Back to Journals » Clinical Ophthalmology » Volume 17
Neuroprotection for Nonarteritic Central Retinal Artery Occlusion: Lessons from Acute Ischemic Stroke
Authors Okonkwo ON , Agweye CT , Akanbi T
Received 3 January 2023
Accepted for publication 19 May 2023
Published 31 May 2023 Volume 2023:17 Pages 1531—1543
DOI https://doi.org/10.2147/OPTH.S403433
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Ogugua Ndubuisi Okonkwo,1 Chineze Thelma Agweye,2 Toyin Akanbi1
1Department of Ophthalmology, Eye Foundation Hospital and Eye Foundation Retina Institute, Ikeja, Lagos, Nigeria; 2Department of Ophthalmology, University of Calabar and University of Calabar Teaching Hospital, Cross River, Nigeria
Correspondence: Ogugua Ndubuisi Okonkwo, Eye Foundation Retina Institute, 27, Isaac John Street, GRA, Ikeja, Lagos, Nigeria, Tel +234 803 502 7308, Email [email protected]
Abstract: Nonarteritic central retinal artery occlusion (NA-CRAO) is a variant of acute ischemic stroke (AIS) and is a cause of sudden severe loss of vision. There are guidelines by the American Heart Association and the American Stroke Association for the care of CRAO patients. This review explores the basis of retinal neuroprotection for CRAO and its potential for improving the outcome of NA-CRAO. Recently, there have been significant advances in research into the use of neuroprotection to treat retinal diseases, including retinal detachment, age-related macular degeneration, and inherited retinal diseases. Also, neuroprotective research in AIS has been extensive, and newer drugs tested, including Uric acid, Nerinetide, and Otaplimastat, with promising results. Progress in cerebral neuroprotection after AIS offers hope for retinal neuroprotection after CRAO; and a possibility of extrapolating research findings from AIS into CRAO. Combining neuroprotection and thrombolysis can extend the therapeutic window for NA-CRAO treatment and potentially improve outcomes. Experimented neuroprotection for CRAO includes Angiopoietin (Comp Ang1), KUS 121, Gene therapy (XIAP), and hypothermia. Efforts in the field of neuroprotection for NA-CRAO should focus on better imaging to delineate the penumbra after an acute episode of NA-CRAO (using a combination of high-definition optical coherence angiography and electrophysiology). Also, research should explore details of pathophysiologic mechanisms involved in NA-CRAO, allowing for further neuroprotective intervention, and closing the gap between preclinical and clinical neuroprotection.
Keywords: neuroprotection, non-arteritic central retinal artery occlusion, thrombolysis, acute ischemic stroke
Introduction
In recent years, significant resources have been invested in the research into treating retinovascular diseases, including diabetic retinopathy (DR), retinal vein occlusion (RVO), and macular diseases including age-related macular degeneration (AMD). This effort has led to remarkably improved outcomes for patients suffering from these diseases. This experience has not been replicated for retinal artery occlusion (RAO). The reason for this is multifactorial and includes fewer RAO patients relative to the other retinovascular diseases and the fact that several central retinal artery occlusion (CRAO) patients present for treatment after the theoretical time limit for retinal neuron survival.1–3 Unlike DR, RVO, and AMD, treatment for CRAO must be instituted within a specified time when neurons are still salvageable.3
CRAO is an ocular variant of acute ischemic stroke (AIS); they share similar predispositions and pathogenetic mechanisms.4 CRAO eyes have been classified into four categories based on etiology, permanency of occlusion of the central retinal artery and preexisting collateral supply (cilioretinal artery). The four known types of CRAO are non-arteritic (NA) CRAO, NA-CRAO with cilioretinal artery sparing, transient NA-CRAO, and arteritic CRAO (occurring secondary to giant cell arteritis-GCA). Hayreh has shown that the natural history of these four types of CRAO defers significantly in terms of spontaneous improvements in vision and visual fields after an acute episode. Upto 82% of transient NA-CRAO and 67% of NA-CRAO with cilioretinal sparing will show spontaneous improvements in vision.5 The NA-CRAO variant is more commonly encountered in patients.3 The pathogenesis of NA-CRAO is thromboembolic. Emboli may occur at the narrowest portion of the central retinal artery (CRA), which is the point at which it penetrates the dura,6 or thrombi may form within the region of the lamina cribrosa, usually posterior to it.7,8 Vascular occlusion in CRAO can be partial or complete. CRAO can also be permanent or transient, depending on the nature and mobility of the vessel occluding emboli.8 Patients who suffer transient CRAO are at increased risk of more permanent NA-CRAO and other ischemic diseases and should be evaluated carefully. In a more significant proportion of patients, when CRAO occurs, it can cause profound and irreversible loss of vision. The reported visual outcome of CRAO using the so-called conservative treatment in most cases is similar to or worse than natural history and unsatisfactory.9 Seventeen percent of CRAO patients regain functional vision. In 80% of patients, vision is counting fingers or worse, while in about 50% of patients, only a tiny peripheral island of vision remains.9–11 Neovascularization of the iris may occur in up to 18.2% of cases.12 Treatment principles for CRAO are similar to that for AIS and include 1. Acute reperfusion of the CRA, 2. Prevention of ocular complications, and 3. Vascular review to prevent further end-organ ischemia.
There are guidelines by the American Heart Association (AHA) and the American Stroke Association (ASA) which guide the management of patients with acute stroke.13 Also, the Retinal and Ophthalmic Artery Occlusions Preferred Practice Pattern® of the American Academy of Ophthalmology (AAO) gives detailed instruction on the holistic care of a patient who suffers a CRAO.14 In addition, local intra-arterial thrombolysis is used by several departments worldwide for the treatment of NA-CRAO. This practice continues despite the finding that intra-arterial thrombolysis was similar in efficacy to conservative therapy for treating NA-CRAO and resulted in a higher rate of adverse events, in the EAGLE study.15 However, significant criticisms and short comings have trailed the EAGLE study design, including that the average time from symptom onset to intra-arterial therapy was >12 hours in the treatment group. In addition, none of the patients received intra-arterial therapy within 4.5 hours of symptom onset.15
This review explores neuroprotection as an additional strategy alongside already-known strategies for the care of NA-CRAO. The role of neuroprotection is often overlooked in ocular stroke. Instead, revascularization techniques are given the spotlight. This manuscript seeks to redirect our attention to neuroprotection which is an important element of ocular stroke management. The need for a different approach arises from the unfortunate fact that outcomes after CRAO over the past three decades have remained the same. `The lack of randomized controlled studies providing guidance or recommendations for treatment makes the situation worse. To fulfil the need for improved outcomes after NA-CRAO, it should be mentioned that there are two ongoing clinical trials investigating the use of intravenous alteplase. These are a Phase 2 trial (Early Reperfusion Therapy With Intravenous Alteplase for Recovery of VISION in Acute Central Retinal Artery Occlusion (REVISION),) [NCT04965038],16 and a Phase 3 trial (Multicenter Study Assessing the Efficacy and Safety of IV THrombolysis (Alteplase) in Patients With acutE Central retInal Artery Occlusion (THEIA)) [NCT03197194].17 These two studies investigate the role of treatment < 4.5 hours after symptom onset in NA-CRAO patients. Similarly, there is a phase 3 study, TENecteplase in Central Retinal Artery Occlusion Study (TenCRAOS) [NCT04526951],18 investigating the role of intravenous Tenecteplase for CRAO. These studies hopefully will provide some information and fill the knowledge gap that currently exist on the use of intravenous thrombolysis for treating NA-CRAO.
