Back to Journals » International Medical Case Reports Journal » Volume 16
Neurosarcoidosis, Coccidioidomycosis, or Both!
Authors Akhavanrezayat A , Matsumiya W, Ongpalakorn P, Ghoraba HH, Or C, Khojasteh Jafari H, Uludag Kirimli G, Yasar C , Than NTT, Karaca I , Zaidi M, Mobasserian A, Yavari N , Bazojoo V, Shin YU, Bromeo AJ, Nguyen QD
Received 21 August 2023
Accepted for publication 19 December 2023
Published 28 December 2023 Volume 2023:16 Pages 887—895
DOI https://doi.org/10.2147/IMCRJ.S434632
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ronald Prineas
Amir Akhavanrezayat,1 Wataru Matsumiya,1,2 Prapatsorn Ongpalakorn,1 Hashem H Ghoraba,1 Chris Or,1 Hassan Khojasteh Jafari,1 Gunay Uludag Kirimli,1 Cigdem Yasar,1 Ngoc Trong Tuong Than,1 Irmak Karaca,1 Moosa Zaidi,1 Azadeh Mobasserian,1 Negin Yavari,1 Vahid Bazojoo,1 Yong Un Shin,1 Albert John Bromeo,1 Quan Dong Nguyen1
1Spencer Center for Vision Research, Byers Eye Institute, Stanford University School of Medicine, Palo Alto, CA, USA; 2Department of Surgery, Division of Ophthalmology, Kobe University Graduate School of Medicine, Kobe, Japan
Correspondence: Quan Dong Nguyen, Spencer Center for Vision Research, Byers Eye Institute at Stanford University, 2452 Watson Court, Suite 200, Palo Alto, CA, 94303, USA, Tel +1 650 723 9386, Email [email protected]
Purpose: To report a case of neurosarcoidosis (NS) who was initially diagnosed as Coccidioidomycosis immitis (CI) infection.
Observations: A 57-year-old diabetic man presented with sudden painless diminution of vision, metamorphopsia, and color vision deficits in the left eye (OS) for one month. His vision was 20/20 in the right eye (OD) and 20/40 OS. Ophthalmic examination revealed left relative afferent pupillary defect, blurred optic nerve margin, creamy chorioretinal infiltration around the optic disc, and mild macular edema. OD examination was non-revealing. Chest CT scan with contrast showed calcified mediastinal lymph nodes, but biopsy of the lymph nodes was normal. Brain and orbit MRI demonstrated soft tissue abnormality with enhancement in left orbital apex with involvement of the extraocular muscles. CSF culture was negative, but complement fixation had positive titer of 1:2 for CI. The patient was diagnosed with CI meningitis, and antifungal therapy was initiated. Slight visual and symptomatic improvement was observed, which was not completely satisfactory. Biopsy of extraocular orbital muscle five months later revealed non-caseating granulomatous inflammation, leading to initiation of prednisone trial therapy. Nine months later, the patient was referred to a tertiary center owing to persistence of optic disc edema OS. PET CT was consistent with a diagnosis of sarcoidosis. Antifungal treatment was discontinued, and oral prednisone with methotrexate was initiated. Subsequently, methotrexate was replaced by infliximab to further manage ocular inflammation and neurologic symptoms which was effective. Vision was 20/20 OD and 20/30 OS at the most recent visit.
Conclusion and Importance: Signs and symptoms of neurosarcoidosis and coccidioidomycosis can be similar and deceiving. The index case underscores importance of considering appropriate differential diagnoses in patients with similar symptoms and signs who may respond to preliminary designated treatment but not to the optimal extent. Considering such possibility could assist clinicians in managing the patients timely and efficiently.
