Back to Journals » Local and Regional Anesthesia » Volume 16
Neuropathies Following an Ultrasound-Guided Axillary Brachial Plexus Block
Authors Koh K , Tatsuki O, Sakuraba S, Yamazaki S, Yako H, Omae T
Received 11 July 2023
Accepted for publication 26 August 2023
Published 4 September 2023 Volume 2023:16 Pages 123—132
DOI https://doi.org/10.2147/LRA.S426515
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Stefan Wirz
Keito Koh, Onishi Tatsuki, Sonoko Sakuraba, Sho Yamazaki, Hajime Yako, Takeshi Omae
Department of Anesthesiology and Pain Medicine, Juntendo University Shizuoka Hospital, Izunokuni, Japan
Correspondence: Keito Koh, Department of Anesthesiology and Pain Medicine, Juntendo University Shizuoka Hospital, 1129 Nagaoka, Izunokuni-shi, Shizuoka, 410-2211, Japan, Tel +81 55 948 3111 ex 7111, Fax +81-55-947-2565, Email [email protected]
Purpose: Ultrasound-guided brachial plexus block (UGBPB) has interscalene, supraclavicular, infraclavicular, and axillary approaches. The axillary block is considered to be the safest and with fewer adverse events compared to the interscalene (eg, phrenic nerve block, spinal cord or vertebral artery puncture) and supraclavicular (eg, pneumothorax). However, with regard to postoperative neurological symptoms (PONS), it is controversial whether its incidence after an axillary block was higher than that after non-axillary approaches”. In this study, we investigated whether the incidence of a neuropathy after an axillary block was higher than that after non-axillary approaches.
Patients and Methods: This was a single-center, retrospective cohort study. All UGBPBs were performed under general anesthesia between January 2014 and March 2020. The outcomes included the overall incidence of PONS and neuropathies for axillary and non-axillary approaches. The etiology, symptoms, and outcomes of patients were investigated.
Results: Of the 992 patients, 143 (14%) and 849 (86%) were subjected to axillary and non-axillary approaches, respectively. Among 19 cases (19.2:1000; 95% confidence interval [CI], 18.2– 20.1) of PONS, four (4.0:1000; 95% CI, 3.8– 4.2) were neuropathies attributed to the UGBPB, three (21.0:1000; 95% CI, 18.1– 23.8) to the axillary and one (2.8:1000; 95% CI, 2.6– 3.1) to non-axillary approaches. The incidence of neuropathies after an axillary block was significantly higher than that after non-axillary approaches (P = 0.005).
Conclusion: The incidence of neuropathies after US-guided axillary block under general anesthesia was significantly higher than that after non-axillary approaches.
Keywords: ultrasound, brachial plexus block, axillary block, neuropathy, nerve injury
Introduction
A brachial plexus block (BPB) is used for effective perioperative analgesia in a wide range of procedures involving the upper extremities and can be performed through four approaches, namely interscalene, supraclavicular, infraclavicular, and axillary. Currently, the ultrasound (US)-guided method is the most used when performing BPBs and is superior to methods such as the landmark and nerve stimulation (NS) in terms of an improved success rate, a shorter time for peak effect, and a lower incidence of vascular puncture.
However, several studies have reported that the US-guided method did not contribute to reducing the incidence of postoperative neurological symptoms (PONS).1–4 Since the injection pressure can be high while administering a local anesthetic, injection pressure monitoring (IPM) may be useful in preventing PONS after a peripheral nerve block.5,6 Among the four approaches, the axillary block is considered the safest and with fewer adverse events compared to the interscalene (eg, phrenic nerve block, spinal cord or vertebral artery puncture) and supraclavicular (eg, pneumothorax).7 However, with regard to PONS, a study has showed its incidence was highest in the axillary block,8 while another has reported no difference for each of the four approaches.9 It is controversial whether the incidence of PONS after an axillary block was higher than that after non-axillary approaches.
In our daily practice, we had the impression that some patients complained of PONS after axillary block. Therefore, we hypothesized that an axillary block could lead to a higher incidence of neuropathies than non-axillary approaches.