In several cases of CRAO, reperfusion of the retina does occur without significant improvement in vision.19 The lack of improvement in vision in CRAO is multifactorial, as elucidated by Jusufovic et al, and a significant contribution is due to the issue of retinal survival time (RST).1,13 Real-world experience suggests that a substantial proportion of CRAO patients eventually seek care after exceeding the RST.
Retinal Survival Time (RST)
Retinal survival time is the time from retinal ischemic insult to the irreversible death of retinal neurons. RST offers a “Therapeutic Window” for the treatment of CRAO. Treatment instituted within RST stands the best chance of a favorable outcome and could significantly improve vision since the retinal neurons are still viable within this time frame. Hayreh et al, from laboratory experiments, proposed an RST of 240 mins (4h).20,21 The four-hour RST has been accepted widely. However, controversies surround Hayreh’s work. Some observed weaknesses of Hayreh’s study questions extrapolation of the study findings to all humans:22
1. The experiments were performed in old atherosclerotic hypertensive “Rhesus monkeys”; therefore, the findings may not apply to younger humans.
2. Various neuroprotective agents used in the experiments were not controlled, such as hypothermia and barbiturates anesthesia, which have been demonstrated to have significant neuroprotective effects in experimental models.
3. Collateral circulation occurring distal to the point of CRA clamp in the monkeys may have played a significant role in retinal perfusion.
We suggest a variable RST for humans. RST may vary significantly from person to person, therefore offering a variable therapeutic window. To buttress the case for a variable RST, it will be appropriate to cite a study investigating intravenous (IV) tissue plasminogen activator (tPA) use for treating CRAO. Study subgroup analysis showed that only those patients who received IV tPA within six hours of symptom onset improved >3 lines. Though a single author’s experience, this study suggests that the maximum retinal tolerance time for effective reperfusion therapy could be up to 6 hours after CRAO in some individuals.23 Furthermore, the preclinical stroke model has shown that in AIS, the neurovascular unit’s response after stroke could be affected by some variables, including age, sex, and comorbidity. For example, the response by an elderly severely hypertensive stroke patient may differ from a younger person with less morbidity.24 Therefore, these variables could significantly alter the RST for an individual, and this effect varies from person to person.
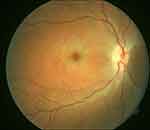
In a clinical context, retinal survival and, by extension, treatment outcome of CRAO could also depend on the degree of occlusion. Retinal survival depends on if the retinal artery occlusion is complete (retinal infarction could occur in as short a time as within 15mins) or partial (would have a more prolonged retinal survival because of residual perfusion and effect of collateral circulation).22 Because of uncertainty regarding the completeness and duration of the retinal artery occlusion, CRAO patients are usually offered treatment even if the timing from the onset of occlusion to presentation is deemed to be several hours. Patients with incomplete occlusion are more likely to have a favorable outcome than eyes with complete occlusion. The clinical dilemma regarding the uncertainty of the completeness of occlusion may explain the unpredictability of response to the treatment of CRAO. Figure 1 presents a probably less severe and incomplete CRAO with seemingly less macular infarction; Figure 2 shows a more severely infarcted retina with significant macular edema and pallor, suggesting a subtotal CRAO. Figure 3 is the fluorescein angiography of Figure 2. Paradoxically visual treatment outcome was better in the patient represented by Figure 2 with a more infarcted retina, supporting a multifactorial effect on treatment outcome.
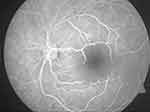 |
Figure 3 Fluorescein angiography of Figure 2, showing significant non perfusion in the foveomacula area. |
Acute Ischemic Stroke (AIS)
Neuroprotection in stroke has been researched extensively for over three decades. Courtesy of the efforts of several researchers whose work spans several decades, we have gained significant understanding and relevant knowledge regarding AIS’s pathophysiologic mechanisms and treatment principles. There are several detailed guidelines on the care of a stroke patient. Reviewing neuroprotection for AIS warrants a definition of neuroprotection for AIS and understanding the concept of the “ischemic penumbra”, a fundamental principle and the basis for neuroprotection.25,26 It is essential to highlight recanalization and reperfusion after the vaso-occlusive event and give an overview of the molecular basis of neuronal death after AIS. This article will provide a brief introduction to each of these concepts.
Neuroprotection for AIS
Neuroprotection’ is a terminology used to describe the effect of interventions to protect the brain from pathological damage and could be pharmacologic or non-pharmacologic.27,28 The basic concept of neuroprotection is that a drug or induced state inhibits pathophysiological events during ischemia when a thromboembolic process occludes the main arterial supply. Research into cerebral neuroprotection after AIS will benefit CRAO since it is an analog and shares similar pathogenetic mechanisms. Therefore, researchers have reasoned that one can extrapolate aspects of neuroprotective research in AIS into CRAO. An example of this is that preclinical experience has shown that neuroprotective therapy for AIS is more effective in reversible ischemic stroke models than permanent occlusion. In both AIS and CRAO, reperfusion must be established before neuroprotection is effective. Therefore, a combination of neuroprotection with reperfusion provides an ideal strategy.
Concept of the Penumbra
The penumbra is the area of hypoperfusion surrounding the area affected directly by an ischemic event in a thrombotic or embolic stroke.26,29,30 During a cerebral vessel occlusion, there is a core area where damage to the brain is irreversible and an area of penumbra where the brain has lost function owing to decreased blood flow but is not irreversibly injured. After the cerebral ischemic event, blood flow and oxygen transport reduce. There is hypoxia of the adjacent cells close to the area of the original insult. With time, hypoxic cell death (infarction) occurs, magnifying the initial damage from the ischemia. Penumbra has been described in CRAO, and areas of anoxic, hypoxic, and normoxic retina are known to occur.31 The penumbra region may remain viable for several hours after an ischemic event due to the collateral arteries that supply the penumbral zone.32–34 Therefore, a penumbra implies salvage of cells is possible, and this is the goal of neuroprotection in the early hours of AIS. Researchers have demonstrated a high correlation between the extent of spontaneous neurological recovery after AIS and the volume of ischemic penumbra that escapes infarction. Saving the penumbra should improve the clinical outcome of AIS. To conclude, Fisher rightly summarized the issues relating to the usefulness of the concept of the ischemic penumbra region and its relationship with neuroprotection into three questions. Firstly, how can this ischemic region be most accurately identified in stroke patients? Secondly, what mechanisms of ischemic cell death are most important for progressing from penumbra toward irreversible injury? Lastly, what therapeutic modalities are most likely to impede the development of infarction?30
Current Research and Techniques in the Study of Ischemic Penumbra
Treating NA CRAO will benefit from imaging modalities that differentiate severely infarcted retina from the viable retina. Such imaging will also benefit the testing of future neuroprotective treatments and research in this area. Therefore, there is a growing need for proper imaging of the ischemic penumbra after acute retina vaso occlusion. Like neuroimaging after AIS, defining by imaging the ischemic penumbra after an acute episode of CRAO provides hope of personalizing treatment and predicting the beneficial response to therapy. Furthermore, in AIS patients, tissue viability-based selection for treatment is safe, effective, and superior to time-based patient selection.35 It allows for treating patients > sixteen hours after the acute episode with good outcomes, making a case for better retina imaging after acute CRAO that can guide treatment and prognosticate the outcome of such treatment.
