic in its own right, with evidence of direct transmission of drug-resistant forms of TB.2,3
The emergence of MDR-TB hampers efforts to effectively prevent and treat TB, and threatens the prospects for success of the new END TB strategy ratified by the World Health Assembly in 2014.4 This is because current treatments for MDR-TB are inadequate: they require patients to undergo lengthy courses of treatment, subject them to severe side effects, are programmatically expensive to implement – and despite all these drawbacks, still show poor success rates, ranging from 48% for MDR-TB to 22% for XDR-TB.1
Therefore, it is of enormous significance that, for the first time in nearly 50 years, two new compounds bedaquiline and delamanid have been approved for the treatment of MDR-TB when an effective treatment regimen is not otherwise available.5–7 Bedaquiline (Janssen, Beerse, Belgium), the first new drug, received accelerated approval from the US Food and Drug Administration in December 2012.7 The second drug, delamanid (Otsuka Pharmaceutical Co, Ltd, Tokyo, Japan), received approval from the European Medicines Agency and Japan’s Pharmaceuticals Medical Devices Agency in 2014.5 Alongside these completely new drugs, there is growing evidence for the potential role of repurposed medicines, including clofazimine and linezolid, which are showing effectiveness against drug-resistant forms of TB.8–10
It is hoped that the new drugs and the repurposed drugs that accompany them offer promise for improving the treatment of drug-resistant forms of TB, in terms of both better outcomes and quality of life for patients. This article reviews current clinical data and guidance on the use of these new drugs, discusses how they might be used to change MDR-TB treatment outcomes, and raises the challenges seen by clinicians and patients with experience using the drugs.
Efficacy and safety evidence from clinical trials of the new drugs
Delamanid, previously OPC-67863, is a drug of the dihydro-nitroimidazole class and has potent anti-TB activity. Delamanid is thought to primarily inhibit synthesis of methoxy-mycolic and keto-mycolic acid, components of the mycobacterial cell wall. As a prodrug, it requires metabolic activation to exert its anti-TB activity.11
Clinical trial data come from three related Phase IIb studies on the same cohort of MDR-TB patients (trial 204, trial 208, and observational study 116).11,12 The key data are from trial 204, which investigated the effect of delamanid, taken with a WHO-recommended MDR-TB optimized background treatment regimen (OBR), on 2-month sputum culture conversion rates in patients with pulmonary MDR-TB (Table 1). Patients were randomized into three arms: delamanid 100 mg or 200 mg twice daily, or placebo, all administered with an OBR.
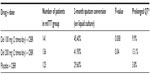  | Table 1 Results of trial 204 for delamanid in MDR-TB patients |
The number of patients withdrawing from trial 204 due to adverse effects was small and was evenly distributed across the three treatment groups. Rates of hepatotoxicity were low across all groups.11
To assess longer term safety and efficacy, trial 208 followed as an open label extension to trial 204. An additional 6 months of delamanid with OBR was given to 213/481 trial 204 participants, a minimum of 4 weeks after the end of study 204. Study 116 studied an observation cohort of 421/481 trial 204 participants who were followed up to the end of their MDR-TB treatment or to 24 months after the first dose of delamanid in trial 204 whichever came first. Despite the weakness of the study designs for trial 208 and study 116,13 most importantly the possibility of selection bias, these studies showed promising results among patients who received 6–8 months of delamanid compared to those who received 0–2 months of delamanid, with significantly higher proportion of favorable outcomes (cure or treatment complete) (75.5% versus 55%) and lower mortality (1% versus 8.3%).12
A multicenter, double-blind Phase III placebo-controlled trial in MDR-TB patients to assess the efficacy and safety of the addition of 6 months of delamanid to a WHO-recommended OBR, has recently completed recruitment.14
Bedaquiline, previously called TMC 207, is a diarylquinoline that acts by specifically inhibiting mycobacterial adenosine triphosphate synthase and has a long half-life of approximately 5 months.15 A Phase IIb A clinical trial (C208 study) compared a second-line background regimen with or without 24 weeks of bedaquiline (Table 2).16
  | Table 2 Results of trial C208 for bedaquiline |
Notably, a higher numbers of deaths were reported in the bedaquiline treatment arm, with ten deaths occurring in bedaquiline-treated patients versus two deaths in the placebo arm (P=0.017).
None of these deaths were attributed to bedaquiline. All but one occurred after bedaquiline had been stopped, although, given the drug’s long half-life, a potential role in these deaths cannot be excluded. Other side effects noted with bedaquiline included liver toxicity and QT prolongation.16 The planned Phase III trial of bedaquiline, STREAM II (Table 3), has yet to start.17
For both delamanid and bedaquiline, there is little information on their use in HIV coinfected patients, including those on antiretroviral treatment. An ongoing pediatric trial, with results expected in 2017, is evaluating pharmacokinetics, safety, tolerability, and antimycobacterial activity of delamanid in children, including some who are HIV infected and others with “probable” TB.18 A pediatric trial for bedaquiline is due to start in 2015.
