Back to Journals » International Journal of Nanomedicine » Volume 18
Neurotoxicity of Titanium Dioxide Nanoparticles: A Comprehensive Review
Authors Zhang X, Song Y, Gong H, Wu C, Wang B, Chen W, Hu J, Xiang H, Zhang K, Sun M
Received 2 October 2023
Accepted for publication 25 November 2023
Published 5 December 2023 Volume 2023:18 Pages 7183—7204
DOI https://doi.org/10.2147/IJN.S442801
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor R.D.K. Misra
Xing Zhang,1,* Yuanyuan Song,1,* Hongyang Gong,1,* Chunyan Wu,1 Binquan Wang,1 Wenxuan Chen,2 Jiawei Hu,2 Hanhui Xiang,2 Ke Zhang,1 Mingkuan Sun1
1Department of Toxicology, School of Public Health, Nanjing Medical University, Nanjing, Jiangsu, People’s Republic of China; 2The Second Clinical Medical School, Nanjing Medical University, Nanjing, Jiangsu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Mingkuan Sun, Department of Toxicology, School of Public Health, Nanjing Medical University, No. 101 Longmian Avenue, Jiangning District, Nanjing, 211166, People’s Republic of China, Email [email protected]
Abstract: The increasing use of titanium dioxide nanoparticles (TiO2 NPs) across various fields has led to a growing concern regarding their environmental contamination and inevitable human exposure. Consequently, significant research efforts have been directed toward understanding the effects of TiO2 NPs on both humans and the environment. Notably, TiO2 NPs exposure has been associated with multiple impairments of the nervous system. This review aims to provide an overview of the documented neurotoxic effects of TiO2 NPs in different species and in vitro models. Following exposure, TiO2 NPs can reach the brain, although the specific mechanism and quantity of particles that cross the blood-brain barrier (BBB) remain unclear. Exposure to TiO2 NPs has been shown to induce oxidative stress, promote neuroinflammation, disrupt brain biochemistry, and ultimately impair neuronal function and structure. Subsequent neuronal damage may contribute to various behavioral disorders and play a significant role in the onset and progression of neurodevelopmental or neurodegenerative diseases. Moreover, the neurotoxic potential of TiO2 NPs can be influenced by various factors, including exposure characteristics and the physicochemical properties of the TiO2 NPs. However, a systematic comparison of the neurotoxic effects of TiO2 NPs with different characteristics under various exposure conditions is still lacking. Additionally, our understanding of the underlying neurotoxic mechanisms exerted by TiO2 NPs remains incomplete and fragmented. Given these knowledge gaps, it is imperative to further investigate the neurotoxic hazards and risks associated with exposure to TiO2 NPs.
Keywords: TiO2 NPs, neurotoxic effects, oxidative stress, neuronal damage, neurotoxic mechanisms
Graphical Abstract:
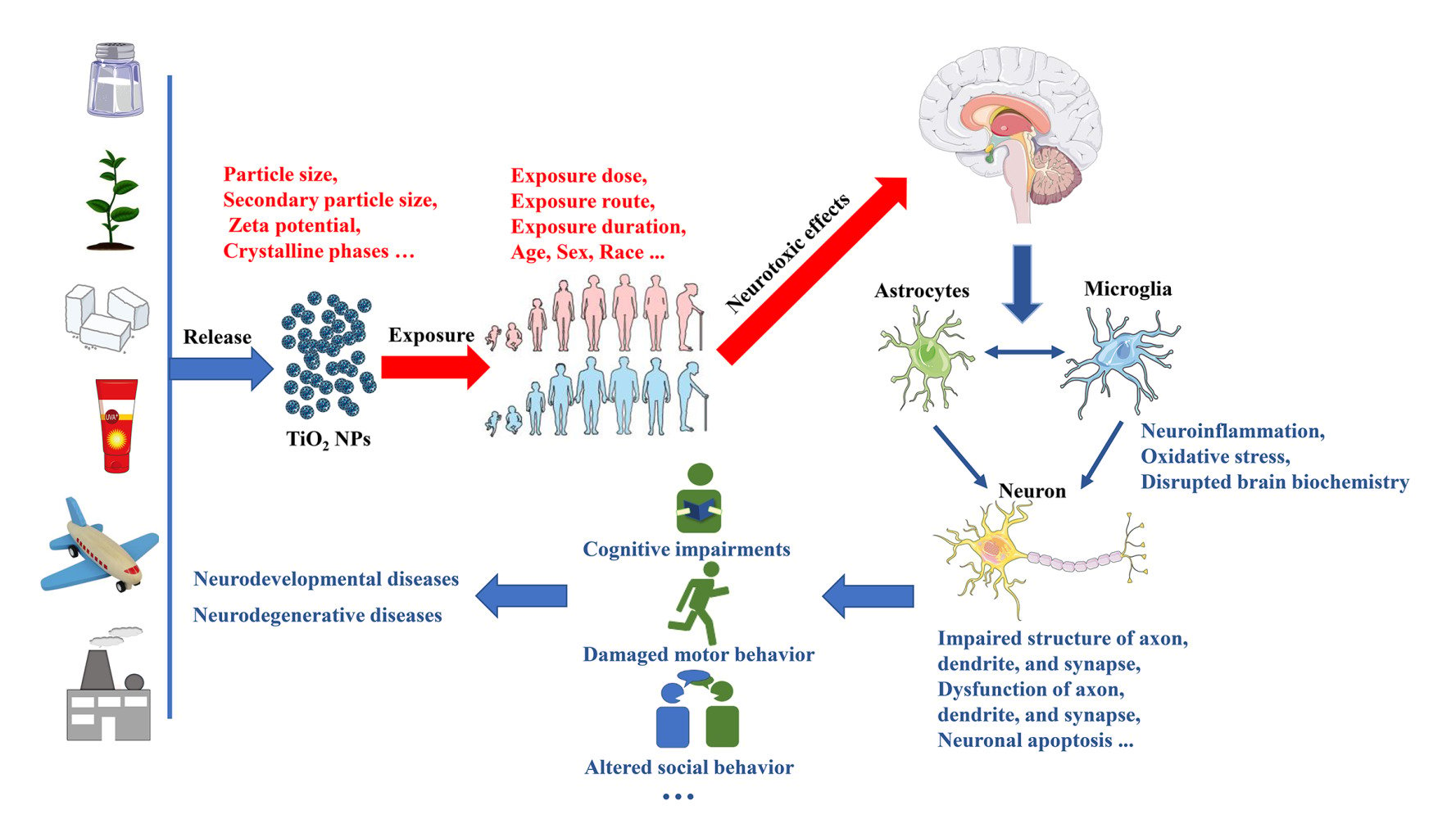
Introduction
Nanomaterials (NMs) are materials defined as having at least one dimension ranging from 1 to 100 nanometers (nm). Titanium dioxide nanoparticles (TiO2 NPs) rank among the top five NMs used in consumer products, such as food additives, toys, cosmetics, electronic products, and pharmaceuticals (Figure 1).1,2 Consequently, the presence of TiO2 NPs in air, water, soil, and other environmental media has gradually increased due to their widespread use.3 This growing application and contamination have made human and animal exposure to TiO2 NPs unavoidable. Apart from skin exposure, inhalation, and oral exposure, other routes of exposure to TiO2 NPs include intraperitoneal injection, subcutaneous injection, and intramuscular injection.4 Importantly, regardless of the route of exposure, TiO2 NPs can ultimately enter the systemic circulation and translocate to various tissues and organs (Figure 2).4 As the accumulation of TiO2 NPs in the body increases, the associated health hazards become more severe.5
 |
Figure 1 Application of TiO2 NPs. |
 |
Figure 2 TiO2 NPs can enter the human body through different ways and accumulate in the body, posing a threat to human health. |
Before the rise of nanotechnology, TiO2 was widely used in the form of fine particles (FPs), which were considered as poor soluble and low toxicity particles.6 However, some studies have contradicted this view, such as lung tumors in rats exposed to high levels of TiO2 FPs for two years.7 Furthermore, TiO2 has been classified as a Group 2B carcinogen (possibly carcinogenic to humans) by the International Agency for Research on Cancer.8 Although the specific carcinogenicity of TiO2 FPs is still debated, there is no doubt that TiO2 FPs pose a health risk.9 Compared to TiO2 FPs, TiO2 NPs present stronger catalytic activity and bioactivity due to their nanoscale dimensions.9 Consequently, the toxicity of TiO2 NPs cannot be solely inferred from the known toxicology of TiO2 FPs, nor can it be determined using conventional methods.10 In recent years, extensive exploration of the effects of TiO2 NPs exposure on human health also reflects the high concern about the safety of TiO2 NPs. In addition to considering all relevant exposure scenarios and biological intermediate steps, understanding the final toxic outcome is critical for human health risk assessment.11 Results from another child cohort study in China indicated that Ti can cross the placental barrier (PB) to harm fetuses that are extremely sensitive to environmental threats.12 Several epidemiological studies have confirmed that Ti exposure increases the risk of adverse birth outcomes, including neural tube defects, preterm birth, fetal distress, and low birth weight.13–15 Moreover, TiO2 NP exposure can have detrimental effects on the health of the population beyond the fetus. Emerging epidemiological evidence suggests that higher levels of urinary or blood Ti are associated with an increased risk of various adverse health effects, including diabetes and cardiopulmonary disorders (Figure 2).16–19 In recent years, laboratory studies on the toxicity of TiO2 NPs have surpassed epidemiological studies. Common animal models such as mice, rats, zebrafish, and Drosophila have been used to study TiO2 NPs. In vivo, studies have shown that TiO2 NPs exposure may be linked to lung inflammation, pneumoconiosis, cardiovascular disease, reproductive toxicity, retinal impairments, etc.20–24 In vitro, studies have also supported these toxic effects of TiO2 NPs.25–28 Given that nanoparticles can enter the brain, concerns regarding their neurotoxic effects, including those of TiO2 NPs, have gained significant attention.29
The entry of TiO2 NPs into the brain mainly occurs through the blood-brain barrier (BBB), via absorption-mediated transversion or intranasal pathways.30,31 However, the mechanisms by which nano-titanium dioxide penetrates and targets different brain regions remain unknown. The degree of TiO2 NPs accumulation in each brain region closely correlates with the extent of neurotoxic effects. Common neurotoxic effects include behavior deficits, nervous system dysfunction, and structural changes induced by oxidative stress, autophagy, inflammation, or the activation of specific signaling pathways.32 Although emerging studies support the role of TiO2 NPs exposure as an environmental risk factor for human health, conscientious and systematic investigations are scarce into the extent of TiO2 NPs translocation to different brain regions and the resulting damage to the neuronal system in relation to particle dose and particle size. The lack of information on the neurotoxicity of TiO2 NPs also complicates risk assessment following exposure. Therefore, this paper will mainly focus on current studies concerning the neurotoxicology of TiO2 NPs, while also reviewing the molecular mechanisms underlying their neurotoxic effects to mitigate potential damage resulting from exposure.
