Back to Journals » Eye and Brain » Volume 15
Neuromodulation: Actions of Dopamine, Retinoic Acid, Nitric Oxide, and Other Substances on Retinal Horizontal Cells
Authors McMahon DG , Dowling JE
Received 5 May 2023
Accepted for publication 18 August 2023
Published 31 October 2023 Volume 2023:15 Pages 125—137
DOI https://doi.org/10.2147/EB.S420050
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Margaret Wong-Riley
Douglas G McMahon,1 John E Dowling2
1Department of Biological Sciences, Vanderbilt University, Nashville, TN, 37235, USA; 2Department of Molecular and Cellular Biology, Harvard University, Cambridge, MA, 02138, USA
Correspondence: Douglas G McMahon, Department of Biological Sciences, Vanderbilt University, Nashville, TN, 37235, USA, Email [email protected]
Abstract: Whereas excitation and inhibition of neurons are well understood, it is clear that neuromodulatory influences on neurons and their synapses play a major role in shaping neural activity in the brain. Memory and learning, emotional and other complex behaviors, as well as cognitive disorders have all been related to neuromodulatory mechanisms. A number of neuroactive substances including monoamines such as dopamine and neuropeptides have been shown to act as neuromodulators, but other substances thought to play very different roles in the body and brain act as neuromodulators, such as retinoic acid. We still understand little about how neuromodulatory substances exert their effects, and the present review focuses on how two such substances, dopamine and retinoic acid, exert their effects. The emphasis is on the underlying neuromodulatory mechanisms down to the molecular level that allow the second order bipolar cells and the output neurons of the retina, the ganglion cells, to respond to different environmental (ie lighting) conditions. The modulation described affects a simple circuit in the outer retina, involves several neuroactive substances and is surprisingly complex and not fully understood.
Keywords: neural network adaptation, vision, synaptic modulation, gap junctions, glutamate receptors, GABA receptors
In addition to rapid synaptic excitation and inhibition, neuromodulation, by slow and sustained influences of neuroactive substances, plays a critical role in determining the responses of neurons in the brain. Beyond the well-known neuroactive substances being involved in neuromodulation such as monoamines and neuropeptides, other substances that play very different roles in various tissues including the brain, such as retinoic acid (RA), have now also been shown to act as neuromodulators.
Having spent many years studying neuromodulation in the retina, and one of us having shown RA is the active metabolite of vitamin A except for the initial step in phototransduction that requires retinaldehyde,1,2 a review of the modulation of retinal function and synaptic plasticity by various agents seemed appropriate and hopefully useful. Here, we will focus on and compare the roles of dopamine and RA as retinal neuromodulators, but also briefly discuss other substances such as nitric oxide, which may also play such a role.
Retinal studies have shed considerable light on the cellular and molecular mechanisms underlying how neuromodulators exert their effects, something we know less about in terms of how these substances work elsewhere in the brain, where most emphasis has been on mapping where neuromodulatory substances are found, the pathways and projections in which they are involved, and the behaviors that relate to these substances. One reason the retina has been yielding in this regard is because of a relatively simple circuit found in the outer retina involved in establishing the center-surround receptive field organization of second-order retinal bipolar cells.3 It has long been known that the center surround organization of ganglion cells, the third order retinal neurons that receive input from bipolar cells, show strikingly different surround responses depending on lighting conditions.4 In the dark, surround antagonism is weak or non-existent, whereas in the light there is significant surround inhibition; the notion being that in the dark with little surround inhibition, the retina is a more efficient photodetector, whereas in the light, with strong surround inhibition, the retina is better at resolving images.
