Back to Journals » International Journal of Nanomedicine » Volume 19
Neutrophil Extracellular Traps in Tumors and Potential Use of Traditional Herbal Medicine Formulations for Its Regulation
Authors Li X , Hu L , Naeem A, Xiao S , Yang M, Shang H, Zhang J
Received 23 November 2023
Accepted for publication 28 February 2024
Published 19 March 2024 Volume 2024:19 Pages 2851—2877
DOI https://doi.org/10.2147/IJN.S449181
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yan Shen
Xiang Li,1,* Lei Hu,1,* Abid Naeem,2,3,* Shanghua Xiao,2 Ming Yang,2 Hongming Shang,4 Jing Zhang1,2
1National Pharmaceutical Engineering Center for Solid Preparation in Chinese Herbal Medicine, Jiangxi University of Chinese Medicine, Nanchang, Jiangxi, 330006, People’s Republic of China; 2Key Laboratory of Modern Preparation of TCM, Ministry of Education, Jiangxi University of Chinese Medicine, Nanchang, Jiangxi, 330004, People’s Republic of China; 3School of Life Science, Advanced Research Institute of Multidisciplinary Science, School of Medical Technology, Key Laboratory of Molecular Medicine and Biotherapy, Key Laboratory of Medical Molecule Science and Pharmaceutics Engineering, Beijing Institute of Technology, Beijing, 100081, People’s Republic of China; 4Department of Biochemistry & Chemical Biology, Vanderbilt University, Nashville, TN, USA
*These authors contributed equally to this work
Correspondence: Jing Zhang, Tel/Fax +86 79187118658, Email [email protected]
Abstract: Neutrophil extracellular traps (NETs) are extracellular fibers composed of deoxyribonucleic acid (DNA) and decorated proteins produced by neutrophils. Recently, NETs have been associated with the development of many diseases, including tumors. Herein, we reviewed the correlation between NETs and tumors. In addition, we detailed active compounds from traditional herbal medicine formulations that inhibit NETs, related nanodrug delivery systems, and antibodies that serve as “guiding moieties” to ensure targeted delivery to NETs. Furthermore, we discussed the strategies used by pathogenic microorganisms to evade NETs.
Keywords: neutrophil extracellular traps, cancer, traditional herbal medicine formulations, nano delivery systems
Introduction
Millions of people worldwide die from cancer each year. The number of new cases is expected to reach 20 million per year by 2025.1 In recent years, various types of cancer have continued to pose a threat to human life, and cancer has increasingly become a major issue for society. In recent years, there has been an increasing interest in the immune system’s role in cancer progression and how it influences treatment response.2 The innate immune system is reported to play a critical role in the fight against cancer by modulating immune function.3
Neutrophils constitute the largest population of innate immune cells. Their functional mechanisms (eg, phagocytosis, degranulation) are important tools in the innate immune response.4,5 Neutrophils are thought to play a major part in cancer progression, the angiogenesis of tumors, and regulation of the immune response in tumors.6 In addition to macrophages, it has also been suggested that polarization occurs in murine neutrophils, where they can also undergo polarization toward a protumor (N2) or antitumor (N1) phenotype.7 The natural antitumor effects of N1 neutrophils are attributed to their production of reactive oxygen species (ROS) and antibody-dependent cellular cytotoxicity.8 In addition, N1 neutrophils can activate cluster of differentiation (CD)8+ T cells as well as dendritic cells (DCs), and may even present tumor-specific antigens to them.9 N2 neutrophils contribute to cancer development by assisting re-building of the extracellular matrix (ECM), accelerating lymphangiogenesis and angiogenesis, and affecting immune function by producing cytokines that promote tumor growth.10 Even though neutrophils are believed to polarize towards N1 or N2 phenotypes in vivo, the specific conditions under which this process occurs are unclear, and the phenotypic differentiation between human neutrophils has not been clearly demonstrated.11
Neutrophil extracellular traps (NETs) are reticular structures with deoxyribonucleic acid (DNA) as a skeleton and various proteins as surface “decorations”.12 NETs were first described by Brinkmann et al in 2004.13 At first, they were found to capture and kill pathogens and bacteria, so were called “NETs”.14 NETs play a crucial role in infectious as well as non-infectious conditions and are also essential to the defense of the host. Although NETs have been described as beneficial in combating pathogens, their detrimental effects are only now becoming evident. NETs are involved in various pathological conditions, such as diabetes, wound healing, atherosclerosis, coagulation disorders, and periodontitis.15,16 Excessive numbers of NETs also have a significant impact on cancer development (Please see the Graphic Abstract).17
NETs formation in human tissues was first demonstrated by histopathological analysis of Ewing sarcoma biopsy samples. Six of the eight tissue samples contained tumor-associated neutrophils, and two had NETs. It was found that the formation of NETs was closely associated with relapses and metastatic complications despite treatment with chemotherapy.18,19 Several subsequent studies have shown the presence of NETs in peripheral blood and tumor samples collected from patients and animals with both primary and metastatic tumor. Neutrophils from mice suffering from lung cancer have been found to release NETs more frequently than those from healthy mice. Overproduction of NETs occurred in conjunction with intravascular coagulation induction and microvascular thrombosis in these animals.20 NETs also are able to wrap and shield tumor cells from interactions with cytotoxic immune cells, resulting in impaired activity of CD8+ T cells and NK cells.21 Of course, NETs do not always play a bad role. The unexpected fact is that NETs themselves have the function of killing tumors. Because of the heterogeneity of tumor, the location of NETs formation matters. In the primary tumor niche, NETs are found to be pro-tumor, like their effects in more aggressive disease states.15,22 Therefore, NETs-targeted therapies become more and more potential for therapeutic implications of cancer treatment.23,24
Right now, most cancers are treated with surgery, chemotherapy, radiotherapy, and immunotherapy. Among them, the use of chemotherapeutic drugs can be effective, however, it may result in adverse effects on normal cells, multidrug resistance, insufficient efficacy, and metastasis.25 The limitations of conventional drugs have prompted researchers to explore alternatives. Accordingly, traditional herbal medicines (THMs) offer numerous advantages over synthetic drugs because of their diverse chemical composition, minimal toxicity to normal cells, low side effects, and accessibility, giving them the advantage of being both an effective and affordable alternative.26 Furthermore, compared with chemical drugs, THMs may work through various mechanisms, including regulating cancer-related signaling pathways, inhibiting cell proliferation, causing cell apoptosis and autophagy. Same trend is also found on active ingredients from THMs, like baicalein and ginsenoside Rg3, not only inducing apoptosis and triggering autophagy of tumor cells, but also reducing the resistance of tumor cells to the chemotherapeutic drugs.27,28 Because of the distinguish characterization of THMs, it was reported that 32% of all small molecule drugs approved between January 1981 and September 2019 were natural products or derivatives of natural products. Furthermore, 51% of all the 1211 small molecule drugs that were approved globally between 1981 and 2014 were derivatives of natural products.29 A report published in 2016 revealed that the US Food and Drug Administration (FDA) approved 547 natural products and their derivatives for use as medications from 1827 to 2013. They are prescribed for treating various diseases, primarily different types of cancers, infections, and hypertension. Similarly, the study found that 68% of all 136 small-molecule drugs approved for cancer treatment between 1940 and 2014 were derived from natural products.30
However, the therapeutic potential of THMs restricted in most cases by their poor solubility in bodily fluids, low permeability, poor stability, and bioavailability. To achieve the maximized advantages of natural compounds, a variety of functional nanocarriers, such as liposomes, dendrimers, micelles, and nanoparticles have been developed,31,32 aiming to improve half-life, especially for volatile compound, and achieve targeting drug delivery.33–35
Taking the NETs contribution to cancer progression into consideration, this review summarizes the relationship and specific molecular mechanisms between NETs and tumor-related events, aiming to provide information about recent advances in the application of THM-original compounds-based nanodelivery systems to target the NETs against tumor progress, invasion, and migration.
What are NETs?
Originally, it was believed that NETs formation occurred due to the death of neutrophil cells, referred to as NETosis. Later, researchers discovered that living neutrophils may also release NETs. Typically, this occurs after neutrophils are activated, then they become flat within a few minutes, their nuclear membranes disintegrate within an hour, chromatin decondensed, and the outer and inner nuclear membranes separate. The nucleoplasm and cytoplasm merge into homogenous clumps after the nuclear membrane separates into individual vesicles. Condensation and rounding of cells cause the cytoplasmic membrane to rupture, forming fibrous bundles called NETs.36
Structure of NETs
NETs have a reticular form which takes the depolymerized chromatin as the basic framework (Figure 1). NETs are strongly negative-charged web-like structures, embedded with various active proteins: histones, myeloperoxidase (MPO), neutrophil elastase (NE), cathepsin G, calreticulin, and proteinase 3 (PR3) (Table 1).37–40
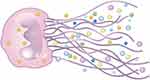 |
Figure 1 Structure of NETs. |
Biomarkers of NETs
Studies have shown that NETs may exist in primary tumor tissue, metastatic tumor tissue, and/or the circulatory system of tumors. Perhaps this is because extracellular DNA enzymes degrade NETs into soluble products that are released into the peripheral bloodstream. Several studies have shown a correlation between infiltrated NETs in tumor tissue and NETs in peripheral blood.41 Many markers are used in studies to visualize and assess NETs production, such as NE, MPO, cell-free DNA (cfDNA), MPO-DNA, high-mobility group protein B1 (HMGB1), nucleosomes, and H3Cit.42–47 However, cfDNA is not NETs-specific. Apoptosis or necrosis can occur in any type of cell. Apoptosis causes extracellular DNA to be released in the form of apoptotic bodies (collapsed cell debris or large fragments), membrane-bound particles, or free DNA.48 Notably, citrullination denotes the conversion of arginine residues in protein–peptide chains into citrulline residues under the action of PAD, which is an important process of protein post-translational modification.49 For example, PAD4 catalyzes the H3Cit arginine residues (Arg 2, 8, 17), which is thought to be a special way to trigger neutrophils to respond to infection (ie, NETs formation). Therefore, H3Cit is the most specific biomarker for NETs.45,46,50–52
Where Does NETs Come from?
The production pathway of NETs is quite unique and different from that of autophagy, apoptosis, and necrosis.53 Neutrophils can be stimulated to release NETs by various means, such as lipopolysaccharide (LPS), phorbol myristate acetate (PMA), complement 5a, complement 3a, interleukin (IL)-6, and IL8 (Table 2).11 A type of cell death known as “NETosis” results in the release of NETs, and the stimulation method determines how NETs are produced.54,55 In addition, recent studies have shown that autophagy is also involved in regulating NETs production.56 The phagocyte receptor separates NE from the nucleus by promoting phagosome formation, so NE drives chromatin depolymerization by processing histones.57 In general, there are two ways of generating NETs.
 |
Table 1 Structure of NETs |
 |
Table 2 Stimulators of NETs Formation |
One way is through suicidal NETosis, in which the rupture of nuclear and plasma membranes and chromatin depolymerization are involved.75 This pathway dependents upon nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX), which produces ROS.76,77 Initial stimulation of neutrophils activates NOX and Raf/mitogen-activated ERK kinase/extracellular signal-regulated kinases (Raf/MEK/ERK) signaling via protein kinase C (PKC) signaling, which subsequently stimulates ROS production. After that, citrullinated histone-3 (H3Cit) is induced by the calcium-dependent enzyme peptidyl arginine deiminase (PAD)4 present in the nucleus of neutrophils,78 resulting in chromatin depolymerization.39,79,80 Neutrophils release NETs within 2–4 h of being activated, and then they usher in their own death.72,81,82
PMA is a classic suicidal NETosis stimulus that activates PKC and Raf/MEK/ERK pathways. During this time, many ROS are generated rapidly in the cell, which rupture and dissolve granule and neutrophil membranes. Rupture of the granule membrane releases many protein components into the cytoplasm, such as NE, MPO, matrix metallopeptidase 9 (MMP9), and cathepsin G. The released NE and MPO contribute to permeabilization of the nuclear membrane and unfolding of intracellular chromatin.83 Some reports have indicated that cyclin-dependent kinase-4 and −6 are also involved in the dissolution and rupture of nuclear and cell membranes.84 Receptor-interacting protein kinase 3 (RIPK3), mixed lineage kinase domain-like protein (MLKL), and necroptotic factors are also thought to mediate NETs formation.85 ROS-dependent neutrophil necroptosis drives NETs generation through the formation of surface MLKL.86 After stimulation, RIPK3 is “turned on” in the nucleus and mediates MLKL activation, rupturing the nuclear envelope and allowing DNA to leak into the cytoplasm.87 RIPK1 and caspase-8 have been shown to have negative regulatory roles in producing RIPK3/MLKL-mediated NETs.86,87 PKC activation also contributes to the activation of peptidyl arginine deiminase 4 (PAD4) and then H3Cit,88 which is considered to be the molecular feature of NETs formation.89 Eventually, this process leads to chromosome depolymerization and the generation of a many DNA fibers. Afterwards, under the combined action of ROS and various proteases, the neutrophil membrane ruptures and releases the chromatin-fiber network attached to various proteins, such as NE and MPO.
The other pathway for generating NETs is called vital NETosis.60 Vital NETosis is a mild method to generate NETs. In this pathway, NETs production is activated by microbe-specific molecular patterns of pathogen-associated molecular patterns or endogenous damage-associated molecular patterns recognized by host recognition receptors.90 When complement proteins or toll-like receptors (TLRs) in neutrophils “sense” the presence of pathogens, they stimulate NETs production.66 This process involves chromatin transportation by vesicles and the “resealing” of the cell membrane,91 which signifies that NETs production does not kill neutrophils.83 Bryan et al92 observed that NETs contain intracellular vesicles and cell-membrane ingredients of human neutrophils upon infection by Gram-positive bacteria, which showed that chromatin was delivered to the extracellular space without cell-membrane rupture.