Evidence from Epidemiological and Human Exposure Studies
In earlier years, population exposure to TiO2 NPs was primarily investigated among occupational populations. Welding fumes, industrial waste combustion, and mineral mining can all result in environmental contamination by TiO2 NPs, thereby increasing the exposure risk for workers.33 Exposure to fumes from metal-inert gas soldering has been found to increase the risk of Parkinson’s disease (PD).34 Although these fumes mainly consist of zinc, copper, and iron, Andujar et al discovered an excessive accumulation of Ti in the lung tissue sections of welders in 2014.35 Industrial waste, pesticides, and automobile exhaust are common sources of environmental pollutants associated with neurotoxic effects.36 Among various environmental pollutants, NPs can easily penetrate the BBB and induce neurotoxicity by activating innate immune responses in astrocytes, microglia, and neurons.36 TiO2 NPs are a major component among environmental pollutants, with up to 760 tons of TiO2 NPs being released into the soil through sewage and sludge each year.37,38 Currently, there is no direct evidence of neurotoxic effects caused by TiO2 NPs exposure in mineral miners, but a previous study suggested a significantly increased inflammatory response in mineral miners exposed to TiO2 NPs.39 It is well known that the occurrence of inflammatory reactions in other organs is closely related to nervous system damage.40 With the increasing application of TiO2 NPs, concerns have also arisen regarding the neurotoxic effects of non-occupational populations exposed to TiO2 NPs. A recent cohort study demonstrated that high levels of urinary Ti during pregnancy were significantly associated with impaired language development, suggesting that TiO2 NPs might act as developmental neurotoxicants.41 Furthermore, elevated levels of Ti in maternal hair were also significantly associated with an increased risk of neural tube defects.42 However, epidemiological studies on the neurotoxic effects caused by TiO2 NPs are still limited. Currently, laboratory studies are the main basis for evaluating the neurotoxicity of TiO2 NPs.
Literature Search
To review the neurotoxic potential of TiO2 NPs, a comprehensive literature search was conducted using the “Pubmed” database, covering articles from 1991 to September 29, 2023. The search utilized combinations of the following keywords: Titanium dioxide nanoparticles exposure; E171 exposure; Titanium dioxide nanoparticles neuron; Titanium dioxide nanoparticles brain; Titanium dioxide nanoparticles behavior; and Titanium dioxide nanoparticles neurotoxicology. Our search strategy involved an initial screening of all titles and abstracts, followed by a full-text review of the pertinent review articles. One hundred forty-seven papers were selected, including one hundred twenty-one research papers. Citations within twenty-six reviews were also screened for additional studies not identified in the electronic search; however, no additional research papers were found through these references. In the end, a total of one hundred and twenty-one research papers were selected, all of which included neurotoxicity endpoints in their experimental designs.
For experimental research, we identified in vitro or in vivo studies involving the administration of TiO2 NPs. The researched organism groups mainly consisted of rodents, zebrafish, and cells. Fifty-five studies were conducted on rodents, twenty on zebrafish, and two on Caenorhabditis elegans (C. elegans), while one each on Pheretima hawayana, Tegillarca granosa, and Drosophila melanogaster. Additionally, forty-one studies used in vitro cells, including various animals and human-derived neuronal cells (Figure 3).
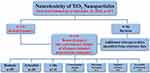 |
Figure 3 Study selection flow diagram. The flow chart illustrates the number of citations and resources that underwent screening, exclusion, and/or inclusion in the review. |
Neurotoxic Effects of TiO2 NPs in Rodents
The results of the literature search demonstrate that rodents were the most commonly used species in neurotoxicity studies of TiO2 NPs, forming the basis of 55 articles. Among these, 23 articles focused on rat models, with the Wistar rat being the most commonly employed. The exposed TiO2 NPs generally had a particle size of less than 20 nm, and the exposure doses ranged below 200 mg/kg (Table 1). Intratracheal instillation, intragastric administration, intravenous injection, and intraperitoneal injection are common methods of rat exposure to TiO2 NPs. Pregnancy and lactation have been identified as critical periods of neurodevelopment and have been selected as exposure windows to toxicants in many neurotoxicological studies, including the neurotoxicity of TiO2 NPs.43–45 Exposure to TiO2 NPs (10 nm, 100 mg/kg) during pregnancy or lactation has been found to impair memory and learning in Wistar offspring rats by reducing hippocampal cell proliferation.44,46 Prenatal exposure to TiO2 NPs (5 nm, 1 μg/μL) via subcutaneous injection has been shown to enhance depressive-like behavior in adult Sprague-Dawley (SD) rats.47 Additionally, Engler-Chiurazzi et al conducted locomotion, learning, and anxiety tests on male adult SD rats and observed significant cognitive impairments in offspring resulting from inhalation exposure to TiO2 NPs (170.9 ± 6.4 nm, 10.4 ± 0.4 mg/m3) during pregnancy.48 In earlier studies, the roles of age and sex in early-life exposure at different developmental stages were often overlooked. However, a recent study demonstrated that female pups were more susceptible to adverse outcomes after early exposure to oral TiO2 NPs (postnatal day, PND 2–5 or PND 7–10) compared to male pups, while male pups exhibited more severe motor deficits following exposure to nano-titanium dioxide during late lactation (PND 17–20).45 The behavioral impairments resulting from TiO2 NPs exposure during pregnancy and lactation involve multiple mechanisms, including oxidative damage, changes in neurotransmitter concentrations, and metabolic disturbances.45,47 Furthermore, exposure to TiO2 NPs during pregnancy has been associated with neuronal apoptosis, decreased neurogenesis, altered expression of brain-derived neurotrophic factor (BDNF), and impaired synaptic plasticity.49–51
 |
Table 1 Overview of Literature Investigating Neurotoxic Effects of TiO2 NPs in Rats |
Adolescence is a transitional period of physical and behavioral development between childhood and adulthood, and it represents a particularly vulnerable neurodevelopmental phase.67,68 In a study by Cui et al exposure to TiO2 NPs (5 nm, 20 mg/kg) during adolescence via intravenous injection induced anxiety-like behavior, cognitive impairment, neuroinflammation, and oxidative damage in the hippocampus.63 Similar results were observed in another study using adult Wistar rats, where subacute exposure to 20 mg/kg TiO2 NPs increased the anxious index.61 Furthermore, motor functional damage and spatial cognitive impairments were observed in adult Wistar rats exposed to TiO2 NPs.54,57,60 Certain regions of the mammalian brain continue to exhibit neurogenesis throughout adulthood, such as the subventricular zone of the lateral ventricles and the subgranular zone of the hippocampal dentate gyrus.69 Adult neurogenesis is a multiple-step process, and abnormalities at any stage can impair neurogenesis and brain function, leading to cognitive impairment and neurodegenerative diseases.70 Exposure to TiO2 NPs in adulthood also induces various neurotoxic effects, including mitochondrial dysfunction, oxidative stress, cell apoptosis, alterations in neuronal architecture, neuroinflammation, decreased neurogenesis, and electrophysiological alterations.52–54,56,58,59,64
To date, slightly more neurotoxicology studies on TiO2 NPs have been conducted using mice as animal models compared to rats, totaling 32 studies. Among them, 21 studies used ICR mice, 7 studies used C57BL/6, 2 studies used Albino mice, while BALB/c mice and Swiss Webster mice were used in 1 study each (Table 2). The exposure methods used in most of the neurotoxicological studies of TiO2 NPs using mice as animal models mimic the actual pathways of human exposure to TiO2 NPs. TiO2 NPs can enter the human body via intentional or unintentional inhalation; therefore, intranasal administration was chosen as the exposure method in 10 studies. Intranasal drug administration is a promising method to bypass BBB, resulting in higher bioavailability and greater brain exposure compared to oral administration at the same dose.71 Several studies have shown that intranasal administration of TiO2 NPs in mice resulted in various neurological damages, including hippocampal cell apoptosis, tissue necrosis, oxidative damage, neuroinflammation, imbalance of glutamate metabolism, and altered gene expression.72–79 These structural and functional impairments in the nervous system are closely related to behavioral deficits. Ze et al found that exposure to TiO2 NPs (208–330 nm, 2.5 mg/kg body weight, BW) for 90 consecutive days via intranasal administration damaged the hippocampus structure, decreased long-term potentiation, altered gene expression, and caused spatial recognition deficits.80 Intragastric administration, a method commonly used to assess the effects of oral exposure in the hazard assessment of environmental toxicants, was employed in 13 neurotoxicological studies of TiO2 NPs using mice as animal models.81 Pregnancy exposure to TiO2 NPs (21 nm, 150 mg/kg) via intragastric administration caused the delayed appearance of neurobehavioral impairments in both dams and offspring, which may be related to disruption of the gut-brain axis.82,83 Furthermore, exposure to low levels of TiO2 NPs (6.5 nm, < 5 mg/kg) via intragastric administration during pregnancy and lactation retarded axonal and dendritic outgrowth, impaired cognitive ability, and increased hippocampal neurons apoptosis.84–86 In addition to common impairments in the nervous system associated with TiO2 NPs exposure, such as neuroinflammation, oxidative stress, and altered gene expression, Wang et al found that exposure to TiO2 NPs (10 nm) increased the risk of PD.87
 |
Table 2 Overview of the Literature Investigating Neurotoxic Effects of TiO2 NPs in Mice |
Neurotoxic Effects of TiO2 NPs in Zebrafish
Zebrafish (Danio rerio) is commonly used as in vivo model system for studying the toxicity of nanomaterials due to its low cost, rapid growth, and significant homology to humans.105 A total of 20 studies have investigated the neurotoxic effects of TiO2 NPs in zebrafish, with 11 of them examining co-exposure to other compounds (Table 3). Among the 11 studies, 8 studies selected the embryonic stage of zebrafish for TiO2 NPs exposure, 2 studies selected adult zebrafish, and one study selected zebrafish larvae. The most commonly used dose of TiO2 NPs in studies involving co-exposure to other compounds was 100 μg/L. So far, TiO2 NPs have been shown to enhance Pb,106,107 decabromodiphenyl oxide (BDE-209),108 cypermethrin,109 triphenyl phosphate,110 bisphenol A,111,112 difenoconazole,113 tetracycline,114 and microcystin-LR115 -induced neurotoxicity. TiO2 NPs mainly enhance the neurotoxicity of these compounds by increasing their bioconcentration and bioavailability in zebrafish. Interestingly, co-exposure with TiO2 NPs did not alter pentachlorophenol-induced neurotoxicity.116
 |
Table 3 Overview of Literature Investigating Neurotoxic Effects of TiO2 NPs in Zebrafish |
Exposure to TiO2 NPs alone is also able to induce a variety of neurotoxic effects in zebrafish. The embryonic stage of zebrafish is the most commonly used exposure stage for TiO2 NPs exposure models, which may be attributed to the incomplete development of the BBB during this period.126 TiO2 NPs exposure during the embryonic stage of zebrafish significantly alters motor behavior, social behavior, and spatial recognition memory.117–120 In addition to behavioral impairments, TiO2 NPs exposure causes oxidative stress, promotes neuronal proliferation, decreases motor neuron axon length, alters gene expression, and increases cell apoptosis.120–123 Two study chose the adult stage of zebrafish for TiO2 NPs exposure, and their results suggested that TiO2 NPs exposure caused cognitive deficit, promoted neuroinflammation, and altered biochemical constituents of the brain.124,125
Neurotoxic Effects of TiO2 NPs in Other Animal Models
In vivo, studies investigating the potential neurotoxic effects of TiO2 NPs exposure on animals other than rodents and zebrafish are relatively scarce. To date, only five studies investigated the neurotoxicity of TiO2 NPs exposure in other animal models, namely C. elegans, Tegillarca granosa, Pheretima hawayana, and Drosophila melanogaster (Table 4).