The outer retinal circuit consists of three neuronal elements: photoreceptors that provide the input to second order bipolar and horizontal cells. The bipolar cells carry a visual message to the inner retina where they synapse on third-order ganglion and amacrine cells, while the horizontal cells are confined to the outer retina and spread their processes widely there (see Figure 1). In addition, the horizontal cells are electrically coupled to one another, via electrical (gap junction) synapses, which greatly extends their receptive field size. The photoreceptors directly activate the bipolar and horizontal cells, whereas the horizontal cells provide inhibition back onto the photoreceptor cells as well as forward inhibition onto the bipolar cells. Thus, the center-surround organization observed in bipolar cells occurs as follows: The center response reflects the direct photoreceptor-bipolar cell synaptic input, whereas the surround response results from inhibitory synaptic interactions mediated by the horizontal cells. The transmitter released from the photoreceptors is L-glutamate,6 but how horizontal cells feedback onto photoreceptors occurs is still uncertain. There is evidence for possible contributions by GABA, for ephaptic signaling through gap junction hemi-channels, and for protons.7,8 The feed-forward inhibition by the horizontal cells onto bipolar cells appears to be mediated by GABA at classic synapses.9
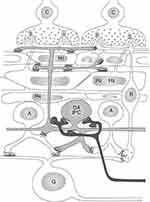 |
Figure 1 Schematic diagram of the synaptic connections of the dopaminergic interplexiform cells (DA IPC) of the fish retina. The input to these neurons is the inner plexiform layer from amacrine cells (A) and centrifugal fibers (black) that originate in the olfactory bulb. The interplexiform cell processes make synapses onto amacrine cell processes in the inner plexiform layer, but they never contact the ganglion cells (G) or their dendrites. In the outer plexiform layer processes of the interplexiform cells surround the cone horizontal cells of all three types (H1, H2, and H3). They make synapses onto the horizontal cell perikarya and onto bipolar (B) cell dendrites, but not on the cone (C) synaptic terminals. With permission from “The Retina: An approachable part of the brain”. J.E. Dowling, Harvard University Press, 2012, figure adapted and modified from Zucker and Dowling (1987).5 |
The above is a bare-bones description of the circuit, and our focus here will be on the horizontal cells. We will start with dopamine modulation of horizontal cells and move on to discuss nitric oxide briefly and then on to the modulation by RA and other substances thought to be involved.
Dopamine
Although dopamine was first synthesized in 1910, its importance as a neuroactive substance in the brain was not appreciated until the late 1950s when it was discovered that there was a loss of dopamine in the brains of Parkinson patients.10 Subsequently, it was shown that L-DOPA, a precursor of dopamine, is an effective therapy for the disease, cementing the importance of dopamine in neural function.
In the 1970s, the first dopamine receptor was identified, and by the 1980s five dopamine receptors had been found.11 All, it was shown, exert their effects by altering the levels of the second messenger molecule cyclic AMP (cAMP). The D1 and D5 receptors couple to Gαs heterotrimeric G-proteins to increase cAMP levels, whereas D2, 3, and 4 receptors couple to Gαi heterotrimeric G-proteins to decrease cAMP levels. Dopamine receptors are found in varying amounts in the brain, with the D1 receptors being the most abundant, and D4 receptors least abundant, although D4 receptors are well-expressed in the retina. There is also variability in which receptors are in various brain regions, although overlap does occur. In some brain regions, where there is co-expression of different dopamine receptors, there is evidence for the formation of heterodimers and novel Gαq coupling to Ca++ and PKC pathways in addition to the canonical Gαs and Gαi coupling to cAMP.12
Dopamine neurons in the retina were first observed using the Falk Hillarp method, which results in neurons containing dopamine to fluoresce.13 Curiously, in teleost fish and New World monkeys, the dopaminergic neurons appeared unique among retinal neurons in that, although their cell perikarya sit among the amacrine cells in the inner retina, they extend abundant processes in both synaptic layers in the retina - the outer plexiform layer in the outer retina and inner plexiform layer in the inner retina – something amacrine cells do not do (Figure 1). Termed interplexiform (IP) cells, they were found to form synapses on horizontal cells in the outer plexiform layer (OPL) and on amacrine cells in the inner plexiform layer (IPL).
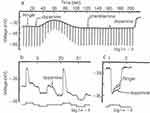 |
Figure 2 The effects of dopamine on intracellularly recorded responses of a horizontal cell, depolarizing bipolar cells, and red cone. (a) Dopamine depolarized the horizontal cell and reduced its light responsiveness. The effects of dopamine were blocked by the antagonist phentolamine. (b) Dopamine hyperpolarized the bipolar cell; following dopamine application the depolarizing response to center illumination (raised abscissa) was increased, while the hyperpolarizing response to annular illumination (decreased abscissa) was decreased. (c) Dopamine caused no change in the resting membrane potential of the cone. It did affect the waveform of the response, however, making the response squarer. The change in waveform reflects, presumably a decrease of horizontal feedback onto the receptor. Log I relative flash intensity. With permission from the “The Retina: An approachable part of the brain”. J. E. Dowling, Harvard University Press, 2012, figure adapted and modified from Hedden and Dowling (1978).24 |
Subsequently, such IP cells were identified in other species by Golgi staining, and so such cells are believed to be a general feature of all vertebrate retinas.14 Curiously, most OPL IP cell processes do not show abundant dopamine fluorescence, although some such OPL fluorescence has now been seen in many retinas. However, in all species, clear dopamine fluorescence is observed in the IPL.