Keywords: neurosarcoidosis, coccidioidomycosis, sarcoidosis, ocular involvement
Introduction
Sarcoidosis is an inflammatory disorder of unknown etiology that can involve multiple systems.1 It is more common in females, with the mean age of onset typically between 33 and 41 years, but it can present in all age groups. The highest incidence of the disease is observed in African-Americans and North European Whites, with an approximate incidence of 20 new cases per 100,000 per year.2
Lung, lymph nodes, skin, liver, eyes, spleen, cardiac, and nervous systems are among the most common sites of sarcoidosis involvement.1 Nervous system involvement is termed neurosarcoidosis (NS) and can involve either central or peripheral nervous systems.3 It occurs in 5–15% of sarcoidosis cases, or even higher, according to a number of postmortem studies.4
Similar to other cases of sarcoidosis, the diagnosis of neurosarcoidosis (NS) is confirmed via biopsy. This can be challenging in some cases of NS due to inaccessibility. Therefore, the diagnosis of NS can be made with positive histological results of another organ (in systemic cases) in addition to compatible clinical, imaging, and laboratory evidence of neural system involvement.2,5 In 1999, Zajicek et al classified NS diagnosis into three categories: definite, probable, and possible NS, based on the presence or absence of histological proof, and suggested sets of criteria for each class, which have been updated over time.3,6
Magnetic resonance imaging (MRI), chest X-ray (CXR), high-resolution computed tomography (CT), and 67Ga Scintigraphy are examples of helpful imaging modalities for the diagnosis of NS.5 Laboratory results, including angiotensin-converting enzyme (ACE) serum titer, CD4/CD8 ratio from bronchoalveolar lavage (BAL) sample, and pleocytosis, level of protein, ACE, or glucose in cerebrospinal fluid (CSF) can also be useful in its diagnosis.5 Ophthalmologic examination, including slit-lamp evaluation, is recommended to be performed for the detection of uveitis and other sarcoidosis-related sequelae.5
Differential diagnoses for NS can be broad. Idiopathic Bell’s palsy or facial neuropathy associated with Lyme disease in a patient with cranial nerve dysfunction, multiple sclerosis (MS), and malignancy in a patient with parenchymal lesions, and viral, bacterial, fungal, or spirochete infection (eg, HIV, tuberculosis (TB), Coccidioidomycosis, and syphilis) in a patient with meningeal disease, can be considered as a potential differential diagnosis for the NS.7,8
Similar to systemic sarcoidosis, the first-line treatment for NS is corticosteroid therapy. However, due to a higher morbidity and mortality rate, treatment of NS should be initiated as early as possible and with a higher dose of steroids.4
We herein report a case of NS in a patient who was initially diagnosed with coccidioidomycosis infection but was found to have neurosarcoidosis afterward.
Case Report
A 57-year-old male with a history of diabetes presented to the ophthalmology clinic with chief complaints of sudden painless diminution of vision, metamorphopsia, and color vision deficits in the left eye (OS) for one month. At that time, his vision was 20/20 in the right eye (OD) and 20/40 in OS, and intraocular pressure (IOP) was 13 mmHg OU. Ophthalmic examination revealed a left relative afferent pupillary defect (RAPD), blurred optic nerve margin, creamy chorioretinal infiltration around the optic disc, and mild macular edema, with no evidence of diabetic retinopathy. Examination of the right eye was non-revealing. The patient denied any history of drug abuse, smoking, or recent travels, and other than type 2 diabetes mellitus with glycated hemoglobin of 7.2%, his past medical history was not contributory.
All laboratory tests including complete blood count (CBC), C-reactive protein (CRP), anti-nuclear antibody (ANA), rheumatoid factor (RF), angiotensin-converting enzyme (ACE), viral hepatitis titers, lysozyme, Histoplasma antibody, Syphilis, HLA-B27, HIV, Bartonella serology, anti-neutrophil cytoplasmic antibody (ANCA), QuantiFERON, and thyroid-stimulating hormone (TSH) were normal except for an erythrocyte sedimentation rate (ESR) of 28 mm/hr. CXR was normal; however, calcified mediastinal lymph nodes were detected on a chest CT with contrast, and their endobronchial ultrasound biopsy results were normal for histology and culture. MRI of the brain and orbit with Gadolinium enhancement displayed soft tissue abnormality with enhancement in the left orbital apex with involvement of the extraocular muscles, anterior aspect of Meckel’s cave, and lateral cavernous sinus. It also showed dural-based lesions along the greater wing of the right sphenoid and inferior frontal convexity with underlying cortical erosion and enhancement of the calvarium and subtle leptomeningeal enhancement along the ventral surface of the pons (Figure 1).