 |
Table 4 Overview of the Literature Investigating Neurotoxic Effects of TiO2 NPs in Other Animal Models |
The nervous system of the C. elegans model is structurally and functionally similar to that of mammals. Its small size, short life cycle, and high reproductive rate make C. elegans an advantageous model in neuroscience.132 Long-term exposure to TiO2 NPs (10 nm) resulted in severe defects in the development of AVL and DVB neurons that control defecation in nematodes.127 Neurons exposed to both anatase and rutile TiO2 NPs exhibited shorter axon growth, which may be the reason for defective locomotion behavior in nematodes.128
Drosophila melanogaster is an excellent animal model for evaluating the neurotoxicity of various NPs due to its low-cost, physiological similarities to humans, and well-known behavioral and developmental characteristics.133,134 The results of a recent study indicated that chronic exposure to TiO2 NPs (approximately 20 nm, 20 mg/kg) induced deficits in motor behavior by disrupting the development of the neuromuscular junction (NMJ) in Drosophila.129
Tegillarca granosa and Pheretima hawayana are rarely studied in neurotoxicological research involving NPs. Tegillarca granosa inhabits intertidal mudflat, where the concentration of NPs is predicted to be higher than in other parts of the ocean.135 Selecting Tegillarca granosa as an animal model provides better insights into the neurotoxicity of NPs in marine bivalve mollusks.130 Exposure to TiO2 NPs (200 nm) increased neurotransmitters concentrations, suppressed the activity of acetylcholinesterase (AChE), and decreased the expression of neurotransmitter-related genes, which may disrupt various physiological processes in Tegillarca granosa.130 While TiO2 NPs are increasingly being released into the soil, their effects on soil biota remain largely unknown.136 The traditional sentinel species for soil toxicity testing is the earthworm, and Pheretima hawayana is a species of Egyptian earthworm. Exposure to TiO2 NPs altered biochemical indices related to the function of the nervous system, such as inhibiting AChE, increasing antioxidant enzymes, and accumulating malondialdehyde (MDA).131
Neurotoxic Effects of TiO2 NPs in vitro Models
In vitro models are widely used to assess neurotoxic effects on cellular functions.137 Several studies have evaluated the neurotoxic effects of TiO2 NPs using in vitro models. Primary hippocampal and cortical neurons are widely used in vitro models for neurotoxicology testing as they are easily polarized and form unique axons and dendrites. In addition, these models are used to study neuronal polarization, axon/dendrite morphology, synaptic formation, and central nervous system (CNS) functions.138 Exposure to TiO2 NPs impairs neuronal function, inhibits neuroblast proliferation, reduces cell viability, and increases cell apoptosis by promoting oxidative stress in both primary hippocampal and cortical neurons.139–144 Furthermore, TiO2 NPs inhibit neurite outgrowth of hippocampal neurons by interfering with glutamate metabolism and impairing N-methyl-D-aspartic acid (NMDA) receptor function.145 According to some previous studies, the suppression of axonal development, dendritic development, and synapse development by TiO2 NPs was associated with decreased expression of axon growth-related factors and inhibition of the Wnt/β-catenin and BDNF-TrkB pathways.146–148 See Table 5 for details.
 |
Table 5 Overview of the Literature on Neurotoxic Effects of TiO2 NPs on Primary Neuron and Nerve Cell Lines |
Rat pheochromocytoma (PC12) cell line and human SH-SY5Y neuroblastoma cell line have been used as models for neurotoxicity testing of TiO2 NPs (Table 5). PC12 cell line shows morphological and functional differentiation similar to sympathetic neurons. PC12 cell line is a suitable model for studying the chemical disruption of neuronal differentiation, synthesis, storage, and release of neurotransmitters, function and regulation of ion channels, and the interaction of compounds with membrane-bound receptors.160 A previous study revealed that treatment of PC12 cells with TiO2 NPs (< 36 nm, < 200 μg/mL) decreased cell viability, increased cell apoptosis via oxidative stress, inhibited the neurite outgrowth, disturbed cell cycle, and disrupted the ubiquitin-proteasome system.149–151 The human-derived SH-SY5Y cell line is preferred over the PC12 cell line as it avoids interspecific differences in chemical action.161 The SH-SY5Y cell line is an excellent model for studying toxicity on proliferating or differentiated cells because it can be maintained as neuroblasts or induced to differentiate into more neuron-like morphologies.161 TiO2 NPs were shown to cause endoplasmic reticulum (ER) stress, autophagy, inhibition of cell proliferation, disturbance of the microtubule dynamics, and membrane damage in SH-SY5Y cells.153–158 Several in vivo studies investigated the neurotoxic effects of TiO2 NPs on mouse hippocampus. However, one in vitro study explored the neurotoxic effects of TiO2 NPs on mouse hippocampal neuronal HT22 cells. The study revealed that TiO2 NPs increased apoptosis of HT22 cells via oxidative stress- and calcium imbalance-mediated ER stress.159
Acute or prolonged exposure to TiO2 NPs is associated with toxic effects on neuronal and glial cells.162 Glial cells are critical cells of the nervous system, which serve as tissue-resident macrophages. Microglia are crucial regulators that influence nervous system development, maintenance of the neural environment, and response to injury and repair.163 The immortalized mouse microglia cell line BV2 is often used as an alternative for primary microglia in cell experiments. Some previous studies showed that exposure of BV2 cells to TiO2 NPs was associated with mitochondrial dysfunction and increased oxidative stress.28,164 Astrocytes play a key role in innate and adaptive immune responses in CNS injury.165 Due to advancements in cell culture technology, primary astrocytes have become a common primary cell model. Previous studies revealed that TiO2 NPs induced mitochondria damage, oxidative stress, autophagy, neuroinflammation, and cell apoptosis in primary rat cortical astrocytes.166–168 Other studies employed human glial cell lines as in vitro models for neurotoxicity studies to eliminate species differences. Some previous studies revealed that TiO2 NPs inhibited cell proliferation, induced morphological changes, decreased immuno-location of F-actin fibers, and increased cell apoptosis in U374 cells.169,170 Furthermore, several studies have investigated the neurotoxic effects of TiO2 NPs in a co-culture of glial cells and other cells. For example, Yang et al showed that TiO2 NPs stimulate the inflammatory reaction in brain microglia and damage neuron using a co-culture model of primary microglia and PC12 cell line.171 Similarly, TiO2 NPs was shown to stimulate the inflammatory reaction in brain microglia and damage neurons in co-culture models of BV2 and N27 mesencephalic neurons, and BV2 and N2a neuroblastoma cells.172,173 See Table 6 for details.
 |
Table 6 Overview of the Literature on Neurotoxic Effects of TiO2 NPs in Primary Glial Cells and Glial Cell Lines |
Most in vivo and in vitro studies have evaluated the neurotoxic effects of TiO2 NPs in the cortex, hippocampus, and cerebellum. However, to the best of our knowledge, no studies have evaluated the neurotoxic effects of TiO2 NPs on other brain regions. The BBB is effective in protecting the brain from chemical damage. Therefore, there is a need to understand the effects of TiO2 NPS on the BBB. A previous study exploring the effects of TiO2 NPs on an in vitro model of BBB established by co-culturing primary human brain microvascular endothelial cells (HBMECs) and primary human astrocytes, revealed that TiO2 NPs increased the permeability of the BBB.31 Another study showed that acute or long-term exposure of an in vitro model of the BBB established by co-culturing primary rat endothelial cells and glial cells to TiO2 NPs was associated with BBB dysfunction related to increased inflammatory response and altered expression of the ABC transporter.174 Moreover, treatment of T98G human glioblastoma cells with TiO2 NPs was associated with changes in the transcriptome, suggesting that exposure to TiO2 NPs could compromise BBB integrity and cause neuroinflammation.175 Furthermore, TiO2 NPs can be internalized by dorsal root ganglion cells (DRG) and cause damage via apoptosis.176,177 Yu et al showed an association between the toxic effects of TiO2 NPs on olfactory bulb neuron cells and its pathogenicity to neurodegenerative diseases.178 Furthermore, exposure to TiO2 NPs was associated with varying degrees of cytotoxicity to the human cerebral endothelial cell line (HCECs), human neural stem cell line (hNSCs), and neuroectodermal stem cell line (1C11) models.179–181 See Table 7 for details.
 |
Table 7 Overview of the Literature on Neurotoxic Effects of TiO2 NPs in Other Cells |
Factors Influencing the Neurotoxic Potential of TiO2 NPs
The neurotoxic effects of TiO2 NPs are influenced by various factors. The exposure characteristics, such as exposure dose, method, duration, and species, can influence the toxic effects of TiO2 NPs in vivo. A review of the literature showed that the exposure dose in vivo and in vitro experiments was larger than the actual exposure dose of the population. According to a previous study, the levels of TiO2 NPs in air and water ranged from 0.7 to 16 μg/L.182 It is estimated that children have an intake of TiO2 NPs of about 2–3 mg/kg/day, while adults have a TiO2 NPs intake of about 1 mg/kg/day.2 Human exposure to TiO2 NPs is mainly through dietary intake and air inhalation. Although the exposure methods selected in animal studies attempted to mimic human exposure closely, there are some gaps. For example, the system for intranasal administration is simple compared to inhalation administration. Furthermore, intranasal administration is significantly affected by the inhalational dose.183 The intranasal administration volumes in rodents at a given time should be limited to approximately 5 μL per nostril since volumes greater than this are likely to become wasted.184,185 Furthermore, ingested TiO2 NPs first interacts with the oral mucosa. However, intragastric administration does not interact with the oral mucosa and is thus associated with significant differences in absorption, bioavailability, and metabolism with implications for assumptions and models of toxicity kinetics.81 In addition, the exposure period and duration also influence the neurotoxic effects of TiO2 NPs.45,94 However, the exposure duration in experiments tends to be shorter than that in humans. Species differences are often unavoidable. Therefore, there is a need to conduct epidemiological studies exploring the neurotoxic effects of TiO2 NPs on humans.
Furthermore, the physical and chemical properties of TiO2 NPs can affect their neurotoxicity. Particle size is key. In general, small particles are more likely to be absorbed and thus exert toxic effects.186 According to some previous studies, the neurotoxic effects of TiO2 NPs depend on particle size.60,168 The hydrodynamic diameter or secondary particle sizes of TiO2 NPs are important with respect to neurotoxicity. While smaller NPs may seem more neurotoxic, they are also more likely to clump together and form aggregates.186 Theoretically, the particle aggregation would increase the effective particle size thus reducing the neurotoxic potential. Several studies have used dynamic light scattering (DLS) to determine the effects of hydrodynamics or secondary particle size of TiO2 NPs on neurotoxicity. However, no studies have explored the effect of aggregate particle size on the neurotoxicity of TiO2 NPs. The zeta potential of TiO2 NPs has also been investigated in most neurotoxicological studies. Since most cell membranes are negatively charged, the zeta potential affects the tendency of NPs to penetrate the membrane, with cationic particles generally exhibiting higher toxicity associated with cell wall damage.187 Furthermore, the surface charge of the nanoparticles can determine the degree of aggregation.122,162,164 However, further studies are needed to investigate whether the zeta potential affects the neurotoxicity of TiO2 NPs. In addition, the toxicity of TiO2 NPs is dependent on crystalline phases. The anatase form of TiO2 NPs is more neurotoxic than that of rutile TiO2 NPs and P25 TiO2 NPs since anatase has a higher ability to induce oxidative stress.152,166,188
Taken together, various factors can affect the neurotoxic potential of TiO2 NPs, including physical and chemical properties of TiO2 NPs, and exposure dose, exposure duration, exposed species. However, the specific effects of these factors on the neurotoxic effects of TiO2 NPs still need to be systematically compared.