That clear and abundant dopaminergic fluorescence is observed prominently in IP cells in teleost fish provided the opportunity to explore in depth the effects of dopamine on a specific type of retinal neuron, the horizontal cell, which mediates lateral inhibition in the outer retina. Furthermore, the horizontal cells in teleost fish are exceptionally large neurons, easily identified both in vivo and in vitro, and are especially convenient for many types of physiological and biophysical studies including intracellular recordings with both sharp and patch electrodes, intracellular injections and perfusions, and the analysis of single-channel properties in both control and drug-treated preparations.
An additional advantage of this system for a detailed analysis was the finding by electron microscopy that the dopaminergic IP neurons in fish make classic synapses on the horizontal cells, whereas their input is all in the IPL. Whereas the cells do form synapses in the IPL, most of their synapses are in the OPL. Thus, IP cells serve as centrifugal neurons within the retina carrying information from the inner to the outer retina. Interestingly, the IP cells in the fish's inner retina get at least some of their input from centrifugal fibers that enter the retina from higher brain regions.5
In the mammalian retina dopaminergic IP neurons also serve as centrifugal neurons – histaminergic centrifugal fibers from the hypothalamus15 also innervate the dopaminergic IP neurons of the rodent retina, where they influence dopamine release. Remarkably, dopaminergic IP neurons also form a retrograde signaling pathway conducting photic signals that originate in novel melanopsin-expressing intrinsically photoreceptive ganglion cells from the inner to the outer retina. A subset of dopaminergic IP neurons receive synaptic contacts from and are driven by the light responses of melanopsin ganglion cells.16,17 Intrinsically, photoreceptive ganglion cells drive sustained spiking of dopaminergic neurons and provide intra-retinal feedback of background light intensity, likely acting on retinal cone adaptation through D4 dopamine receptors.18 Interestingly, this reciprocal microcircuit – in which melanopsin ganglion cells drive dopamine neurons, and dopamine neurons inhibit melanopsin ganglion cells – is tuned and modulated by somatostatin-expressing amacrine cells.19 Light drive on retinal dopamine neurons by melanopsin ganglion cells likely functions in parallel with the drive from outer retinal photoreceptors20–22 to provide a sustained component to light-induced dopamine release similar to the way melanopsin ganglion cell signaling mediates sustained components of circadian photoreception and the pupillary light response.23
These findings reveal a novel function of retinal ganglion cells – intraretinal photic signaling – in addition to their canonical role as projection neurons projecting visual signals to the rest of the brain. With photoreception originating in both the inner and outer retina, and with reciprocal signaling in each direction – now one can truly say that the retina is a two-way street, with IP neurons providing the “centrifugal lane”! Overall, the IP cells then represent an intraretinal feedback system for neuromodulation based on light intensity, as well as the final stage of a centrifugal system from central brain regions to the outer reaches of the retina.
The first physiological experiments examining the effects of dopamine on retinal neurons were carried out in the goldfish retina.24 Intracellular responses were recorded from cone photoreceptors, bipolar cells, and horizontal cells (Figure 2). In horizontal cells, dopamine induced a slow depolarization of the membrane potential and a decrease in light-evoked responses. Important to note is that the outer retinal neurons respond to light only with graded potentials; they do not generate action potentials, again a feature that aids in the analysis of the effects of a neuroactive substance on a neuron. Furthermore, the photoreceptors hyperpolarize in response to light, as do the horizontal cells. Bipolar cells, on the other hand, are of two basic types – ON-bipolar cells that depolarize to a central light spot and OFF-cells that hyperpolarize to a central spot. Both bipolar cell types show surround inhibition induced by an annulus of light - ON-cells hyperpolarize; OFF-cells depolarize. Thus, bipolar cells are the first cell types in the visual system to show clear center-surround receptive field organization.25
 |
Figure 3 Effects of dopamine on an electrically coupled pair of white perch horizontal cells maintained in culture (left). Both cells were voltage-clamped at −60mV with patch electrodes, and current pulses were applied to the driver cell (lower trace) to shift the membrane potential +20mV. Ringer solution containing dopamine was applied briefly (0.5 sec) to the cell pair. The cells uncoupled, as shown by the decrease in magnitude of the current pulses required to depolarize the driver cell by 20mV, which reflects the increase of resistance of the driver cell. The decrease in the magnitude of the current pulses passed into the follower cell (upper trace), reflects the decreased conductance of the junctional membrane. With permission from “The Retina: An approachable part of the brain”, J.E. Dowling, Harvard University Press, 2012, figure adapted and modified from Lasater and Dowling (1985).27 |
The dopamine-induced depolarization and reduction in light-evoked responses in horizontal cells are slow and long-lasting; a brief pulse of exogenously applied DA to the retina resulted in membrane potential changes and reduction in light responsiveness that lasted for many seconds. Why DA induces these changes in horizontal cells will be explained later, but recordings from bipolar and photoreceptor cells are more revealing in terms of one of the primary effects of DA on this simple circuit. That is, DA in both cases reduces the effects of surround illumination. In the case of bipolar cells, since one can elicit center and surround responses separately, dopamine increased the central response while decreasing the surround response. In the photoreceptor response, DA reduced the rapid partial recovery of the response following its initial peak. Both the surround response of bipolar cells and the partial depolarization of the photoreceptor following the initial peak response are thought to be mediated by inhibition from the horizontal cells, suggesting that DA was reducing the effectiveness of this inhibition.