Additionally, lumbar puncture showed normal opening pressure (11 cm H2O), and CSF analysis demonstrated a white blood cell (WBC) count of 45 cells/µL (88% lymphocyte), protein of 174 mg/dL, and glucose of 59 mg/dL. Complement fixation (CF) test for Coccidioides immitis was positive (titer of 1:2); however, the culture was negative. Flow cytometry excluded lymphoma. According to these findings and the patient’s previously vague history of flu-like symptoms, headaches, intermittent nausea/vomiting, and neck pain (2–3 months before), a tentative diagnosis of Coccidioidomycosis meningitis was made. Fluconazole (800 mg) once daily was commenced. However, due to its adverse effects of intractable nausea and vomiting, it was later switched to posaconazole 100 mg three times daily.
Three months later, the patient stated a mild improvement in his vision, which was in line with the change in his BCVA (20/25 OS). He also tested negative for Coccidioides immitis antibodies in CSF and blood. A repeat orbital and head MRI scan also displayed stable enhancement of the left orbit cavernous sinus, both optic nerves and meninges.
Although the follow-up testing was negative, the patient was maintained on a lower dose of posaconazole (100 mg twice daily) according to the expert advice of three Infectious Disease specialists who believed Coccidioides immitis meningitis was the most probable diagnosis for the patient based on the existing evidence.
Five months later, the patient presented with acute onset of anterior uveitis (3+ anterior chamber cells) in OD. In addition to increasing the posaconazole to the previous dosage (300 mg), he underwent an extraocular orbital muscle biopsy for further investigation. The biopsy revealed non-caseating granulomatous inflammation with negative polymerase chain reaction (PCR) results for Coccidioides immitis. To manage possible concurrent inflammatory etiology, his rheumatologist then attempted a trial of 60 mg of oral prednisone daily. New treatment improved his fundoscopic findings, including regression of the previously noted creamy choroidal infiltrate and resolution of optic nerve edema and subretinal fluid. However, his vision and visual field remain unchanged.
Seven months later, a neuro-ophthalmologist noted significant active left optic disc edema, which had worsened due to poor compliance with his corticosteroid therapy. According to a diagnosis of presumed sarcoidosis flare-up, the patient was restarted on 20 mg prednisone daily and was referred to the Uveitis Clinic for further management.
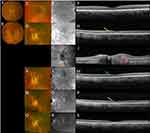
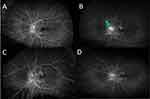
At that time, his vision was 20/40 OD and 20/60 OS, and IOP was 9 and 10 mmHg OD and OS, respectively. Ophthalmic examination revealed disc swelling with a blurred optic nerve margin, peripapillary chorioretinal atrophy, and 1+ cell in anterior vitreous OS and unremarkable findings OD (Figure 2). Although the spectral-domain optical coherence tomography (SD-OCT) image was normal OD (Figure 2), preserved foveal contour with intraretinal edema and ellipsoid zone disruption in the macular region were detected in OS (Figure 2). Moreover, SD-OCT of the left eye optic nerve showed elevated disc and subretinal fluid at the temporal side (Figure 2). Wide-angle fundus fluorescein angiography (FA) at the late phase showed moderate optic disc hyperfluorescence and window defect on the lesion with chorioretinal atrophy OS (Figure 3). A follow-up MRI displayed stable soft tissue abnormalities. As the constellations of findings were consistent with the diagnosis of NS, prednisone 20 mg was restarted for the patient, leading to the resolution of his ocular symptoms in OS. At the time, chest CT showed nonspecific solid and ground glass pulmonary nodules. Positron emission tomography (PET) CT demonstrated multiple fluorodeoxyglucose (FDG) avid lymph nodes above and below the diaphragm, consistent with a diagnosis of sarcoidosis (Figure 1). Multiple follow-up CSF testing showed no evidence of coccidioidomycosis, which made it unlikely for the patient to have active coccidioidomycosis meningitis, further supporting NS as our primary diagnosis. At that point, according to the highly probable diagnosis of NS, the Infectious Disease specialist discontinued posaconazole.