Fundus photography, fluorescein angiography (FA), and optical coherence tomography (OCT) have been the conventional methods for imaging vaso occlusion in NA CRAO. However, newer methods that assess and image the structural and functional damage in CRAO have been researched and developed.35 These newer methods hold promise for accurately determining infarcted and healthy retina, providing objective viewing of the ischemic penumbra area. Nowadays, the researched structural imaging techniques include optical coherence tomography angiography (OCTA),36–38 retinal function imager (RFI),39 and photoacoustic microscopy (PAM).40,41 RFI and PAM can image both structure and function after CRAO. Laser speckle contrast analysis was experimented in CRAO with promising results in evaluating blood flow after endovascular administration of tissue plasminogen activator.42,43
OCTA uses motion contrast provided by the moving erythrocytes within the retinal blood vessels to detect flow. It gives images of the superficial and deep capillary plexi and the foveal avascular zone. FA cannot usually image the deep capillary plexus. OCTA is dye less, noninvasive, and reproducible. It, however, is limited by the small size of the retina area scanned and requires proper fixation, lacking in several CRAO patients, for scan acquisition. High-resolution images of more expansive areas of the retina will be possible with faster scans that will be commercially available soon. Software technology can remove artifacts that are a significant challenge of OCTA.
The RFI, like the OCTA, offers noninvasive, high-resolution imaging of retinal microvasculature by creating capillary perfusion maps. It measures retinal blood velocity directly and performs functional imaging with retinal blood oximetry. Lengthy image acquisition times are a significant challenge to its widespread adoption and use.39
Photoacoustic microscopy (PAM) is a hybrid in vivo imaging technique that acoustically detects optical contrast via the photoacoustic effect.40 PAM can simultaneously image anatomical, functional, molecular, flow dynamic and metabolic contrasts in vivo. Compared with backscattering-based confocal microscopy and optical coherence tomography, PAM provides absorption contrast instead of scattering contrast.
Laser speckle contrast techniques use changes in speckle pattern to visualize tissue perfusion.42 The speckle pattern is noticed as the bright and dark areas brought about by the backscattering light, which occurs when coherent laser light illuminates a diffuse object. This forms an interference pattern on the detector. Using this technology, blood flow can be imaged.
Retinal blood velocity and flow can be measured with high precision using adaptive optics scanning laser ophthalmoscopy (AOSLO).44,45 It has been used in studies comparing retinal blood flow in diabetics and controls. In vivo, retinal blood flow measurement is obtained by measuring the blood velocity of erythrocytes and lumen diameters of the blood vessels using AOSLO. Erythrocyte velocity is measured by tracking erythrocytes moving across a horizontal scanning line.
Multimodal imaging is now commonly used in imaging after CRAO. Multimodal imaging using FA, Fundus autofluorescence, OCT, OCTA, and adaptive optics was used to image a case of branch retinal artery occlusion (BRAO) in which the causative embolus was present at the inferior bifurcation of the nasal and temporal branches of the central retinal artery.46
The ischemic penumbra that occurs following partial CRAO has two components a homogenous annular penumbra in the mid-periphery and a heterogenous macular penumbra. It has been suggested that this enduring ischemic penumbral tissue can be identified using hypoxia tracers such as fluoromisonidazole.47 To conclude, with further developments in these imaging techniques, the future of CRAO will see more objective parameters for assessing and prognosticating treatment.
Recanalization and Reperfusion in AIS and CRAO
Early reperfusion therapy to restore blood flow to salvageable ischemic cerebral tissue can prevent cell death and facilitate neurological recovery. After AIS, perfusion is re-established using intravenous thrombolysis, intra-arterial thrombolysis, mechanical thrombectomy, ultrasound-enhanced thrombolysis, and a combination of these approaches.48–50 To re-establish perfusion after NA-CRAO, the following have been utilized, anterior chamber paracentesis, digital massage, use of ocular hypotensive, inhalation of carbogen, intra-arterial cannulation and thrombolysis, vitrectomy with adjunctive techniques including intra-arterial injection of thrombolytics, optic disc massage, bloodletting, and manipulation of intraocular and arterial pressure.3,8,51–53
Questions continue to be raised concerning the efficacy of the conservative treatment approach for CRAO since the visual outcome is not better than natural history and could worsen.54 The most promising CRAO treatments are reports that suggest that fibrinolysis for fibrin thrombus within the specified time frame (4.5 hours) is associated with reperfusion and improvements in vision.9,55–59 Therefore, the use of intraarterial (IA) or intravenous (IV) thrombolysis (IV-tPA) in CRAO though controversial,60 could hold some promise for a category of patients who can have treatment early and perhaps suffer from incomplete arterial occlusion.57,58 Concerns about patient safety have been raised with the use of thrombolysis for CRAO, including the reported 2% risk of intracerebral/intracranial hemorrhage (ICH) and hematuria. That reperfusion occurs spontaneously has also been demonstrated. The poor visual outcome often experienced after reperfusion can be attributed to irreversible infarction and ischemia’s complex biochemical and electrical consequences. Understanding these mechanisms is essential in providing neuroprotection for the ischemic retina.
Molecular Basis of Neuronal Death
Combinations of molecular mechanisms are known to induce eventual neuronal cell death following AIS. These mechanisms include but are not limited to calcium influx and overload, apoptosis, excitotoxicity, inflammation, oxidative stress, glutathione decrease, reperfusion Injury, hemorrhagic transformation (secondary bleeding which can be tPA induced), and autophagy.24,61 Each mechanism presents a possible target for neuroprotective intervention. Furthermore, some of these mechanisms can be biphasic.24 In other words, the mechanism has a deleterious effect in the acute state but can become a reparative mechanism afterward. An example is neuroinflammation, which is initially deleterious but later becomes a reparative mechanism.62
Neuroprotective Therapeutics and Strategies
Mechanisms for treating CRAO have focused on re-establishing retinal vascular perfusion. However, a review of stroke management shows that multiple strategies are employed to manage both the acute and after-effects of cerebral injury. The multipronged approach used in managing AIS should also be employed in CRAO patients to modify the disease outcome favorably. It is known that secondary injuries occur after the acute stroke event.62,63 The secondary injuries are due to other mechanisms that occur several hours and days after the acute injury and involve further loss of cells in the penumbra. Neuroprotection aims to rescue and preserve those viable cells in the hypoperfused area marked for delayed cell death.