Reflections on Neurotoxicity Induced by TiO2 NPs
Most studies to date have focused on rodents, and most experimental exposures used are not very realistic for human exposure. In addition, there is currently limited information on the levels of TiO2 NPs in the environment, consumer goods, and food products. For humans, more accurate monitoring is needed to determine daily exposure levels, particle characteristics and exposure route, all of which affect the neurotoxic potential of TiO2 NPs. Evaluating and availing data on TiO2 NPs levels in different environmental media helps to reliably estimate human exposure and thus assess the risk of TiO2 NPs. Furthermore, the degree of uptake through the digestive system, respiratory system, potential BBB crossing, and potential translocation to or even accumulation in nervous system should be further investigated. This information will indicate which route of exposure mitigation is most valuable for human health protection. However, apart from the recommended exposure limits (REL) established by the National Institute for Occupational Safety and Health (NIOSH), no other regulatory agencies have set occupational or environmental exposure limits for TiO2 NPs.9 There are limitations in the monitoring methods of TiO2 NPs. There is an urgent need to develop appropriate methods for reducing TiO2 NPs in environmental media and food to prevent their potentially harmful health effects.189
The specific mechanisms behind the neurotoxic effects of TiO2 NPs have only been explored through animal and cell experiments. TiO2 NPs increase the formation of reactive oxygen species (ROS) in the brain, thus inducing oxidative stress. Ze et al reported that TiO2 NPs induced oxidative stress thus causing brain damage through overactivation of the p38-Nrf-2 signaling pathway.78 Oxidative stress can induce neuroinflammation, thus further aggravating cell damage.63,73,92 Cell damage, including structural and functional damage, is associated with increased onset and development of neurodevelopmental or neurodegenerative diseases, such as autism spectrum disorder (ASD) and PD.87,100 Cell damage is also linked to behavioral deficits.46,57 Abnormal motor ability could be caused by a decrease in the axon length of motor neurons.120 In addition, changes in hippocampal synaptic plasticity could lead to decreased spatial recognition.80 The development of axons, dendrites and synapses is regulated by various signaling pathways. TiO2 NPs impair the growth of axons and dendrites through excessive activation of the ERK1/2/MAPK signaling pathway.84 In addition, impairment of dendritic growth by TiO2 NPs is also related to inhibition of the Wnt/β-catenin signaling pathway.147 Moreover, suppression of the neuronal synaptic outgrowth by TiO2 NPs is linked to the inhibition of the BDNF-TrkB signaling pathway.148 Furthermore, the accumulation of TiO2 NPs in the brain could cause alterations in brain biochemistry and changes in neurotransmitter levels, contributing to behavioral changes.45,57,117 Although all of these studies confirm that TiO2 NPs cause neurotoxic effects through different mechanisms, most of the evidence on the neurotoxic effects of TiO2 NPs is fragmentary and is obtained from different species. Furthermore, few of these mechanism studies have explored whether the neurotoxic effects of TiO2 NPs are mediated through synergistic interactions of multiple brain regions, organs, and systems. Whether TiO2 NPs with different characteristics cause different degrees of toxic effects through different mechanisms remains be further explored. Extensive systematic studies are needed to fully elucidate the neurotoxic mechanisms of TiO2 NPs, which will be helpful for the prevention and treatment of neurotoxic effects of TiO2 NPs.
Conclusion
Animals and humans can be exposed to TiO2 NPs through different exposure pathways, thus posing health hazards. At present, the neurotoxic effects of TiO2 NPs have only been evaluated through animal models, including rats, mice, and zebrafish, and cell studies, including primary neurons, PC12, and SH-SY5Y cell lines. TiO2 NPs can induce oxidative stress, promote neuroinflammation, alter brain biochemistry, or damage neurons. Neuronal damage can further lead to various behavioral disorders and is closely associated with increased onset and development of neurodevelopmental or neurodegenerative diseases. However, due to the lack of relevant epidemiological studies, whether TiO2 NPs are linked to neurodevelopmental or neurodegenerative diseases in humans remains unknown. Furthermore, the neurotoxic potential of TiO2 NPs can be affected by various factors. There is a need for researchers to understand the neurotoxic effects of TiO2 NPs on humans and develop strategies for mitigating the effects of TiO2 NPs on human health.
Abbreviations
NMs, nanomaterials; TiO2 NPs, titanium dioxide nanoparticles; FPs, fine particles; PB, placental barrier; BBB, blood-brain barrier; C. elegans, Caenorhabditis elegans; SD rats, Sprague-Dawley rats; PND, postnatal day; BDNF, brain-derived neurotrophic factor; BW, body weight; BDE-209, decabromodiphenyl oxide; NMJ, neuromuscular junction; AchE, acetylcholinesterase; MDA, malondialdehyde; CNS, central nervous system; NMDA, N-methyl-D-aspartic acid; ER, endoplasmic reticulum; HBMECs, human brain microvascular endothelial cells; DRG, dorsal root ganglion; HCECs, human cerebral endothelial cell line; HNSCs, human neural stem cell line; 1C11, neuroectodermal stem cell line; DLS, Dynamic light scattering; REL, recommended exposure limit; NIOSH, National Institute for Occupational Safety and Health; ROS, reactive oxygen species; ASD, autism spectrum disorder; PD, Parkinson’s disease.
Acknowledgment
The authors would like to thank all the reviewers who participated in the review, as well as MJEditor (www.mjeditor.com) for providing English editing services during the preparation of this manuscript.
Funding
This work was supported by the Research Foundation for Talented Scholars, Nanjing Medical University (NMUR20210002).
Disclosure
The authors declare that they have no known competing interests.
References
1. Grande F, Tucci P. Titanium dioxide nanoparticles: a risk for human health? Mini Rev Med Chem. 2016;16(9):762–769. doi:10.2174/1389557516666160321114341
2. Weir A, Westerhoff P, Fabricius L, et al. Titanium dioxide nanoparticles in food and personal care products. Environ Sci Technol. 2012;46(4):2242–2250. doi:10.1021/es204168d
3. Mueller NC, Nowack B. Exposure modeling of engineered nanoparticles in the environment. Environ Sci Technol. 2008;42(12):4447–4453. doi:10.1021/es7029637
4. Shakeel M, Jabeen F, Shabbir S, et al. Toxicity of Nano-Titanium Dioxide (TiO2-NP) through various routes of exposure: a review. Biol Trace Elem Res. 2016;172(1):1–36. doi:10.1007/s12011-015-0550-x
5. Ali SA, Rizk MZ, Hamed MA, et al. Assessment of titanium dioxide nanoparticles toxicity via oral exposure in mice: effect of dose and particle size. Biomarkers. 2019;24(5):492–498. doi:10.1080/1354750X.2019.1620336
6. Institute IRS. The relevance of the rat lung response to particle overload for human risk assessment: a workshop consensus report. Inhal Toxicol. 2000;12(1–2):1–17. doi:10.1080/08958370050029725
7. Lee KP, Trochimowicz HJ, Reinhardt CF. Pulmonary response of rats exposed to titanium dioxide (TiO2) by inhalation for two years. Toxicol Appl Pharmacol. 1985;79(2):179–192. doi:10.1016/0041-008x(85)90339-4
8. Racovita AD. Titanium dioxide: structure, impact, and toxicity. Int J Environ Res Public Health. 2022;19(9):5681. doi:10.3390/ijerph19095681
9. Shi H, Magaye R, Castranova V, et al. Titanium dioxide nanoparticles: a review of current toxicological data. Part Fibre Toxicol. 2013;10:15. doi:10.1186/1743-8977-10-15
10. Song B, Liu J, Feng X, et al. A review on potential neurotoxicity of titanium dioxide nanoparticles. Nanoscale Res Lett. 2015;10(1):1042. doi:10.1186/s11671-015-1042-9
11. Boyes WK, van Thriel C. Neurotoxicology of nanomaterials. Chem Res Toxicol. 2020;33(5):1121–1144. doi:10.1021/acs.chemrestox.0c00050
12. Li A, Zhuang T, Shi J, et al. Heavy metals in maternal and cord blood in Beijing and their efficiency of placental transfer. J Environ Sci (China). 2019;80:99–106. doi:10.1016/j.jes.2018.11.004
13. Jin Y, Li Z, An H, et al. Environmental titanium exposure and reproductive health: risk of low birth weight associated with maternal titanium exposure from a nested case-control study in northern China. Ecotoxicol Environ Saf. 2021;208:111632. doi:10.1016/j.ecoenv.2020.111632
14. Zheng G, Zhong H, Guo Z, et al. Levels of heavy metals and trace elements in umbilical cord blood and the risk of adverse pregnancy outcomes: a population-based study. Biol Trace Elem Res. 2014;160(3):437–444. doi:10.1007/s12011-014-0057-x
15. Kinghorn KJ, Grönke S, Castillo-Quan JI, et al. A Drosophila model of neuronopathic gaucher disease demonstrates lysosomal-autophagic defects and altered mTOR signalling and is functionally rescued by rapamycin. J Neurosci. 2016;36(46):11654–11670. doi:10.1523/JNEUROSCI.4527-15.2016
16. Feng W, Cui X, Liu B, et al. Association of urinary metal profiles with altered glucose levels and diabetes risk: a population-based study in China. PLoS One. 2015;10(4):e0123742. doi:10.1371/journal.pone.0123742
17. Xiao Y, Yuan Y, Liu Y, et al. Circulating multiple metals and incident stroke in Chinese adults. Stroke. 2019;50(7):1661–1668. doi:10.1161/STROKEAHA.119.025060
18. Yang L, Jiang Y, Zhao L, et al. Multiple metals exposure and arterial stiffness: a panel study in China. Chemosphere. 2021;263:128217. doi:10.1016/j.chemosphere.2020.128217
19. Yuan Y, Xiao Y, Feng W, et al. Plasma metal concentrations and incident coronary heart disease in Chinese adults: the Dongfeng-Tongji cohort. Environ Health Perspect. 2017;125(10):107007. doi:10.1289/EHP1521
20. Yamano S, Goto Y, Takeda T, et al. Pulmonary dust foci as rat pneumoconiosis lesion induced by titanium dioxide nanoparticles in 13-week inhalation study. Part Fibre Toxicol. 2022;19(1):58. doi:10.1186/s12989-022-00498-3
21. Sagawa T, Honda A, Ishikawa R, et al. Role of necroptosis of alveolar macrophages in acute lung inflammation of mice exposed to titanium dioxide nanoparticles. Nanotoxicology. 2021;15(10):1312–1330. doi:10.1080/17435390.2021.2022231
22. Wu Y, Chen L, Chen F, et al. A key moment for TiO(2): prenatal exposure to TiO(2) nanoparticles may inhibit the development of offspring. Ecotoxicol Environ Saf. 2020;202:110911. doi:10.1016/j.ecoenv.2020.110911
23. Chen Z, Wang Y, Zhuo L, et al. Effect of titanium dioxide nanoparticles on the cardiovascular system after oral administration. Toxicol Lett. 2015;239(2):123–130. doi:10.1016/j.toxlet.2015.09.013
24. Savi M, Rossi S, Bocchi L, et al. Titanium dioxide nanoparticles promote arrhythmias via a direct interaction with rat cardiac tissue. Part Fibre Toxicol. 2014;11(1):63. doi:10.1186/s12989-014-0063-3
25. Hong F, Zhao X, Chen M, et al. TiO2 nanoparticles-induced apoptosis of primary cultured Sertoli cells of mice. J Biomed Mater Res A. 2016;104(1):124–135. doi:10.1002/jbm.a.35548
26. Hussain S, Thomassen LC, Ferecatu I, et al. Carbon black and titanium dioxide nanoparticles elicit distinct apoptotic pathways in bronchial epithelial cells. Part Fibre Toxicol. 2010;7:10. doi:10.1186/1743-8977-7-10
27. Li X, Kang B, Eom Y, et al. Comparison of cytotoxicity effects induced by four different types of nanoparticles in human corneal and conjunctival epithelial cells. Sci Rep. 2022;12(1):155. doi:10.1038/s41598-021-04199-3
28. Rihane N, Nury T, M’rad I, et al. Microglial cells (BV-2) internalize titanium dioxide (TiO2) nanoparticles: toxicity and cellular responses. Environ Sci Pollut Res Int. 2016;23(10):9690–9699. doi:10.1007/s11356-016-6190-7
29. Teleanu DM, Chircov C, Grumezescu A, et al. Neurotoxicity of nanomaterials: an up-to-date overview. Nanomaterials. 2019;9(1):96. doi:10.3390/nano9010096
30. Liu Y, He Q. The route of nanomaterials entering brain. In: Neurotoxicity of Nanomaterials and Nanomedicine. Elsevier; 2017:33–57.