Current guidance for clinical use
WHO has issued interim guidance for both bedaquiline in 201319 and delamanid in 201420 and expanded on this guidance in the companion guidelines.21 These guidelines specify five conditions that must be in place prior to the introduction of each drug (Table 4).
  | Table 4 Criteria for introduction of new TB drugs |
Interim policy guidance states that bedaquiline can be used when an effective treatment regimen containing four second-line drugs (in addition to pyrazinamide) including a FQ and a second-line injectable agent cannot be designed. This includes patients with known resistance, adverse drug reactions, poor tolerance, or contraindication to any component of the regimen.19
The interim policy guidance for delamanid is similar to that of bedaquiline, recommending its use when an effective treatment regimen cannot be designed due to resistance or intolerance to existing drugs. However, it goes further, recommending delamanid use in patients with high risk of poor outcome (ie, extensive or advanced disease), meaning that delamanid is potentially suitable for a larger number of patients.20
Caution is recommended when using these new drugs in people living with HIV or other comorbidities (ie, diabetes, renal, or liver dysfunction) and in people reporting alcohol or substance abuse, due to a lack of data. A single drug should not be added to a failing MDR-TB regimen, and this also applies to new drugs. Today, they cannot be combined with one another. With regard to concerns over potential impact on QTc, baseline testing and monitoring for QTc prolongation are recommended for both drugs. Bedaquiline and delamanid are classed as group 5 drugs in the WHO classification system.
Importance of accompanying drugs
A growing body of evidence indicates a potential role for some existing antibiotics in the treatment of complex drug-resistant patients also classed by WHO as group 5.21,22 The main drugs of interest are clofazimine, linezolid, and imipenem (Table 5).
  | Table 5 Characteristics of clofazimine, linezolid, and imipenem |
Clofazimine is thought to have promise as an antimycobacterial due to its long half-life (65–70 days), slow metabolic elimination, high concentration in macrophages, and rapid localization within phagocytes.23 Recent in vitro and in vivo trials in mice have shown low toxicity and good efficacy against drug-resistant TB (DR-TB) strains.24 Several systematic review articles have highlighted the potential beneficial role of clofazimine in DR-TB regimens.8,23 Additionally, recent studies noted successful outcomes of shortened DR-TB treatment regimes containing clofazimine;25,26 these findings are being further investigated as part of the larger STREAM randomized control trial27 (Table 3). However, a recent EBA study showed no efficacy at all at 14 days in humans.28
Linezolid is thought to act by competitive inhibition of the enzyme that binds incoming transfer RNA to messenger RNA.29 A systematic review found that treatment outcomes with linezolid-containing regimens for complicated cases of MDR-TB were equal to or better than those reported for uncomplicated MDR-TB,30 and also better than those reported among patients treated for XDR-TB.31 A randomized controlled study in patients with XDR-TB not responding to treatment who had linezolid added into their regimen showed that 87% had a negative sputum culture within 6 months after linezolid had been added to their drug regimen.32
Carbapenems (imipenem/cilastatin or meropenem) have an extremely wide spectrum of activity that includes Gram-positive, Gram-negative, aerobic, and anaerobic bacteria. Data on carbapenem use against drug-resistant forms of TB are slim, but the limited evidence available from mouse models suggests anti-TB activity; several case reports33,34 are consistent with this view. However, these drugs must be administered intravenously, which would limit their large-scale use against MDR-TB.
Experience with the new drugs in compassionate use programs
Despite an estimated 48,000 patients globally with XDR-TB and at least double that with pre-XDR-TB and MDR-TB that would fulfill the WHO criteria for new drugs, the unfortunate reality is that fewer than 1,000 patients have received bedaquiline outside of clinical trials;35 the majority of whom accessed the drug through compassionate use or equivalent programs rather than through routine programmatic use. To our knowledge, delamanid – despite its much broader indication – has been used outside clinical trials by approximately 100 patients.
From both a patient and a public health perspective, access through compassionate use pathways has several restrictions and additional requirements compared with typical routine programmatic use. Compassionate use (also referred to as “clinical access” or “expanded access” programs) is intended as a way of providing lifesaving experimental treatments to patients suffering from a disease for which no satisfactory approved therapy is available, or who cannot enter a clinical trial. However, to take advantage of this access route, patients must reside in a country with appropriate legislation or authorization must be given allowing the compassionate use of new, unregistered therapies. Another restriction is that the drug developer makes final decisions on whether the drug will be supplied for compassionate use and under which conditions. No data collection is required except for the reporting of adverse events. Janssen first provided bedaquiline for compassionate use in 2011, and Otsuka provided delamanid in 2014.
The three countries with the most patients to benefit from the use of bedaquiline via compassionate use programs were France, South Africa, and Armenia. France, which has a well-developed mechanism for expanded-access use, began compassionate use of bedaquiline in 2011. An interim analysis of the first 35 patients in a French cohort was published in 2014.36 South Africa first needed to establish the appropriate legislative framework for compassionate use of bedaquiline, but after approval of a clinical access program it quickly overtook France as the biggest user of bedaquiline. After South Africa and France, Armenia has the third largest compassionate use cohort, through a program supported by Médecins Sans Frontières (MSF).
Although all these programs have very different patient profiles and programmatic means, they all are starting to share very similar early outcome results. Published data is available from the French cohort, and very recently from South Africa.37 Data from Armenia has been presented at the 4th Annual TB symposium in Yerevan, hosted by the Ministry of Health of Armenia and MSF.38 Table 6 summarizes the profiles of patients from these three cohorts.
All three programs have treated adult pulmonary XDR-TB or pre-XDR-TB patients, most of whom were resistant to FQ. The French cohort was able to include patients without FQ or injectable resistance (2/35 patients) in the published analysis; although the South African Clinical Access Program inclusion criteria included MDR-TB patients without additional resistance to second-line drugs but with intolerance to other group 1–4 drugs (such as ototoxicity or renal toxicity), it did not report any cases benefiting from this access.
The majority of treated patients in all cohorts were male. The most important difference among cohorts was the rate of HIV coinfection: very high (59.3%) in South Africa, compared with no HIV-positive patients in France and only 2 (4%) in Armenia. Accompanying drugs in the regimen also varied: linezolid was used extensively in Armenia (100%) and France (94%), and in 70% of patients in South Africa. Imipenem was not used in South Africa, and clofazamine is used much less in France (14%) than in Armenia (74%) or South Africa (74.7%).