Understanding the current state of research into neuroprotection for AIS and its limitations is essential to neuroprotection for CRAO. Despite several decades of preclinical and clinical studies exploring neuroprotection for stroke, no neuroprotective therapy has made it into clinical guidelines or been generally adopted. The success of preclinical trials has yet to be replicated in clinical studies for cerebral neuroprotection after stroke. One can summarize the translational failures of preclinical trials reported by Chamorro as primarily due to different forms of bias, such as selecting study participants, assessment, attrition, generalizability, and study relevance.24 There was a need for more methodological agreement between preclinical and clinical studies.64 Issues with the therapeutic window, power of the study, the certainty of recanalization, and heterogeneity of the human stroke and participating sample weakened previous studies.24,64 These known causes of translational failure in AIS are relevant considerations in CRAO too.
Some Promising Researched Pharmacotherapies for Neuroprotection After AIS
Recently, there have been randomized trials of potential neuroprotective drugs combined with reperfusion therapy. Some tested drugs with multiple mechanisms of action include Nerinetide, Uric Acid, Otaplimastat and Neu2000. The following studies ESCAPE-NA-1,65–67 URICO-ICTUS,68,69 SAFE-TPA,70 and SONIC,71 respectively investigated the safety and efficacy of the listed drugs as neuroprotectants after AIS and reported promising results. This offers hope of bed side neuroprotection for AIS soon.
Drugs That Have Been Researched for Potential Retinal Neuroprotection After CRAO
We performed a nonsystematic search using popular search engines, including PubMed, Embase, Cochrane database, and Google scholar, to discover any therapy that has been experimented with as a neuroprotectant after acute retinal ischemia in CRAO. There were no date limits to our search. We found that four therapies had been investigated as neuroprotection for CRAO, including Angiopoietin, KUS 121, Gene therapy (XIAP), and Pentoxifylline. All four showed a favorable response.
- Angiopoietin: Researchers have used Angiopoietin as a potential retinal neuroprotectant after CRAO. Ang 1 increases blood vessel stabilization and survival. It has a demonstratable neuroprotective effect, positively impacting neuronal cells. COMP-Ang1 is a more soluble and potent recombinant form of Ang1. It improves neurological deficits by promoting neuronal survival in CRAO. In a preclinical study, CRAO mice treated with it experienced better visual tracking response than controls.72 COMP- Ang1 also improved ERG responses after CRAO.
- Kyoto University Substance (KUS) 121: The Kyoto University Substance inhibits ATPase, ameliorates, and suppresses endoplasmic reticulum (ER) stress.73,74 ER stress suppression has been targeted in neuroprotection for glaucoma and other neurodegenerative diseases. In one study, intravitreal KUS was given to preclinical mice models.73 KUS was noted to suppress inner retinal thinning and retinal cell death and maintain visual function. Intravitreal KUS could be a promising novel therapeutic strategy for CRAO and possibly other ischemic retinal diseases.
- Gene therapy: Gene therapy targeting apoptosis has also been experimented with as a potential neuroprotective strategy. Death of retinal ganglion cells has been shown to be through apoptotic mechanisms. Blocking the activation of apoptotic mechanisms may be an effective strategy to prevent death of retinal ganglion cells following NA-CRAO. The X-linked inhibitor of apoptosis (XIAP) gene is a potent caspase inhibitor that has been experimented on to protect retinal ganglion cells in models of glaucoma.75 XIAP therapy was shown to provide functional and structural protection for retinal ganglion cells and axons in model of glaucoma. In situations of retinal ischemia XIAP-mediated gene therapy has been also shown to protect neuronal cells from apoptosis. Adeno Associated Virus (AAV) vector expressing XIAP was injected intravitreally into rat eyes, and after six weeks, the retina was rendered ischemic by raising the intraocular pressure.76 The expression of XIAP resulted in a positive response with a larger b-wave amplitude compared to controls. The results further demonstrated the preservation of the inner nuclear layer cells and inner retina thickness compared to control eyes. In conclusion, researchers found XIAP-mediated gene therapy to produce functional and structural retina protection after transient ischemia.
- Pentoxifylline (PTX): It is a methylxanthine derivative with an immune modulation effect, affects wound healing, and has hemorheological properties. Pentoxifylline improves hypercoagulable states by decreasing platelet aggregation and adhesion, increases plasminogen activator, plasmin, and antithrombin III, and decreases fibrinogen.77 Pentoxifylline treated ischemic rat retina had a favorable response compared to non-treated rat retina after retinal reperfusion. PTX treatment significantly reduced overall retinal thickness loss and inner retinal layer thinning. This benefit is because of the decreased up-regulated activation of NF-kappa B and the antagonistic effect on pro-inflammatory cytokines such as TNF-alpha and IL-1beta.78
Corticosteroid therapy has a significant positive effect on arteritic CRAO. Immediate aggressive treatment with high-dose systemic corticosteroid, administered either as intravenous or oral preparation, could improve vision or prevent vision loss in patients with the arteritic variant of CRAO associated with giant cell arteritis (GCA).79 Lastly, Brimonidine is an alpha-2 adrenergic agonist that may have a protective effect on the retina by inducing the release of neurotrophic substances that protect the cells in the outer retina, ie, RPE and photoreceptor cells.80,81 Animal studies have shown that Brimonidine also has a protective effect on retinal ganglion cells and may positively affect the inner layer cells and therefore be effective in ischemia affecting the retina as in CRAO.82
Non-Pharmacologic Neuroprotective Strategies for CRAO
- Hypothermia (Hypothermic Vitrectomy): Hypothermia has been widely studied and used as a neuroprotective strategy. There is evidence that moderate hypothermia can confer high-grade neuroprotection in focal and global cerebral ischemia by impeding a host of harmful metabolic and biochemical injury mechanisms. Hypothermia has been used to control malignant brain edema in stroke patients.83–85
Two landmark clinical trials, published in the New England Journal of Medicine (NEJM) in 2002, demonstrated that mild therapeutic hypothermia (within the range of 32–34 deg) instituted after cardiac arrest significantly reduces mortality and improves neurological function.86,87 These findings led to hypothermia becoming the first neuroprotective strategy recommended by the American Heart Association (AHA) in comatose patients after cardiac arrest.