31. Liu X, Sui B, Sun J. Size- and shape-dependent effects of titanium dioxide nanoparticles on the permeabilization of the blood-brain barrier. J Mater Chem B. 2017;5(48):9558–9570. doi:10.1039/c7tb01314k
32. Jiang X, Gao H. Neurotoxicity of Nanomaterials and Nanomedicine. Academic Press; 2016.
33. Bencsik A, Lestaevel P, Guseva Canu I. Nano- and neurotoxicology: an emerging discipline. Prog Neurobiol. 2018;160:45–63. doi:10.1016/j.pneurobio.2017.10.003
34. Teschke K, Marion SA, Tsui JKC, et al. Parkinson’s disease and occupation: differences in associations by case identification method suggest referral bias. Am. J. Ind. Med. 2014;57(2):163–171. doi:10.1002/ajim.22272
35. Andujar P, Simon-Deckers A, Galateau-Sallé F, et al. Role of metal oxide nanoparticles in histopathological changes observed in the lung of welders. Part Fibre Toxicol. 2014;11(1):23. doi:10.1186/1743-8977-11-23
36. Iqubal A, Ahmed M, Ahmad S, et al. Environmental neurotoxic pollutants: review. Environ Sci Pollut Res Int. 2020;27(33):41175–41198. doi:10.1007/s11356-020-10539-z
37. Gottschalk F, Sonderer T, Scholz RW, et al. Modeled environmental concentrations of engineered nanomaterials (TiO 2, ZnO, Ag, CNT, Fullerenes) for different regions. Environ Sci Technol. 2009;43(24):9216–9222. doi:10.1021/es9015553
38. Zhang R, Zhang H, Tu C, et al. Facilitated transport of titanium dioxide nanoparticles by humic substances in saturated porous media under acidic conditions. J Nanopart Res. 2015;17(4):1–11. doi:10.1007/s11051-015-2972-y
39. Gosset P, Lassalle P, Vanhée D, et al. Production of tumor necrosis factor-α and Interleukin-6 by human alveolar macrophages exposed in vitro to coal mine dust. Am J Respir Cell Mol Biol. 1991;5(5):431–436. doi:10.1165/ajrcmb/5.5.431
40. Mao Y, Bajinka O, Tang Z, et al. Lung-brain axis: metabolomics and pathological changes in lungs and brain of respiratory syncytial virus-infected mice. J Med Virol. 2022;94(12):5885–5893. doi:10.1002/jmv.28061
41. Jiang Y, Wei Y, Guo W, et al. Prenatal titanium exposure and child neurodevelopment at 1 year of age: a longitudinal prospective birth cohort study. Chemosphere. 2023;311(Pt 1):137034. doi:10.1016/j.chemosphere.2022.137034
42. Li Z, Huo W, Li Z, et al. Association between titanium and silver concentrations in maternal hair and risk of neural tube defects in offspring: a case-control study in north China. Reprod Toxicol. 2016;66:115–121. doi:10.1016/j.reprotox.2016.10.006
43. Zhang X, Mei D, Li Y, et al. Arsenic exposure via drinking water during pregnancy and lactation induces autism-like behaviors in male offspring mice. Chemosphere. 2022;290:133338. doi:10.1016/j.chemosphere.2021.133338
44. Mohammadipour A, Hosseini M, Fazel A, et al. The effects of exposure to titanium dioxide nanoparticles during lactation period on learning and memory of rat offspring. Toxicol Ind Health. 2016;32(2):221–228. doi:10.1177/0748233713498440
45. Mortensen NP, Pathmasiri W, Snyder RW, et al. Oral administration of TiO(2) nanoparticles during early life impacts cardiac and neurobehavioral performance and metabolite profile in an age- and sex-related manner. Part Fibre Toxicol. 2022;19(1):3. doi:10.1186/s12989-021-00444-9
46. Mohammadipour A, Fazel A, Haghir H, et al. Maternal exposure to titanium dioxide nanoparticles during pregnancy; impaired memory and decreased hippocampal cell proliferation in rat offspring. Environ Toxicol Pharmacol. 2014;37(2):617–625. doi:10.1016/j.etap.2014.01.014
47. Cui Y, Chen X, Zhou Z, et al. Prenatal exposure to nanoparticulate titanium dioxide enhances depressive-like behaviors in adult rats. Chemosphere. 2014;96:99–104. doi:10.1016/j.chemosphere.2013.07.051
48. Engler-Chiurazzi EB, Stapleton PA, Stalnaker JJ, et al. Impacts of prenatal nanomaterial exposure on male adult Sprague-Dawley rat behavior and cognition. J Toxicol Environ Health A. 2016;79(11):447–452. doi:10.1080/15287394.2016.1164101
49. Asghari A, Hosseini M, Beheshti F, et al. Inducible nitric oxide inhibitor aminoguanidine, ameliorated oxidative stress, interleukin-6 concentration and improved brain-derived neurotrophic factor in the brain tissues of neonates born from titanium dioxide nanoparticles exposed rats. J Matern Fetal Neonatal Med. 2019;32(23):3962–3973. doi:10.1080/14767058.2018.1480602
50. Ebrahimzadeh Bideskan A, Mohammadipour A, Fazel A, et al. Maternal exposure to titanium dioxide nanoparticles during pregnancy and lactation alters offspring hippocampal mRNA BAX and Bcl-2 levels, induces apoptosis and decreases neurogenesis. Exp Toxicol Pathol. 2017;69(6):329–337. doi:10.1016/j.etp.2017.02.006
51. Gao X, Yin S, Tang M, et al. Effects of developmental exposure to TiO2 nanoparticles on synaptic plasticity in hippocampal dentate gyrus area: an in vivo study in anesthetized rats. Biol Trace Elem Res. 2011;143(3):1616–1628. doi:10.1007/s12011-011-8990-4
52. Grissa I, Guezguez S, Ezzi L, et al. The effect of titanium dioxide nanoparticles on neuroinflammation response in rat brain. Environ Sci Pollut Res Int. 2016;23(20):20205–20213. doi:10.1007/s11356-016-7234-8
53. Grissa I, ElGhoul J, Mrimi R, et al. In deep evaluation of the neurotoxicity of orally administered TiO(2) nanoparticles. Brain Res Bull. 2020;155:119–128. doi:10.1016/j.brainresbull.2019.10.005
54. Nalika N, Waseem M, Kaushik P, et al. Role of melatonin and quercetin as countermeasures to the mitochondrial dysfunction induced by titanium dioxide nanoparticles. Life Sci. 2023;328:121403. doi:10.1016/j.lfs.2023.121403
55. Krawczynska A, Dziendzikowska K, Gromadzka-Ostrowska J, et al. Silver and titanium dioxide nanoparticles alter oxidative/inflammatory response and renin-angiotensin system in brain. Food Chem Toxicol. 2015;85:96–105. doi:10.1016/j.fct.2015.08.005
56. Valentini X, Deneufbourg P, Paci P, et al. Morphological alterations induced by the exposure to TiO(2) nanoparticles in primary cortical neuron cultures and in the brain of rats. Toxicol Rep. 2018;5:878–889. doi:10.1016/j.toxrep.2018.08.006
57. Naima R, Imen M, Mustapha J, et al. Acute titanium dioxide nanoparticles exposure impaired spatial cognitive performance through neurotoxic and oxidative mechanisms in Wistar rats. Biomarkers. 2021;26(8):760–769. doi:10.1080/1354750X.2021.1999501
58. Horvath T, Papp A, Kovács D, et al. Electrophysiological alterations and general toxic signs obtained by subacute administration of titanium dioxide nanoparticles to the airways of rats. Ideggyogy Sz. 2017;70(3–4):127–135. doi:10.18071/isz.70.0127
59. Papp A, Horváth T, Igaz N, et al. Presence of titanium and toxic effects observed in rat lungs, kidneys, and central nervous system in vivo and in cultured astrocytes in vitro on exposure by titanium dioxide nanorods. Int J Nanomedicine. 2020;15:9939–9960. doi:10.2147/IJN.S275937
60. Horvath T, Vezér T, Kozma G, et al. Functional neurotoxicity and tissue metal levels in rats exposed subacutely to titanium dioxide nanoparticles via the airways. Ideggyogy Sz. 2018;71(1–02):35–42. doi:10.18071/isz.71.0035
61. Younes NR, Amara S, Mrad I, et al. Subacute toxicity of titanium dioxide (TiO2) nanoparticles in male rats: emotional behavior and pathophysiological examination. Environ Sci Pollut Res Int. 2015;22(11):8728–8737. doi:10.1007/s11356-014-4002-5
62. Nalika N, Parvez S. Mitochondrial dysfunction in titanium dioxide nanoparticle-induced neurotoxicity. Toxicol Mech Methods. 2015;25(5):355–363. doi:10.3109/15376516.2015.1020183
63. Cui Y, Che Y, Wang H. Nono-titanium dioxide exposure during the adolescent period induces neurotoxicities in rats: ameliorative potential of bergamot essential oil. Brain Behav. 2021;11(5):e02099. doi:10.1002/brb3.2099
64. Latif MA, Jabeen F, Ali M, et al. Neurotoxic effects of titanium dioxide nanoparticles on the brain of male sprague dawley rats. Pak J Pharm Sci. 2019;32(5(Supplementary)):2311–2316.