Vital NETosis is distinct from suicidal NETosis dependent upon the production of NADPH-mediated ROS.93 Pilsczek et al94 reported that neutrophils rapidly release vesicles containing nuclear DNA in vitro. Speziale described the pathway of NETs production by Staphylococcus aureus infection through complement receptors and TLR-2 ligands, which are independent of activation of NADPH enzymes.95 In addition to releasing nuclear DNA, neutrophils release mitochondrial DNA (mtDNA) to generate NETs.90,96 Mitochondria also play an important part in this NADPH-independent pathway97 because they can release mitochondrial-derived ROS,72 which then mediate the extracellular release of mtDNA to generate NETs without cell rupture.63,98 Vital NETosis occurs much faster than suicidal NETosis: vital NETosis takes only 5–60 min to release NETs. Interestingly, after generating NETs by neutrophils through vital NETosis, they continue to survive and retain relevant immune activity.92,99
NETs in Cancer
The relationship between NETs and cancer is incompletely understood. Many NETs were first detected in the tumor tissue of patients with Ewing’s sarcoma and found to be strongly associated with a poor prognosis.19 Pastor et al100 used MPO, NE, and circulating DNA as markers for NETs. They found that the levels of these markers were positively correlated with the probability of a diagnosis of metastatic colorectal cancer, suggesting that NETs are important players in the occurrence and development of cancer. In one study, enzyme-linked immunosorbent assays of MPO-double-stranded DNA were used to visualize NETs. These NETs were found to be related to the disease stage of patients with lung or upper-gastrointestinal (esophagogastric) adenocarcinoma. The level of NETs in patients with advanced (stage III and IV for lung adenocarcinoma, and stage II and III for upper-gastrointestinal adenocarcinoma) was significantly higher than that in patients with local tumors (stage I and II).22 One study observed more NETs in the peripheral blood of patients with ventricular aneurysm.101 Simultaneously, multiple signaling pathways (eg, Smad, mitogen-activated protein kinase (MAPK), Ras homolog gene family, member A (RhoA)) were activated, and the development of cardiac fibrosis was observed, which was considered to denote aggravation of ventricular aneurysms.101
In addition, certain factors in the tumor microenvironment associated with NETs, such as IL-8, IL-17, granulocyte colony-stimulating factor (G-CSF), and CXC chemokine receptor (CXCR), are also directly or indirectly involved in cancer progression.102–104 For example, IL-8 stimulates the aggregation and apoptosis of neutrophils but also participates in NETs generation, resulting in deterioration of the disease.104,105 Nie et al106 found that IL-8 secreted by diffuse large B cell lymphoma (DLBCL) stimulated NETs production through Src, p38, and ERK pathways, and NETs, in turn, upregulated TLR9 expression from DLBCL, which finally activated nuclear factor-kappa B (NF-κB), signal transducer and activator of transcription 3 (STAT3), and p38 pathways to promote tumor progression.
Some proteins of NETs are also thought to be involved in tumor progression. NE and MMP9 have been shown to be actively involved in cancer progression.62,107,108 NE is a serine protease. It is an important part of NETs but also involved in NETs formation.109 NE is actively involved in the genesis and growth of tumors.110 The NE-specific small-molecule inhibitor sivelestat has been shown to reduce the growth of prostate-cancer xenografts in athymic mice.111
NETs have been suggested as potential therapeutic targets in several tumor types (Table 3). Accordingly, various approaches have emerged to fight against NETosis or eliminate NETs, such as degrading the DNA skeleton, inhibiting expression of PAD4 or NE, targeting cathepsin C, blocking or interfering with signaling pathways.112–121 However recent studies have also revealed another side of NETs: blockade of tumor progression. NETs have been reported to induce the killing of immune cells. NETs induced by a cigarette-smoke extract in vitro can drive the maturation and activation of plasmacytoid DCs (eg, CD40, CD86) and initiate a T cell-mediated immune response.122 It was reported that the injection of NETs-DNA and NETs protein into subcutaneous tumors resulted in greater recruitment of T cells, which led to cancer-cell death and a reduced tumor volume.123 NETs may also be directly involved in the killing of tumor cells.124 Few reports have focused on the active anti-tumor effect of NETs, but it cannot be ignored because it suggests a “third way” to treat NETs-related tumors (ie, reliance on NETs) in addition to inhibiting NETosis and eliminating existing NETs.
 |
Table 3 Effect of NETs on the Occurrence and Development of Tumors |
Relationship with Tumor Metastasis
NETs are actively involved in tumor metastasis, which is the main factor of tumor-associated death (Figure 2).22,140–142 Studies have suggested that tumor metastasis is related to epithelial–mesenchymal transition (EMT). Specifically, epithelial tumor cells lose their ability to adhere and gain the ability to migrate to mesenchymal cells through EMT.143 NETs have been reported to enhance the migration of and invasion by tumor cells by inducing EMT,144,145 but thrombomodulin can degrade HMGB1 and NETs components to inhibit EMT, thereby reducing the risk of tumor-cell migration.61 NETs also promote tumor metastasis by degrading thrombospondin-1 (TSP-1), which is an important inhibitor of metastasis.114 In addition, due to their special structure, NETs have an outstanding ability to capture tumor cells and promote adhesion between tumor cells and endothelial cells (ECs).11,112 Then, NE derived from NETs increases the permeability of ECs through vascular endothelial-cadherin proteolysis,145 and causes damage to ECs,146 making it easier for tumor cells to break through the vascular wall and exudate to distant organs.11,112 Capture of tumor cells by NETs could also enable them to avoid immune surveillance by CD4+ T cells, further preventing them from being destroyed by CD8+ T cells and natural killer (NK) cells.128,147
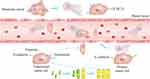 |
Figure 2 How NETs promote tumor metastasis. |
Furthermore, NETs, tumor cells, NETs-related cells, factors, signaling pathways, and genes, may form an optimized positive loop that promotes tumor metastasis. In a mouse model of spontaneous breast cancer, researchers found that NETs mediated NF-κB activation and upregulated expression of downstream genes related to the growth and metastasis of tumor cells, such as cyclin D1, zinc finger protein SNAI1 (SNAIL), IL-8, and IL-1β. Expression of these factors activated neutrophils to release NETs.68
Some proteins expressed by tumors interact with NETs and promote metastasis. The transmembrane protein coiled-coil domain containing 25 (CCDC25) on tumor cells was thought to be attracted by NETs and cause distal metastasis of tumor cells because NETs-mediated metastasis of tumor cells did not occur after knockout of CCDC25 expression.148 Circulating tumor cells (CTCs) derived from primary tumors have also been considered to be important factors in promoting metastasis.62,149 Specifically, tumor-derived CTCs activate expression of the serine PR3 on neutrophil membranes, which leads to IL-1β activation in lung neutrophils and stimulates ROS generation through the p38/MAPK signaling pathway to induce NETs.114 In addition, CTCs in blood undergo mesenchymal–epithelial transition after reaching other body parts through the blood circulation, regain the adhesion ability of epithelial tumor cells, and form metastases.150
Relationship with Dormant Tumor Cells
Tumor cells from a primary tumor tend to spread to other locations. During this process, T cells and NK cells destroy them if they start to proliferate and may lead them into a dormant state. However, tumor cells in the dormant state cannot be detected clinically.151 NETs have been shown to “awaken” dormant tumor cells to cause tumor recurrence and promote their malignant growth and spread.18,146,152 Albrengues et al144 found that inflammation induced by tobacco smoke and LPS promoted the invasive lung metastasis of dormant tumor cells. NE and MMP9 from NETs induced the hydrolysis of laminin (important component of the ECM). This process involves activating the signal transduction of integrin α3β1 and awakening dormant tumor cells to proliferate through the focal adhesion kinase/ extracellular regulated protein kinases/mixed lineage kinase domain-like protein/yes-associated protein 1 (FAK/ERK/MLCK/YAP) signaling pathway.144 In addition, they also found that NE and MMP9 hydrolyzed TSP-1, which inhibits tumor awakening and metastasis.144 Treatment with PAD4 inhibitors or DNA enzymes inhibited NETs formation and prevented the activation and metastasis of dormant tumor cells (Figure 3).144 Unfortunately, data on NETs awakening and promoting the development of dormant tumor cells are scarce, and the research in this area must be deepened.
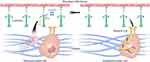 |
Figure 3 NETs awakening tumor dormant cells. |
Relationship with Cancer-Associated Thrombosis (CAT)
CAT is a major indicator of patient mortality, and NETs are active participants in CAT (Figure 4).153–156 Thrombosis depends upon the adhesion, activation, and aggregation of platelets. Fibrin and von Willebrand factor (vWF) provide a “scaffold” for platelet adhesion.157,158 The stability of a thrombus in CAT events is also dependent upon the support provided by NETs. NETs can damage ECs,15 which release vWF.159 Adhesion of many NETs onto the vascular system (vWF-mediated) can cause thrombosis by providing “stents” for the adhesion and activation of platelets and thrombin production.112 In addition, NETs bind to plasma proteins such as fibrinogen, vWF, and fibronectin; they also contribute to the development of arterial thrombosis.160,161 Fuchs et al initially reported the relationship between NETs and thrombosis. For the first time, they disclosed that NETs provide stents for thrombus formation.162 Evidence for the prothrombotic capacity of NETs was based on the finding that NETs entangle tumor cells and allow them to spread.153 The G-CSF level was found to be increased significantly (NETosis) in mice bearing human pancreatic BxPc-3 tumors.163 Moreover, an increased thrombin density and denser fibrin were observed in tumor-bearing mice; unsurprisingly, DNase I administration reduced the thrombus weight significantly.163
 |
Figure 4 Role of NETs in cancer-related thrombosis. |
Furthermore, tissue factors (TFs) are important participants in CAT. TFs trigger thrombosis by activating the coagulation cascade.162,164 Tumor cells are thought to have high expression of TFs,165–167 which are the key to platelet activation.168 Activated platelets can promote NETs production through an interaction between P-selectin (P-SEL) expressed on the surface on cells and P-selectin glycoprotein ligand-1 expressed by neutrophils,169 but also release stored HMGB1, which can combine with TLR4 expressed by neutrophils to stimulate NETs production.71 Surprisingly, NETs also mediate the release of TFs to accelerate thrombosis.170,171 The specific mechanism may be that NE and cathepsin G derived from NETs inhibit the signaling pathway that inhibits TFs.172 In receptor for advanced glycation end products (RAGE)-knockout mice bearing pancreatic cancer, the serum levels of TFs were reduced, and NET-induced platelet accumulation was decreased significantly.173 Therefore, NETs are expected to become new targets for treating cancer-related thrombosis, hopefully improving the prognosis and survival chances of patients suffering from cancer.
Relationship with Cancer Drug Resistance
In addition to actively participating in tumor development, metastasis, and awakening dormant tumors, NETs also mediate drug resistance during tumor chemotherapy, radiotherapy, and immunotherapy (Figure 5). Dying cancer cells induce neutrophil recruitment and release IL-1β, which immediately stimulates neutrophils to release NETs. NETs-related integrin-αvβ1 and MMP9 lead to the emergence of subsequent EMT and drug resistance through TGF-β activation.174 The clinical use of DNase (Pulmozyme®) targeting extracellular DNA restored the sensitivity of myeloma cells to doxorubicin, thus prolonging the survival time of tumor-bearing animals.175 Besides of chemo drug-related resistance, it was found that radiation induced NETs production in a TLR4-dependent manner by stimulating the release of HMGB1 from bladder tumor cells, while NETs may prevent the infiltration of CD8+ T cells in tumor cells to promote radiotherapy resistance, which could be attenuated by administration of NE inhibitors (NEi) and DNase I.176 IL-17 was uncovered to be the main cause of drug resistance in pancreatic cancer treatment, which is responsible for recruiting neutrophils and triggering NETs. IL-17 promotes the inactivation of CD8+ T cells by stimulating NETs production, thus reducing the sensitivity of checkpoint blockade, that is, drug resistance.105
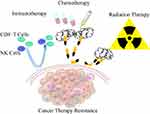 |
Figure 5 Role of NETs in cancer drug resistance. |
Targeting NETs
THMs Targeting NETs
Several studies have reported that THMs can be used to treat immune system-related diseases.177–179 The anti-NETs active substances from THMs have been structurally characterized (Table 4). These active ingredients are categorized into three groups based on their reactions.
 |
Table 4 Traditional Herbal Medicine Targeting NETs |
The first group of active ingredients inhibits the proteins of NETs (histone, NE and MPO) or proteins that affect the production of NETs.194 Using PAD4 (mediating histone citrullination), NE or MPO inhibitors have been observed conclusive evidence of the reduction of NETs.195–199 An example is andrographolide, the active component of Andrographis paniculate. Andrographolide can inhibit PMA-induced upregulation of PAD4 expression, reverse citrullination of histone-3, and inhibit autophagy of neutrophils and NETs production. The second group is to inhibit NETs generation by blocking ROS from NADPH or mitochondria, which is an important mediator in NETs generation. Flavonoids were found to inhibit ROS formation and block the formation pathway of NOX-dependent NETosis. The third group is signaling-pathway inhibitors in NETs generation. The signaling pathways involved in the generation of NETs are relatively complex. Typical examples are safflor yellow A, salvianolic acid B, and anhydrosafflor yellow B, which are the main three active ingredients of Xuebijing injection. They inhibit NETs generation by blocking the Raf/MEK/EPK signaling pathway.