While induction of systemic hypothermia can be associated with significant adverse effects such as shivering, hypotension, cardiac arrhythmias, pneumonia, and thrombocytopenia, locally induced ocular hypothermia could prevent these.88 In the eye and retina, localized hypothermia can be achieved during vitrectomy using hypothermic saline at the desired temperature; one can use the infusion fluid during vitrectomy to achieve this hypothermia if a known pre-operative temperature target is determined. We have called this “Hypothermic Vitrectomy”. Hypothermic vitrectomy will positively reduce retinal metabolism and consequent demand for oxygen and improve ischemia and reperfusion injury. Also, vitrectomy improves oxygenation within the vitreous cavity for an extended period, benefiting retinal oxygen saturation and improving retinal ischemia.89
Multiple researchers have shown that lowering the retina’s temperature to between 4–8 degrees by cooling the infusion fluid during vitrectomy increases retinal resistance and decreases retinal damage.90–94 However, research has yet to conclude how much lowering is safe for the retina. One study suggests that moderate hypothermia to 22 degrees was safe in rabbit eyes. Furthermore, induced localized retinal hypothermia has increased retinal survival time to 12 hours at 21 degrees and up to 50 hours at 4 degrees.91 The mechanism for hypothermic neuroprotection may be related to decreased energy requirements.95
Despite these reports and several others of improvements in visual outcome after HBO treatment for CRAO,101,102 Rosignoli et al reported no visual benefit for HBO treated patients and felt HBO did not enhance the final visual outcome.103
Through the modest efforts made in neuroprotective research, we know that neuroprotection combined with early reperfusion can be safe and effective for AIS and CRAO. Some researched neuroprotective agents have shown promise and require further research into efficacy. Future research into neuroprotection research should employ stringent research protocols to eliminate bias.24,104 Furthermore, research into the transition from “injury to repair” mechanisms and a better understanding of this transition process could provide even more neuroprotective modification or amplification targets. In designing clinical studies to test the effectiveness of neuroprotection for CRAO, such trials should have defined primary and secondary endpoints. The visual function should be assessed using visual acuity measurement, visual fields, and electrophysiology.105 Optical coherence tomography (OCT) and high-resolution OCT Angiography (OCTA) should be used to assess retinal structure and vasculature. The choice of clinical endpoint must reflect a shift from functional blindness to significant improvement. Candidates for such research should have a good initial vision before vision loss from the CRAO. It is crucial to prevent ineligibility due to delays and late admission after CRAO. Neuroimaging to check for cerebral ischemia and intracranial hemorrhage is warranted. Neuroprotection for CRAO could answer several patients’ unmet need for improved vision. However, efforts should focus on closing the gap between preclinical and clinical outcomes for this to happen.96
Conclusion
To conclude, immediate treatment of acute CRAO is currently focused on reperfusion of the ischemic retina. A combination of reperfusion and neuroprotection may result in better functional outcomes.106 Such a multipronged approach deserves further investigation. Retinal neuroprotection after CRAO can extend retinal survival time, extending the therapeutic window. With the increasing hope of therapeutic neuroprotection for stroke patients being a reality, intravenous thrombolysis could be combined with neuroprotection for CRAO treatment. Intervention should be offered within a pre-specified period of expected therapeutic response (4.5 hours). This therapeutic window could be longer for healthier, younger adults with less comorbidity. Neuroprotective research for CRAO should also focus on better imaging to delineate the penumbra (using high-definition optical coherence angiography, other researched imaging technologies mentioned earlier in this review and electrophysiology). Imaging studies of the retinal penumbra, as in cerebral perfusion, can be incorporated into better thrombolysis algorithms, as in the management of AIS.59 Lastly, using an intravitreal route for drug administration in CRAO, the local administration of available neuroprotective pharmacotherapy may avoid blood-brain barrier issues and problems encountered with drug delivery to the target site experienced in AIS patients.
Acknowledgment
We gratefully acknowledge the support of Dr. Adekunle Hassan for the advice and support given to us during the preparation of this manuscript. Written informed consent was obtained from the patients whose figures were used in this manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chronopoulos A, Schutz JS. Central retinal artery occlusion-a new, provisional treatment approach. Surv Ophthalmol. 2019;64(4):443–451. doi:10.1016/j.survophthal.2019.01.011
2. Rumelt S, Brown GC. Update on treatment of retinal arterial occlusions. Curr Opin Ophthalmol. 2003;14(3):139–141. doi:10.1097/00055735-200306000-00005
3. Cugati S, Varma DD, Chen CS, Lee AW. Treatment options for central retinal artery occlusion. Curr Treat Options Neurol. 2013;15(1):63–77. doi:10.1007/s11940-012-0202-9
4. Dattilo M, Biousse V, Newman NJ. Update on the management of central retinal artery occlusion. Neurol Clin. 2017;35(1):83–100. doi:10.1016/j.ncl.2016.08.013
5. Hayreh SS. Central retinal artery occlusion. Indian J Ophthalmol. 2018;66(12):1684–1694. doi:10.4103/ijo.IJO_1446_18
6. Hayreh SS. Acute retinal arterial occlusive disorders. Prog Retin Eye Res. 2011;30(5):359–394. doi:10.1016/j.preteyeres.2011.05.001
7. Mangat HS. Retinal artery occlusion. Surv Ophthalmol. 1995;40(2):145–156. doi:10.1016/S0039-6257(95)80004-2
8. Sharma RA, Dattilo M, Newman NJ, Biousse V. Treatment of nonarteritic acute central retinal artery occlusion. Asia Pac J Ophthalmol. 2018;7(4):235–241.
9. Schrag M, Youn T, Schindler J, Kirshner H, Greer D. Intravenous fibrinolytic therapy in central retinal artery occlusion: a patient-level meta-analysis. JAMA Neurol. 2015;72(10):1148–1154. doi:10.1001/jamaneurol.2015.1578
10. Mac Grory B, Nackenoff A, Poli S, et al. Intravenous fibrinolysis for central retinal artery occlusion: a cohort study and updated patient-level meta-analysis. Stroke. 2020;51(7):2018–2025. doi:10.1161/STROKEAHA.119.028743
11. Hayreh SS, Zimmerman MB. Central retinal artery occlusion: visual outcome. Am J Ophthalmol. 2005;140(3):376–391. doi:10.1016/j.ajo.2005.03.038
12. Rudkin AK, Lee AW, Chen CS. Ocular neovascularization following central retinal artery occlusion: prevalence and timing of onset. Eur J Ophthalmol. 2010;20(6):1042–1046. doi:10.1177/112067211002000603
13. Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021;52(7):e364–e467.
14. Retinal and ophthalmic artery occlusions preferred practice pattern® from AAO. Available from: www.aaojournal.org/article/S0161-6420(19)32095-0/pdf.
15. Schumacher M, Schmidt D, Jurklies B, et al; EAGLE-Study Group. Central retinal artery occlusion: local intra-arterial fibrinolysis versus conservative treatment, a multicenter randomized trial. Ophthalmology. 2010;117(7):1367–1375. doi:10.1016/j.ophtha.2010.03.061
16. Early Reperfusion Therapy With Intravenous Alteplase for Recovery of VISION in Acute Central Retinal Artery Occlusion (REVISION). Available from: https://clinicaltrials.gov/ct2/show/NCT04965038.
17. A Phase III Randomized, Blind, Double Dummy Multicenter Study Accessing the Efficacy and Safety of IV THrombolysis (Alteplase) in Patients With acutE Central retinal Artery Occlusion (THEIA). Available from: https://clinicaltrials.gov/ct2/show/NCT03197194.
18. TENectaplase in Central Artery Occlusion Study (TenCRAOS). Available from: https://clinicaltrials.gov/ct2/show/NCT04526951.