To date, literature comparing the incidence of neuropathies after axillary blocks in UGBPBs with the non-axillary approaches is sparse.10,11
In this study, we investigated whether the incidence of neuropathies due to nerve injury after an axillary block was higher than that after non-axillary approaches under general anesthesia.
Materials and Methods
Study Design and Data Collection
This single-center retrospective cohort study was conducted at the Juntendo University Shizuoka Hospital in Shizuoka, Japan. The study protocol was approved by the institutional review board of Juntendo University Shizuoka Hospital (ID: S19-0707). Patient consent was not required because this was a retrospective study analyzing data collected in daily clinical practice. All patient data were kept anonymized by the authors of this study. All procedures in this study were in accordance with the Declaration of Helsinki (1964). The patients who were aged >18 years and underwent perioperative UGBPBs between January 2014 (UGBPB was introduced at our institution) and March 2020 (UGBPB has been performed under general anesthesia instead of the awake condition) were included. Patients who were administered nerve blocks through an unknown technique or more than two nerve blocks and patients with trauma and preoperative complaints of neurologic deficits at the surgical site were excluded from this study.
PONS following peripheral nerve blocks has various causes such as the original injury, surgical manipulation, nerve injury caused by block manipulation, neurotoxicity of administered solutions, surgical position, and undetermined etiology.12 A neuropathy due to a UGBPB in this study was defined as a nerve injury caused by block manipulation with a new onset of paresthesia, hypoesthesia, numbness, or muscle weakness occurring within 48 h postoperatively and lasting for more than 5 days;8,13,14 the sensory deficit area was consistent with the nerve distribution targeted by a BPB. This period was chosen because previous studies have shown that the time of action after a BPB, using 10 mL of 0.25% levobupivacaine, was of approximately 10 h.15 As for interscalene blocks, most patients reported symptoms within 48 h postoperatively.13,14 We excluded cases of neurological symptoms that could be explained by the original injury, surgical manipulation, surgical position, or tourniquet use and those with an undetermined etiology from PONS cases. An original injury was defined as a pre-existing deficit detected before surgery. Surgical manipulation was defined as a deficit that was more likely to be associated with the surgical approach than with the preoperative deficit and UGBPB. Surgical position and tourniquet use were defined as deficits associated with the injured site due to the intraoperative position and tourniquet use, respectively. An undetermined etiology was defined as other neurological symptoms. Cases of PONS were investigated by an anesthesiologist who reviewed the patient’s electronic medical records. PONS factors were classified based on the consensus of the neurologist, attending orthopedic surgeon, and anesthesiologist.
In this study, the overall incidence of PONS and neuropathies for axillary and non-axillary approaches attributable to UGBPBs were investigated as the primary outcomes. Age; body mass index; sex; surgical position; block laterality; surgery location; tourniquet use (time and pressure); local anesthetics (amount and type); etiology; symptoms, and outcomes of patients with PONS were investigated as the secondary outcomes.
UGBPB Procedures
First, nerve pre-scanning was performed using a SonoSite S-Nerve US device (SonoSite, Bothell, WA, USA), with a SonoSite linear probe (HFL50x/15–6 MHz). After disinfection under aseptic conditions, single-dose nerve blocks were administered using a 20 G × 80 mm Tuohy needle (Uniever, Unisys Corp, Tokyo, Japan). All nerve blocks were administered under general anesthesia, which was performed using a parallel approach by an attending anesthesiologist or supervised trainee before the surgery; they were not combined with NS or IPM.
The UGBPB techniques for each approach are as follows:
- Interscalene: The patient was placed in a semi-lateral position with the blocked side up, and a local anesthetic was administered between the C5 and C6 vertebrae, following US-guided identification of the C6 nerve root.
- Supraclavicular: The patient was placed in a supine position, and a US probe was placed parallel to the clavicle. The first rib, subclavian artery, and brachial plexus were identified, and a local anesthetic was administered around the entire brachial plexus.