The active ingredients of THMs are worth investigating. However, combining these active ingredients of THMs to develop new preparations with higher drug loading, higher bioavailability, stronger targeting, and more convenient drug delivery is a major challenge.
Nanomedicine Preparations for Targeting NETs
An excessive number of NETs is closely related to the occurrence and development of tumors and various autoimmune diseases. Hence, inhibiting NETosis or eliminating existing NETs could be a strategy against NETs. Since NETs were found to be linked to tumors, multiple approaches have been employed to inhibit NETs to curb tumor metastasis. These include DNase I (degrades the skeleton of existing NETs) or inhibitors of PAD4, NE, MPO, or H3cit (inhibit NETs production). However, the use of most of these inhibitors has ceased in the clinical stage due to low oral bioavailability and poor targeting. Therefore, improving the inhibitory effect and targeting ability of these inhibitors is a considerable challenge.
However, the rapid development of nanotechnology has overcome most of the shortcomings of non-specific chemotherapeutic drugs. The use of nanoparticles in cancer treatment is advantageous because it can increase the half-life of drugs in circulation and delivery to tumor cells in a precise and controlled manner while sparing healthy cells from toxicity.200–202 Nanoparticles (size > 5 nm) can accumulate passively at the tumor site due to weakened barrier integrity, a condition referred to as enhanced permeability and retention (EPR).203,204 Furthermore, nanoparticles conjugated with active targeting ligands dramatically increase the concertation of formulation in the targeting sites.205
In recent years, functional nanoparticles have become a better choice for anti-tumor therapies.206 For example, DNase I degrades the structure of NETs and is hoped to resist NETs-mediated tumor progression. However, its short half-life in plasma and poor stability hampers the ability of DNase I to block NETs-mediated tumor progression.197,207 To address this issue, researchers encapsulated DNase on the surface of nanoparticles. They achieved a more stable and longer plasma half-life than those in the free-DNase group. Under identical conditions, compared with the free-DNase group, the nanoparticle group had a higher drug concentration in the blood and lead to tumor inhibition.141 The research status of nanomedicine preparations targeting NETs is summarized in Table 5. Three main strategies for combating NETs have been reported: elimination of existing NETs, inhibition of NETosis and inhibition of neutrophils (the cells that produce NETs).
 |
Table 5 Nanomedicine Preparations Used to Target NETs |
Besides, multidrug-combination nanomedicine preparations against NETs-related tumors are also an option. DNase–chemotherapy agent multifunction nanomedicine preparations could achieve synergistic antitumor effects. Recently, preparations with paclitaxel (as the core) and a polymorphic sequence of cell-penetrating DNase I-substrate peptides (as the shell) have achieved good results in inhibiting the growth and metastasis of malignant tumors.209
However, most anti-NETs nanomedicine preparations are aimed at degrading the DNA structure of NETs. In contrast, research on nanomedicine preparations for some proteins, TFs, and signaling pathways involved in NETosis and multidrug combination therapy is scarce. The study of preparations targeting NETs specifically is even rarer. Hence, some examples of antibodies targeting NETs are provided below. We hope that these antibodies can be used for studying specifically targeted NETs preparations in the future.
Progress in Antibody Research for Targeting NETs
Antibodies that target NETs specifically for application in against NETs-involved diseases are summarized in Table 6. These antibodies can be divided into three groups. One group targets histone, such as antibody 7C10, which targets H3cit. The latter is a specific marker to produce NETs. Mouse-derived antibody 7C10 can bind specifically to H3cit at positions 2, 8, and 17. More importantly, 7C10 does not react with mono- or bis-H3cit. The second group targets proteins on NETs, such as PAD4. The participation of PAD4 is essential for generating NETs. PAD4 is involved in catalyzing the arginine residues of H3cit. The rabbit polyclonal antibody ab50247 can bind specifically to the 74-kDa PAD4 protein and does not cross-react with other subtypes. The third group binds specifically to the intact nucleosome (the main component of NETs). Monoclonal antibody 2C5 can elicit nucleosome-restricted specificity. Conjugating antibodies to nanoparticles can lead to a better effect in degrading NETs. 2C5-modified DNase I micelles can target and eliminate NETs; this is a good example of employing antibodies as targeting moieties for nanomedicines against NETs.229
 |
Table 6 Potential Antibody Targets of NETs |
Taking Advantage of NETs Anti-Tumor Properties
In tumor-related diseases, neutrophils can exert positive and negative effects depending on the neutrophil subtype (N1 or N2). N1 shows anti-tumor activity, while N2 shows tumor-promoting properties. TGF-β and interferon-β have a serious impact on the expression of neutrophil subtypes. TGF-β leads to transformation from the N1 phenotype to the N2 phenotype. Interferon-β induces the change in the opposite direction. Moreover, NETs are the most important way for N2 neutrophils to exert negative effects.75 Previously, it has been reported that NETs promote tumor development, and high levels of NETs have been associated with a low chance of survival and poor prognosis in patients with cancer.244,245 Hence, must NETs be synonymous with bad outcomes? Some components of NETs, such as NE and MPO,246,247 have been shown to have antitumor activity. Therefore, developing therapies that employ NETs to fight against tumors may be a feasible strategy.248
A recent study by Schedel et al found that melanoma cells adhered to NETs via integrins, and that NETs inhibited melanoma metastasis because co-cultured NETs had a cytotoxic effect on melanomas.124 Another study showed that NETs induced by bacillus Calmette–Guerin (BCG) inhibited tumors by attacking tumor cells and increasing the infiltration of immune cells.135 NETs may be cytotoxic to capture hepatocellular carcinoma cells and may limit their metastasis.134,249 Arelaki et al conducted in vitro experiments to study the effect of NETs on the progression of solid tumors when they are co-cultured with a colon tumor cell line (Caco-2).250 NETs were induced by PMA and septic serum-induced apoptosis in a concentration-dependent manner, and DNase I or heparin could attenuate this effect.
The “ideal” drug carrier should have good biocompatibility, be non-toxic, biodegradable, and avoid rapid clearance in vivo. Employing living cells as drug carriers have recently attracted attention. Natural cells have incomparable biocompatibility and targeting ability. Using the functional properties of neutrophils to release NETs in a targeted manner to deliver and release endocytosed drugs to the target site (hijacking NETosis) is a novel and efficacious therapeutic approach.251–253 Cheng et al254 prepared a liposome fused by a mycoplasma membrane to inhibit tumor development. This liposome preparation retained the characteristics of the mycoplasma membrane so that it would be recognized and “swallowed” by neutrophils. When neutrophils recognize inflammatory signals, NETs are released. With the release of NETs, the drugs were also excreted to tumor tissue. Wu et al255 prepared TLiplT nanoparticles by encapsulating IR780 molecules and tyrosinase-related protein 2 (TRP-2) peptides (both promoted T-cell activation and induced a potent immune response) in cell-penetrating peptide-TAT functionalized liposomes. Then, the preparation was endocytosed by neutrophils (NEs) to form TLiplT/NEs. When neutrophils migrated actively to inflammatory tumor sites, IR780 molecules and TRP-2 peptide were liberated through the release of NETs and exerted a curative effect. In addition, in recent years, some “biological micromotors” have been reported to enhance the natural targeting ability of cells and shorten the time of drug delivery to target sites.
Escape of Microorganisms from NETs
Certain bacteria, parasites, and fungi have developed strategies to resist NETs or elimination. Additionally, some pathogens have even been shown to be capable of modifying NETs in order to utilize them for nutrition or to avoid being recognized by other immune cells.55 Most microorganisms employ three main strategies to avoid NETs: degradation of NETs, modification of their surface structures to increase resistance, and suppression or inhibition of formation of NETs (Table 7).55,256 This concept provides great inspiration, and we may be able to learn from this experience and combine it with preparation technology to avoid or counteract the adverse effects of NETs on tumors.
 |
Table 7 Escape of Microorganisms from Killing by NETs |
Typical examples of pathogenic microorganisms evading NETs are summarized in Table 7. There are three ways that microorganisms evade NETs. The first one is excreting DNase to degrade the DNA skeleton of NETs. Group-A streptococci contain two genes that encode DNA enzymes. In vivo studies have found that DNase can resist bactericidal effects by degrading the DNA skeleton of NETs. The second way is inhibiting NETosis. A typical example is hepatitis B virus (HBV), which reduces ROS production and NETosis by inhibiting ERK, p38, and MAPK signaling pathways with HBV-C and HBV-E proteins. The third way is utilizing the surface structure and adjusting the surface charge. Streptococcus pneumoniae can acylate the lipoteichoic acid on the surface through the surface DLT gene locus to make its surface carry the same charge as that of the cationic peptide in NETs to repel its capture. In addition, the N-terminal region of the surface protein A of S. pneumoniae can show a negative charge to repel the negatively charged NETs-DNA to escape its killing.
Targeting Microorganisms Using NETs Mimicking Strategy
Excretion of NETs is a special type of neutrophil immune response. NETs adhere to the surface of bacteria to prevent infection. Inhibition of infection through the bionic pathway of NETs is a relatively new research direction. Recently, Huang et al277 developed a supramolecular assembly system, BQH-GGFF, to mimic NETs. This supramolecular assembly system was used to capture Methicillin-resistant Staphylococcus epidermidis and prevent its transmission in vivo. The supramolecular assembly system they prepared had good antibacterial activity. Chen et al278 developed a nano-gel using zinc oxide nanoparticles and DNA to mimic the structure of NETs. Compared with the control group, this NETs-like structure alleviated the clinical symptoms of mice stimulated by LPS. They investigated the anti-inflammatory properties of this NETs-like structure in vivo. Compared with the ZnO group, this preparation inhibited the proliferation of more bacteria, inhibited the entry of Escherichia coli into the circulation effectively, and greatly prolonged the survival time of mice infected with E. coli.
Conclusions and Future Prospectives
In recent years, increased attention has been given to NETs and their role in the poor prognosis of tumors. The structural components targeting NETs can effectively inhibit the growth and metastasis of tumor cells. This review elaborates the relationship between NETs and tumors and explores targeting strategies to reduce the adverse effects of NETs. The active components of THMs and nanomedicine-preparation technology were combined to be able to combat the adverse effects of NETs. However, further studies are needed to improve the anti-NETs efficiency.
Firstly, NETs inhibition-related studies are limited to animal or cell experiments, there is a paucity of formal clinical trials against NETs and related tumors, including NCT00536952 and NCT02462265 against NETs-DNA, NCT03400332 to improve sensitivity to immune checkpoint blockade, NCT03161431, NCT03473925 that blocks CXCR1 and CXCR2 to impede neutrophil recruitment to generate NETs, and NCT02370238, which combines the traditional herbal extract paclitaxel with a molecular drug (Reparixin) targeting CXCR1/2 for the treatment of metastatic triple-negative breast cancer. Accelerating the development of proteins and pathway inhibitors in the process of NETosis will be an effective way to block tumor development.
Secondly, the mechanism of the relationship between NETs and tumor is very complex and incompletely understood. Often, tumor-cell migration accelerates the death of a patient. The reticular nature of “camouflage” NETs enables them to capture tumor cells and makes the immune surveillance of the “sheriff” CD4+ T cells ineffective, making them unable to transmit the killing signal to the “executioner” NK cells and CD8+ T cells. NETs act on a tumor through the intermediate bridge (upstream and downstream cells, signaling pathways, and proteins) to accelerate its metastasis which, in turn, activates neutrophils to release NETs. Such layer-by-layer optimization necessitates vigilance, and finding ways to block such optimization may become the focus of treating NETs-related tumors. NETs can also awaken dormant tumor cells and make them proliferate. Two NETs-derived proteins, NE and MMP9, sequentially cut and remodel laminin in the ECM and degrade TSP-1, which may be the culprits of this awakening mechanism. Cancer-associated thrombosis has also attracted attention. NETs provide platelets and coagulation factors for thrombus formation-stents. Unfortunately, research on NETs and tumor-associated thrombi is relatively scarce.
Thirdly, exploration of the relationship between the N1 subtype neutrophils and NETs that exert anti-tumor activity is an important research area. Two subtypes of neutrophils, N1 and N2, have important roles in tumor development. CD16highCD26Lbim with anti-tumor activity may be mediated by NETs and could be an excellent candidate for N1 neutrophils. Research on the relationship between N1 neutrophils and anti-tumor NETs is still insufficient. Most of literatures are focused on the anti-tumor activity of certain proteins of NETs. Moreover, only a few of studies have examined the interaction between NPs and neutrophils. Due to the complexity of this interaction, further research is needed to gain a more comprehensive understanding of the mechanisms underlying the potential toxicity of NPs toward neutrophils, as well as emphasizing neutrophils and NET roles in cancer biology. Signaling pathways and chemokines play various roles in the regulation process, but many of their functions are still poorly understood. Researchers are still investigating the underlying regulatory mechanisms of neutrophil functions, and new properties are being discovered as research into neutrophils continues.