While the end-of-treatment outcomes for MDR-TB patients are in general poor, close to 50% success, the treatment of the difficult-to-treat subgroup, XDR-TB patients, is even worse in general. Although a retrospective study of XDR-TB patient treatment outcomes from Tomsk showed 14/29 (48.3%) patients had a successful end of treatment outcome,39 most other analyses reported much lower success rates. For example, only 12/107 (11%) XDR-TB patients from South Africa had a favorable outcome at 60 months,40 while WHO has reported 22% favorable end of treatment outcomes among 1,269 XDR-TB patients from 40 countries.1 Although there are no published results on the subgroup of pre-XDR-TB (FQ) patients, given that resistance to FQ is associated with failure and lack of culture conversion, the outcomes are likely to be closer to XDR-TB patients than MDR-TB patients without FQ resistance.41,42
One of the challenges to developing better treatment for MDR-TB is the long treatment duration and therefore the long time needed to analyze end of treatment outcomes. Clinicians use the conversion of culture from positive to negative to guide their clinical management of patients. In MDR-TB patients, it has been reported that no culture conversion by the third month of treatment is an independent predictor of death and failure41 and that treatment outcomes were worse in patients who did not convert by 2 months.43 In the subgroup of XDR-TB patients, Pietersen et al40 found net culture conversion was associated with survival, whereas O’Donnell et al44 found culture conversion by 2 months associated with survival, although time to conversion was not a good predictor of favorable outcomes. Therefore, while acknowledging the weakness of this indicator, culture conversion at 6 months has been used as an early proxy for the assessment of treatment outcomes.
Previous published data on cohorts of treated XDR-TB patients have shown low culture conversion rates at 6 months. O’Donnell et al44 found 36.8% of their cohort of 114 XDR-TB patients culture converted and Pietersen et al40 found only 9.3% (10/107) of 107 patients had culture converted by 6 months. In contrast, the 6-month culture conversion rates for the new bedaquiline compassionate use cohorts (Table 7) show a vast improvement over these earlier results using other treatment regimens: 77% (33/43) for patients treated in South Africa, 84% (22/26) in Armenia, and 97% (28/29) in France. Although the patients in all three of these cohorts were not all XDR-TB patients, they are almost all FQ-resistant MDR-TB patients for whom we could anticipate similar outcomes.45
Early mortality in the 24 weeks of bedaquiline treatment was low in general, and all deaths were evaluated as being unrelated to bedaquiline.
None of the projects has identified significant adverse events, although in all cases patients were managed with enhanced monitoring. As expected, an increase, although small in QTcB/F was reported by the French and South African cohorts, but without adverse outcomes for the patients. Elevated liver enzymes were reported in 14% of patients in France, which is similar to the findings from the clinical trials.16,36 No reported adverse events linked to hepatotoxicity were reported the South African cohort which is very reassuring considering the majority of patients were also taking anti-retro viral drugs in particular nevarapine, a known hepatotoxic drug. Armenia did not report on hepatotoxicity although their analyses may not be complete.
The South African cohort analysis has significantly contributed to reducing the lack of data on the use of bedaquline in HIV infected persons, and in combination with antiretroviral drugs. The excellent results, both in terms of safety and effectiveness, are reassuring for both clinicians and patients.37 These results must be interpreted with caution because they are only interim findings and because culture conversion is only a first indication of treatment response; clinical outcomes can only be known when patients have completed their treatment course and been followed up for at least a year post-treatment in order to detect relapses.
Because of the very recent introduction of bedaquiline into routine programmatic use, there are no routine results to report so far. Similarly, for delamanid there are no published or presented data on delamanid use outside clinical trials, although the manufacturing company reports that approximately 100 patients around the world have benefited from this drug. MSF began a compassionate use program using delamanid with the first patients starting treatment in February 2015.
However, as stated earlier, compassionate use programs provide a useful interim step to providing access and experience in using new drugs, but the conditions attached to these programs limit the number of patients who can be included. For the tens of thousands of patients who urgently need these new drugs, and for the drugs’ full public health benefit to be realized, it is essential that they become incorporated into routine programmatic use. The reasons why this may not be happening are discussed later in the article.
Maximizing the benefit of the new drugs
Another key step in realizing the promise of these new drugs is to incorporate them into better combination regimens, since no single drug can cure TB. New regimens for drug-resistant forms of TB must be shorter, more effective, and safer (fewer adverse events).46
Several planned clinical trials will investigate new TB regimens that incorporate new drugs, including delamanid and bedaquiline (Table 3). These trials are crucial for establishing the best ways to use these new drugs and maximize their potential. However, results are likely to be at least 5 years away, and the use of the new drugs should not be delayed until these data are available. In the meantime, countries can and should add these new drugs to the arsenal available in their programs as per WHO guidance.
Duration
Clinical trials data for bedaquiline and delamanid are currently available for only 24 weeks of use. For this reason, WHO and stringent regulatory authorities have recommended limiting treatment with both drugs to a 24-week maximum,19,21 a strategy that potentially compromises their effectiveness in certain patients. Considering the very positive early responses of XDR-TB and pre-XDR-TB patients treated with bedaquiline in compassionate use programs, is it appropriate to stop an effective drug, potentially weakening the treatment regimen? The other effective drugs in these therapeutic regimens also have major drawbacks: linezolid is associated with serious side effects, especially hematological and neurological toxicity,30,47,48 while imipenem is difficult to administer – it must be given intravenously twice daily through central catheters. Given these serious limitations for the drugs now in use, bedaquiline may be essential for maintaining an effective regimen over the duration of treatment.