Two months later, his vision was 20/30 and 20/60 in OD and OS, respectively. Fundus photo showed milder optic disc edema and SD-OCT manifested improvement, such as decreased size and echogenicity of intraretinal fluid OS (Figure 2). Likewise, FA showed improvement in the optic disc inflammation as reduced hyperfluorescence (Figure 3). Subsequently, prednisone was tapered to 10 mg daily, and methotrexate at 20 mg weekly was initiated with folic acid supplementation. Follow-up MRI showed improvement in soft tissue thickening and enhancement within the intraconal soft tissues near the orbital apex as well as the peripheral enhancement of the proximal intraorbital segment of the optic nerve sheath complex (Figure 1). However, three months later, methotrexate was replaced with monthly infliximab infusion (7.5 mg/kg for 4 months) to manage the worsening of his neurologic symptoms, such as severe headaches as well as ocular inflammation OU (+1 anterior chamber flare and cells) (Figure 2). In the meanwhile, the patient underwent bilateral cataract surgery as well. During the most recent visit, the patient indicated an improvement in blurriness, light sensitivity, and eye pain OU, which was in line with the resolution of ocular inflammation (no anterior chamber flare and cells) and BCVA of 20/20 and 20/30 in OD and OS, respectively (Figure 2).
Discussion
Herein, we have presented a case of a 57-year-old Caucasian male with NS and evidence of fungal meningitis. Initially, the patient was diagnosed and treated for coccidioidomycosis due to positive CSF results. However, subsequent evaluations, including repeated CSF evaluations, were negative following two months of antifungal therapy. Given this observation, non-caseating granulomas on histopathology, and the patient’s positive clinical response to corticosteroids, a clinical diagnosis of NS was favored in our case.
Coccidioides immitis is a fungus that is commonly found in the Southwest region of the United States.9 Both coccidioidomycosis and sarcoidosis are granulomatous diseases. Although the etiology of coccidioidomycosis (fungal infection) and sarcoidosis (unknown/autoimmune) is different, sharing similar clinical presentations makes it quite challenging to discriminate between these two entities as alternative differential diagnoses.7,10
The diagnosis of NS usually requires a combination of neuroimaging, serologic and CSF data, and tissue biopsy.11,12 Specifically, PET-CT scans play an important role in the diagnosis of NS.13 Imaging with FDG-PET is useful in collecting information with regard to pulmonary and extrapulmonary sarcoidosis due to its high sensitivity (between the range of 78–100% depending on the involved organ).13–17 In our case, PET-CT showed multiple FDG avid lymph nodes above and below the diaphragm, which are characteristic of active sarcoidosis. Akaike et al have noted the importance of using PET-CT in diagnosing and managing sarcoidosis. However, due to the high cost of the process, they only recommended it for the following occasions: assessing reversible granuloma, treatment response, disease extent, occult disease, and suitable biopsy sites.17 In addition to PET-CT, previous literature has noted enhancement of basilar meninges and optic nerve findings in MRI of NS cases,11 which was also present in our patients.
The primary goal of treatment in NS patients is to prevent or decrease the progression of granulomatous inflammation to the other organs.18 Glucocorticoids are the first therapeutic option in most cases; however, a significant percentage of patients (29%) may not respond well to the treatment.19,20 Pulse-dose IV methylprednisolone, 1 g daily for 3–5 days, followed by a prolonged oral glucocorticoid taper, has been suggested in the literature for management of moderate to severe NS.21–23 To date, our patient’s response to the glucocorticoids and infliximab has been satisfactory and promising.
In addition to NS, optic disc swelling can develop in patients with coccidioidal meningitis. Similar to our case with optic disc involvement, Garoon et al have reported a patient who presented with headaches and intermittent blurred vision. Ophthalmic examination revealed bilateral optic disc swelling and macular hard exudates without vitreous inflammation.24 Non-contrast CT of the brain showed no compressive lesions. Following a positive coccidioidal titer in the CSF, fluconazole 400 mg daily was prescribed. Three months later, the symptoms improved.