19. Jusufovic M, Elsais A, Kerty E. Seven points that explain the lack of effect from reperfusion therapy in central retinal artery occlusion. Ophthalmol Retina. 2019;3(9):713–715. doi:10.1016/j.oret.2019.05.014
20. Hayreh SS, Kolder HE, Weingeist TA. Central retinal artery occlusion and retinal tolerance time. Ophthalmology. 1980;87(1):75–78. doi:10.1016/S0161-6420(80)35283-4
21. Hayreh SS, Zimmerman MB, Kimura A, Sanon A. Central retinal artery occlusion. Retinal survival time. Exp Eye Res. 2004;78(3):723–736. doi:10.1016/S0014-4835(03)00214-8
22. Tobalem S, Schutz JS, Chronopoulos A. Central retinal artery occlusion - rethinking retinal survival time. BMC Ophthalmol. 2018;18(1):101. doi:10.1186/s12886-018-0768-4
23. Hattenbach LO, Kuhli-Hattenbach C, Scharrer I, Baatz H. Intravenous thrombolysis with low-dose recombinant tissue plasminogen activator in central retinal artery occlusion. Am J Ophthalmol. 2008;146(5):700–706. doi:10.1016/j.ajo.2008.06.016
24. Chamorro Á, Lo EH, Renú A, van Leyen K, Lyden PD. The future of neuroprotection in stroke. J Neurol Neurosurg Psychiatry. 2021;92(2):129–135. doi:10.1136/jnnp-2020-324283
25. Paciaroni M, Caso V, Agnelli G. The concept of ischemic penumbra in acute stroke and therapeutic opportunities. Eur Neurol. 2009;61(6):321–330. doi:10.1159/000210544
26. Ramos-Cabrer P, Campos F, Sobrino T, Castillo J. Targeting the ischemic penumbra. Stroke. 2011;42(1 Suppl):S7–11. doi:10.1161/STROKEAHA.110.596684
27. Ginsberg MD. Neuroprotection for ischemic stroke: past, present and future. Neuropharmacology. 2008;55(3):363–389. doi:10.1016/j.neuropharm.2007.12.007
28. Rajah GB, Ding Y. Experimental neuroprotection in ischemic stroke: a concise review. Neurosurg Focus. 2017;42(4):E2. doi:10.3171/2017.1.FOCUS16497
29. Heiss WD, Graf R. The ischemic penumbra. Curr Opin Neurol. 1994;7(1):11–19. doi:10.1097/00019052-199402000-00004
30. Fisher M. The ischemic penumbra: identification, evolution and treatment concepts. Cerebrovasc Dis. 2004;17(Suppl 1):1–6. doi:10.1159/000074790
31. McLeod D, Beatty S. Evidence for an enduring ischaemic penumbra following central retinal artery occlusion, with implications for fibrinolytic therapy. Prog Retin Eye Res. 2015;49:82–119. doi:10.1016/j.preteyeres.2015.06.001
32. Rusanen H, Saarinen JT, Sillanpää N. Collateral circulation predicts the size of the infarct core and the proportion of salvageable penumbra in hyperacute ischemic stroke patients treated with intravenous thrombolysis. Cerebrovasc Dis. 2015;40(3–4):182–190. doi:10.1159/000439064
33. Winship IR. Cerebral collaterals and collateral therapeutics for acute ischemic stroke. Microcirculation. 2015;22(3):228–236. doi:10.1111/micc.12177
34. Wufuer A, Wubuli A, Mijiti P, et al. Impact of collateral circulation status on favorable outcomes in thrombolysis treatment: a systematic review and meta-analysis. Exp Ther Med. 2018;15(1):707–718. doi:10.3892/etm.2017.5486
35. Mac Grory B, Schrag M, Poli S, et al. Structural and functional imaging of the retina in central retinal artery occlusion - current approaches and future directions. J Stroke Cerebrovasc Dis. 2021;30(7):105828. doi:10.1016/j.jstrokecerebrovasdis.2021.105828
36. Baumal CR. Optical coherence tomography angiography of retinal artery occlusion. Dev Ophthalmol. 2016;56:122–131.
37. Bonini Filho MA, Adhi M, de Carlo TE, et al. Optical coherence tomography angiography in retinal artery occlusion. Retina. 2015;35(11):2339–2346. doi:10.1097/IAE.0000000000000850
38. Yang S, Liu X, Li H, Xu J, Wang F. Optical coherence tomography angiography characteristics of acute retinal arterial occlusion. BMC Ophthalmol. 2019;19(1):147. doi:10.1186/s12886-019-1152-8
39. Su D, Garg S. The retinal function imager and clinical applications. Eye Vis. 2018;5:20. doi:10.1186/s40662-018-0114-1
40. Yao J, Wang LV. Photoacoustic microscopy. Laser Photon Rev. 2013;7(5):758–778. doi:10.1002/lpor.201200060
41. Yao J, Wang LV. Sensitivity of photoacoustic microscopy. Photoacoustics. 2014;2(2):87–101. doi:10.1016/j.pacs.2014.04.002
42. Draijer M, Hondebrink E, van Leeuwen T, Steenbergen W. Review of laser speckle contrast techniques for visualizing tissue perfusion. Lasers Med Sci. 2009;24(4):639–651. doi:10.1007/s10103-008-0626-3
43. Heeman W, Steenbergen W, van Dam G, Boerma EC. Clinical applications of laser speckle contrast imaging: a review. J Biomed Opt. 2019;24(8):1–11. doi:10.1117/1.JBO.24.8.080901
44. Zhong Z, Petrig BL, Qi X, Burns SA. In vivo measurement of erythrocyte velocity and retinal blood flow using adaptive optics scanning laser ophthalmoscopy. Opt Express. 2008;16(17):12746–12756. doi:10.1364/OE.16.012746
45. Romero-Borja F, Venkateswaran K, Roorda A, Hebert T. Optical slicing of human retinal tissue in vivo with the adaptive optics scanning laser ophthalmoscope. Appl Opt. 2005;44(19):4032–4040. doi:10.1364/AO.44.004032
46. Venkatesh R, Mutalik D, Reddy NG, Akkali MC, Yadav NK, Chhablani J. Retinal vessel wall imaging using fluorescein angiography and adaptive optics imaging in acute branch retinal artery occlusion. Eur J Ophthalmol. 2022;5:11206721221113202.