While guidelines for WHO do not allow extension of bedaquiline beyond 24 weeks, the French compassionate use program allows clinicians extensions on a case-by-case basis. This exception has been made for patients with fewer than three effective drugs in the regimen, resulting in a current median treatment time of 36 weeks as of February 2015 and increasing as bedaquiline is continued in these patients.49 The US Centers for Disease Control50 and European Medical Agency51 have also left open the possibility of case-by-case extension of bedaquiline beyond 24 weeks. Despite these agencies allowing flexibility in treatment duration, WHO guidance preserves the 24-week limitation in both the interim policy guidance and the more recent implementation plan for bedaquiline.19,21,52 Although at the time of the initial expert recommendations in 2013 this conservatism was understandable, it may be unnecessarily restrictive in 2015 considering both the accumulated experience with these drugs since then (and specifically with extended use of bedaquiline) and the positive initial outcomes data. While it is obviously preferable to have a strong evidence base for recommending extension of treatment, the risks and benefits must be appropriately evaluated, along with the availability of alternatives on a case-by-case basis. Furthermore, many other drugs used today for treating MDR-TB also lack a strong evidence base: for example, the effectiveness of the group 5 drugs linezolid, imipenem, and clofazamine against MDR-TB has never been tested in a clinical trial, nor has clinical experience on their extended use been published.21 In our view, these factors, along with the very poor outcomes of current treatment for XDR-TB, shift the risk–benefit in favor of extending bedaquiline and delamanid use beyond 24 weeks in patients who have limited alternatives, a good response to treatment, and no adverse effects.
Continuation of both bedaquiline and delamanid treatment for selected patients is a crucial next step in the learning curve on these drugs. For support in these efforts, providers in most countries can access expert advice on patients, either through the ERS/WHO TB Consilium forum53 or from other such committees. With access to technical support from such groups, countries should have the flexibility to extend treatment durations, as appropriate, to ensure that MDR-TB patients can remain on the strongest regimen.
Drug combinations
Since successful TB treatment depends on successful regimens, and not individual drugs, the most important new drug combination with the greatest potential to really make inroads into improving DR-TB treatment of drug-resistant forms of TB would be the combination of delamanid and bedaquiline, two completely new classes of drugs. In patients with limited treatment options, such as XDR-TB patients, the use of these drugs together could be an option. However, due to the potential overlap in the two drugs’ toxicities, particularly QTc prolongation, and the lack of any studies investigating this combination, at this point it is not recommended to combine these two drugs.21 A drug–drug interaction study that has been planned by NIH has yet to commence, but until the results are available and the concerns about the prolonged QT interval caused by both drugs have been resolved, it is unlikely that the guidance will be changed. However, could certain exceptions be considered, where the benefits of combining these two drugs outweigh the possible risks? Another option is to use the two drugs sequentially, and WHO gives guidance on this.21 Considering the long half-life of bedaquiline, patients could be started on delamanid for 24 weeks and then switched to bedaquiline for an additional 24 weeks, giving just over a year of benefit from the new drugs. The long half-life of bedaquiline makes reverse sequence (bedaquiline followed by delamanid) a more risky combination.
Concerns about QT interval prolongation also apply to the combination of the FQ moxifloxicin with bedaquiline or delamanid. Moxifloxacin has the greatest impact on QT among the FQ;54 WHO recommends that the use of the combination of moxifloxacin with bedaquiline and clofazimine (three drugs that strongly prolong the QT interval) should be avoided. In the French cohort of XDR-TB and pre-XDR-TB (FQ) patients, bedaquiline was combined with either moxifloxacin at standard (400 mg/day) or high dose (800 mg/day) or with levofloxacin. Their analysis found that the presence of a FQ in the treatment regimen was the only independent factor associated with a faster time to culture conversion. No cardiac arrhythmias were recorded, and three patients (9%) had a QTcB greater than 500 ms, and two patients had bedaquiline discontinued (6%) due to persistent QTcB prolongation, one who received concomitant moxifloxacin and 1 who received clofazamine and amiodarone.36 In South Africa and Armenia, moxifloxacin was not used in combination with bedaquiline. As the higher generation FQ (Lfx, Mfx) may have residual effectiveness in a small percentage of patients resistant to ofloxacin, it is essential to be able to identify these patients in whom the possible benefits of the combination of moxifloxacin with new drugs could outweigh the risks. Identifying these patients would require changes to diagnostic capacity in many countries where the only DST available for FQ is DST to ofloxacin.
Who should get what drug?
How to choose between bedaquiline and delamanid for an individual patient? WHO has given some practical advice in the most recent updated guidelines.21 Currently, there is more experience of using bedaquiline in XDR-TB and pre-XDR-TB (FQ) patients due to the early availability through compassionate use or equivalent programs. However, the safety profile of delamanid is better, and the data from the trials show lower mortality in patients who received delamanid.12 This would make delamanid the drug of choice for MDR-TB patients with adverse events requiring the substitution of a drug in the treatment regimen or those with high risk of a poor outcome. Other factors apart from resistance profile should be taken into account, in particular comorbidities; delamanid has less interactions with antiretrovirals and other drugs metabolized by the cytochrome P450 enzymes like CYP3A4 and less hepatic effects, which is important in coinfected hepatitis B and C patients.5,55,56
Of concern, there is recent evidence of a possible cross-resistance between clofazimine and bedaquiline.57,58 With the lack of accessible DST for bedaquiline and clofazimine, delamanid may be preferred in patients with previous use of clofazimine. Further studies are required to know if the presence of the mutation Rv0678 is clinically important in predicting resistance to bedaquiline and clofazimine, only then will the significance of this finding be known.