Our case had positive Coccidioides immitis complement fixation (CF) result in his CSF analysis with a titer of 1:2. After two months of antifungal treatment, the follow-up antibody (Ab) titer was negative. The seroconversion could be attributed to the patient’s recovery or the initial false positivity of the test. There are several case reports that showed the persistence of coccidioidal titer despite antifungal treatment.10,25 However, one case report showed a negative serology test after treatment.10
The positive titer of 1:2 for IgM and IgG test of CSF is often interpreted as a high likelihood of false positivity.26 Although complement fixation (CF) test of CSF is more accurate and reliable than Ab tests with a similar titer of 1:2 and the clinical presentations of the index case were partially in support of initial coccidioidomycosis infection, after a comprehensive review and reevaluation of the patient’s records, negative CSF culture, repeated negative Ab tests, and disease trajectory, we strongly believe that most of the findings can also be attributed to sarcoidosis and false positivity of the CF test.26 However, the evidence was not completely clear at the time of management, which convinced three Infectious Disease specialists to agree on the diagnosis of coccidioidomycosis for the patient and kept him on antifungal treatment for 18 months. According to the revised criteria of International Workshop on Ocular Sarcoidosis (IWOS) for the diagnosis of ocular sarcoidosis, based on our patient’s histology specimen result and compatible finding of uveitis, he can be considered as a definite ocular sarcoidosis case.27
There are studies that hypothesized the relationship between fungal infection and sarcoidosis, which causes granulomatous inflammation.10,25 In assessing the pathophysiology of both diseases, CD4 seems to be the crucial common factor between the two entities.28,29 Although a reduced level of CD4 predisposes patients to contract coccidioidomycosis, the situation for sarcoidosis is enigmatic.28,29 Nevertheless, the high CD4/CD8 ratio of BAL specimen is one of the main characteristics of sarcoidosis. A recent study showed that the level of peripheral CD4 decreased, probably due to their involvement in the granulomatous areas.30 Therefore, the unbalanced regulation of CD4, which can occur in patients who contract coccidioidomycosis, can be a potential trigger for developing sarcoidosis in susceptible patients.
We have found 4 cases with an ultimate diagnosis of sarcoidosis who had prior coccidioidomycosis infection (Table 1).10,25,31–34 One case was a 34-year-old African American male with fever, pain, and elbow swelling with a positive culture of elbow aspiration and CF serology (1:8). The patient had been taking antifungal therapy; however, three years later, his situation deteriorated, and lung biopsy confirmed the diagnosis of sarcoidosis.10 In another report, a 29-year-old black male was diagnosed with coccidioidomycosis based on the culture results from lymph node and right tibia biopsy and CF serology (1:128). Similarly, three years later, a biopsy of his lymph nodes was in support of sarcoidosis.32 Two other reports had a similar trajectory for the development of sarcoidosis in coccidioidomycosis-positive patients with the interval of one and five years between the first and second diagnosis.25,31 Interestingly, all cases were male and lived in California.
 |
Table 1 Studies That Diagnosed Both Sarcoidosis and Coccidioidomycosis in Their Cases at Different Time Points |
Having a CF positive test in a patient who lives in California, which is endemic for coccidioidomycosis, should bring the antifungal drugs to the top of the therapeutic list.35 However, we would like to underscore the importance of achieved response. If following the course of antifungal treatment, the patient’s response is not significant or is suboptimal, sarcoidosis as a probable alternative diagnosis should be considered, despite the evidence of coccidioides infection. Our patient was on antifungal therapy for 18 months and had a mild extent of improvement; however, the rate and level of improvement were not comparable to when the NS treatment plan was commenced. It should also be noted that posaconazole has a robust anti-inflammatory feature, which may justify the slight response we observed in our patient when he was on antifungal treatment.36
In summary, we believe the presentation and trajectory of the disease course in the index patient could magnify the importance of considering these two entities (coccidioidomycosis and sarcoidosis) as differential diagnoses in similar cases. Subsequently, it will assist clinicians in designating the most efficient therapeutic plan in a timely manner.