47. Read SJ, Hirano T, Abbott DF, et al. Identifying hypoxic tissue after acute ischemic stroke using PET and 18F-fluoromisonidazole. Neurology. 1998;51(6):1617–1621. doi:10.1212/WNL.51.6.1617
48. Nam J, Jing H, O’Reilly D. Intra-arterial thrombolysis vs. standard treatment or intravenous thrombolysis in adults with acute ischemic stroke: a systematic review and meta-analysis. Int J Stroke. 2015;10(1):13–22. doi:10.1111/j.1747-4949.2012.00914.x
49. Alonso de Leciñana M, Egido JA, Casado I, et al. Spanish neurological society. Guidelines for the treatment of acute ischaemic stroke. Neurologia. 2014;29(2):102–122. doi:10.1016/j.nrl.2011.09.012
50. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344–e418. doi:10.1161/STR.0000000000000211
51. Mac Grory B, Lavin P, Kirshner H, Schrag M. Thrombolytic therapy for acute central retinal artery occlusion. Stroke. 2020;51(2):687–695. doi:10.1161/STROKEAHA.119.027478
52. Okonkwo ON, Hassan AO, Akanbi T, Umeh VC, Ogunbekun OO. Vitrectomy and manipulation of intraocular and arterial pressures for the treatment of nonarteritic central retinal artery occlusion. Taiwan J Ophthalmol. 2020;11(3):305–311. doi:10.4103/tjo.tjo_51_20
53. Kadonosono K, Yamane S, Inoue M, Yamakawa T, Uchio E. Intra-retinal arterial cannulation using a microneedle for central retinal artery occlusion. Sci Rep. 2018;8(1):1360. doi:10.1038/s41598-018-19747-7
54. Sharma RA, Newman NJ, Biousse V. Conservative treatments for acute nonarteritic central retinal artery occlusion: do they work? Taiwan J Ophthalmol. 2020;11(1):16–24. doi:10.4103/tjo.tjo_61_20
55. Schultheiss M, Härtig F, Spitzer MS, et al. Intravenous thrombolysis in acute central retinal artery occlusion - a prospective interventional case series. PLoS One. 2018;13(5):e0198114. doi:10.1371/journal.pone.0198114
56. Dumitrascu OM, Shen JF, Kurli M, et al. Is intravenous thrombolysis safe and effective in central retinal artery occlusion? A critically appraised topic. Neurologist. 2017;22(4):153–156. doi:10.1097/NRL.0000000000000129
57. Ko SJ, Shin IC, Kim DW, Choi SS, Yang YS. Safety and efficacy of selective intra-arterial thrombolysis for central retinal artery occlusion. Korean J Ophthalmol. 2021;35(4):261–271. doi:10.3341/kjo.2020.0082
58. Ahn SJ, Kim JM, Hong JH, et al. Efficacy and safety of intra-arterial thrombolysis in central retinal artery occlusion. Invest Ophthalmol Vis Sci. 2013;54(12):7746–7755. doi:10.1167/iovs.13-12952
59. Dumitrascu OM, Newman NJ, Biousse V. Thrombolysis for central retinal artery occlusion in 2020: time Is Vision! J Neuroophthalmol. 2020;40(3):333–345. doi:10.1097/WNO.0000000000001027
60. Hakim N, Hakim J. Intra-arterial thrombolysis for central retinal artery occlusion. Clin Ophthalmol. 2019;13:2489–2509. doi:10.2147/OPTH.S232560
61. Zhu T, Wang L, Feng Y, Sun G, Sun X. Classical active ingredients and extracts of Chinese herbal medicines: pharmacokinetics, pharmacodynamics, and molecular mechanisms for ischemic stroke. Oxid Med Cell Longev. 2021;13(2021):8868941.
62. Endres M, Engelhardt B, Koistinaho J, et al. Improving outcome after stroke: overcoming the translational roadblock. Cerebrovasc Dis. 2008;25(3):268–278. doi:10.1159/000118039
63. Mergenthaler P, Dirnagl U, Meisel A. Pathophysiology of stroke: lessons from animal models. Metab Brain Dis. 2004;19(3–4):151–167. doi:10.1023/B:MEBR.0000043966.46964.e6
64. Sutherland BA, Minnerup J, Balami JS, Arba F, Buchan AM, Kleinschnitz C. Neuroprotection for ischaemic stroke: translation from the bench to the bedside. Int J Stroke. 2012;7(5):407–418. doi:10.1111/j.1747-4949.2012.00770.x
65. Hill MD, Goyal M, Menon BK, et al. Efficacy and safety of nerinetide for the treatment of acute ischaemic stroke (ESCAPE-NA1): a multicentre, double-blind, randomised controlled trial. Lancet. 2020;395(10227):878–887. doi:10.1016/S0140-6736(20)30258-0
66. Baron JC. Nerinetide: a potential neuroprotectant as adjunct to thrombectomy for acute stroke. Can J Neurol Sci. 2021;48(1):138. doi:10.1017/cjn.2020.183
67. Zhou XF. ESCAPE-NA1 trial brings hope of neuroprotective drugs for acute ischemic stroke: highlights of the phase 3 clinical trial on nerinetide. Neurosci Bull. 2021;37(6):579–581. doi:10.1007/s12264-020-00627-y
68. Aliena-Valero A, Rius-Pérez S, Baixauli-Martín J, et al. Uric acid neuroprotection associated to IL-6/STAT3 signaling pathway activation in rat ischemic stroke. Mol Neurobiol. 2021;58(1):408–423. doi:10.1007/s12035-020-02115-w
69. Chamorro A, Amaro S, Castellanos M, et al; URICO-ICTUS Investigators. Safety and efficacy of uric acid in patients with acute stroke (URICO-ICTUS): a randomised, double-blind phase 2b/3 trial. Lancet Neurol. 2014;13(5):453–460. doi:10.1016/S1474-4422(14)70054-7
70. Kim JS, Lee KB, Park JH, et al; SAFE-TPA Investigators. Safety and efficacy of otaplimastat in patients with acute ischemic stroke requiring tPA (SAFE-TPA): a multicenter, randomized, double-blind, placebo-controlled phase 2 study. Ann Neurol. 2020;87(2):233–245. doi:10.1002/ana.25644
71. Hong JM, Choi MH, Sohn SI, et al; on the behalf of the SONIC investigators. Safety and optimal neuroprotection of neu2000 in acute ischemic stroke with reCanalization: study protocol for a randomized, double-blinded, placebo-controlled, phase-II trial. Trials. 2018;19(1):375. doi:10.1186/s13063-018-2746-9
72. Ahmed F, Cahoon JM, Archer B, Ambati B. COMP-Ang1 has retinal neuroprotective properties after hypoxic and vascular insults. Invest Ophthalmol Vis Sci. 2014;55(13):5745.