Barriers to accessing new and repurposed drugs
Any discussion regarding how best to use these new drugs must also consider their availability in countries with a high burden of MDR-TB patients, which cannot be taken for granted. Access to new and repurposed TB drugs for programmatic use requires that several processes are in place, and several existing barriers removed.
For the drugs without an indication for TB (clofazimine, linezolid, and imipenem/cilastatin), this may present problems with importing the drug into a country as well as issues arising around using a drug as it becomes off label use. (Of note this also applies to FQ and second-line injectable agents). The main barrier to the scaling up and use of imipenem/cilastatin is its formulation. It is only available as a twice-daily injectable agent, which creates many programmatic barriers to its widespread use.
Importation can be possible immediately in the short term through importation waivers while registration processes are under way, although registration is desirable in the long term. Janssen has submitted a number of dossiers for the registration of bedaquiline, with rapid approval processes leading to successful registrations in Russia and South Africa, two key high-burden countries. Otsuka has not currently submitted registration dossiers for delamanid in any countries outside the European Union.
The price of the drugs needs to be considered as affordability is a crucial aspect of access for patients. Price for a drug cannot be viewed in isolation but must be considered in addition to a background regimen that already has a price range of USD1,500–USD7,000.59 For bedaquiline, Janssen has implemented a tiered pricing strategy in which low-income countries pay USD900 for a 6-month course, middle-income countries pay USD3,000, and high-income countries USD30,000.60
However, up to now the scale-up of routine programmatic use of bedaquiline (where this pricing mechanism would come into play) has been very slow; to date, the majority of patients who received bedaquiline have done so through compassionate use programs, where the drug is usually free to patients. It is unclear why scale-up in routine programs has been so slow. The tiered pricing structure will eventually require middle-income countries with high MDR-TB and XDR-TB burdens to make a considerable financial commitment to provide access bedaquiline to all those who would benefit from it.
For delamanid there is no global price structure available, and it is not marketed or available in any low- or middle-income countries. Where it is marketed in Europe, it has a similar price to that of the high-income countries price of bedaquiline. For linezolid, there are increasing numbers of quality-assured suppliers and this is having a positive impact on the price of the product, which had until recently been priced out of reach of widespread use.
The looming price barrier for bedaquiline and delamanid has been temporarily removed in some settings with the announcements of various donation programs. Janssen has established a program with USAID61 to make 30,000 courses of bedaquiline available to global fund Global Fund-eligible countries over the next 4 years. Otsuka has announced a targeted access donation program for delamanid. The details of this donation program are yet to be announced but are focused on the 27 high-burden countries.62
Such donation programs can potentially enable short-term scale-up of new drugs. However, they are not a sustainable method of ensuring access to drugs, since they are usually limited in duration and geographical scope (as in this case) – leaving patients who are ineligible for the program with few or no options for gaining affordable access. In the case of delamanid, there is no information on how countries that are not eligible for the donation program will be able to procure the drug. Nevertheless, if the donation process is straightforward and has a wide scope, then even short-term removal of the price barrier creates an opening to address additional barriers to widespread, sustainable programmatic implementation.
Another challenge for scaling up programmatic use of bedaquiline and delamanid is the issue of pharmacovigilance (PV). Since these drugs are completely new and were approved with only limited clinical trial data, it is important to collect additional safety data. This can be accomplished in a number of ways. WHO guidance21 on the new drugs states that “Active pharmacovigilance measures must be in place to ensure early detection and proper management of adverse drug reactions and potential interactions with other drugs”. Many countries with high MDR-TB burdens have spontaneous PV models in place and do not have extensive experience of running cohort event monitoring, which is the most robust forms of PV. Although it is critical to have a system for detecting unexpected adverse events, it is also important to recognize that all countries cannot be expected to have a functioning comprehensive cohort event monitoring system before the new drugs are introduced. The WHO companion guidelines have template CEM forms that would allow clinicians using the new drugs to report any adverse events, while the national program is setting up a central PV system, which is a longer-term process.
Barriers, including PV and regulatory strengthening, will also require technical support and adequate funding beyond the cost of the drugs, in order to be addressed. It is therefore important that global fund monies, along with national and donor funding, are used to strengthen all aspects of the programmatic management of DR-TB programs including the supply of quality companion drugs for the new drugs.
Finally, the countries need to have the necessary political will and support to ensure that these drugs are made available to all those who need them with minimal delay.
Conclusion
For clinicians and patients alike, the development of bedaquiline and delamanid after so many decades without new TB drugs is an historic opportunity to gain ground in the fight against TB. The promise of these new medicines applies not only to patients with the most urgent, immediate needs – those with additional drug resistance, with side effects from existing drugs, or with expected poor outcomes – but also to all DR-TB patients, for whom these drugs offer the possibility of shorter, safer, and better treatment. There is work to be done by many: national programs to introduce the new drugs under reasonable conditions; governments, researchers, and manufacturers to support clinical trials of new regimens; and technical assistance from WHO and other actors.
At this point in time, there are clear recommendations from WHO on when, how, and for whom to use these new drugs, as well as both practical clinical experience from programs that have already introduced them and technical support for implementation. Yet despite all these, few patients have actually received these new drugs. This must change. New drugs should not be kept as a last resort; on the contrary, they should be introduced as soon as possible, to improve treatment outcomes and patient adherence to long and toxic regimens. Countries that have not yet introduced these drugs may be proceeding with caution, intimidated by the need for PV reporting or by their lack of experience with the new drugs. However, these factors should be weighed against the poor outcomes and toxicity of today’s treatment regimens.