Patient Consent and Institutional Approval
A written consent form of publishing case details was acquired from the patient. The study imaging and data gathering procedures were approved by the Institutional Review Board (IRB) (or Ethics Committee) of Stanford University (protocol code IRB-41266). However, IRB approval was not required for the publication of this case report.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This project was funded by an unrestricted grant from Research to Prevent Blindness, and the National Eye Institute P30-EY02687.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bathla G, Freeman CW, Moritani T, et al. Retrospective, dual-centre review of imaging findings in neurosarcoidosis at presentation: prevalence and imaging sub-types. Clin Radiol. 2020;75(10):796.e791–796.e799. doi:10.1016/j.crad.2020.05.008
2. Smith JK, Matheus MG, Castillo M. Imaging manifestations of neurosarcoidosis. AJR Am J Roentgenol. 2004;182(2):289–295. doi:10.2214/ajr.182.2.1820289
3. Stern BJ, Royal W 3rd, Gelfand JM, et al. Definition and Consensus Diagnostic Criteria for Neurosarcoidosis: from the Neurosarcoidosis Consortium Consensus Group. JAMA Neurol. 2018;75(12):1546–1553. doi:10.1001/jamaneurol.2018.2295
4. Hoitsma E, Faber CG, Drent M, Sharma OP. Neurosarcoidosis: a clinical dilemma. Lancet Neurol. 2004;3(7):397–407. doi:10.1016/S1474-4422(04)00805-1
5. Lacomis D. Neurosarcoidosis. Curr Neuropharmacol. 2011;9(3):429–436. doi:10.2174/157015911796557975
6. Zajicek JP, Scolding NJ, Foster O, et al. Central nervous system sarcoidosis--diagnosis and management. Qjm. 1999;92(2):103–117. doi:10.1093/qjmed/92.2.103
7. Jamilloux Y, Néel A, Lecouffe-Desprets M, et al. Progressive multifocal leukoencephalopathy in patients with sarcoidosis. Neurology. 2014;82(15):1307–1313. doi:10.1212/WNL.0000000000000318
8. Nguyen JT, Green A, Wilson MR, DeRisi JL, Gundling K. Neurologic Complications of Common Variable Immunodeficiency. J Clin Immunol. 2016;36(8):793–800. doi:10.1007/s10875-016-0336-8
9. Johnson RH, Sharma R, Kuran R, Fong I, Heidari A. Coccidioidomycosis: a review. J Investig Med. 2021;69(2):316–323. doi:10.1136/jim-2020-001655
10. Kuberski T, Yourison I. Coccidioidomycosis A Cause of Sarcoidosis. Open Forum Infect Dis. 2017;4(3):ofw117. doi:10.1093/ofid/ofw117
11. Baldwin KJ, Zunt JR. Evaluation and treatment of chronic meningitis. Neurohospitalist. 2014;4(4):185–195. doi:10.1177/1941874414528940
12. Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160(2):736–755. doi:10.1164/ajrccm.160.2.ats4-99
13. Vettiyil B, Gupta N, Kumar R. Positron emission tomography imaging in sarcoidosis. World J Nucl Med. 2013;12(3):82–86. doi:10.4103/1450-1147.136731
14. Ohira H, Tsujino I, Ishimaru S, et al. Myocardial imaging with 18F-fluoro-2-deoxyglucose positron emission tomography and magnetic resonance imaging in sarcoidosis. Eur J Nucl Med Mol Imaging. 2008;35(5):933–941. doi:10.1007/s00259-007-0650-8
15. Kim HR, Lee KH, Park SJ, et al. Anti-cancer activity and mechanistic features of a NK cell activating molecule. Cancer Immunol Immunother. 2009;58(10):1691–1700. doi:10.1007/s00262-009-0680-0
16. Wang Y, Andrews J, Jenkins Colon P, Wundes A. FDG-PET abnormalities leading to the diagnosis of an unusual case of probable neurosarcoidosis. Neurol Neuroimmunol Neuroinflamm. 2018;5(6):e506. doi:10.1212/NXI.0000000000000506