How might this occur? Shortly after these initial studies, Negishi and Drujan26 in Japan elegantly showed that DA uncouples horizontal cells from one another. Recall that horizontal cells are coupled by electrical (gap junction) synapses, and what Negishi and Drujan did was to compare horizontal cell responses to light spots and annuli. Upon DA application, the annular responses declined in amplitude, whereas the spot responses grew in size. That this is due to electrical uncoupling of the cells was subsequently demonstrated by isolating pairs of horizontal cells that were electrically coupled to one another and applying DA to them (Figure 3).27 Current pulses to depolarize the membrane by 20 mV were injected into one of the two cells, the driver cell, and the current pulses then passed into the follower cell via the gap junctions. The voltages generated across the driver cell’s membrane reflect the resistance of its membrane, whereas the voltages induced in the follower cell’s membrane reflect how much current is passing from one cell to the other. As seen in the figure, the voltages generated across the follower cell’s membrane decreased substantially after DA application, indicating that the gap junctions were admitting much less current. The current pulses in the driver cell decreased because the resistance of its cell membrane substantially increased and much less current was now needed to depolarize the membrane by 20 mV in accord with Ohm’s Law (V=IR).
 |
Figure 4 (a) Intracellular records of carp horizontal cell responses to spot and full-field white-light stimuli before, during, and after the addition of dopamine (20µM) to the superfusion medium. The spot (0.8mm in diameter) and full-field stimuli were presented as an alternating pair and adjusted before dopamine application to generate responses of approximately equal amplitudes. Dopamine caused the responses to the spot stimuli to increase in amplitude and the responses to the full-field stimuli to decrease in amplitude. Recovery from these drug effects required an average of about 15 min. Note that dopamine also caused the horizontal cell to depolarize slightly. (b) Average carp horizontal cell response amplitudes as a function of stimulus spot diameter. The stimuli were centered on receptors in the middle of the cell’s receptive field and were at an intensity that generated a half-maximal response when a full-field stimulus was used. Dopamine application cause average response amplitudes to small spot stimuli to be significantly larger and average response to large spot and full-field stimuli to be significantly smaller. With permission from “The Retina: An approachable part of the brain”, J.E.Dowling, Harvard University Press, 2012, figure adapted and modified from Mangel and Dowling, (1985).30 |
Why were the gap junctions less effective in allowing current to flow from one cell to the other? This was studied by examining single-channel kinetics in pairs of horizontal cells in culture as well as by noise analysis following DA application.28 The single channel unitary conductance did not change (~50 ps) in response to DA nor the number of channels in the junctions, but because of a reduction in the open probability of the channels after DA application (~0.75–0.14) brought about by a decrease in the open time of the channels by 2–3-fold.
As noted earlier, DA exerts its effects on neurons by altering levels of the second-messenger molecule, cAMP, and that this occurs in teleost horizontal cells was shown by isolating and purifying batches of them by velocity sedimentation and applying DA to them, which resulted in cAMP accumulation.29 The horizontal cells possess D1 receptors, which when activated by DA, stimulate a G-protein that in turn activates the enzyme adenylate cyclase, which converts ATP to cAMP. Cyclic AMP exerts its effects by activating protein kinase A (PKA) and injections of PKA into fish horizontal cells uncouples them from one another.