73. Hata M, Ikeda HO. Modulation of valosin-containing protein by Kyoto University Substances (KUS) as a novel therapeutic strategy for ischemic neuronal diseases. Neural Regen Res. 2017;12(8):1252–1255. doi:10.4103/1673-5374.213540
74. Ikeda HO, Muraoka Y, Hata M, et al. Safety and effectiveness of a novel neuroprotectant, KUS121, in patients with non-arteritic central retinal artery occlusion: an open-label, non-randomized, first-in-humans, Phase 1/2 trial. PLoS One. 2020;15(2):e0229068. doi:10.1371/journal.pone.0229068
75. Visuvanathan S, Baker AN, Lagali PS, et al. XIAP gene therapy effects on retinal ganglion cell structure and function in a mouse model of glaucoma. Gene Ther. 2022;29(3–4):147–156. doi:10.1038/s41434-021-00281-7
76. Renwick J, Narang MA, Coupland SG, et al. XIAP-mediated neuroprotection in retinal ischemia. Gene Ther. 2006;13(4):339–347. doi:10.1038/sj.gt.3302683
77. Samlaska CP, Winfield EA. Pentoxifylline. J Am Acad Dermatol. 1994;30(4):603–621. doi:10.1016/S0190-9622(94)70069-9
78. Ji Q, Zhang L, Lv R, Jia H, Xu J. Pentoxifylline decreases up-regulated nuclear factor kappa B activation and cytokine production in the rat retina following transient ischemia. Ophthalmologica. 2006;220(4):217–224. doi:10.1159/000093074
79. Hayreh SS, Zimmerman B. Management of giant cell arteritis. Our 27-year clinical study: new light on old controversies. Ophthalmologica. 2003;217(4):239–259. doi:10.1159/000070631
80. Rajagopalan L, Ghosn C, Tamhane M, et al. A nonhuman primate model of blue light-induced progressive outer retina degeneration showing brimonidine drug delivery system-mediated cyto- and neuroprotection. Exp Eye Res. 2021;209:108678. doi:10.1016/j.exer.2021.108678
81. Wubben TJ, Zacks DN, Besirli CG. Retinal neuroprotection: current strategies and future directions. Curr Opin Ophthalmol. 2019;30(3):199–205. doi:10.1097/ICU.0000000000000558
82. Danesh-Meyer HV. Neuroprotection in glaucoma: recent and future directions. Curr Opin Ophthalmol. 2011;22(2):78–86. doi:10.1097/ICU.0b013e32834372ec
83. Arrich J, Holzer M, Havel C, Müllner M, Herkner H. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst Rev. 2016;2(2):CD004128. doi:10.1002/14651858.CD004128.pub4
84. Freeman WD. Management of intracranial pressure. Continuum. 2015;21(5 Neurocritical Care):1299–1323. doi:10.1212/CON.0000000000000235
85. Jeon SB, Koh Y, Choi HA, Lee K. Critical care for patients with massive ischemic stroke. J Stroke. 2014;16(3):146–160. doi:10.5853/jos.2014.16.3.146
86. Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346(8):557–563. doi:10.1056/NEJMoa003289
87. Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549–556. doi:10.1056/NEJMoa012689
88. Xi L, Kozlov AV. Research progress of the application of hypothermia in the eye. Oxid Med Cell Longev. 2020;2020:3897168. doi:10.1155/2020/3897168
89. Simpson AR, Dowell NG, Jackson TL, Tofts PS, Hughes EH. Measuring the effect of pars plana vitrectomy on vitreous oxygenation using magnetic resonance imaging. Invest Ophthalmol Vis Sci. 2013;54(3):2028–2034. doi:10.1167/iovs.12-11258
90. Faberowski N, Stefansson E, Davidson RC. Local hypothermia protects the retina from ischemia. A quantitative study in the rat. Invest Ophthalmol Vis Sci. 1989;30(11):2309–2313.
91. Reinhard K, Mutter M, Gustafsson E, et al. Hypothermia promotes survival of ischemic retinal ganglion cells. Invest Ophthalmol Vis Sci. 2016;57(2):658–663. doi:10.1167/iovs.15-17751
92. Salido EM, Dorfman D, Bordone M, Chianelli M, González Fleitas MF, Rosenstein RE. Global and ocular hypothermic preconditioning protect the rat retina from ischemic damage. PLoS One. 2013;8(4):e61656. doi:10.1371/journal.pone.0061656
93. Tamai K, Toumoto E, Majima A. Local hypothermia protects the retina from ischaemic injury in vitrectomy. Br J Ophthalmol. 1997;81(9):789–794. doi:10.1136/bjo.81.9.789
94. Wang X, Niwa M, Hara A, et al. Neuronal degradation in mouse retina after a transient ischemia and protective effect of hypothermia. Neurol Res. 2002;24(7):730–735. doi:10.1179/016164102101200663
95. Quiñones-Hinojosa A, Malek JY, Ames A, et al. Metabolic effects of hypothermia and its neuroprotective effects on the recovery of metabolic and electrophysiological function in the ischemic retina in vitro. Neurosurgery. 2003;52(5):1178–86; discussion <pg>1186–1187.
96. Kim SH, Cha YS, Lee Y, Kim H, Yoon IN. Successful treatment of central retinal artery occlusion using hyperbaric oxygen therapy. Clin Exp Emerg Med. 2018;5(4):278–281. doi:10.15441/ceem.17.271
97. Celebi ARC. Hyperbaric oxygen therapy for central retinal artery occlusion: patient selection and perspectives. Clin Ophthalmol. 2021;15:3443–3457. doi:10.2147/OPTH.S224192
98. Hadanny A, Maliar A, Fishlev G, et al. Reversibility of retinal ischemia due to central retinal artery occlusion by hyperbaric oxygen. Clin Ophthalmol. 2016;11:115–125. doi:10.2147/OPTH.S121307
99. Sunny CL, Steffi SY, Leow PL, Callie KL. Efficacy and safety of hyperbaric oxygen therapy for acute central retinal artery occlusion in Hong Kong: results of the first 3 years. Hong Kong J Ophthalmol. 2022;26(1):1.
100. Rozenberg A, Hadad A, Peled A, et al. Hyperbaric oxygen treatment for non-arteritic central retinal artery occlusion retrospective comparative analysis from two tertiary medical centres. Eye. 2022;36(6):1261–1265. doi:10.1038/s41433-021-01617-8
101. Elder MJ, Rawstron JA, Davis M. Hyperbaric oxygen in the treatment of acute retinal artery occlusion. Diving Hyperb Med. 2017;47(4):233–238. doi:10.28920/dhm47.4.233-238
102. Cope A, Eggert JV, O’Brien E. Retinal artery occlusion: visual outcome after treatment with hyperbaric oxygen. Diving Hyperb Med. 2011;41(3):135–138.
103. Rosignoli L, Chu ER, Carter JE, Johnson DA, Sohn JH, Bahadorani S. The effects of hyperbaric oxygen therapy in patients with central retinal artery occlusion: a retrospective study, systematic review, and meta-analysis. Korean J Ophthalmol. 2022;36(2):108–113. doi:10.3341/kjo.2021.0130
104. Wubben TJ, Besirli CG, Johnson MW, Zacks DN. Retinal neuroprotection: overcoming the translational roadblocks. Am J Ophthalmol. 2018;192:xv–xxii. doi:10.1016/j.ajo.2018.04.012
105. Yotsukura J, Adachi-Usami E. Correlation of electroretinographic changes with visual prognosis in central retinal artery occlusion. Ophthalmologica. 1993;207(1):13–18. doi:10.1159/000310400
106. Gutiérrez M, Merino JJ, Alonso de Leciñana M, Díez-Tejedor E. Cerebral protection, brain repair, plasticity and cell therapy in ischemic stroke. Cerebrovasc Dis. 2009;27(Suppl 1):177–186. doi:10.1159/000200457
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.