Finally, future research efforts should be made to discover effective herbal compounds and elucidate the mechanisms of action as well as the safety and efficacy of them. Although several studies reported the improved safety of THMs, the intrinsic toxicity associated with certain herbs should still be given due consideration. For the nanocarriers, to improve drug-targeting ability, antibodies could be employed as accurate targeting moieties. A nanomedicine preparation delivering active compounds modified with NETs-specific antibodies could help to reduce the adverse effects of NETs in tumors. Moreover, the NETs structure can be simulated to prepare drug-delivery systems and to hijack NETosis to deliver antineoplastic drugs. Hence, if faced with NETs-related cancer, an alternative method to fight NETs, that is, relying on NETs, will be available.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 82260695, China), Jiangxi Provincial Natural Science Foundation (NO. 20232ACB206062 and 20212ACB206004, China), Innovation Team for Safety and Development of Chinese Medicine (NO. ZYYCXTD-D-202207, China), Young Jinggang Scholar of Jiangxi Province (J.Z.), and New Century Talents Project of Jiangxi Province (2017082, X.L. and 2020028, J.Z.), Jiangxi University of Chinese Medicine Science and Technology Innovation Team Development Program (CXTD22001 and CXTD22006), and Project of college students’ innovation and entrepreneurship training program of Jiangxi University of Chinese Medicine (L. Hu, [2022]2).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Zugazagoitia J, Guedes C, Ponce S, Ferrer I, Molina-Pinelo S, Paz-Ares L. Current challenges in cancer treatment. Clin Ther. 2016;38(7):1551–1566. doi:10.1016/j.clinthera.2016.03.026
2. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331(6024):1565–1570. doi:10.1126/science.1203486
3. Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14(10):1014–1022. doi:10.1038/ni.2703
4. Rogers T, DeBerardinis RJ. Metabolic plasticity of neutrophils: relevance to pathogen responses and cancer. Trends Cancer. 2021;7(8):700–713. doi:10.1016/j.trecan.2021.04.007
5. Nicolás-ávila J, Adrover JM, Hidalgo A. Neutrophils in homeostasis, immunity, and cancer. Immunity. 2017;46(1):15–28. doi:10.1016/j.immuni.2016.12.012
6. Liang W, Ferrara N. The complex role of neutrophils in tumor angiogenesis and metastasis. Cancer Immunol Res. 2016;4(2):83–91. doi:10.1158/2326-6066.CIR-15-0313
7. Wang X, Qiu L, Li Z, Wang XY, Yi H. Understanding the multifaceted role of neutrophils in cancer and autoimmune diseases. Front Immunol. 2018;9:2456. doi:10.3389/fimmu.2018.02456
8. Xiong S, Dong L, Cheng L. Neutrophils in cancer carcinogenesis and metastasis. J Hematol Oncol. 2021;14(1):173. doi:10.1186/s13045-021-01187-y
9. Beauvillain C, Delneste Y, Scotet M, et al. Neutrophils efficiently cross-prime naive T cells in vivo. Blood. 2007;110(8):2965–2973. doi:10.1182/blood-2006-12-063826
10. Aloe C, Wang H, Vlahos R, Irving L, Steinfort D, Bozinovski S. Emerging and multifaceted role of neutrophils in lung cancer. Translational Lung Cancer Res. 2021;10(6):2806–2818. doi:10.21037/tlcr-20-760
11. Cristinziano L, Modestino L, Antonelli A, et al. Neutrophil extracellular traps in cancer. Semin Cancer Biol. 2022;79:91–104. doi:10.1016/j.semcancer.2021.07.011
12. Wang C, Liu X, Han Z, et al. Nanosilver induces the formation of neutrophil extracellular traps in mouse neutrophil granulocytes. Ecotoxicol Environ Saf. 2019;183:109508. doi:10.1016/j.ecoenv.2019.109508
13. Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. doi:10.1126/science.1092385
14. Martínez-Alberquilla I, Gasull X, Pérez-Luna P, Seco-Mera R, Ruiz-Alcocer J, Crooke A. Neutrophils and neutrophil extracellular trap components: emerging biomarkers and therapeutic targets for age-related eye diseases. Ageing Res Rev. 2022;74:101553. doi:10.1016/j.arr.2021.101553
15. Adrover JM, McDowell SAC, He XY, Quail DF, Egeblad M. NETworking with cancer: the bidirectional interplay between cancer and neutrophil extracellular traps. Cancer Cell. 2023;41(3):505–526. doi:10.1016/j.ccell.2023.02.001
16. Li X, Xiao S, Filipczak N, et al. Role and therapeutic targeting strategies of neutrophil extracellular traps in inflammation. Int J Nanomed. 2023;18:5265–5287. doi:10.2147/IJN.S418259
17. De Meo ML, Spicer JD. The role of neutrophil extracellular traps in cancer progression and metastasis. Semin Immunol. 2021;57:101595. doi:10.1016/j.smim.2022.101595
18. Demkow U. Neutrophil extracellular traps (NETs) in cancer invasion, evasion and metastasis. Cancers. 2021;13(17):4495. doi:10.3390/cancers13174495
19. Berger-Achituv S, Brinkmann V, Abed UA, et al. A proposed role for neutrophil extracellular traps in cancer immunoediting. Front Immunol. 2013;4:48. doi:10.3389/fimmu.2013.00048
20. Demers M, Krause DS, Schatzberg D, et al. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc Natl Acad Sci USA. 2012;109(32):13076–13081. doi:10.1073/pnas.1200419109
21. Ireland AS, Oliver TG. Neutrophils create an impeNETrable shield between tumor and cytotoxic immune cells. Immunity. 2020;52(5):729–731. doi:10.1016/j.immuni.2020.04.009
22. Rayes RF, Mouhanna JG, Nicolau I, et al. Primary tumors induce neutrophil extracellular traps with targetable metastasis promoting effects. JCI Insight. 2019;5(16):e128008. doi:10.1172/jci.insight.128008
23. Yang D, Liu J. Neutrophil extracellular traps: a new player in cancer metastasis and therapeutic target. J Exp Clin Cancer Res. 2021;40(1):233. doi:10.1186/s13046-021-02013-6
24. X-Y H, Ng D, Egeblad M. Caught in a web: emerging roles of neutrophil extracellular traps in cancer. Ann Rev Cancer Biol. 2022;6(1):223–243. doi:10.1146/annurev-cancerbio-080421-015537
25. Gao Q, Feng J, Liu W, et al. Opportunities and challenges for co-delivery nanomedicines based on combination of phytochemicals with chemotherapeutic drugs in cancer treatment. Adv Drug Delivery Rev. 2022;188:114445. doi:10.1016/j.addr.2022.114445
26. Naeem A, Hu P, Yang M, et al. Natural products as anticancer agents: current status and future perspectives. Molecules. 2022;27(23):8367. doi:10.3390/molecules27238367
27. Wei Z, Chen J, Zuo F, et al. Traditional Chinese medicine has great potential as candidate drugs for lung cancer: a review. J Ethnopharmacol. 2023;300:115748. doi:10.1016/j.jep.2022.115748
28. Sun CY, Zhu Y, Li XF, et al. Scutellarin increases cisplatin-induced apoptosis and autophagy to overcome cisplatin resistance in non-small cell lung cancer via ERK/p53 and c-met/AKT signaling pathways. Front Pharmacol. 2018;9:92. doi:10.3389/fphar.2018.00092
29. Newman DJ, Cragg GM. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Natural Prod. 2020;83(3):770–803. doi:10.1021/acs.jnatprod.9b01285
30. Patridge E, Gareiss P, Kinch MS, Hoyer D. An analysis of FDA-approved drugs: natural products and their derivatives. Drug Discov Today. 2016;21(2):204–207. doi:10.1016/j.drudis.2015.01.009
31. Zhang J, Shen L, Li X, Song W, Liu Y, Huang L. Nanoformulated codelivery of quercetin and alantolactone promotes an antitumor response through synergistic immunogenic cell death for microsatellite-stable colorectal cancer. ACS Nano. 2019;13(11):12511–12524. doi:10.1021/acsnano.9b02875
32. Zhang J, Hu K, Di L, et al. Traditional herbal medicine and nanomedicine: converging disciplines to improve therapeutic efficacy and human health. Adv Drug Delivery Rev. 2021;178:113964. doi:10.1016/j.addr.2021.113964
33. Serini S, Trombino S, Curcio F, Sole R, Cassano R, Calviello G. Hyaluronic acid-mediated phenolic compound nanodelivery for cancer therapy. Pharmaceutics. 2023;15(6):1751. doi:10.3390/pharmaceutics15061751
34. Ashique S, Chawla P, Upadhyay A, Chawla V. One-dimensional polymeric nanocomposites in drug delivery systems. Curr Nanosci. 2023;19(6):825–839. doi:10.2174/1573413719666230110110706
35. Ashique S, Sandhu NK, Chawla V, Chawla PA. Targeted drug delivery: trends and perspectives. Curr Drug Delivery. 2021;18(10):1435–1455. doi:10.2174/1567201818666210609161301
36. Zhao Z, Pan Z, Zhang S, et al. Neutrophil extracellular traps: a novel target for the treatment of stroke. Pharmacol Ther. 2023;241:108328. doi:10.1016/j.pharmthera.2022.108328
37. Tabrizi ZA, Khosrojerdi A, Aslani S, et al. Multi-facets of neutrophil extracellular trap in infectious diseases: moving beyond immunity. Microb Pathog. 2021;158:105066. doi:10.1016/j.micpath.2021.105066
38. Dąbrowska D, Jabłońska E, Garley M, Ratajczak-Wrona W, Iwaniuk A. New aspects of the biology of neutrophil extracellular traps. Scand J Immunol. 2016;84(6):317–322. doi:10.1111/sji.12494
39. Xie L, Ma Y, Opsomer G, Pascottini OB, Guan Y, Dong Q. Neutrophil extracellular traps in cattle health and disease. Res Vet Sci. 2021;139:4–10. doi:10.1016/j.rvsc.2021.06.019
40. Urban CF, Ermert D, Schmid M, et al. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against candida albicans. PLoS Pathogens. 2009;5(10):e1000639. doi:10.1371/journal.ppat.1000639
41. Wang M, Lv X, Wang Y, et al. Biomarkers of peripheral blood neutrophil extracellular traps in the diagnosis and progression of malignant tumors. Cancer Med. 2024;00:1–16.
42. Maruchi Y, Tsuda M, Mori H, et al. Plasma myeloperoxidase-conjugated DNA level predicts outcomes and organ dysfunction in patients with septic shock. Critical Care. 2018;22(1):176. doi:10.1186/s13054-018-2109-7
43. de Frutos L L, Serrano-Gonzalo I, Menendez-Jandula B, et al. Assessment of macrophage inflammatory biomarkers and neutrophil extracellular traps associated proteins in COVID-19 patients. Blood. 2021;138(Suppl 1):567.
44. Whittall-García LP, Torres-Ruiz J, Zentella-Dehesa A, et al. Neutrophil extracellular traps are a source of extracellular HMGB1 in lupus nephritis: associations with clinical and histopathological features. Lupus. 2019;28(13):1549–1557. doi:10.1177/0961203319883936
45. Grilz E, Mauracher LM, Posch F, et al. Citrullinated histone H3, a biomarker for neutrophil extracellular trap formation, predicts the risk of mortality in patients with cancer. Br J Haematol. 2019;186(2):311–320. doi:10.1111/bjh.15906
46. Nomura K, Miyashita T, Yamamoto Y, et al. Citrullinated histone H3: early biomarker of neutrophil extracellular traps in septic liver damage. J Surg Res. 2019;234:132–138. doi:10.1016/j.jss.2018.08.014
47. Mołek P, Ząbczyk M, Malinowski KP, Natorska J, Undas A. Markers of NET formation and stroke risk in patients with atrial fibrillation: association with a prothrombotic state. Thromb Res. 2022;213:1–7. doi:10.1016/j.thromres.2022.02.025
48. Pisetsky DS. Anti-DNA antibodies--quintessential biomarkers of SLE. Nat Rev Rheumatol. 2016;12(2):102–110. doi:10.1038/nrrheum.2015.151
49. Wang R, Wang G. Protein modification and autophagy activation. Adv Exp Med Biol. 2019;1206:237–259.
50. Mauracher LM, Posch F, Martinod K, et al. Citrullinated histone H3, a biomarker of neutrophil extracellular trap formation, predicts the risk of venous thromboembolism in cancer patients. J Thrombosis Haemostasi. 2018;16(3):508–518. doi:10.1111/jth.13951
51. Thålin C, Lundström S, Seignez C, et al. Citrullinated histone H3 as a novel prognostic blood marker in patients with advanced cancer. PLoS One. 2018;13(1):e0191231. doi:10.1371/journal.pone.0191231
52. Thålin C, Daleskog M, Göransson SP, et al. Validation of an enzyme-linked immunosorbent assay for the quantification of citrullinated histone H3 as a marker for neutrophil extracellular traps in human plasma. Immunol Res. 2017;65(3):706–712. doi:10.1007/s12026-017-8905-3