The current clinical experience with bedaquiline in combination with other group 5 drugs offers real hope to MDR-TB patients. Wide-scale introduction of the new and group 5 drugs has the potential to radically improve the outcomes of MDR-TB patients. Let us not miss this historic opportunity.
Acknowledgment
The authors thank Patricia Kahn for critical editorial input.
Disclosure
The authors report no conflicts of interest in this work.
References
World Health Organisation. Global Tuberculosis Report 2014. Geneva, Switzerland: World Health Organization; 2014. Available from: http://www.who.int/tb/publications/global_report/en/. Accessed July 11, 2015. | |
World Health Organisation. Towards Universal Access to Diagnosis and Treatment of Multidrug-resistant and Extensively Drug-resistant Tuberculosis by 2015: WHO Progress Report 2011. Geneva, Switzerland: World Health Organization; 2011. | |
World Health Organisation. Global Tuberculosis Report, Supplement Drug Resistant TB, Surveillance and Response. Geneva, Switzerland: World Health Organization; 2014. doi: WHO/HQ/TB/2014.12. | |
World Health Organisation. WHO End TB Strategy. Geneva: World Health Organization. Available from: http://www.who.int/tb/post2015_strategy/en/. Accessed July 11, 2015. | |
European Medicines Agency C for MP for HU. European Medicines Agency: Assessment Report: Deltyba. London, England: European Medicines Agency C for MP for HU; 2013. Procedure No EMEA/H/C/002552. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002552/smops/Positive/human_smop_000572.jsp&mid=WC0b01ac058001d127. Accessed July 11, 2015. | |
European Medicine Agency. Situro. London, England: European Medicine Agency. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2013/12/news_detail_001999.jsp&mid=WC0b01ac058004d5c1. | |
Food and Drug Administration. Sirturo (bedaquiline) Product Insert. Silver Spring, MD: Food and Drug Administration. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/204384s000lbl.pdf. Accessed July 11, 2015. | |
Dooley KE, Obuku EA, Durakovic N, Belitsky V, Mitnick C, Nuermberger EL. World Health Organization group 5 drugs for the treatment of drug-resistant tuberculosis: unclear efficacy or untapped potential? J Infect Dis. 2013;207(9):1352–1358. doi:10.1093/infdis/jis460. | |
Padayatchi N. Clofazimine in the treatment of extensively drug-resistant tuberculosis with HIV coinfection in South Africa: a retrospective cohort study. J Antimicrob Chemother. 2014;69(11):3103–3107. doi:101093/jac/dku235. | |
Gopal M, Padayatchi N, Metcalfe JZ, O’Donnell MR. Systematic review of clofazimine for the treatment of drug-resistant tuberculosis. Int J Tuberc Lung Dis. 2013;17(8):1001–1007. doi:10.5588/ijtld.12.0144. | |
Gler MT, Skripconoka V, Sanchez-Garavito E, et al. Delamanid for multidrug-resistant pulmonary tuberculosis. N Engl J Med. 2012;366(23):2151–2160. doi:10.1056/NEJMoa1112433. | |
Skripconoka V, Danilovits M, Pehme L, et al. Delamanid improves outcomes and reduces mortality in multidrug-resistant tuberculosis. Eur Respir J. 2013;41(6):1393–1400. doi:10.1183/09031936.00125812. | |
Szumowski JD, Lynch JB. Profile of delamanid for the treatment of multidrug-resistant tuberculosis. Drug Des Devel Ther. 2015;9: 677–682. doi:10.2147/DDDT.S60923. | |
Otsuka Pharmaceutical Development and Commercialization, Inc. Safety and efficacy trial of delamanid for 6 months in patients with multidrug resistant tuberculosis (NCT01424670). Available from: https://clinicaltrials.gov/ct2/show/NCT01424670. Accessed July 11, 2015. | |
Matteelli A, Carvalho ACC, Dooley KE, et al. TMC207: the first compound of a new class of potent anti-tuberculosis drugs. Future Microbiol. 2010;5(6):849–858. | |
Diacon AH, Pym A, Grobusch MP, et al. Multidrug-resistant tuberculosis and culture conversion with bedaquiline for the TMC207-C208 study group*. N Engl J Med. 2014;8371(21):723–732. doi:10.1056/NEJMoa1313865. | |
Treatment Action Group. HIV-HCV- TB Pipeline Report. New York, NY: Treatment Action Group; 2014. | |
Otsuka Pharmaceutical Development and Commercialization, Inc. A 6-month safety, efficacy, and pharmacokinetic trial of delamanid in pediatric patients with multidrug resistant tuberculosis (NCT01859923). Available from: http://clinicaltrials.gov/show/NCT01859923. Accessed July 11, 2015. | |
World Health Organisation. WHO interim guidance on the use of bedaquiline to treat MDR-TB. Geneva, Switzerland: World Health Organisation; 2013. Available from: http://www.who.int/mediacentre/news/notes/2013/bedaquiline_mdr_tb_20130613/en/. Accessed July 11, 2015. | |
World Health Organisation. The Use of Delamanid in the Treatment of Multidrug-Resistant Tuberculosis. Geneva, Switzerland: World Health Organisation; 2014. Available from: http://apps.who.int/iris/bitstream/10665/137334/1/WHO_HTM_TB_2014.23_eng.pdf?ua=1. Accessed July 11, 2015. | |