- Infraclavicular: The patient was placed in the supine position, and the axillary artery was identified using a US probe under the clavicle, just medial to the coracoid process and parallel to the long axis of the trunk. The lateral, medial, and posterior nerve bundles around the axillary artery were identified, and a local anesthetic was administered.
- Axillary: The blocked side arm was abducted 90° and the elbow flexed 90°. A US probe was applied perpendicular to the long axis of the arm; as the musculocutaneous, radial, ulnar, and median nerves were identified, a local anesthetic was administered around each nerve.
Stastical Analysis
The demographic characteristics, type of BPB applied, and complication details were summarized using descriptive statistics. Categorical variables are described as absolute and relative frequencies. The incidence of PONS and neuropathies were described as means (cases out of 1000) and 95% confidence intervals (CIs). The association of categorical variables with the presence of PONS and neuropathies was analyzed using the Shapiro or Fligner test. Statistical significance was set at P < 0.05. The data were analyzed using the Python software (Python Software Foundation, Delaware, United States of America) and its corresponding packages.
Results
Of the 1022 patients initially included in this study, 12 (1.2%) underwent a combination of two nerve block approaches, and 18 (1.8%), an unknown nerve block approach. Of the 992 patients remaining, 143 (14%) and 849 (86%) underwent axillary and non-axillary approaches, respectively (Figure 1). Table 1 shows the demographic data of these patients. Levobupivacaine was the most common anesthetic used in all procedures. There were 19 cases of PONS (19.2:1000; 95% CI, 18.2–20.1), of which neuropathies caused by UGBPB were reported in four (4.0:1000; 95% CI, 3.8–4.2); the incidence of neuropathies was reported in three cases (21.0:1000; 95% CI, 18.1–23.8) for the axillary and in one case (1.20:1000; 95% CI, 1.10–1.20) for the non-axillary approaches, which included one case for the interscalene approach (Table 2). The incidence of neuropathies after the axillary block was significantly higher than that after non-axillary approaches (P = 0.005) (Figure 2).
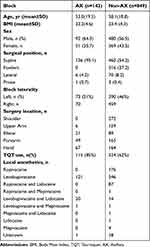 |
Table 1 Demographic Data |
 |
Table 2 Incidence of PONS and Neuropathy |
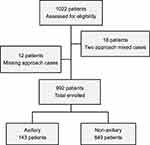 |
Figure 1 Flow diagram of the study. |
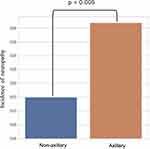 |
Figure 2 Comparison of the incidence of postoperative neuropathies between axillary (n=143) and non-axillary approaches (n=849); total (n=992). |
Considering the 19 PONS cases, 11 (60%), four (20%), two (10%), one (5%), and one (5%) were attributed to surgical manipulation, neuropathies caused by UGBPBs, original injuries, surgical position, and an undetermined etiology, respectively (Table 3).
 |
Table 3 Report of Cases with Postoperative Neurological Symtoms: Background, Diagnosis, Cause and Outcome |
Discussion
In this study of 992 UGBPB cases, there were 19 cases of PONS. Among the four cases of neuropathies caused by UGBPBs, three were with the axillary and one with the non-axillary approaches. The incidence of neuropathies after an axillary block was significantly higher than that after non-axillary approaches.