65. Disdier C, Chalansonnet M, Gagnaire F, et al. Brain inflammation, blood brain barrier dysfunction and neuronal synaptophysin decrease after inhalation exposure to titanium dioxide nano-aerosol in aging rats. Sci Rep. 2017;7(1):12196. doi:10.1038/s41598-017-12404-5
66. Halawa A, Elshopakey G, El‐Adl M, et al. Chitosan attenuated the neurotoxicity-induced titanium dioxide nanoparticles in brain of adult rats. Environ Toxicol Int J. 2022;37(3):612–626. doi:10.1002/tox.23429
67. Herting MM, Sowell ER. Puberty and structural brain development in humans. Front Neuroendocrinol. 2017;44:122–137. doi:10.1016/j.yfrne.2016.12.003
68. Lees B, Meredith LR, Kirkland AE, et al. Effect of alcohol use on the adolescent brain and behavior. Pharmacol Biochem Behav. 2020;192:172906. doi:10.1016/j.pbb.2020.172906
69. Jiang M, Jang SE, Zeng L. The effects of extrinsic and intrinsic factors on neurogenesis. Cells. 2023;12(9):1285. doi:10.3390/cells12091285
70. Ribeiro FF, Xapelli S. An overview of adult neurogenesis. Adv Exp Med Biol. 2021;1331:77–94. doi:10.1007/978-3-030-74046-7_7
71. Erdo F, Bors LA, Farkas D, Bajza Á, Gizurarson S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res Bull. 2018;143:155–170. doi:10.1016/j.brainresbull.2018.10.009
72. Ze Y, Hu R, Wang X, et al. Neurotoxicity and gene-expressed profile in brain-injured mice caused by exposure to titanium dioxide nanoparticles. J Biomed Mater Res A. 2014;102(2):470–478. doi:10.1002/jbm.a.34705
73. Wang J, Liu Y, Jiao F, et al. Time-dependent translocation and potential impairment on central nervous system by intranasally instilled TiO(2) nanoparticles. Toxicology. 2008;254(1–2):82–90. doi:10.1016/j.tox.2008.09.014
74. Zhang L, Bai R, Li B, et al. Rutile TiO(2) particles exert size and surface coating dependent retention and lesions on the murine brain. Toxicol Lett. 2011;207(1):73–81. doi:10.1016/j.toxlet.2011.08.001
75. Ze X, Su M, Zhao X, et al. TiO2 nanoparticle-induced neurotoxicity may be involved in dysfunction of glutamate metabolism and its receptor expression in mice. Environ Toxicol: Int J. 2016;31(6):655–662. doi:10.1002/tox.22077
76. Ze Y, Sheng L, Zhao X, et al. TiO2 nanoparticles induced hippocampal neuroinflammation in mice. PLoS One. 2014;9(3):e92230. doi:10.1371/journal.pone.0092230
77. Wang J, Chen C, Liu Y, et al. Potential neurological lesion after nasal instillation of TiO(2) nanoparticles in the anatase and rutile crystal phases. Toxicol Lett. 2008;183(1–3):72–80. doi:10.1016/j.toxlet.2008.10.001
78. Ze Y, Zheng L, Zhao X, et al. Molecular mechanism of titanium dioxide nanoparticles-induced oxidative injury in the brain of mice. Chemosphere. 2013;92(9):1183–1189. doi:10.1016/j.chemosphere.2013.01.094
79. Tachibana K, Kawazoe S, Onoda A, et al. Effects of prenatal exposure to titanium dioxide nanoparticles on DNA methylation and gene expression profile in the mouse brain. Front Toxicol. 2021;3:705910. doi:10.3389/ftox.2021.705910
80. Ze Y, Sheng L, Zhao X, et al. Neurotoxic characteristics of spatial recognition damage of the hippocampus in mice following subchronic peroral exposure to TiO2 nanoparticles. J Hazard Mater. 2014;264:219–229. doi:10.1016/j.jhazmat.2013.10.072
81. Vandenberg LN, Welshons WV, Vom Saal FS, Toutain PL, Myers JP. Should oral gavage be abandoned in toxicity testing of endocrine disruptors? Environ Health. 2014;13(1):46. doi:10.1186/1476-069X-13-46
82. Su J, Duan X, Qiu Y, et al. Pregnancy exposure of titanium dioxide nanoparticles causes intestinal dysbiosis and neurobehavioral impairments that are not significant postnatally but emerge in adulthood of offspring. J Nanobiotechnology. 2021;19(1):234. doi:10.1186/s12951-021-00967-5
83. Yang C, Xue J, Qin Q, et al. Prenatal exposure to titanium dioxide nanoparticles induces persistent neurobehavioral impairments in maternal mice that is associated with microbiota-gut-brain axis. Food Chem Toxicol. 2022;169:113402. doi:10.1016/j.fct.2022.113402
84. Zhou Y, Ji J, Chen C, Hong F. Retardation of axonal and dendritic outgrowth is associated with the MAPK signaling pathway in offspring mice following maternal exposure to nanosized titanium dioxide. J Agric Food Chem. 2019;67(9):2709–2715. doi:10.1021/acs.jafc.8b06992
85. Hong F, Zhou Y, Ji J, et al. Nano-TiO(2) inhibits development of the central nervous system and its mechanism in offspring mice. J Agric Food Chem. 2018;66(44):11767–11774. doi:10.1021/acs.jafc.8b02952
86. Zhou Y, Hong F, Tian Y, et al. Nanoparticulate titanium dioxide-inhibited dendritic development is involved in apoptosis and autophagy of hippocampal neurons in offspring mice. Toxicol Res. 2017;6(6):889–901. doi:10.1039/c7tx00153c
87. Heidari Z, Mohammadipour A, Haeri P, Ebrahimzadeh-Bideskan A. The effect of titanium dioxide nanoparticles on mice midbrain substantia nigra. Iran J Basic Med Sci. 2019;22(7):745–751. doi:10.22038/ijbms.2019.33611.8018
88. Umezawa M, Tainaka H, Kawashima N, et al. Effect of fetal exposure to titanium dioxide nanoparticle on brain development − brain region information. J Toxicol Sci. 2012;37(6):1247–1252. doi:10.2131/jts.37.1247
89. Shimizu M, Tainaka H, Oba T, et al. Maternal exposure to nanoparticulate titanium dioxide during the prenatal period alters gene expression related to brain development in the mouse. Part Fibre Toxicol. 2009;6(1):20. doi:10.1186/1743-8977-6-20
90. Takahashi Y, Mizuo K, Shinkai Y, et al. Prenatal exposure to titanium dioxide nanoparticles increases dopamine levels in the prefrontal cortex and neostriatum of mice. J Toxicol Sci. 2010;35(5):749–756. doi:10.2131/jts.35.749
91. Tarlan M, Sajedianfard J, Fathi M. Effect of titanium dioxide nanoparticles administered during pregnancy on depression-like behavior in forced swimming and tail suspension tests in offspring mice. Toxicol Ind Health. 2020;36(4):297–304. doi:10.1177/0748233720925707
92. Ma L, Liu J, Li N, et al. Oxidative stress in the brain of mice caused by translocated nanoparticulate TiO2 delivered to the abdominal cavity. Biomaterials. 2010;31(1):99–105. doi:10.1016/j.biomaterials.2009.09.028
93. Habibi P, Ostad SN, Monazzam MR, et al. Thermal stress and TiO(2) nanoparticle-induced oxidative DNA damage and apoptosis in mouse hippocampus. Environ Sci Pollut Res Int. 2022;29(60):90128–90139. doi:10.1007/s11356-022-21796-5
94. Wang J-X, Li Y-F, Zhou G-Q, et al. [Influence of intranasal instilled titanium dioxide nanoparticles on monoaminergic neurotransmitters of female mice at different exposure time]. Zhonghua Yu Fang Yi Xue Za Zhi. 2007;41(2):91–95. Chinese.