53. Rai G. Chapter 3 - Factors regulating NETosis. In: Rai G, editor. Netosis. Academic Press; 2019:57–88.
54. Kenny EF, Herzig A, Krüger R, et al. Diverse stimuli engage different neutrophil extracellular trap pathways. Elife. 2017;6:e24437. doi:10.7554/eLife.24437
55. Ríos-López AL, González GM, Hernández-Bello R, Sánchez-González A. Avoiding the trap: mechanisms developed by pathogens to escape neutrophil extracellular traps. Microbiol Res. 2021;243:126644. doi:10.1016/j.micres.2020.126644
56. Guo Y, Gao F, Wang X, et al. Spontaneous formation of neutrophil extracellular traps is associated with autophagy. Sci Rep. 2021;11(1):24005. doi:10.1038/s41598-021-03520-4
57. Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol. 2018;18(2):134–147. doi:10.1038/nri.2017.105
58. Ravindran M, Khan MA, Palaniyar N. Neutrophil extracellular trap formation: physiology, pathology, and pharmacology. Biomolecules. 2019;9(8):365. doi:10.3390/biom9080365
59. Jablonska E, Garley M, Surazynski A, et al. Neutrophil extracellular traps (NETs) formation induced by TGF-β in oral lichen planus - Possible implications for the development of oral cancer. Immunobiology. 2020;225(2):151901. doi:10.1016/j.imbio.2019.151901
60. Yipp BG, Kubes P. NETosis: how vital is it? Blood. 2013;122(16):2784–2794. doi:10.1182/blood-2013-04-457671
61. Kajioka H, Kagawa S, Ito A, et al. Targeting neutrophil extracellular traps with thrombomodulin prevents pancreatic cancer metastasis. Cancer Lett. 2021;497:1–13. doi:10.1016/j.canlet.2020.10.015
62. Tohme S, Yazdani HO, Al-Khafaji AB, et al. Neutrophil extracellular traps promote the development and progression of liver metastases after surgical stress. Cancer Res. 2016;76(6):1367–1380. doi:10.1158/0008-5472.CAN-15-1591
63. Yousefi S, Mihalache C, Kozlowski E, Schmid I, Simon HU. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 2009;16(11):1438–1444. doi:10.1038/cdd.2009.96
64. Mahajan A, Hasíková L, Hampel U, et al. Aggregated neutrophil extracellular traps occlude Meibomian glands during ocular surface inflammation. Ocul Surf. 2021;20:1–12. doi:10.1016/j.jtos.2020.12.005
65. Ortiz-Espinosa S, Morales X, Senent Y, et al. Complement C5a induces the formation of neutrophil extracellular traps by myeloid-derived suppressor cells to promote metastasis. Cancer Lett. 2022;529:70–84. doi:10.1016/j.canlet.2021.12.027
66. Drury B, Hardisty G, Gray RD, Ho GT. Neutrophil extracellular traps in inflammatory bowel disease: pathogenic mechanisms and clinical translation. Cell Mol Gastroenterol Hepatol. 2021;12(1):321–333. doi:10.1016/j.jcmgh.2021.03.002
67. Du J, Zhang J, Chen X, et al. Neutrophil extracellular traps induced by pro-inflammatory cytokines enhance procoagulant activity in NASH patients. Clin Res Hepatol Gastroenterol. 2022;46(1):101697. doi:10.1016/j.clinre.2021.101697
68. Zhu B, Zhang X, Sun S, Fu Y, Xie L, Ai P. NF-κB and neutrophil extracellular traps cooperate to promote breast cancer progression and metastasis. Exp Cell Res. 2021;405(2):112707. doi:10.1016/j.yexcr.2021.112707
69. Barnado A, Crofford LJ, Oates JC. At the Bedside: neutrophil extracellular traps (NETs) as targets for biomarkers and therapies in autoimmune diseases. J Leukoc Biol. 2016;99(2):265–278. doi:10.1189/jlb.5BT0615-234R
70. Hoste E, Maueröder C, van Hove L, et al. Epithelial HMGB1 delays skin wound healing and drives tumor initiation by priming neutrophils for NET formation. Cell Rep. 2019;29(9):2689–2701.e4. doi:10.1016/j.celrep.2019.10.104
71. Tadie J-M, Bae H-B, Jiang S, et al. HMGB1 promotes neutrophil extracellular trap formation through interactions with Toll-like receptor 4. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2013;304(5):L342–L349. doi:10.1152/ajplung.00151.2012
72. Douda DN, Khan MA, Grasemann H, Palaniyar N. SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proc Natl Acad Sci USA. 2015;112(9):2817–2822. doi:10.1073/pnas.1414055112
73. Wang L, Zhou X, Yin Y, Mai Y, Wang D, Zhang X. Hyperglycemia induces neutrophil extracellular traps formation through an NADPH Oxidase-dependent pathway in diabetic retinopathy. Front Immunol. 2018;9:3076. doi:10.3389/fimmu.2018.03076
74. Rawat S, Vrati S, Banerjee A. Neutrophils at the crossroads of acute viral infections and severity. Mol Aspect Med. 2021;81:100996. doi:10.1016/j.mam.2021.100996
75. Wolach O, Martinod K. Casting a NET on cancer: the multiple roles for neutrophil extracellular traps in cancer. Curr Opin Hematol. 2022;29(1):53–62. doi:10.1097/MOH.0000000000000690
76. Fuchs TA, Abed U, Goosmann C, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176(2):231–241. doi:10.1083/jcb.200606027
77. Yost CC, Cody MJ, Harris ES, et al. Impaired neutrophil extracellular trap (NET) formation: a novel innate immune deficiency of human neonates. Blood. 2009;113(25):6419–6427. doi:10.1182/blood-2008-07-171629
78. Zhou Y, Chen B, Mittereder N, et al. Spontaneous secretion of the citrullination enzyme PAD2 and cell surface exposure of PAD4 by neutrophils. Front Immunol. 2017;8:1200. doi:10.3389/fimmu.2017.01200
79. Masuda S, Nakazawa D, Shida H, et al. NETosis markers: quest for specific, objective, and quantitative markers. Int j Clin Chem. 2016;459:89–93. doi:10.1016/j.cca.2016.05.029
80. Hakkim A, Fuchs TA, Martinez NE, et al. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat Chem Biol. 2011;7(2):75–77. doi:10.1038/nchembio.496
81. Brinkmann V. Neutrophil extracellular traps in the second decade. J Innate Immun. 2018;10(5–6):414–421. doi:10.1159/000489829
82. Amini P, Stojkov D, Felser A, et al. Neutrophil extracellular trap formation requires OPA1-dependent glycolytic ATP production. Nat Commun. 2018;9(1):2958. doi:10.1038/s41467-018-05387-y
83. Jorch SK, Kubes P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat Med. 2017;23(3):279–287. doi:10.1038/nm.4294
84. Amulic B, Knackstedt SL, Abu Abed U, et al. Cell-cycle proteins control production of neutrophil extracellular traps. Dev Cell. 2017;43(4):449–462.e5. doi:10.1016/j.devcel.2017.10.013
85. D’Cruz AA, Speir M, Bliss-Moreau M, et al. Padi4 regulates NET formation and inflammatory cell death downstream of Mlkl. Blood. 2018;132(Suppl 1):45.
86. D’Cruz AA, Bliss-Moreau M, Ericcson M, Croker BA. Mlkl pores release neutrophil extracellular traps in necroptotic neutrophils. Blood. 2015;126(23):2200. doi:10.1182/blood.V126.23.2200.2200
87. Zhang T, Yin C, Boyd DF, et al. Influenza virus Z-RNAs induce ZBP1-mediated necroptosis. Cell. 2020;180(6):1115–1129.e13. doi:10.1016/j.cell.2020.02.050
88. Neeli I, Radic M. Opposition between PKC isoforms regulates histone deimination and neutrophil extracellular chromatin release. Front Immunol. 2013;4:38. doi:10.3389/fimmu.2013.00038
89. Koga T, Morotomi-Yano K, Sakugawa T, Saitoh H, Yano KI. Nanosecond pulsed electric fields induce extracellular release of chromosomal DNA and histone citrullination in neutrophil-differentiated HL-60 cells. Sci Rep. 2019;9(1):8451. doi:10.1038/s41598-019-44817-9
90. Rai G. Chapter 1 - Neutrophil extracellular trap formation: an introduction. In: Rai G, editor. Netosis. Academic Press; 2019:1–21.
91. Van Avondt K, Hartl D. Mechanisms and disease relevance of neutrophil extracellular trap formation. Eur J Clin Invest. 2018;48(Suppl 2):e12919. doi:10.1111/eci.12919
92. Yipp BG, Petri B, Salina D, et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat Med. 2012;18(9):1386–1393. doi:10.1038/nm.2847
93. Parker H, Dragunow M, Hampton MB, Kettle AJ, Winterbourn CC. Requirements for NADPH oxidase and myeloperoxidase in neutrophil extracellular trap formation differ depending on the stimulus. J Leukocyte Biol. 2012;92(4):841–849. doi:10.1189/jlb.1211601
94. Pilsczek FH, Salina D, Poon KK, et al. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J Iimmunol. 2010;185(12):7413–7425. doi:10.4049/jimmunol.1000675
95. Speziale P, Pietrocola G. Staphylococcus aureus induces neutrophil extracellular traps (NETs) and neutralizes their bactericidal potential. Comput Struct Biotechnol J. 2021;19:3451–3457. doi:10.1016/j.csbj.2021.06.012
96. Lood C, Blanco LP, Purmalek MM, et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat Med. 2016;22(2):146–153. doi:10.1038/nm.4027
97. Takishita Y, Yasuda H, Shimizu M, et al. Formation of neutrophil extracellular traps in mitochondrial DNA-deficient cells. J Clin Biochem Nutr. 2020;66(1):15–23. doi:10.3164/jcbn.19-77
98. Kim J, Gupta R, Blanco LP, et al. VDAC oligomers form mitochondrial pores to release mtDNA fragments and promote lupus-like disease. Science. 2019;366(6472):1531–1536. doi:10.1126/science.aav4011
99. Ramos-Martínez E, Hernández-González L, Ramos-Martínez I, et al. Multiple origins of extracellular DNA traps. Front Immunol. 2021;12:621311. doi:10.3389/fimmu.2021.621311
100. Pastor B, Abraham JD, Pisareva E, et al. Association of neutrophil extracellular traps with the production of circulating DNA in patients with colorectal cancer. iScience. 2022;25(2):103826. doi:10.1016/j.isci.2022.103826
101. He L, Liu R, Yue H, et al. NETs promote pathogenic cardiac fibrosis and participate in ventricular aneurysm formation after ischemia injury through the facilitation of perivascular fibrosis. Biochem Biophys Res Commun. 2021;583:154–161. doi:10.1016/j.bbrc.2021.10.068
102. Cedervall J, Zhang Y, Olsson AK. Tumor-induced NETosis as a risk factor for metastasis and organ failure. Cancer Res. 2016;76(15):4311–4315. doi:10.1158/0008-5472.CAN-15-3051
103. Cai Z, Zhang M, Boafo Kwantwi L, et al. Breast cancer cells promote self-migration by secreting interleukin 8 to induce NET formation. Gene. 2020;754:144902. doi:10.1016/j.gene.2020.144902
104. Lee KH, Kronbichler A, Park DD, et al. Neutrophil extracellular traps (NETs) in autoimmune diseases: a comprehensive review. Autoimmun Rev. 2017;16(11):1160–1173. doi:10.1016/j.autrev.2017.09.012
105. Yang L, Liu L, Zhang R, et al. IL-8 mediates a positive loop connecting increased neutrophil extracellular traps (NETs) and colorectal cancer liver metastasis. J Cancer. 2020;11(15):4384–4396. doi:10.7150/jca.44215
106. Nie M, Yang L, Bi X, et al. Neutrophil extracellular traps induced by IL8 promote diffuse large B-cell lymphoma progression via the TLR9 signaling. Clin Cancer Res. 2019;25(6):1867–1879. doi:10.1158/1078-0432.CCR-18-1226
107. Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103(3):481–490. doi:10.1016/S0092-8674(00)00139-2
108. Yan C, Sun C, Lu D, et al. Estimation of associations between MMP9 gene polymorphisms and breast cancer: evidence from a meta-analysis. Int J Biol Markers. 2022;37(1):13–20. doi:10.1177/17246008221076145
109. Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol. 2010;191(3):677–691. doi:10.1083/jcb.201006052
110. Huang H, Zhang H, Onuma AE, Tsung A. Neutrophil elastase and neutrophil extracellular traps in the tumor microenvironment. Adv Exp Med Biol. 2020;1263:13–23.