Of the 19 PONS cases, 11 were classified as secondary to surgical manipulation (cases 2–5, 7–12, and 14), which is generally the most frequent cause of PONS.16,17 The symptoms of four patients (cases 2–5), who were operated on using an arthroscopic rotator cuff repair, were centered on the ulnar nerve area. The orthopedic surgeon diagnosed these cases to be caused by postoperative difficulty in elevating the affected limb. Though PONS after shoulder surgery is multifactorial, it may occur when the elbow remains extended because of postoperative difficulty in raising the affected limb, resulting in an increased pressure in the elbow canal.18 Case 7 showed ulnar nerve palsy owing to a fixation pin and fully recovered after it was removed 40 days post-surgery. Case 8 showed numbness in the thumb and index finger due to intraoperative injury to the radial nerve, with full recovery 4 months post-surgery. Case 9 demonstrated ulnar nerve palsy after open reduction with internal fixation of the humerus, with partial recovery 10 days post-surgery. Case 10 showed radial nerve palsy due to an intraoperative radial nerve injury, which was diagnosed as an axonal impairment using electromyography. Case 11 demonstrated paralysis of the ring and little fingers owing to intraoperative ulnar nerve injury; however, some symptoms were relieved 1 month postoperatively. Case 14 showed numbness in the thumb, index, and middle fingers due to intraoperative nerve suture; however, the numbness resolved 10 days post-surgery. Case 1 was attributed to the surgical position, which was the beach chair position, and ulnar nerve palsy was observed. Two other PONS cases (cases 17 and 19) were attributed to the original injury. Case 17 had ossification of the posterior longitudinal ligament before surgery, and case 19 had poorly controlled diabetes mellitus for 35 years, with neurological symptoms observed in the same area before surgery.
It is difficult to compare the incidence of PONS after nerve blocks among different studies as they include other nerve blocks in addition to BPBs. Moreover, the definition of PONS, follow-up periods, and implemented methods differed among institutions. Our study investigated neuropathies after UGBPBs; therefore, it was compared with analogous previous studies.
Pablo et al reported the incidence of PONS after UGBPBs as 2.81:1000 (95% CI, 1.70–4.63). Of this, the incidence of neuropathies due to UGBPBs was 0.0:1000 (95% CI, 0.0–15.4) for axillary, 5.16:1000 (95% CI, 2.21–12.0) for interscalene, 2.79:1000 (95% CI, 1.52–5.13) for supraclavicular, and 0.0:1000 (95% CI, 0.0–13.4) for infraclavicular approaches.9
Brian et al reported the incidence of PONS after UGBPBs to be 1.8:1000 (95% CI, 1.1–2.7). Of this, the incidence of neuropathies due to UGBPBs was 23.0:1000 (95% CI, 0.6–125.7), 3.5:1000 (95% CI, 1.4–7.3), and 2.0:1000 (95% CI, 0.4–5.8) for the axillary, interscalene, and supraclavicular blocks, respectively.8
A former report9 regarding whether UGBPBs were performed under sedation or an awake condition is ambiguous, whereas a latter study,8 which presented a higher incidence of PONS in the axillary, performed UGBPBs under sedation, similarly to our study. The ASRA recommends that nerve blocks should be performed under awake conditions to prevent PONS.19 We hypothesized the following as the possible causes for the higher incidence of neuropathies after axillary blocks compared to that after non-axillary approaches: 1) individual differences in the brachial plexus anatomy; 2) a greater number of needle punctures; and 3) a longer subcutaneous needle length. Of the four BPB approaches, the axillary approach was the most peripheral block. Therefore, the brachial plexus and blood vessels are already branched in the axilla, with significantly varying patterns.18,20 The branches of the ulnar, radial, median, and musculocutaneous nerves are already present at a level that can be discerned using US. Anatomically, the ulnar, radial, and median nerves are located apart from the musculocutaneous nerve; consequently, more than two punctures are often required to individually block all these nerves using a parallel technique.7 In conventional axillary nerve blocks, the arterial penetration method is often combined with NS,21 and the block needle is inserted vertically under the axillary artery.22 Therefore, the subcutaneous needle length is shorter than that used in a US-guided block, and one puncture is typically sufficient.
In contrast, in the US-guided axillary block used, the total subcutaneous needle length was visible in all cases, and a parallel technique was used. Accordingly, the subcutaneous needle length was greater than that used in conventional methods, and the number of punctures was likely to be ≥ 2; however, the number of axillary punctures was not recorded. We typically block the musculocutaneous nerve and ulnar, radial, and median nerves separately;8 thus, the number of punctures per block was also possibly ≥ 2. Consequently, multiple punctures, long subcutaneous needle lengths, and individual differences in brachial plexus anatomy may cause neuropathies in a US-guided axillary block due to direct nerve injury and unexpected intraneural injections of local anesthetics.