95. Hu R, Gong X, Duan Y, et al. Neurotoxicological effects and the impairment of spatial recognition memory in mice caused by exposure to TiO2 nanoparticles. Biomaterials. 2010;31(31):8043–8050. doi:10.1016/j.biomaterials.2010.07.011
96. Hu R, Zheng L, Zhang T, et al. Molecular mechanism of hippocampal apoptosis of mice following exposure to titanium dioxide nanoparticles. J Hazard Mater. 2011;191(1–3):32–40. doi:10.1016/j.jhazmat.2011.04.027
97. Jia X, Wang S, Zhou L, et al. The potential liver, brain, and embryo toxicity of titanium dioxide nanoparticles on mice. Nanoscale Res Lett. 2017;12(1):478. doi:10.1186/s11671-017-2242-2
98. Zhang S, Jiang X, Cheng S, et al. Titanium dioxide nanoparticles via oral exposure leads to adverse disturbance of gut microecology and locomotor activity in adult mice. Arch Toxicol. 2020;94(4):1173–1190. doi:10.1007/s00204-020-02698-2
99. Shin JA, Lee EJ, Seo SM, et al. Nanosized titanium dioxide enhanced inflammatory responses in the septic brain of mouse. Neuroscience. 2010;165(2):445–454. doi:10.1016/j.neuroscience.2009.10.057
100. Notter T, Aengenheister L, Weber-Stadlbauer U, et al. Prenatal exposure to TiO(2) nanoparticles in mice causes behavioral deficits with relevance to autism spectrum disorder and beyond. Transl Psychiatry. 2018;8(1):193. doi:10.1038/s41398-018-0251-2
101. Sofranko A, Wahle T, Heusinkveld HJ, et al. Evaluation of the neurotoxic effects of engineered nanomaterials in C57BL/6J mice in 28-day oral exposure studies. Neurotoxicology. 2021;84:155–171. doi:10.1016/j.neuro.2021.03.005
102. Mohamadzadeh N, Zirak Javanmard M, Karimipour M, et al. Developmental toxicity of the neural tube induced by titanium dioxide nanoparticles in mouse embryos. Avicenna J Med Biotechnol. 2021;13(2):74–80. doi:10.18502/ajmb.v13i2.5524
103. Shrivastava R, Raza S, Yadav A, et al. Effects of sub-acute exposure to TiO 2, ZnO and Al2 O3 nanoparticles on oxidative stress and histological changes in mouse liver and brain. Drug Chem Toxicol. 2014;37(3):336–347. doi:10.3109/01480545.2013.866134
104. Mohamed HR, Hussien NA. Genotoxicity studies of Titanium Dioxide Nanoparticles (TiO2NPs) in the brain of mice. Scientifica. 2016;2016:6710840. doi:10.1155/2016/6710840
105. Liu W, Huang G, Su X, et al. Zebrafish: a promising model for evaluating the toxicity of carbon dot-based nanomaterials. ACS Appl Mater Interfaces. 2020;12(43):49012–49020. doi:10.1021/acsami.0c17492
106. Hu S, Han J, Yang L, et al. Impact of co-exposure to titanium dioxide nanoparticles and Pb on zebrafish embryos. Chemosphere. 2019;233:579–589. doi:10.1016/j.chemosphere.2019.06.009
107. Miao W, Zhu B, Xiao X, et al. Effects of titanium dioxide nanoparticles on lead bioconcentration and toxicity on thyroid endocrine system and neuronal development in zebrafish larvae. Aquat Toxicol. 2015;161:117–126. doi:10.1016/j.aquatox.2015.02.002
108. Wang Q, Chen Q, Zhou P, et al. Bioconcentration and metabolism of BDE-209 in the presence of titanium dioxide nanoparticles and impact on the thyroid endocrine system and neuronal development in zebrafish larvae. Nanotoxicology. 2014;8 Suppl 1:196–207. doi:10.3109/17435390.2013.875232
109. Li M, Wu Q, Wang Q, Xiang D, Zhu G. Effect of titanium dioxide nanoparticles on the bioavailability and neurotoxicity of cypermethrin in zebrafish larvae. Aquat Toxicol. 2018;199:212–219. doi:10.1016/j.aquatox.2018.03.022
110. Fan B, Dai L, Liu C, et al. Nano-TiO(2) aggravates bioaccumulation and developmental neurotoxicity of triphenyl phosphate in zebrafish larvae. Chemosphere. 2022;287(Pt 3):132161. doi:10.1016/j.chemosphere.2021.132161
111. Fu J, Guo Y, Yang L, Han J, Zhou B. Nano-TiO(2) enhanced bioaccumulation and developmental neurotoxicity of bisphenol a in zebrafish larvae. Environ Res. 2020;187:109682. doi:10.1016/j.envres.2020.109682
112. Guo Y, Chen L, Wu J, et al. Parental co-exposure to bisphenol A and nano-TiO(2) causes thyroid endocrine disruption and developmental neurotoxicity in zebrafish offspring. Sci Total Environ. 2019;650(Pt 1):557–565. doi:10.1016/j.scitotenv.2018.09.007
113. Zhu R, Liu C, Wang J, et al. Nano-TiO(2) aggravates bioaccumulation and developmental neurotoxicity of difenoconazole in zebrafish larvae via oxidative stress and apoptosis: protective role of vitamin C. Ecotoxicol Environ Saf. 2023;251:114554. doi:10.1016/j.ecoenv.2023.114554
114. Xu L, Yang X, He Y, Hu Q, Fu Z. Combined exposure to titanium dioxide and tetracycline induces neurotoxicity in zebrafish. Comp Biochem Physiol C. 2023;267:109562. doi:10.1016/j.cbpc.2023.109562
115. Wu Q, Yan W, Liu C, et al. Co-exposure with titanium dioxide nanoparticles exacerbates MCLR-induced brain injury in zebrafish. Sci Total Environ. 2019;693:133540. doi:10.1016/j.scitotenv.2019.07.346
116. Lei L, Qiao K, Guo Y, et al. Titanium dioxide nanoparticles enhanced thyroid endocrine disruption of pentachlorophenol rather than neurobehavioral defects in zebrafish larvae. Chemosphere. 2020;249:126536. doi:10.1016/j.chemosphere.2020.126536
117. Sheng L, Wang L, Su M, et al. Mechanism of TiO2 nanoparticle-induced neurotoxicity in zebrafish (D anio rerio). Environ Toxicol Int J. 2016;31(2):163–175. doi:10.1002/tox.22031
118. Chen J, Li J, Jiang H, et al. Developmental co-exposure of TBBPA and titanium dioxide nanoparticle induced behavioral deficits in larval zebrafish. Ecotoxicol Environ Saf. 2021;215:112176. doi:10.1016/j.ecoenv.2021.112176
119. Chen J, Lei L, Mo W, et al. Developmental titanium dioxide nanoparticle exposure induces oxidative stress and neurobehavioral changes in zebrafish. Aquat Toxicol. 2021;240:105990. doi:10.1016/j.aquatox.2021.105990
120. Gu J, Guo M, Huang C, et al. Titanium dioxide nanoparticle affects motor behavior, neurodevelopment and axonal growth in zebrafish (Danio rerio) larvae. Sci Total Environ. 2021;754:142315. doi:10.1016/j.scitotenv.2020.142315
121. Sun X, Yang Q, Jing M, et al. Environmentally relevant concentrations of organic (benzophenone-3) and inorganic (titanium dioxide nanoparticles) UV filters co-exposure induced neurodevelopmental toxicity in zebrafish. Ecotoxicol Environ Saf. 2023;249:114343. doi:10.1016/j.ecoenv.2022.114343
122. Hu Q, Guo F, Zhao F, et al. Effects of titanium dioxide nanoparticles exposure on parkinsonism in zebrafish larvae and PC12. Chemosphere. 2017;173:373–379. doi:10.1016/j.chemosphere.2017.01.063
123. Caruso G, Scalisi EM, Pecoraro R, et al. Effects of carnosine on the embryonic development and TiO(2) nanoparticles-induced oxidative stress on Zebrafish. Front Vet Sci. 2023;10:1148766. doi:10.3389/fvets.2023.1148766
124. Palaniappan PR, Pramod KS. The effect of titanium dioxide on the biochemical constituents of the brain of Zebrafish (Danio rerio): an FT-IR study. Spectrochim Acta A Mol Biomol Spectrosc. 2011;79(1):206–212. doi:10.1016/j.saa.2011.02.038
125. Kaur K, Narang RK, Singh S. Glabridin mitigates TiO(2)NP induced cognitive deficit in adult zebrafish. Neurochem Int. 2023;169:105585. doi:10.1016/j.neuint.2023.105585
126. Bauer B, Mally A, Liedtke D. Zebrafish embryos and larvae as alternative animal models for toxicity testing. Int J Mol Sci. 2021;22(24):13417. doi:10.3390/ijms222413417
127. Zhao Y, Wu Q, Tang M, et al. The in vivo underlying mechanism for recovery response formation in nano-titanium dioxide exposed Caenorhabditis elegans after transfer to the normal condition. Nanomedicine. 2014;10(1):89–98. doi:10.1016/j.nano.2013.07.004
128. Hu CC, Wu G-H, Hua T-E, et al. Uptake of TiO 2 nanoparticles into C. elegans neurons negatively affects axonal growth and worm locomotion behavior. ACS Appl Mater Interfaces. 2018;10(10):8485–8495. doi:10.1021/acsami.7b18818
129. Zhang X, Song Y, Wang J, et al. Chronic exposure to titanium dioxide nanoparticles induces deficits of locomotor behavior by disrupting the development of NMJ in Drosophila. Sci Total Environ. 2023;888:164076. doi:10.1016/j.scitotenv.2023.164076
130. Guan X, Shi W, Zha S, et al. Neurotoxic impact of acute TiO(2) nanoparticle exposure on a benthic marine bivalve mollusk, Tegillarca granosa. Aquat Toxicol. 2018;200:241–246. doi:10.1016/j.aquatox.2018.05.011
131. Khalil AM. Neurotoxicity and biochemical responses in the earthworm Pheretima hawayana exposed to TiO2NPs. Ecotoxicol Environ Saf. 2015;122:455–461. doi:10.1016/j.ecoenv.2015.09.010
132. Ruszkiewicz JA, Pinkas A, Miah MR, et al. C. elegans as a model in developmental neurotoxicology. Toxicol Appl Pharmacol. 2018;354:126–135. doi:10.1016/j.taap.2018.03.016
133. Chifiriuc MC, Ratiu A, Popa M, et al. Drosophotoxicology: an emerging research area for assessing nanoparticles interaction with living organisms. Int J Mol Sci. 2016;17(2):36. doi:10.3390/ijms17020036
134. Ong C, Yung L-YL, Cai Y, et al. Drosophila melanogaster as a model organism to study nanotoxicity. Nanotoxicology. 2015;9(3):396–403. doi:10.3109/17435390.2014.940405
135. Sun TY, Bornhöft NA, Hungerbühler K, et al. Dynamic probabilistic modeling of environmental emissions of engineered nanomaterials. Environ Sci Technol. 2016;50(9):4701–4711. doi:10.1021/acs.est.5b05828
136. McShane H, Sarrazin M, Whalen JK, et al. Reproductive and behavioral responses of earthworms exposed to nano-sized titanium dioxide in soil. Environ Toxicol Chem. 2012;31(1):184–193. doi:10.1002/etc.714
137. Tiffany-Castiglioni E, Ehrich M, Dees L, et al. Bridging the gap between in vitro and in vivo models for neurotoxicology. Toxicol Sci. 1999;51(2):178–183. doi:10.1093/toxsci/51.2.178
138. Viesselmann C, Ballweg J, Lumbard D, Dent EW. Nucleofection and primary culture of embryonic mouse hippocampal and cortical neurons. J Vis Exp. 2011;47:e2373.