111. Lerman I, Garcia-Hernandez ML, Rangel-Moreno J, et al. Infiltrating myeloid cells exert protumorigenic actions via neutrophil elastase. Mol Cancer Res. 2017;15(9):1138–1152. doi:10.1158/1541-7786.MCR-17-0003
112. Kolaczkowska E, Jenne CN, Surewaard BG, et al. Molecular mechanisms of NET formation and degradation revealed by intravital imaging in the liver vasculature. Nat Commun. 2015;6:6673. doi:10.1038/ncomms7673
113. Yang C, Dong ZZ, Zhang J, et al. Peptidylarginine deiminases 4 as a promising target in drug discovery. Eur J Med Chem. 2021;226:113840. doi:10.1016/j.ejmech.2021.113840
114. Xiao Y, Cong M, Li J, et al. Cathepsin C promotes breast cancer lung metastasis by modulating neutrophil infiltration and neutrophil extracellular trap formation. Cancer Cell. 2021;39(3):423–437.e7. doi:10.1016/j.ccell.2020.12.012
115. Okeke EB, Louttit C, Fry C, et al. Inhibition of neutrophil elastase prevents neutrophil extracellular trap formation and rescues mice from endotoxic shock. Biomaterials. 2020;238:119836. doi:10.1016/j.biomaterials.2020.119836
116. Changelian PS, Flanagan ME, Ball DJ, et al. Prevention of organ allograft rejection by a specific Janus kinase 3 inhibitor. Science. 2003;302(5646):875–878. doi:10.1126/science.1087061
117. Pérez-Sánchez C, Ruiz-Limón P, Aguirre MA, et al. Diagnostic potential of NETosis-derived products for disease activity, atherosclerosis and therapeutic effectiveness in rheumatoid arthritis patients. J Autoimmun. 2017;82:31–40. doi:10.1016/j.jaut.2017.04.007
118. Mastellos DC, Pires da Silva BGP, Fonseca BAL, et al. Complement C3 vs C5 inhibition in severe COVID-19: early clinical findings reveal differential biological efficacy. Clin Immunol. 2020;220:108598. doi:10.1016/j.clim.2020.108598
119. Ali RA, Gandhi AA, Meng H, et al. Adenosine receptor agonism protects against NETosis and thrombosis in antiphospholipid syndrome. Nat Commun. 2019;10(1):1916. doi:10.1038/s41467-019-09801-x
120. Zhang Y, Han K, Du C, et al. Carboxypeptidase B blocks ex vivo activation of the anaphylatoxin-neutrophil extracellular trap axis in neutrophils from COVID-19 patients. Critical Care. 2021;25(1):51. doi:10.1186/s13054-021-03482-z
121. Strich JR, Ramos-Benitez MJ, Randazzo D, et al. Fostamatinib inhibits neutrophils extracellular traps induced by COVID-19 patient plasma: a potential therapeutic. J Infect Dis. 2021;223(6):981–984. doi:10.1093/infdis/jiaa789
122. Qiu SL, Zhang H, Tang QY, et al. Neutrophil extracellular traps induced by cigarette smoke activate plasmacytoid dendritic cells. Thorax. 2017;72(12):1084–1093. doi:10.1136/thoraxjnl-2016-209887
123. Tillack K, Breiden P, Martin R, Sospedra M. T lymphocyte priming by neutrophil extracellular traps links innate and adaptive immune responses. J Iimmunol. 2012;188(7):3150–3159. doi:10.4049/jimmunol.1103414
124. Schedel F, Mayer-Hain S, Pappelbaum KI, et al. Evidence and impact of neutrophil extracellular traps in malignant melanoma. Pigm Cell Melanoma Res. 2020;33(1):63–73. doi:10.1111/pcmr.12818
125. Albrengues J, Shields Mario A, Ng D, et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science. 2018;361(6409):eaao4227. doi:10.1126/science.aao4227
126. Zhang Y, Wang C, Yu M, et al. Neutrophil extracellular traps induced by activated platelets contribute to procoagulant activity in patients with colorectal cancer. Thromb Res. 2019;180:87–97. doi:10.1016/j.thromres.2019.06.005
127. Jin W, Yin H, Li H, Yu XJ, Xu HX, Liu L. Neutrophil extracellular DNA traps promote pancreatic cancer cells migration and invasion by activating EGFR/ERK pathway. J Cell Mol Med. 2021;25(12):5443–5456. doi:10.1111/jcmm.16555
128. Wang H, Zhang H, Wang Y, et al. Regulatory T-cell and neutrophil extracellular trap interaction contributes to carcinogenesis in non-alcoholic steatohepatitis. J Hepatol. 2021;75(6):1271–1283. doi:10.1016/j.jhep.2021.07.032
129. Tohme S, Kaltenmeier C, Yazdani H, Geller D, Tsung A. Pre-surgery neutrophil extracellular trap levels predict recurrence-free and overall survival for hepatic malignancies. HPB. 2020;22:S22. doi:10.1016/j.hpb.2020.04.819
130. Xia X, Zhang Z, Zhu C, et al. Neutrophil extracellular traps promote metastasis in gastric cancer patients with postoperative abdominal infectious complications. Nat Commun. 2022;13(1):1017. doi:10.1038/s41467-022-28492-5
131. Zhu T, Zou X, Yang C, et al. Neutrophil extracellular traps promote gastric cancer metastasis by inducing epithelial‑mesenchymal transition. Int J Mol Med. 2021;48(1):127. doi:10.3892/ijmm.2021.4960
132. Lee W, Ko SY, Mohamed MS, Kenny HA, Lengyel E, Naora H. Neutrophils facilitate ovarian cancer premetastatic niche formation in the omentum. J Exp Med. 2019;216(1):176–194. doi:10.1084/jem.20181170
133. Pedersen S, Kristensen AF, Falkmer U, Christiansen G, Kristensen SR. Increased activity of procoagulant factors in patients with small cell lung cancer. PLoS One. 2021;16(7):e0253613. doi:10.1371/journal.pone.0253613
134. Yang LY, Luo Q, Lu L, et al. Increased neutrophil extracellular traps promote metastasis potential of hepatocellular carcinoma via provoking tumorous inflammatory response. J Hematol Oncol. 2020;13(1):3. doi:10.1186/s13045-019-0836-0
135. Liu K, Sun E, Lei M, et al. BCG-induced formation of neutrophil extracellular traps play an important role in bladder cancer treatment. Clin Immunol. 2019;201:4–14. doi:10.1016/j.clim.2019.02.005
136. Meher AK, Spinosa M, Davis JP, et al. Novel role of IL (Interleukin)-1β in neutrophil extracellular trap formation and abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2018;38(4):843–853. doi:10.1161/ATVBAHA.117.309897
137. Zha C, Meng X, Li L, et al. Neutrophil extracellular traps mediate the crosstalk between glioma progression and the tumor microenvironment via the HMGB1/RAGE/IL-8 axis. Cancer Biol Med. 2020;17(1):154–168. doi:10.20892/j.issn.2095-3941.2019.0353
138. Cristinziano L, Modestino L, Loffredo S, et al. Anaplastic thyroid cancer cells induce the release of mitochondrial extracellular DNA traps by viable neutrophils. J Iimmunol. 2020;204(5):1362–1372. doi:10.4049/jimmunol.1900543
139. Li Y, Yuan R, Ren T, et al. Role of Sciellin in gallbladder cancer proliferation and formation of neutrophil extracellular traps. Cell Death Dis. 2021;12(1):30. doi:10.1038/s41419-020-03286-z
140. Teijeira Á, Garasa S, Gato M, et al. CXCR1 and CXCR2 chemokine receptor agonists produced by tumors induce neutrophil extracellular traps that interfere with immune Cytotoxicity. Immunity. 2020;52(5):856–871.e8. doi:10.1016/j.immuni.2020.03.001
141. Park J, Wysocki RW, Amoozgar Z, et al. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci Transl Med. 2016;8(361):361ra138. doi:10.1126/scitranslmed.aag1711
142. Cedervall J, Zhang Y, Huang H, et al. Neutrophil extracellular traps accumulate in peripheral blood vessels and compromise organ function in tumor-bearing animals. Cancer Res. 2015;75(13):2653–2662. doi:10.1158/0008-5472.CAN-14-3299
143. Liu X, Li J, Cadilha BL, et al. Epithelial-type systemic breast carcinoma cells with a restricted mesenchymal transition are a major source of metastasis. Sci Adv. 2019;5(6):eaav4275. doi:10.1126/sciadv.aav4275
144. Albrengues J, Shields MA, Ng D, et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science. 2018;361(6409):eaao4227.
145. Pieterse E, Rother N, Garsen M, et al. Neutrophil extracellular traps drive endothelial-to-mesenchymal transition. Arterioscler Thromb Vasc Biol. 2017;37(7):1371–1379. doi:10.1161/ATVBAHA.117.309002
146. Snoderly HT, Boone BA, Bennewitz MF. Neutrophil extracellular traps in breast cancer and beyond: current perspectives on NET stimuli, thrombosis and metastasis, and clinical utility for diagnosis and treatment. Breast Cancer Res. 2019;21(1):145. doi:10.1186/s13058-019-1237-6
147. Liao P, Wang W, Wang W, et al. CD8(+) T cells and fatty acids orchestrate tumor ferroptosis and immunity via ACSL4. Cancer Cell. 2022;40(4):365–378.e6. doi:10.1016/j.ccell.2022.02.003
148. Yang L, Liu Q, Zhang X, et al. DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature. 2020;583(7814):133–138. doi:10.1038/s41586-020-2394-6
149. Ruffell B, Affara NI, Cottone L, et al. Cathepsin C is a tissue-specific regulator of squamous carcinogenesis. Genes Dev. 2013;27(19):2086–2098. doi:10.1101/gad.224899.113
150. Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2019;20(2):69–84. doi:10.1038/s41580-018-0080-4
151. Malladi S, Macalinao DG, Jin X, et al. Metastatic latency and immune evasion through autocrine inhibition of WNT. Cell. 2016;165(1):45–60. doi:10.1016/j.cell.2016.02.025
152. Chen Q, Zhang L, Li X, Zhuo W. Neutrophil extracellular traps in tumor metastasis: pathological functions and clinical applications. Cancers. 2021;13(11).
153. Abdol Razak N, Elaskalani O, Metharom P. Pancreatic cancer-induced neutrophil extracellular traps: a potential contributor to cancer-associated thrombosis. Int J Mol Sci. 2017;18(3):487. doi:10.3390/ijms18030487
154. Subhan MA, Torchilin VP. Neutrophils as an emerging therapeutic target and tool for cancer therapy. Life Sci. 2021;285:119952. doi:10.1016/j.lfs.2021.119952
155. Leal AC, Mizurini DM, Gomes T, et al. Tumor-derived exosomes induce the formation of neutrophil extracellular traps: implications for the establishment of cancer-associated thrombosis. Sci Rep. 2017;7(1):6438. doi:10.1038/s41598-017-06893-7
156. Chen Y, Hu H, Tan S, et al. The role of neutrophil extracellular traps in cancer progression, metastasis and therapy. Exp Hematol Oncol. 2022;11(1):99. doi:10.1186/s40164-022-00345-3
157. Chandrashekar A, Singh G, Jonah G, Sikalas N, Labropoulos N. Mechanical and biochemical role of fibrin within a venous thrombus. Eur j Vascular Endovascular Surgery. 2018;55(3):417–424. doi:10.1016/j.ejvs.2017.12.002
158. Reininger AJ. VWF attributes – impact on thrombus formation. Thrombosis Res. 2008;122:S9–S13. doi:10.1016/S0049-3848(08)70028-8
159. Fuchs TA, Brill A, Wagner DD. Neutrophil extracellular trap (NET) impact on deep vein thrombosis. Arterioscler Thromb Vasc Biol. 2012;32(8):1777–1783. doi:10.1161/ATVBAHA.111.242859
160. Dhanesha N, Nayak MK, Doddapattar P, et al. Targeting myeloid-cell specific integrin α9β1 inhibits arterial thrombosis in mice. Blood. 2020;135(11):857–861. doi:10.1182/blood.2019002846
161. Moschonas IC, Tselepis AD. The pathway of neutrophil extracellular traps towards atherosclerosis and thrombosis. Atherosclerosis. 2019;288:9–16. doi:10.1016/j.atherosclerosis.2019.06.919
162. Fuchs TA, Brill A, Duerschmied D, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci USA. 2010;107(36):15880–15885. doi:10.1073/pnas.1005743107
163. Hisada Y, Grover SP, Maqsood A, et al. Neutrophils and neutrophil extracellular traps enhance venous thrombosis in mice bearing human pancreatic tumors. Haematologica. 2020;105(1):218–225. doi:10.3324/haematol.2019.217083
164. Witkowski M, Landmesser U, Rauch U. Tissue factor as a link between inflammation and coagulation. Trend Cardiovasc Med. 2016;26(4):297–303. doi:10.1016/j.tcm.2015.12.001
165. Geddings JE, Mackman N. Tumor-derived tissue factor-positive microparticles and venous thrombosis in cancer patients. Blood. 2013;122(11):1873–1880. doi:10.1182/blood-2013-04-460139
166. Koizume S, Miyagi Y. Tissue factor in cancer-associated thromboembolism: possible mechanisms and clinical applications. Br J Cancer. 2022;127(12):2099–2107. doi:10.1038/s41416-022-01968-3
167. Li H, Yu Y, Gao L, Zheng P, Liu X, Chen H. Tissue factor: a neglected role in cancer biology. J Thrombosis Thrombolysis. 2022;54(1):97–108. doi:10.1007/s11239-022-02662-0
168. Puhm F, Allaeys I, Lacasse E, et al. Platelet activation by SARS-CoV-2 implicates the release of active tissue factor by infected cells. Blood Adv. 2022;6(12):3593–3605. doi:10.1182/bloodadvances.2022007444
169. Etulain J, Martinod K, Wong SL, Cifuni SM, Schattner M, Wagner DD. P-selectin promotes neutrophil extracellular trap formation in mice. Blood. 2015;126(2):242–246. doi:10.1182/blood-2015-01-624023
170. Stakos DA, Kambas K, Konstantinidis T, et al. Expression of functional tissue factor by neutrophil extracellular traps in culprit artery of acute myocardial infarction. Eur Heart J. 2015;36(22):1405–1414. doi:10.1093/eurheartj/ehv007
171. Day SM, Reeve JL, Pedersen B, et al. Macrovascular thrombosis is driven by tissue factor derived primarily from the blood vessel wall. Blood. 2005;105(1):192–198. doi:10.1182/blood-2004-06-2225
172. Massberg S, Grahl L, von Bruehl ML, et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med. 2010;16(8):887–896. doi:10.1038/nm.2184
173. Boone BA, Murthy P, Miller-Ocuin J, et al. Chloroquine reduces hypercoagulability in pancreatic cancer through inhibition of neutrophil extracellular traps. BMC Cancer. 2018;18(1):678. doi:10.1186/s12885-018-4584-2
174. Mousset A, Lecorgne E, Bourget I, et al. Neutrophil extracellular traps formed during chemotherapy confer treatment resistance via TGF-β activation. Cancer Cell. 2023;41(4):757–775.e10. doi:10.1016/j.ccell.2023.03.008
175. Lin C, Herlihy SE, Li M, et al. Abstract 2103: nETs promote tumor resistance to anthracyclines. Cancer Res. 2019;79(Suppl 13).