This is an elegant story, but it is just the beginning. Figure 4 shows an experiment in which DA is applied to horizontal cells in a teleost retina and responses to small spots of light increase in amplitude, whereas responses to full field illumination decrease in size.30 This is similar to the Negishi experiments noted earlier showing uncoupling of horizontal cells in response to DA, but with one important difference. Here, rather than using annular stimulation to show the decrease in coupling, full field illumination was used. With full field illumination, little current flow should occur between adjacent horizontal cells because they all receive equal input from the photoreceptors and should all be at the same membrane potential. Thus, altering uncoupling strength should not have an effect on response amplitude. Thus, this experiment indicates that DA is having another effect on the horizontal cells in addition to reducing gap junctional conductance.
What was then found was that DA significantly enhances the response of isolated horizontal cells maintained in culture to L-glutamate.31 The glutamate receptors found on horizontal cells are of the AMPA type and are strongly activated by glutamate as well as by kainate, a non-desensitizing glutamate analogue. Cyclic AMP when injected into the cells mimicked the effect of DA as did the injection of PKA.32 For cAMP and PKA to enhance the response of the cell to kainate, ATP needed to be present in the injection pipette, indicating that phosphorylation was involved.
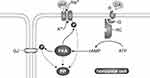
Subsequent analysis of single-channel currents in horizontal cells in response to glutamate or kainate showed that DA increased the frequency of channel opening to a given concentration of glutamate or kainate.33 Thus, the channels were more sensitive to the photoreceptor transmitter glutamate, and this explains why DA depolarizes horizontal cells and reduces their light-evoked responses (Figure 2). That is, by increasing the frequency of opening of the glutamate channels, the cell depolarizes, and because the channels are now more sensitive to glutamate, light that reduces the amount of glutamate released from the photoreceptors (because they hyperpolarize in response to light) will not be as effective; hence, the full-field light responses are smaller. Figure 5 summarizes the biochemical events that occur in a horizontal cell in response to dopamine. The cAMP generated in the cell exerts its effects via phosphorylation by PKA, or possibly by activation of a phosphatase activated by PKA that dephosphorylates proteins in the cell. In All amacrine cells of the IPL, D1 receptors have been shown to modulate gap junctional coupling via activation of phosphatase 2A;34 however, whether D1 stimulation in horizontal cells acts on gap junctions and glutamate receptors via phosphorylation or dephosphorylation has not been determined.
 |
Figure 6 The diverse and multiple effects of dopamine on the cells and synapses in vertebrate retinas. Most of the effects serve to alter the strength of the retinal synapses and reconfigure the retinal circuitry or are on ion channels that contribute to the response of the neurons. The synapses modified by dopamine are indicated, as the ion channels (eg Ina). En passant synapses of bipolar cells to DA neurons and ipGs are indicated.72,73 With permission from “The Retina: An approachable part of the brain” J.E. Dowling, Harvard University Press,3 2012, adapted and modified by D.G. McMahon. |
Interestingly, recent work by Mangel et al demonstrated that ON cone bipolar cells also express dopamine D1 receptors and that there they also play a role in light-adapted receptive field organization.35 Here, dopamine acting through D1 receptors rapidly influences the expression of GABAR on cone bipolar terminals to modulate the strength of HC-driven surrounds in light adaptation.35 Thus, dopamine acting through D1 receptors, cAMP and PKA mediates modulatory changes in both the kinetics and expression of ion channels and receptors in both horizontal cells and bipolar cells in the outer retina.
As D1 dopamine receptors act on both horizontal cells and bipolar cells in the outer retina, this begs the question of the role of dopamine modulation in each cell type with regard to modulation of ganglion cell receptive fields. Addressing the cell-specific roles of dopaminergic modulation experiments using cell-specific knockout of dopamine receptors followed by analysis of receptive field responses to DA could reveal mechanisms of receptive field modulation in intact retinal circuits.
Nitric Oxide
About a decade after the original studies suggesting that dopamine uncouples gap junctions in the retina, DeVries and Schwartz36 found that the gas, nitric oxide (NO), also modulates gap junctions between horizontal cells. Nitric oxide synthase is found in both inner and outer retinal cells including the horizontal cells themselves and has effects on many retinal cell types. NO also activates the synthesis of a second messenger, in this case cGMP, by activating guanylate cyclase. With methods similar to those used to analyze the effects of DA, Lu and McMahon37 showed by applying the NO donor sodium nitro-prusside (SNP) to teleost horizontal cells that the coupling between pairs of horizontal cells decreased by ~60%. To reach maximum uncoupling required 1–2 min and the uncoupling effect lasted for 5–10 min. As with DA, SNP reduced the junctional open probability of the channels, from a control value of ~17 openings/second to ~7.1 openings/sec. Open duration did not change significantly nor did unitary conductance, however there was an increase in the channel closed time.