139. Sheng L, Ze Y, Wang L, et al. Mechanisms of TiO2 nanoparticle-induced neuronal apoptosis in rat primary cultured hippocampal neurons. J Biomed Mater Res A. 2015;103(3):1141–1149. doi:10.1002/jbm.a.35263
140. Gerber LS, Heusinkveld HJ, Langendoen C, et al. Acute, sub-chronic and chronic exposures to TiO(2) and Ag nanoparticles differentially affects neuronal function in vitro. Neurotoxicology. 2022;93:311–323. doi:10.1016/j.neuro.2022.10.010
141. Strickland JD, Lefew WR, Crooks J, et al. In vitro screening of metal oxide nanoparticles for effects on neural function using cortical networks on microelectrode arrays. Nanotoxicology. 2016;10(5):619–628. doi:10.3109/17435390.2015.1107142
142. Sun Y, Wang S, Zheng J. Biosynthesis of TiO(2) nanoparticles and their application for treatment of brain injury-an in-vitro toxicity study towards central nervous system. J Photochem Photobiol B. 2019;194:1–5. doi:10.1016/j.jphotobiol.2019.02.008
143. Gramowski A, Flossdorf J, Bhattacharya K, et al. Nanoparticles induce changes of the electrical activity of neuronal networks on microelectrode array neurochips. Environ Health Perspect. 2010;118(10):1363–1369. doi:10.1289/ehp.0901661
144. Winter M, Beer H-D, Hornung V, et al. Activation of the inflammasome by amorphous silica and TiO2 nanoparticles in murine dendritic cells. Nanotoxicology. 2011;5(3):326–340. doi:10.3109/17435390.2010.506957
145. Hong F, Sheng L, Ze Y, et al. Suppression of neurite outgrowth of primary cultured hippocampal neurons is involved in impairment of glutamate metabolism and NMDA receptor function caused by nanoparticulate TiO2. Biomaterials. 2015;53:76–85. doi:10.1016/j.biomaterials.2015.02.067
146. Mu X, Li W, Ze X, et al. Molecular mechanism of nanoparticulate TiO2 induction of axonal development inhibition in rat primary cultured hippocampal neurons. Environ Toxicol: Int J. 2020;35(8):895–905. doi:10.1002/tox.22926
147. Hong F, Ze Y, Zhou Y, et al. Nanoparticulate TiO 2-mediated inhibition of the Wnt signaling pathway causes dendritic development disorder in cultured rat hippocampal neurons. J Biomed Mater Res A. 2017;105(8):2139–2149. doi:10.1002/jbm.a.36073
148. Hong F, Ze X, Mu X, et al. Titanium dioxide inhibits hippocampal neuronal synapse growth through the brain-derived neurotrophic factor-tyrosine kinase receptor B signaling pathway. J Biomed Nanotechnol. 2021;17(1):37–52. doi:10.1166/jbn.2021.2999
149. Liu S, Xu L, Zhang T, et al. Oxidative stress and apoptosis induced by nanosized titanium dioxide in PC12 cells. Toxicology. 2010;267(1–3):172–177. doi:10.1016/j.tox.2009.11.012
150. Wu J, Xie H. Effects of titanium dioxide nanoparticles on alpha-synuclein aggregation and the ubiquitin-proteasome system in dopaminergic neurons. Artif Cells Nanomed Biotechnol. 2016;44(2):690–694. doi:10.3109/21691401.2014.980507
151. Irie T, Kawakami T, Sato K, et al. Sub-toxic concentrations of nano-ZnO and nano-TiO2 suppress neurite outgrowth in differentiated PC12 cells. J Toxicol Sci. 2017;42(6):723–729. doi:10.2131/jts.42.723
152. Wu J, Sun J, Xue Y. Involvement of JNK and P53 activation in G2/M cell cycle arrest and apoptosis induced by titanium dioxide nanoparticles in neuron cells. Toxicol Lett. 2010;199(3):269–276. doi:10.1016/j.toxlet.2010.09.009
153. Ferraro SA, Domingo MG, Etcheverrito A, et al. Neurotoxicity mediated by oxidative stress caused by titanium dioxide nanoparticles in human neuroblastoma (SH-SY5Y) cells. J Trace Elem Med Biol. 2020;57:126413. doi:10.1016/j.jtemb.2019.126413
154. Lojk J, Repas J, Veranič P, et al. Toxicity mechanisms of selected engineered nanoparticles on human neural cells in vitro. Toxicology. 2020;432:152364. doi:10.1016/j.tox.2020.152364
155. Valdiglesias V, Costa C, Sharma V, et al. Comparative study on effects of two different types of titanium dioxide nanoparticles on human neuronal cells. Food Chem Toxicol. 2013;57:352–361. doi:10.1016/j.fct.2013.04.010
156. Mao Z, Xu B, Ji X, et al. Titanium dioxide nanoparticles alter cellular morphology via disturbing the microtubule dynamics. Nanoscale. 2015;7(18):8466–8475. doi:10.1039/c5nr01448d
157. Rosario F, Costa C, Lopes CB, et al. In vitro hepatotoxic and neurotoxic effects of titanium and cerium dioxide nanoparticles, arsenic and mercury co-exposure. Int J Mol Sci. 2022;23(5):2737. doi:10.3390/ijms23052737
158. Zhou T, Huang W-K, Xu Q-Y, et al. Nec-1 attenuates neurotoxicity induced by titanium dioxide nanomaterials on Sh-Sy5y cells through RIP1. Nanoscale Res Lett. 2020;15(1):65. doi:10.1186/s11671-020-03300-5
159. He Q, Zhou X, Liu Y, et al. Titanium dioxide nanoparticles induce mouse hippocampal neuron apoptosis via oxidative stress- and calcium imbalance-mediated endoplasmic reticulum stress. Environ Toxicol Pharmacol. 2018;63:6–15. doi:10.1016/j.etap.2018.08.003
160. Shafer TJ, Atchison WD. Transmitter, ion channel and receptor properties of pheochromocytoma (PC12) cells: a model for neurotoxicological studies. Neurotoxicology. 1991;12(3):473–492.
161. Lopez-Suarez L, Awabdh SA, Coumoul X, et al. The SH-SY5Y human neuroblastoma cell line, a relevant in vitro cell model for investigating neurotoxicology in human: focus on organic pollutants. Neurotoxicology. 2022;92:131–155. doi:10.1016/j.neuro.2022.07.008
162. Coccini T, Grandi S, Lonati D, et al. Comparative cellular toxicity of titanium dioxide nanoparticles on human astrocyte and neuronal cells after acute and prolonged exposure. Neurotoxicology. 2015;48:77–89. doi:10.1016/j.neuro.2015.03.006
163. Orihuela R, McPherson CA, Harry GJ. Microglial M1/M2 polarization and metabolic states. Br J Pharmacol. 2016;173(4):649–665. doi:10.1111/bph.13139
164. Long TC, Saleh N, Tilton RD, et al. Titanium dioxide (P25) produces reactive oxygen species in immortalized brain microglia (BV2): implications for nanoparticle neurotoxicity. Environ Sci Technol. 2006;40(14):4346–4352. doi:10.1021/es060589n
165. Colombo E, Farina C. Astrocytes: key regulators of neuroinflammation. Trends Immunol. 2016;37(9):608–620. doi:10.1016/j.it.2016.06.006
166. Wilson CL, Natarajan V, Hayward SL, et al. Mitochondrial dysfunction and loss of glutamate uptake in primary astrocytes exposed to titanium dioxide nanoparticles. Nanoscale. 2015;7(44):18477–18488. doi:10.1039/c5nr03646a
167. Perez-Arizti JA, Ventura-Gallegos JL, Galván Juárez RE, et al. Titanium dioxide nanoparticles promote oxidative stress, autophagy and reduce NLRP3 in primary rat astrocytes. Chem Biol Interact. 2020;317:108966. doi:10.1016/j.cbi.2020.108966
168. Liu Y, Xu Z, Li X. Cytotoxicity of titanium dioxide nanoparticles in rat neuroglia cells. Brain Inj. 2013;27(7–8):934–939. doi:10.3109/02699052.2013.793401
169. Marquez-Ramirez SG, Delgado-Buenrostro NL, Chirino YI, et al. Titanium dioxide nanoparticles inhibit proliferation and induce morphological changes and apoptosis in glial cells. Toxicology. 2012;302(2–3):146–156. doi:10.1016/j.tox.2012.09.005
170. Huerta-Garcia E, Pérez-Arizti JA, Márquez-Ramírez SG, et al. Titanium dioxide nanoparticles induce strong oxidative stress and mitochondrial damage in glial cells. Free Radic Biol Med. 2014;73:84–94. doi:10.1016/j.freeradbiomed.2014.04.026
171. Xue Y, Wu J, Sun J. Four types of inorganic nanoparticles stimulate the inflammatory reaction in brain microglia and damage neurons in vitro. Toxicol Lett. 2012;214(2):91–98. doi:10.1016/j.toxlet.2012.08.009
172. Long TC, Tajuba J, Sama P, et al. Nanosize titanium dioxide stimulates reactive oxygen species in brain microglia and damages neurons in vitro. Environ Health Perspect. 2007;115(11):1631–1637. doi:10.1289/ehp.10216
173. Hsiao I-L, Chang -C-C, Wu C-Y, et al. Indirect effects of TiO2 nanoparticle on neuron-glial cell interactions. Chem Biol Interact. 2016;254:34–44. doi:10.1016/j.cbi.2016.05.024
174. Brun E, Carriere M, Mabondzo A. In vitro evidence of dysregulation of blood-brain barrier function after acute and repeated/long-term exposure to TiO(2) nanoparticles. Biomaterials. 2012;33(3):886–896. doi:10.1016/j.biomaterials.2011.10.025
175. Fuster E, Candela H, Estévez J, et al. Titanium dioxide, but not zinc oxide, nanoparticles cause severe transcriptomic alterations in T98G human glioblastoma cells. Int J Mol Sci. 2021;22(4):2084. doi:10.3390/ijms22042084
176. Erriquez J, Bolis V, Morel S, et al. Nanosized TiO2 is internalized by dorsal root ganglion cells and causes damage via apoptosis. Nanomedicine. 2015;11(6):1309–1319. doi:10.1016/j.nano.2015.04.003
177. Bolis V, Busco C, Ciarletta M, et al. Hydrophilic/hydrophobic features of TiO2 nanoparticles as a function of crystal phase, surface area and coating, in relation to their potential toxicity in peripheral nervous system. J Colloid Interface Sci. 2012;369(1):28–39. doi:10.1016/j.jcis.2011.11.058
178. Yu Y, Ren W, Ren B. Nanosize titanium dioxide cause neuronal apoptosis: a potential linkage between nanoparticle exposure and neural disorder. Neurol Res. 2008;30(10):1115–1120. doi:10.1179/0161641215Z.000000000587
179. Halamoda Kenzaoui B, Chapuis Bernasconi C, Guney-Ayra S, et al. Induction of oxidative stress, lysosome activation and autophagy by nanoparticles in human brain-derived endothelial cells. Biochem J. 2012;441(3):813–821. doi:10.1042/BJ20111252
180. Fujioka K, Hanada S, Inoue Y, et al. Effects of silica and titanium oxide particles on a human neural stem cell line: morphology, mitochondrial activity, and gene expression of differentiation markers. Int J Mol Sci. 2014;15(7):11742–11759. doi:10.3390/ijms150711742
181. Ribeiro LW, Pietri M, Ardila-Osorio H, et al. Titanium dioxide and carbon black nanoparticles disrupt neuronal homeostasis via excessive activation of cellular prion protein signaling. Part Fibre Toxicol. 2022;19(1):48. doi:10.1186/s12989-022-00490-x
182. Heringa MB, Peters RJB, Bleys RLAW, et al. Detection of titanium particles in human liver and spleen and possible health implications. Part Fibre Toxicol. 2018;15(1):15. doi:10.1186/s12989-018-0251-7
183. Wolff RK. Toxicology studies for inhaled and nasal delivery. Mol Pharm. 2015;12(8):2688–2696. doi:10.1021/acs.molpharmaceut.5b00146
184. Miller MA, Stabenow JM, Parvathareddy J, et al. Visualization of murine intranasal dosing efficiency using luminescent Francisella tularensis: effect of instillation volume and form of anesthesia. PLoS One. 2012;7(2):e31359. doi:10.1371/journal.pone.0031359
185. Southam DS, Dolovich M, O’Byrne PM, et al. Distribution of intranasal instillations in mice: effects of volume, time, body position, and anesthesia. Am J Physiol Lung Cell Mol Physiol. 2002;282(4):L833–L839. doi:10.1152/ajplung.00173.2001
186. Prust M, Meijer J, Westerink RHS. The plastic brain: neurotoxicity of micro- and nanoplastics. Part Fibre Toxicol. 2020;17(1):24. doi:10.1186/s12989-020-00358-y
187. Clogston JD, Patri AK. Zeta potential measurement. Methods Mol Biol. 2011;697:63–70. doi:10.1007/978-1-60327-198-1_6
188. Jin C, Tang Y, Yang FG, et al. Cellular toxicity of TiO2 nanoparticles in anatase and rutile crystal phase. Biol Trace Elem Res. 2011;141(1–3):3–15. doi:10.1007/s12011-010-8707-0
189. Abbott Chalew TEA, Ajmani GS, Huang H, et al. Evaluating nanoparticle breakthrough during drinking water treatment. Environ Health Perspect. 2013;121(10):1161–1166. doi:10.1289/ehp.1306574
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