A meta-analysis examining various types of nerve blocks reported that the incidence of neuropathies was not reduced by combining the US-guided and NS methods.2 Several randomized controlled trials have compared the success rate of axillary blocks performed with a combination of US and NS guidance against those with NS alone, as well as those with US versus NS guidance; however, these trials have not investigated the incidence of postoperative neuropathies.23–26 In contrast, a prospective study reported 60 successful axillary blocks with zero incidences of postoperative neuropathies by combining US and NS guidance.27 Additionally, for blocks with difficulty in nerve visualization such as the adductor canal and posterior clavicular blocks, double-monitoring using combined US and NS guidance was effective in preventing neuropathies.28,29 Since neuropathies are rare, chance variation was possibly detected. Therefore, there may be little differences in the incidence of neuropathies between US with NS and NS guidance alone.30
With a high-pressure injection during the administration of a local anesthetic, IPM may prevent its administration into undesired intraneural spaces, thereby reducing the incidence of neuropathies,31 as IPM reportedly prevents nerve injury following an interscalene block.32,33 Considering the mechanism of neuropathies after US-guided axillary blocks, the use of triple-monitoring as a combination of US, NS guidance, and IPM31 may be particularly effective in preventing postoperative axillary neuropathies.
While a uniform block procedure application at a single institution was one of the strengths of our study, there were several limitations. First, this study was designed as a retrospective cohort study, which is known to be associated with potential confounding factors and a lack of randomization. Specifically, the different operator experiences in practicing the blocks, the different local anesthetic concentrations and/ or volume are considered as confounding factors. Second, although the cases of PONS should have been investigated by at least two anesthesiologists to avoid any bias and subjective errors, it have being investigated by only one. Third, the definition and assessment of neuropathies were based on the physician’s diagnosis as determined by the patient’s self-report. Hence, the incidence of neuropathies may have been underestimated, unless it was documented in medical records. Fourth, the etiology of PONS was diagnosed mainly based on clinical symptoms, without using objective measures (electromyography and nerve conduction study). Therefore, the role of the injured nerve may not be accurate. Finally, as UGBPBs were performed under general anesthesia, the incidence of neuropathies may differ from that in studies in which they were performed under awake conditions.
Conclusion
The incidence of neuropathies after US-guided axillary block under general anesthesia was significantly higher than that after non-axillary approaches. Considering the anatomical characteristics of the brachial plexus and subcutaneous needle length in the axillary region, triple-monitoring using US and NS guidance with IPM may be useful for preventing neuropathies following US-guided axillary blocks. The findings of this study may be useful to advance the knowledge of neuropathies secondary to UGBPBs.
Abbreviations
BPB, brachial plexus block; US, ultrasound; NS, nerve stimulation; UGBPB, ultrasound-guided brachial plexus block; PONS, postoperative neurological symptom; IPM, injection pressure monitoring; ASRA, American Society of Regional Anesthesia and Pain Medicine.