17. Akaike G, Itani M, Shah H, et al. PET/CT in the Diagnosis and Workup of Sarcoidosis: focus on Atypical Manifestations. Radiographics. 2018;38(5):1536–1549. doi:10.1148/rg.2018180053
18. Bradshaw MJ, Pawate S, Koth LL, Cho TA, Gelfand JM. Neurosarcoidosis: pathophysiology, diagnosis, and treatment. Neurology Neuroimmunology Neuroinflammation. 2021;8(6). doi:10.1212/NXI.0000000000001084
19. Judson MA. Corticosteroids in sarcoidosis. Rheumatic Dis Clin. 2016;42(1):119–135. doi:10.1016/j.rdc.2015.08.012
20. Fritz D, van de Beek D, Brouwer MC. Clinical features, treatment and outcome in neurosarcoidosis: systematic review and meta-analysis. BMC Neurol. 2016;16(1):220. doi:10.1186/s12883-016-0741-x
21. Anthony J, Esper GJ, Ioachimescu A. Hypothalamic–pituitary sarcoidosis with vision loss and hypopituitarism: case series and literature review. Pituitary. 2016;19(1):19–29. doi:10.1007/s11102-015-0678-x
22. Hebel R, Dubaniewicz-Wybieralska M, Dubaniewicz A. Overview of neurosarcoidosis: recent advances. J Neurol. 2015;262(2):258–267. doi:10.1007/s00415-014-7482-9
23. West SG. Current management of sarcoidosis I: pulmonary, cardiac, and neurologic manifestations. Curr Opinion Rheumatol. 2018;30(3):243–248. doi:10.1097/BOR.0000000000000489
24. Garoon RB, Foroozan R, Vaphiades MS. Don’t drink in The Valley. Surv Ophthalmol. 2017;62(3):383–386. doi:10.1016/j.survophthal.2016.04.002
25. Sharma OP, Arora A. Coccidioidomycosis and sarcoidosis. Multiple recurrences. West J Med. 1997;166(5):345–347.
26. Crum NF, Lederman ER, Stafford CM, Parrish JS, Wallace MR. Coccidioidomycosis: a Descriptive Survey of a Reemerging Disease. Clinical Characteristics and Current Controversies. Medicine. 2004;83(3):149–175. doi:10.1097/01.md.0000126762.91040.fd
27. Mochizuki M, Smith JR, Takase H, Kaburaki T, Acharya NR, Rao NA. Revised criteria of International Workshop on Ocular Sarcoidosis (IWOS) for the diagnosis of ocular sarcoidosis. Br J Ophthalmol. 2019;103(10):1418–1422. doi:10.1136/bjophthalmol-2018-313356
28. Van Dyke MCC, Thompson GR, Galgiani JN, Barker BM. The Rise of Coccidioides: forces Against the Dust Devil Unleashed. Front Immunol. 2019;10:2188. doi:10.3389/fimmu.2019.02188
29. Grunewald J, Eklund A. Role of CD4+ T cells in sarcoidosis. Proc Am Thorac Soc. 2007;4(5):461–464. doi:10.1513/pats.200606-130MS
30. Baughman RP, Hurtubise PE. Systemic immune response of patients with active pulmonary sarcoidosis. Clin Exp Immunol. 1985;61(3):535–541.
31. Bacharach T, Zalis EG. Sarcoid Syndrome Associated With Coccidioidomycosis. Am Rev Respir Dis. 1963;88:248–251. doi:10.1164/arrd.1963.88.2.248
32. Ellis FW. Coexistent arrested disseminated coccidioidomycosis and Boeck’s sarcoid. Calif Med. 1955;82(5):400–404.
33. Lord G. Pulmonary sarcoidosis complicated by cryptococcosis and coccidioidomycosis. The changing spectrum of fungus disease in Maine. J Maine Med Assoc. 1974;65(10):236–240.
34. Lipschultz BM, Liston HE. Steroid induced disseminated coccidioidomycosis: report of two cases. Dis Chest. 1964;46(3):355–359. doi:10.1378/chest.46.3.355
35. Shewry S Epidemiologic Summary Of Valley Fever (Coccidioidomycosis) In California; 2019. Available form: https://www.cdph.ca.gov/Programs/CID/DCDC/CDPH%20Document%20Library/CocciEpiSummary2019.pdf.
36. Yang L, Yang M, Li S, Zhao Z. S-allylmercaptocysteine attenuates posaconazole-induced adverse effects in mice through antioxidation and anti-inflammation. Int Immunopharmacol. 2018;58:9–14. doi:10.1016/j.intimp.2018.03.006
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.