176. Shinde-Jadhav S, Mansure JJ, Rayes RF, et al. Role of neutrophil extracellular traps in radiation resistance of invasive bladder cancer. Nat Commun. 2021;12(1):2776. doi:10.1038/s41467-021-23086-z
177. Wang Y, Chen S, Du K, et al. Traditional herbal medicine: therapeutic potential in rheumatoid arthritis. J Ethnopharmacol. 2021;279:114368. doi:10.1016/j.jep.2021.114368
178. Yamaguchi H, Kimura Y, Imamura M, et al. Effect of Rikkunshito, a traditional Japanese herbal medicine, on delayed gastric emptying and oral dietary intake after pancreaticoduodenectomy: a prospective, randomized, single-center, open-labeled study. Clin exp gastroenterol. 2020;13:577–587. doi:10.2147/CEG.S252913
179. Xu Z, Dong M, Yin S, et al. Why traditional herbal medicine promotes wound healing: research from immune response, wound microbiome to controlled delivery. Adv Drug Delivery Rev. 2023;195:114764. doi:10.1016/j.addr.2023.114764
180. Li X, Yuan K, Zhu Q, et al. Andrographolide ameliorates rheumatoid arthritis by regulating the apoptosis-NETosis balance of neutrophils. Int J Mol Sci. 2019;20(20):5035. doi:10.3390/ijms20205035
181. Xu KH, Zhou M, Wu FL, et al. Identification and characterization of a novel elastase inhibitor from Hirudinaria manillensis. Chin J Nat Med. 2021;19(7):540–544. doi:10.1016/S1875-5364(21)60054-7
182. Liu J, Jiang M, Jin Q, et al. Modulation of HMGB1 release in APAP-induced liver injury: a possible strategy of Chikusetsusaponin V targeting NETs formation. Front Pharmacol. 2021;12:723881. doi:10.3389/fphar.2021.723881
183. Chen Y-L, Hwang T-L, Fang J-Y, Lan Y-H, Chong K-Y, Hsieh P-W. Polysaccharides from Kochia scoparia fruits protect mice from lipopolysaccharide-mediated acute lung injury by inhibiting neutrophil elastase. Journal of Functional Foods. 2017;38:582–590. doi:10.1016/j.jff.2017.09.060
184. Ma Z, Xue X, Bai J, et al. Si-Wu-Tang ameliorates bile duct ligation-induced liver fibrosis via modulating immune environment. Biomed pharmacothe. 2022;155:113834. doi:10.1016/j.biopha.2022.113834
185. Yang F, Luo X, Luo G, et al. Inhibition of NET formation by polydatin protects against collagen-induced arthritis. Int Immunopharmacol. 2019;77:105919. doi:10.1016/j.intimp.2019.105919
186. Tao L, Xu M, Dai X, et al. Polypharmacological profiles underlying the antitumor property of salvia miltiorrhiza root (Danshen) interfering with NOX-dependent neutrophil extracellular traps. Oxid Med Cell Longev. 2018;2018:4908328. doi:10.1155/2018/4908328
187. Ali RA. Natural gingerols inhibit neutrophil extracellular trap release elicited by lupus autoantibodies. ACR. 2018.
188. Tao L, Xu M, Liu Y. The total terpenoids of Celastrus orbiculatus (TTC) inhibit NOX-dependent formation of PMA-induced neutrophil extracellular traps (NETs). Eur J Inflammation. 2018;16:2058739218805667. doi:10.1177/2058739218805667
189. Kirchner T, Hermann E, Möller S, et al. Flavonoids and 5-aminosalicylic acid inhibit the formation of neutrophil extracellular traps. Mediators Inflamm. 2013;2013:710239. doi:10.1155/2013/710239
190. Yuan K, Zhu Q, Lu Q, et al. Quercetin alleviates rheumatoid arthritis by inhibiting neutrophil inflammatory activities. J Nutr Biochem. 2020;84:108454. doi:10.1016/j.jnutbio.2020.108454
191. Yang C, Song C, Liu Y, et al. Re-Du-Ning injection ameliorates LPS-induced lung injury through inhibiting neutrophil extracellular traps formation. Phytomedicine. 2021;90:153635. doi:10.1016/j.phymed.2021.153635
192. Wang YP, Guo Y, Wen PS, et al. Three ingredients of safflower alleviate acute lung injury and inhibit NET release induced by lipopolysaccharide. Mediators Inflamm. 2020;2020:2720369. doi:10.1155/2020/2720369
193. Wang Q, Wu GR, Cui YB, Wang JF. Ephedra-Almond herbal pair inhibits formation of NETs by inhibiting the HMGB1/TLR4 signaling pathway. Int J Clin Exp Med. 2019;12(6):6917–6924.
194. Dwyer M, Shan Q, D’Ortona S, et al. Cystic fibrosis sputum DNA has NETosis characteristics and neutrophil extracellular trap release is regulated by macrophage migration-inhibitory factor. J Innate Immun. 2014;6(6):765–779. doi:10.1159/000363242
195. Chen YR, Xiang XD, Sun F, et al. Simvastatin reduces NETosis to attenuate severe asthma by inhibiting PAD4 expression. Oxid Med Cell Longev. 2023;2023:1493684. doi:10.1155/2023/1493684
196. Zhu D, Lu Y, Hu B, et al. Highly-tumor-targeted PAD4 inhibitors with PBA modification inhibit tumors in vivo by specifically inhibiting the PAD4-H3cit-NETs pathway in neutrophils. Eur J Med Chem. 2023;258:115619. doi:10.1016/j.ejmech.2023.115619
197. Cools-Lartigue J, Spicer J, McDonald B, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. 2013;123(8):3446–3458. doi:10.1172/JCI67484
198. Gan T, Yang Y, Hu F, et al. TLR3 regulated poly I:C-induced neutrophil extracellular traps and acute lung injury partly through p38 MAP kinase. Front Microbiol. 2018;9:3174. doi:10.3389/fmicb.2018.03174
199. Zhang L, Zheng B, Bai Y, et al. Exosomes-transferred LINC00668 contributes to thrombosis by promoting NETs formation in inflammatory bowel disease. Adv Sci. 2023;10(28):e2300560. doi:10.1002/advs.202300560
200. Ashique S, Afzal O, Hussain A, et al. It’s all about plant derived natural phytoconstituents and phytonanomedicine to control skin cancer. J Drug Delivery Sci Technol. 2023;84:104495. doi:10.1016/j.jddst.2023.104495
201. Ashique S, Almohaywi B, Haider N, et al. siRNA-based nanocarriers for targeted drug delivery to control breast cancer. Adv Cancer Biol. 2022;4:100047. doi:10.1016/j.adcanc.2022.100047
202. Ashique S, Upadhyay A, Hussain A, et al. Green biogenic silver nanoparticles, therapeutic uses, recent advances, risk assessment, challenges, and future perspectives. J Drug Delivery Sci Technol. 2022;77:103876. doi:10.1016/j.jddst.2022.103876
203. Ashique S, Garg A, Mishra N, et al. Nano-mediated strategy for targeting and treatment of non-small cell lung cancer (NSCLC). Naunyn-Schmiedeberg’s Arch Pharmacol. 2023;396(11):2769–2792. doi:10.1007/s00210-023-02522-5
204. Fang J, Nakamura H, Maeda H. The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev. 2011;63(3):136–151. doi:10.1016/j.addr.2010.04.009
205. Li X, Zhou X, Liu J, et al. Liposomal co-delivery of PD-L1 siRNA/Anemoside B4 for enhanced combinational immunotherapeutic effect. ACS Appl Mater Interfaces. 2022;14(25):28439–28454. doi:10.1021/acsami.2c01123
206. Piktel E, Niemirowicz K, Wątek M, Wollny T, Deptuła P, Bucki R. Recent insights in nanotechnology-based drugs and formulations designed for effective anti-cancer therapy. J Nanobiotechnology. 2016;14(1):39. doi:10.1186/s12951-016-0193-x
207. Prince WS, Baker DL, Dodge AH, Ahmed AE, Chestnut RW, Sinicropi DV. Pharmacodynamics of recombinant human DNase I in serum. Clin Exp Immunol. 1998;113(2):289–296. doi:10.1046/j.1365-2249.1998.00647.x
208. Dong W, Liu D, Zhang T, You Q, Huang F, Wu J. Oral delivery of staphylococcal nuclease ameliorates DSS induced ulcerative colitis in mice via degrading intestinal neutrophil extracellular traps. Ecotoxicol Environ Saf. 2021;215:112161. doi:10.1016/j.ecoenv.2021.112161
209. Yin H, Lu H, Xiong Y, et al. Tumor-associated neutrophil extracellular traps regulating nanocarrier-enhanced inhibition of malignant tumor growth and distant metastasis. ACS Appl Mater Interfaces. 2021;13(50):59683–59694. doi:10.1021/acsami.1c18660
210. Cheng Y, Gong Y, Chen X, et al. Injectable adhesive hemostatic gel with tumor acidity neutralizer and neutrophil extracellular traps lyase for enhancing adoptive NK cell therapy prevents post-resection recurrence of hepatocellular carcinoma. Biomaterials. 2022;284:121506. doi:10.1016/j.biomaterials.2022.121506
211. Lee YY, Park HH, Park W, et al. Long-acting nanoparticulate DNase-1 for effective suppression of SARS-CoV-2-mediated neutrophil activities and cytokine storm. Biomaterials. 2021;267:120389. doi:10.1016/j.biomaterials.2020.120389
212. Zhu L, Li Z, Liu N, Sun H, Wang Y, Sun M. Dynamically deformable protein delivery strategy disassembles neutrophil extracellular traps to prevent liver metastasis. Adv Funct Mater. 2021;31:2105089. doi:10.1002/adfm.202105089
213. Hosseinnejad A, Ludwig N, Wienkamp AK, et al. DNase I functional microgels for neutrophil extracellular trap disruption. Biomater Sci. 2021;10(1):85–99. doi:10.1039/D1BM01591E
214. Chen J, Hou S, Liang Q, et al. Localized degradation of neutrophil extracellular traps by photoregulated enzyme delivery for cancer immunotherapy and metastasis suppression. ACS Nano. 2022;16(2):2585–2597. doi:10.1021/acsnano.1c09318
215. Park HH, Park W, Lee YY, et al. Bioinspired DNase-I-coated melanin-like nanospheres for modulation of infection-associated NETosis dysregulation. Adv Sci. 2020;7(23):2001940. doi:10.1002/advs.202001940
216. Chen Y, Wang Y, Jiang X, et al. Dimethylamino group modified polydopamine nanoparticles with positive charges to scavenge cell-free DNA for rheumatoid arthritis therapy. Bioact Mater. 2022;18:409–420. doi:10.1016/j.bioactmat.2022.03.028
217. Penaloza Arias LC, Huynh DN, Babity S, Marleau S, Brambilla D. Optimization of a liposomal DNase I formulation with an extended circulating half-Life. Mol Pharm. 2022;19(6):1906–1916. doi:10.1021/acs.molpharmaceut.2c00086
218. Liang H, Peng B, Dong C, et al. Cationic nanoparticle as an inhibitor of cell-free DNA-induced inflammation. Nat Commun. 2018;9(1):4291. doi:10.1038/s41467-018-06603-5
219. Zhu D, Lu Y, Gui L, et al. Self-assembling, pH-responsive nanoflowers for inhibiting PAD4 and neutrophil extracellular trap formation and improving the tumor immune microenvironment. Acta pharmaceutica Sinica B. 2022;12(5):2592–2608. doi:10.1016/j.apsb.2021.11.006
220. Bornhöfft KF, Viergutz T, Kühnle A, Galuska SP. Nanoparticles equipped with α2,8-linked sialic acid chains inhibit the release of neutrophil extracellular traps. Nanomaterials. 2019;9(4):610. doi:10.3390/nano9040610
221. Zhang CY, Dong X, Gao J, Lin W, Liu Z, Wang Z. Nanoparticle-induced neutrophil apoptosis increases survival in sepsis and alleviates neurological damage in stroke. Sci Adv. 2019;5(11):eaax7964. doi:10.1126/sciadv.aax7964
222. Liu F, Sheng S, Shao D, et al. Targeting multiple mediators of sepsis using multifunctional tannic acid-Zn2+-gentamicin nanoparticles. Matter. 2021;4(11):3677–3695. doi:10.1016/j.matt.2021.09.001
223. Lu T, Zhang J, Cai J, et al. Extracellular vesicles derived from mesenchymal stromal cells as nanotherapeutics for liver ischaemia-reperfusion injury by transferring mitochondria to modulate the formation of neutrophil extracellular traps. Biomaterials. 2022;284:121486. doi:10.1016/j.biomaterials.2022.121486
224. Tan Q, He L, Meng X, et al. Macrophage biomimetic nanocarriers for anti-inflammation and targeted antiviral treatment in COVID-19. J Nanobiotechnology. 2021;19(1):173. doi:10.1186/s12951-021-00926-0
225. Lu Z, Long Y, Li J, et al. Simultaneous inhibition of breast cancer and its liver and lung metastasis by blocking inflammatory feed-forward loops. J Control Release. 2021;338:662–679. doi:10.1016/j.jconrel.2021.08.047
226. Mei J, Zhou J, Kong L, et al. An injectable photo-cross-linking silk hydrogel system augments diabetic wound healing in orthopaedic surgery through spatiotemporal immunomodulation. J Nanobiotechnology. 2022;20(1):232. doi:10.1186/s12951-022-01414-9
227. Li Y, Lin F. Decoy nanoparticles bearing native C5a receptors as a new approach to inhibit complement-mediated neutrophil activation. Acta Biomater. 2019;99:330–338. doi:10.1016/j.actbio.2019.08.033
228. Hou M, Wu X, Zhao Z, Deng Q, Chen Y, Yin L. Endothelial cell-targeting, ROS-ultrasensitive drug/siRNA co-delivery nanocomplexes mitigate early-stage neutrophil recruitment for the anti-inflammatory treatment of myocardial ischemia reperfusion injury. Acta Biomater. 2022;143:344–355. doi:10.1016/j.actbio.2022.02.018
229. Filipczak N, Li X, Saawant GR, Yalamarty SSK, Luther E, Torchilin VP. Antibody-modified DNase I micelles specifically recognize the neutrophil extracellular traps (NETs) and promote their degradation. J Control Release. 2023;354:109–119. doi:10.1016/j.jconrel.2022.12.062
230. Neeli I, Radic M. Current challenges and limitations in antibody-based detection of citrullinated histones. Front Immunol. 2016;7:528. doi:10.3389/fimmu.2016.00528
231. Mendes LP, Rostamizadeh K, Gollomp K, et al. Monoclonal antibody 2C5 specifically targets neutrophil extracellular traps. mAbs. 2020;12(1):1850394. doi:10.1080/19420862.2020.1850394
232. Chirivi R, Jenniskens G, Raats J. Anti-citrullinated protein antibodies as novel therapeutic drugs in rheumatoid arthritis. J Clin Cell Immunol. 2013;S6:1–13.