Acknowledgments
The authors would like to thank the anesthesiology medical staff of Juntendo University Hospital for their assistance and Honyaku Center Inc. for English language editing.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Abrahams MS, Aziz MF, Fu RF, Horn JL. Ultrasound guidance compared with electrical neurostimulation for peripheral nerve block: a systematic review and meta-analysis of randomized controlled trials. Br J Anaesth. 2009;102:408–417. doi:10.1093/bja/aen384
2. Munirama S, McLeod G. A systematic review and meta-analysis of ultrasound versus electrical stimulation for peripheral nerve location and blockade. Anaesthesia. 2015;70(9):1084–1091. doi:10.1111/anae.13098
3. Neal JM. Ultrasound-guided regional anesthesia and patient safety: update of an evidence-based analysis. Reg Anesth Pain Med. 2016;41(2):195–204. doi:10.1097/AAP.0000000000000295
4. Orebaugh SL, Kentor ML, Williams BA. Adverse outcomes associated with nerve stimulator-guided and ultrasound-guided peripheral nerve blocks by supervised trainees: update of a single-site database. Reg Anesth Pain Med. 2012;37(6):577–582. doi:10.1097/AAP.0b013e318263d396
5. Barrington MJ, Lirk P. Reducing the risk of neurological complications after peripheral nerve block: what is the role of pressure monitoring? Anaesthesia. 2019;74(1):9–12. doi:10.1111/anae.14469
6. Weisman RS, Bhavsar NP, Schuster KA, Gebhard RE. Evaluation of the B-Smart manometer and the CompuFlo computerized injection pump technology for accurate needle-tip injection pressure measurement during peripheral nerve blockade. Reg Anesth Pain Med. 2019;44(1):86–90. doi:10.1136/rapm-2018-000026
7. Ranganath A, Srinivasan KK, Iohom G. Ultrasound guided axillary brachial plexus block. Med Ultrason. 2014;16(3):246–251. doi:10.11152/mu.2013.2066.163.2kks
8. Sites BD, Taenzer AH, Herrick MD, et al. Incidence of local anesthetic systemic toxicity and postoperative neurologic symptoms associated with 12,668 ultrasound-guided nerve blocks: an analysis from a prospective clinical registry. Reg Anesth Pain Med. 2012;37(5):478–482. doi:10.1097/AAP.0b013e31825cb3d6
9. Oliver-Fornies P, Ortega Lahuerta JP, Gomez Gomez R, et al. Postoperative neurological complications after brachial plexus block: a retrospective study conducted at a teaching hospital. J Anesth. 2021;35(6):844–853. doi:10.1007/s00540-021-02989-7
10. Stav A, Reytman L, Stav MY, et al. Comparsion of the Supraclavicular, Infraclavicular and Axillary Approaches for Ultrasound-Guided Brachial Plexus Block for Surgical Anesthesia. Rambam Maimondies Med J. 2016;7:57.
11. Tran DQ, Russo G, Munoz L, et al. A prospective, randomized comparsion between ultrasound-guided supraclavicular, infraclavicular, and axillary brachial plexus blocks. Reg Anesth Pain Med. 2009;34:366–371. doi:10.1097/AAP.0b013e3181ac7d18
12. Brull R, Hadzic A, Reina MA, Barrington MJ. Pathophysiology and etiology of nerve injury following peripheral nerve blockade. Reg Anesth Pain Med. 2015;40(5):479–490. doi:10.1097/AAP.0000000000000125
13. Candido KD, Sukhani R, R D Jr, et al. Neurologic sequelae after interscalene brachial plexus block for shoulder/upper arm surgery: the association of patient, anesthetic, and surgical factors to the incidence and clinical course. Anesth Analg. 2005;100:1489–1495. doi:10.1213/01.ANE.0000148696.11814.9F
14. Borgeat A, Ekatodramis G, Kalberer F, Benz C. Acute and nonacute complications associated with interscalene block and shoulder surgery: a prospective study. Anesthesiology. 2001;95(4):875–880. doi:10.1097/00000542-200110000-00015
15. Maruishi Pharmaceutical Co Ltd. In-House Document: A Phase-III Comparative Clinical Study (Conduction Anesthesia [Brachial Plexus Block]) (MR8A2-13) (Document Evaluated for the Approval); 2012. Japanese.