World Health Organisation. Companion Handbook to the WHO Guidelines for the Programmatic Management of Drug-Resistant Tuberculosis. Geneva, Switzerland: World Health Organisation; 2014. Available from: http://apps.who.int/iris/bitstream/10665/130918/1/9789241548809_eng.pdf?ua=1&ua=1. Accessed July 11, 2015. | |
World Health Organisation. World Health Organisation Prequalification. WH0 Prequalification. Geneva, Switzerland: World Health Organisation; 2012. Available from: http://apps.who.int/prequal/query/ProductRegistry.aspx?list=all. Accessed July 11, 2015. | |
Dey T, Brigden G, Cox H, Shubber Z, Cooke G, Ford N. Outcomes of clofazimine for the treatment of drug-resistant tuberculosis: a systematic review and meta-analysis. J Antimicrob Chemother. 2013;68(2):284–293. doi:10.1093/jac/dks389. | |
Jagannath C, Reddy MV, Kailasam S, O’Sullivan JF, Gangadharam PR. Chemotherapeutic activity of clofazimine and its analogs against mycobacterium tuberculosis. In vitro, intracellular, and in vivo studies. Am J Respir Crit Care Med. 1995;151(4):1083–1086. | |
Aung KJM, Van Deun A, Declercq E, et al. Successful “9-month Bangladesh regimen” for multidrug- resistant tuberculosis among over 500 consecutive patients. Int J Tuberc Lung Dis. 2014;18(10):1180–1187. doi:10.5588/ijtld.14.0100. | |
Piubello A, Harouna SH, Souleymane MB, et al. High cure rate with standardised short-course multidrug- resistant tuberculosis treatment in Niger: no relapses. Int J Tuberc Lung Dis. 2014;18(10):1188–1194. doi:10.5588/ijtld.13.0075. | |
Nunn AJ, Rusen I, Van Deun A, et al. Evaluation of a standardized treatment regimen of anti-tuberculosis drugs for patients with multi-drug-resistant tuberculosis (STREAM): study protocol for a randomized controlled trial. Trials. 2014;15:353. Available from: http://www.trialsjournal.com/content/15/1/353. Accessed July 11, 2015. | |
Diacon SM, Dawson R, Niekerk CV, et al. 14 Day EBA Study of PA-824 bedaquiline *pyrazinamide and clofazimine in smear positive TB patients. In: CROI; 2014; Boston, MA. Available from: http://www.croiconference.org/sessions/14-day-eba-study-pa-824-bedaquiline-pyrazinamide-and-clofazimine-smear-positive-tb-patients. Accessed July 11, 2015. | |
Grosset JH, Singer TG, Bishai WR, Grosset JH. New drugs for the treatment of tuberculosis: hope and reality. Int J Tuberc Lung Dis. 2012;16(8):1005–1014. doi:10.5588/ijtld.12.0277. | |
Cox H, Ford N. Linezolid for the treatment of complicated drug-resistant tuberculosis: a systematic review and meta-analysis. Int J Tuberc Lung Dis. 2012;16(4):447–454. doi:10.5588/ijtld.11.0451. | |
Dheda K, Shean K, Zumla A, et al. Early treatment outcomes and HIV status of patients with extensively drug-resistant tuberculosis in South Africa: a retrospective cohort study. Lancet. 2010;375(9728):1798–1807. doi:10.1016/S0140-6736(10)60492-8. | |
Lee M, Lee J, Carroll MW, et al. Linezolid for treatment of chronic extensively drug-resistant tuberculosis. N Engl J Med. 2012;367:1508–1518. Available from: http://www.nejm.org/doi/full/10.1056/NEJMoa1201964. Accessed July 11, 2015. | |
Chambers HF, Turner J, Schecter GF, Kawamura M, Hopewell PC. Imipenem for treatment of tuberculosis in mice and humans. Antimicrob Agents Chemother. 2005;49(7):2816–2821. doi:10.1128/AAC.49.7.2816-2821.2005. | |
De Lorenzo S, Alffenaar JW, Sotgiu G, et al. Efficacy and safety of meropenem-clavulanate added to linezolid-containing regimens in the treatment of MDR-/XDR-TB. Eur Respir J. 2013; 41(6):1386–1392. doi:10.1183/09031936.00124312. | |
Médecins Sans Frontières Access Campaign. Ready set slow down. [Issue brief]. Available from: https://www.msfaccess.org/sites/default/files/MSF_assets/TB/Docs/MSF_IssueBrief_DRTB_ReadySet Slowdown.pdf. Accessed July 11, 2015. | |
Guglielmetti L, Le Dû D, Jachym M, et al. Compassionate use of bedaquiline for the treatment of multidrug-resistant and extensively drug-resistant tuberculosis: interim analysis of a French cohort. Clin Infect Dis. 2015;60(2):188–194. doi:10.1093/cid/ciu786. | |
Ndjeka, N, Conradie F, Schnippel K, et al. Treatment of drug-resistant tuberculosis with bedaquiline in a high HIV prevalence setting: an interim cohort analysis. Int J Tuberc Lung Dis. 2015; 19(8):979–985. | |
Yeghiazaryan L. Armenian experience on treatment of XDR and pre-XDR patients with new drugs under compassionate use program. Presented at: Tuberculosis Symposium – Eastern Europe and Central Asia; February 17–18, 2015. Available at: http://www.tb-symposium.org/documents/en/presentations/Lusine_Yeghiazaryan_BDQ_practical_use_eng.pdf. Accessed July 11, 2015. | |
Keshavjee S, Gelmanova IY, Farmer PE, et al. Treatment of extensively drug-resistant tuberculosis in Tomsk, Russia: a retrospective cohort study. Lancet. 2008;372(9647):1403–1409. doi:10.1016/S0140-6736 (08)61204-0. | |
Pietersen E, Ignatius E, Streicher EM, et al. Long-term outcomes of patients with extensively drug-resistant tuberculosis in South Africa: a cohort study. Lancet. 2014;383(9924):1230–1239. doi:10.1016/S0140-6736(13)62675-6. | |