233. Chirivi RGS, van Rosmalen JWG, van der Linden M, et al. Therapeutic ACPA inhibits NET formation: a potential therapy for neutrophil-mediated inflammatory diseases. Cell. Mol. Immunol. 2021;18(6):1528–1544. doi:10.1038/s41423-020-0381-3
234. Corsiero E, Bombardieri M, Carlotti E, et al. Single cell cloning and recombinant monoclonal antibodies generation from RA synovial B cells reveal frequent targeting of citrullinated histones of NETs. Ann Rheumatic Dis. 2016;75(10):1866–1875. doi:10.1136/annrheumdis-2015-208356
235. Pratesi F, Dioni I, Tommasi C, et al. Antibodies from patients with rheumatoid arthritis target citrullinated histone 4 contained in neutrophils extracellular traps. Ann Rheumatic Dis. 2014;73(7):1414–1422. doi:10.1136/annrheumdis-2012-202765
236. Wang Y, Pritchard G, Kimber M. A general convergent strategy for the synthesis of tetra-substituted furan fatty acids (FuFAs). Eur J Org Chem. 2020;2020(19):2914–2922. doi:10.1002/ejoc.202000234
237. Sofoluwe A, Bacchetta M, Badaoui M, Kwak BR, Chanson M. ATP amplifies NADPH-dependent and -independent neutrophil extracellular trap formation. Sci Rep. 2019;9(1):16556. doi:10.1038/s41598-019-53058-9
238. Deng Q, Pan B, Alam HB, et al. Citrullinated histone H3 as a therapeutic target for endotoxic shock in mice. Front Immunol. 2019;10:2957. doi:10.3389/fimmu.2019.02957
239. Shi L, Aymonnier K, Wagner DD. Neutrophil stimulation with citrullinated histone H4 slows down calcium influx and reduces NET formation compared with native histone H4. PLoS One. 2021;16(5):e0251726. doi:10.1371/journal.pone.0251726
240. Ai P, Pan H, Chen K, Zheng J, Gao Z, Jin G. Viral mimetic poly(I:C) induces neutrophil extracellular traps via PAD4 to promote inflammation and thrombosis. Biochem Biophys Res Commun. 2021;565:64–71. doi:10.1016/j.bbrc.2021.05.091
241. Cao M, Yu M, Zhang Y, et al. Neutrophil extracellular traps exacerbate inflammatory responses and thrombotic tendency in both a murine colitis model and patients with inflammatory bowel disease. Blood. 2017;130:994. doi:10.1182/blood.V130.Suppl_1.994.994
242. Wu X, You D, Cui J, et al. Reduced neutrophil extracellular trap formation during ischemia reperfusion injury in C3 KO mice: C3 requirement for NETs release. Front Immunol. 2022;13:781273. doi:10.3389/fimmu.2022.781273
243. Panda R, Krieger T, Hopf L, et al. Neutrophil extracellular traps contain selected antigens of anti-neutrophil cytoplasmic antibodies. Front Immunol. 2017;8:439. doi:10.3389/fimmu.2017.00439
244. Cools-Lartigue J, Spicer J, Najmeh S, Ferri L. Neutrophil extracellular traps in cancer progression. Cell Mol Life Sci. 2014;71(21):4179–4194. doi:10.1007/s00018-014-1683-3
245. Gentles AJ, Newman AM, Liu CL, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015;21(8):938–945. doi:10.1038/nm.3909
246. Cui C, Chakraborty K, Tang XA, et al. Neutrophil elastase selectively kills cancer cells and attenuates tumorigenesis. Cell. 2021;184(12):3163–3177.e21. doi:10.1016/j.cell.2021.04.016
247. Ali M, Fulci G, Grigalavicius M, et al. Myeloperoxidase exerts anti-tumor activity in glioma after radiotherapy. Neoplasia. 2022;26:100779. doi:10.1016/j.neo.2022.100779
248. Adrover JM, Aroca-Crevillén A, Crainiciuc G, et al. Programmed ‘disarming’ of the neutrophil proteome reduces the magnitude of inflammation. Nat Immunol. 2020;21(2):135–144. doi:10.1038/s41590-019-0571-2
249. Saffarzadeh M, Juenemann C, Queisser MA, et al. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One. 2012;7(2):e32366. doi:10.1371/journal.pone.0032366
250. Arelaki S, Arampatzioglou A, Kambas K, et al. Gradient infiltration of neutrophil extracellular traps in colon cancer and evidence for their involvement in tumour growth. PLoS One. 2016;11(5):e0154484. doi:10.1371/journal.pone.0154484
251. Meyer D, Telele S, Zelená A, et al. Transport and programmed release of nanoscale cargo from cells by using NETosis. Nanoscale. 2020;12(16):9104–9115. doi:10.1039/D0NR00864H
252. Wu M, Zhang H, Tie C, et al. MR imaging tracking of inflammation-activatable engineered neutrophils for targeted therapy of surgically treated glioma. Nat Commun. 2018;9(1):4777. doi:10.1038/s41467-018-07250-6
253. Walczak M, Brady RA, Mancini L, et al. Responsive core-shell DNA particles trigger lipid-membrane disruption and bacteria entrapment. Nat Commun. 2021;12(1):4743. doi:10.1038/s41467-021-24989-7
254. Cheng X, Yu P, Zhou X, et al. Enhanced tumor homing of pathogen-mimicking liposomes driven by R848 stimulation: a new platform for synergistic oncology therapy. Acta pharmaceutica Sinica B. 2022;12(2):924–938. doi:10.1016/j.apsb.2021.08.018
255. Wu Y, Han X, Zheng R, et al. Neutrophil mediated postoperative photoimmunotherapy against melanoma skin cancer. Nanoscale. 2021;13(35):14825–14836. doi:10.1039/D1NR04002B
256. Bouchery T, Moyat M, Sotillo J, et al. Hookworms evade host immunity by secreting a deoxyribonuclease to degrade neutrophil extracellular traps. Cell Host Microbe. 2020;27(2):277–289.e6. doi:10.1016/j.chom.2020.01.011
257. Buchanan JT, Simpson AJ, Aziz RK, et al. DNase expression allows the pathogen group A Streptococcus to escape killing in neutrophil extracellular traps. Curr Biol. 2006;16(4):396–400. doi:10.1016/j.cub.2005.12.039
258. Mitiku F, Hartley CA, Sansom FM, et al. The major membrane nuclease MnuA degrades neutrophil extracellular traps induced by Mycoplasma bovis. Vet Microbiol. 2018;218:13–19. doi:10.1016/j.vetmic.2018.03.002
259. Yamamoto T, Kida Y, Sakamoto Y, Kuwano K. Mpn491, a secreted nuclease of Mycoplasma pneumoniae, plays a critical role in evading killing by neutrophil extracellular traps. Cellular Microbiology. 2017;19(3):e12666. doi:10.1111/cmi.12666
260. Zhang K, Jiang N, Chen H, et al. TatD DNases of African trypanosomes confer resistance to host neutrophil extracellular traps. Sci China Life Sci. 2021;64(4):621–632. doi:10.1007/s11427-020-1854-2
261. Peterson EJ, Kireev D, Moon AF, et al. Inhibitors of Streptococcus pneumoniae surface endonuclease EndA discovered by high-throughput screening using a PicoGreen fluorescence assay. J Biomol Screen. 2013;18(3):247–257. doi:10.1177/1087057112461153
262. Winstel V, Schneewind O, Missiakas D. Staphylococcus aureus exploits the host apoptotic pathway to persist during infection. mBio. 2019;10(6):e02270–19. doi:10.1128/mBio.02270-19
263. Afonso M, Mestre AR, Silva G, et al. Candida extracellular nucleotide metabolism promotes neutrophils extracellular traps escape. Front Cell Infect Microbiol. 2021;11:678568. doi:10.3389/fcimb.2021.678568
264. Freitas-Mesquita AL, Dick CF, Dos-Santos ALA, et al. Cloning, expression and purification of 3’-nucleotidase/nuclease, an enzyme responsible for the Leishmania escape from neutrophil extracellular traps. Mol Biochem Parasitol. 2019;229:6–14. doi:10.1016/j.molbiopara.2019.02.004
265. Banerjee A, Dasgupta R, Ray S. A computational study to prevent HIV invasion by bovine LF in mucosal-layer via blocking of DC-SIGN_GP120 interaction. Curr Proteomics. 2019;17(5):413–424. doi:10.2174/1570164617666191206162237
266. Rai G. Chapter 6 - NETosis in other diseases and therapeutic approaches. In: Rai G, editor. Netosis. Academic Press; 2019:131–169.
267. Rajeeve K, Das S, Prusty BK, Rudel T. Chlamydia trachomatis paralyses neutrophils to evade the host innate immune response. Nature Microbiol. 2018;3(7):824–835. doi:10.1038/s41564-018-0182-y
268. Shao S, Hao C, Zhan B, et al. Trichinella spiralis calreticulin S-Domain binds to human complement C1q to interfere with C1q-mediated immune functions. Front Immunol. 2020;11:572326. doi:10.3389/fimmu.2020.572326
269. Eby JC, Gray MC, Hewlett EL. Cyclic AMP-mediated suppression of neutrophil extracellular trap formation and apoptosis by the bordetella pertussis adenylate cyclase toxin. Infect Immun. 2014;82(12):5256–5269. doi:10.1128/IAI.02487-14
270. Uchiyama S, Döhrmann S, Timmer AM, et al. Streptolysin O rapidly impairs neutrophil oxidative burst and antibacterial responses to Group A Streptococcus. Front Immunol. 2015;6:581. doi:10.3389/fimmu.2015.00581
271. Khatua B, Bhattacharya K, Mandal C. Sialoglycoproteins adsorbed by Pseudomonas aeruginosa facilitate their survival by impeding neutrophil extracellular trap through siglec-9. J Leukoc Biol. 2012;91(4):641–655. doi:10.1189/jlb.0511260
272. Ratitong B, Pearlman E. Pathogenic Aspergillus and Fusarium as important causes of blinding corneal infections - the role of neutrophils in fungal killing, tissue damage and cytokine production. Curr Opin Microbiol. 2021;63:195–203. doi:10.1016/j.mib.2021.07.018
273. Zafar MA, Hammond AJ, Hamaguchi S, et al. Identification of pneumococcal factors affecting pneumococcal shedding shows that the dlt locus promotes inflammation and transmission. mBio. 2019;10(3):e01032–19. doi:10.1128/mBio.01032-19
274. Martinez PJ, Farhan A, Mustafa M, et al. PspA facilitates evasion of pneumococci from bactericidal activity of neutrophil extracellular traps (NETs). Microb Pathog. 2019;136:103653. doi:10.1016/j.micpath.2019.103653
275. Bhattacharya M, Berends ETM, Zheng X, et al. Leukocidins and the nuclease nuc prevent neutrophil-mediated killing of Staphylococcus aureus biofilms. Infect Immun. 2020;88(10):e00372–20. doi:10.1128/IAI.00372-20
276. Islam EA, Anipindi VC, Francis I, et al. Specific binding to differentially expressed human carcinoembryonic antigen-related cell adhesion molecules determines the outcome of Neisseria gonorrhoeae infections along the female reproductive tract. Infect Immun. 2018;86(8):e00092–18. doi:10.1128/IAI.00092-18
277. Huang Z, Liu Y, Wang L, et al. Supramolecular assemblies mimicking neutrophil extracellular traps for MRSE infection control. Biomaterials. 2020;253:120124.
278. Chen YF, Chiou YH, Chen YC, Jiang YS, Lee TY, Jan JS. ZnO-loaded DNA nanogels as neutrophil extracellular trap-like structures in the treatment of mouse peritonitis. Mater Sci Eng C Mater Biol Appl. 2021;131:112484. doi:10.1016/j.msec.2021.112484
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