16. Dwyer T, Henry PDG, Cholvisudhi P, Chan VWS, Theodoropoulos JS, Brull R. Neurological complications related to elective orthopedic surgery: part 1: common shoulder and elbow procedures. Reg Anesth Pain Med. 2015;40(5):431–442. doi:10.1097/AAP.0000000000000178
17. Thomasson BG, Matzon JL, Pepe M, Tucker B, Maltenfort M, Austin L. Distal peripheral neuropathy after open and arthroscopic shoulder surgery: an under-recognized complication. J Shoulder Elbow Surg. 2015;24(1):60–66. doi:10.1016/j.jse.2014.08.007
18. Yang HJ, Gil YC, Lee HY. Intersegmental origin of the axillary artery and accompanying variation in the brachial plexus. Clin Anat. 2009;22(5):586–594. doi:10.1002/ca.20811
19. Neal JM, Barrington MJ, Brull R, et al. The Second ASRA Practice Advisory on Neurologic Complications Associated With Regional Anesthesia and Pain Medicine: executive Summary 2015. Reg Anesth Pain Med. 2015;40(5):401–430. doi:10.1097/AAP.0000000000000286
20. Lee H, Bang J, Kim S, Yang H. The axillary vein and its tributaries are not in the mirror image of the axillary artery and its branches. PLoS One. 2019;14:e0210464. doi:10.1371/journal.pone.0210464
21. Hewson DW, Bedforth NM, Hardman JG. Peripheral nerve injury arising in anaesthesia practice. Anaesthesia. 2018;73:51–60. doi:10.1111/anae.14140
22. Youssef MS, Desgrand DA. Comparison of two methods of axillary brachial plexus anaesthesia. Br J Anaesth. 1988;60(7):841–844. doi:10.1093/bja/60.7.841
23. Casati A, Danelli G, Baciarello M, et al. A prospective, randomized comparison between ultrasound and nerve stimulation guidance for multiple injection axillary brachial plexus block. Anesthesiology. 2007;106(5):992–996. doi:10.1097/01.anes.0000265159.55179.e1
24. Barrington MJ, Gledhill SR, Kluger R, et al. A randomized controlled trial of ultrasound versus nerve stimulator guidance for axillary brachial plexus block. Reg Anesth Pain Med. 2016;41(6):671–677. doi:10.1097/AAP.0000000000000486
25. Wang H, Li L, Xu C, Qu X, Qu Z, Wang G. The efficacy of simultaneous bilateral axillary brachial plexus block under the guidance of neurostimulator or ultrasound: a prospective study. J Anesth. 2016;30:596–602. doi:10.1007/s00540-016-2193-2
26. Demirelli G, Baskan S, Karabeyoglu I, et al. Comparison of ultrasound and ultrasound plus nerve stimulator guidance axillary plexus block. J Pak Med Assoc. 2017;67(4):508–512.
27. Gili S, Abreo A, GóMez-Fernández M, Solà R, Morros C, Sala-Blanch X. Patterns of distribution of the nerves around the axillary artery evaluated by ultrasound and assessed by nerve stimulation during axillary block. Clin Anat. 2019;32(1):2–8. doi:10.1002/ca.23225
28. Dooley J, Bullock WM, Kumar AH, MacLeod DB, Gadsden J. Systematic sonographic and evoked motor identification of the nerve to vastus medialis during adductor canal block. Reg Anesth Pain Med. 2020;45(11):937–938. doi:10.1136/rapm-2019-101232
29. Sancheti SF, Uppal V, Sandeski R, Kwofie MK, Szerb JJ. A cadaver study investigating structures encountered by the needle during a retroclavicular approach to infraclavicular brachial plexus block. Reg Anesth Pain Med. 2018;43(7):752–755. doi:10.1097/AAP.0000000000000826
30. Gadsden JC. The role of peripheral nerve stimulation in the era of ultrasound-guided regional anaesthesia. Anaesthesia. 2021;76(S1):65–73. doi:10.1111/anae.15257
31. Rambhia M, Gadsden J. Pressure monitoring: the evidence so far. Best Pract Res Clin Anaesthesiol. 2019;33(1):47–56. doi:10.1016/j.bpa.2019.03.001
32. Gadsden JC, Choi JJ, Lin E, Robinson A. Opening injection pressure consistently detects needle-nerve contact during ultrasound-guided interscalene brachial plexus block. Anesthesiology. 2014;120(5):1246–1253. doi:10.1097/ALN.0000000000000133
33. Pascarella G, Strumia A, Costa F, et al. Triple monitoring may avoid intraneural injection during interscalene brachial plexus block for arthroscopic shoulder surgery: a prospective preliminary study. J Clin Med. 2021;10(4):781. doi:10.3390/jcm10040781
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