Kurbatova EV, Taylor A, Gammino VM, et al. Predictors of poor outcomes among patients treated for multidrug-resistant tuberculosis at DOTS-plus projects. Tuberculosis. 2012;92(5):397–403. | |
Johnston JC, Shahidi NC, Sadatsafavi M, Fitzgerald JM. Treatment outcomes of multidrug-resistant tuberculosis: a systematic review and meta-analysis. PLoS One. 2009;4(9):e6914. doi:10.1371/journal.pone.0006914. | |
Holtz TH, Sternberg M, Kammerer S, et al. Time to sputum culture conversion in multidrug-resistant tuberculosis: predictors and relationship to treatment outcome. Ann Intern Med. 2006;144(9):650–659. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16670134. Accessed July 11, 2012. | |
O’Donnell MR, Padayatchi N, Kvasnovsky C, Werner L, Master I, Horsburgh CR. Treatment outcomes for extensively drug-resistant tuberculosis and HIV co-infection. Emerg Infect Dis. 2013;19(3):416–424. doi:10.3201/eid1903.120998. | |
Bonnet M. Effectiveness of the WHO regimen for treatment of multidrug resistant tuberculosis. Presented at: European Respiratory Society Annual Congress, 2013. Available from: http://erj.ersjournals.com/content/42/Suppl_57/190.short. Accessed July 11, 2015. | |
Brigden G, Nyang ’wa B-T, Du Cros P, et al. Policy and practice principles for designing future regimens for multidrug-resistant tuberculosis. Bull World Heal Organ. 2014;92:68–74. doi:10.2471/BLT.13.122028. | |
Sotgiu G, Centis R, D’Ambrosio L, et al. Efficacy, safety and tolerability of linezolid containing regimens in treating MDR-TB and XDR-TB: systematic review and meta-analysis. Eur Respir J. 2012;40(6):1430–1442. doi:10.1183/09031936.00022912. | |
Schecter GF, Scott C, True L, Raftery A, Flood J, Mase S. Linezolid in the treatment of multidrug-resistant tuberculosis. Clin Infect Dis. 2010;50(1):49–55. doi:10.1086/648675. | |
Jachym M. Bedaquiline: practical use and interim results in France . In: 4th Tuberculosis Symposium for Central Asia and Eastern Europe, Yerevan, Armenia; February 17–18, 2015. | |
Centre for Disease Control U. Provisional CDC Guidelines for the Use and Safety Monitoring of Bedaquiline Fumarate (Sirturo) for the Treatment of Multidrug-Resistant Tuberculosis, 2013. Available from: http://www.cdc.gov/mmwr/cme/conted.html. Accessed March 01, 2015. | |
Bedaquiline (Sirturo): Summary of Product Characteristics. European Medicines Agency. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002614/human_med_001730.jsp&mid=WC0b01ac058001d124. Accessed March 01, 2015. | |
World Health Organisation. Introduction of Bedaquiline for the Treatment of Multidrug-Resistant Tuberculosis at Country Level: Implementation Plan. Geneva, Switzerland: World Health Organization; 2015. Available from: http://www.who.int/tb/publications/WHO_BDQimplementationplan.pdf. Accessed July 11, 2015. | |
ERS/WHO Tuberculosis Consilium [homepage on the Internet]. Available from: https://www.tbconsilium.org/. Accessed July 11, 2015. | |
Briasoulis A, Agarwal V, Pierce WJ. QT prolongation and torsade de pointes induced by fluoroquinolones: infrequent side effects from commonly used medications. Cardiology. 2011;120(22):103–110. | |
Shimokawa Y, Sasahara K, Yoda N, Mizuno K, Umehara K. Delamanid does not inhibit or induce cytochrome p450 enzymes in vitro. Biol Pharm Bull. 2014;37(11):1727–1735. | |
Paccaly AJ, Petersen C, Patil S, et al. Absence of clinically relevant drug interaction between delamanid, a new drug for multidrug-resistant tuberculosis (MDR-TB) and tenofovir or lopinavir/ritonavir in healthy subjects. In: 19th International AIDS Conference; 2012; Washington, DC. | |
Hartkoorn RC, Uplekar S, Cole ST. Cross-resistance between clofazimine and bedaquiline through upregulation of mmpl5 in mycobacterium tuberculosis. Antimicrob Agents Chemother. 2014;58(5):2979–2981. doi:10.1128/AAC.00037-14. | |
Somoskovi A. A mutation associated with clofazimine and bedaquiline cross-resistance in MDR-TB following bedaquiline treatment. Eur Respir Soc J. 2015;45(2):554–557. | |
Global Drug Facility. GDF Impact on SLD Dynamics: 2011 vs 2013 Treatment Cost Comparison. Presentation, 2014. Available at: http://www.stoptb.org/assets/documents/gdf/whatis/SecondLineDrugs.pdf. Accessed July 11, 2015. | |
Médecins Sans Frontières. Under the Microscope. 3rd ed. Paris, France: Médecins Sans Frontières; 2013. | |
USAID. USAID and Johnson & Johnson to tackle antibiotic resistant TB. Available from: http://www.usaid.gov/news-information/press-releases/dec-11-2014-usaid-and-johnson-johnson-tackle-antibiotic-resistant-tuberculosis. Accessed July 11, 2015. | |
Tuberculosis (TB). An initiative to extend access to a new TB drug. World Health Organization. Available from: http://www.who.int/tb/features_archive/otsuka_2015/en/. Accessed July 11, 2015. | |
RESIST-TB. DR-TB Clinical Trial Progress Report. Updated May 2015. Available from: http://www.resisttb.org/?page_id=1602. Accessed July 11, 2015. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