That the NO acts through cGMP was shown by applying membrane permeable 8-bromo cGMP to the cells, as well as an inhibitor of PKG that, interestingly, not only blocked the effect of SNP but also increased the basal coupling level, suggesting that NO regulates the basal coupling of the junctions. NO action on gap junction coupling in the retina is not limited to horizontal cells but occurs in AII amacrine cells as well.38
What about the effect of NO on horizontal cell glutamate receptors? What was found was NO and cGMP do modulate the glutamate receptors but in a different way to that of DA. NO reduced the horizontal cell responsiveness to relatively low concentrations of glutamate and kainate39 but increased the maximal current at higher glutamate concentrations thought to reflect synaptic levels of glutamate.40 Quantitative modeling of the dose–response effects of NO on horizontal cell glutamate currents showed that they predict the observed increase in horizontal cell light response amplitudes of NO application in the intact retina.40 Interestingly, NO and DA applied together act synergistically to greatly increase the magnitude of glutamate-evoked currents in horizontal cells.40 Thus, horizontal cell glutamate receptors can be independently, or synergistically affected by second messengers – cAMP increases their affinity to glutamate, whereas cGMP increases maximal current, again by PKA and PKG, respectively. While we have focused here on the effects of nitric oxide on retinal neurons, it is important to note that NO also regulates blood flow in vascularized retinas, as well as modulating immune responses, and therefore has potentially important impacts on a range of eye diseases.41
Retinoic Acid
Retinoic acid (RA) is generally viewed as a morphogen, regulating many aspects of development and maintenance of many tissues including the eye, by the activation of retinoic acid receptors, RARs, and RXRs, which in turn operate mainly by increasing or decreasing the transcription of genes. Indeed, it is estimated that RA alters the expression of hundreds of genes.42 Retinoic acid is involved in early patterning of the retina, establishing dorsal and ventral characteristics in the zebrafish,43,44 in the maturation of retinal cells and circuits45 and in the formation of high-acuity regions of the retina like the fovea.46 Thus, it was a considerable surprise when it was shown by Weiler et al in the late 1990s that RA modulates gap junctional permeability between horizontal cells in adult retinas, not only in fish but also in the mammalian retina.47
How RA does this was elegantly shown by recording from pairs of coupled fish horizontal cells in culture. Micromolar concentrations of RA reduced junctional conductance by up to more than 90%, and a half-maximal inhibition of conductance required just ~2.5 uM of RA. RA did not affect glutamate or kainate-induced currents; indeed, RA seems to have no effect on horizontal cell glutamate channels. A corollary finding to the above was the demonstration that light increases the level of RA in mammalian and fish retinas relative to the dark retina, suggesting how the effects of RA are mediated.48,49 However, these studies did not establish absolute concentrations of RA in the retina.
All-trans RA and 9-cis RA are equipotent in activating the RARs, but all-trans RA is about 50-fold less potent in activating RXRs.11 All-trans RA and 9-cis RA are equally potent in uncoupling horizontal cell gap junctions, suggesting that the uncoupling effect is mediated by an RAR-like protein. When all-trans RA was injected into horizontal cells, or a PKA or PKC inhibitor, there was no uncoupling. On the other hand, application of 1 μM RA applied externally, reduced the cells coupling by ~60%, as did application of RAR-alpha agonists but not RARβ and RARγ-specific agonists, indicating that the uncoupling was being mediated by an external binding site, presumably by an RAR-alpha-like receptor.50 No second messenger was involved nor was a transcriptional mechanism. Remarkably, RA modulates gap junction channels in cell-free excised membrane patches, suggesting direct modulation by the receptor. Single-channel analysis indicated that RA reduced channel opening probability, but not unitary conductance. In addition, RA also induced morphological plasticity in horizontal cells by a non-transcriptional mechanism – stimulating the growth of horizontal cell spinules (see discussion below).
The surprising and unconventional way that RA uncouples horizontal cells via an RA-activated protein without the involvement of a second messenger, PKA, PKG, or PKC, suggests that RA could potentially affect other neural circuits in similar unique ways. Indeed, such has been found in the hippocampus where one RAR iso-form, RAR-alpha, directly enhances the expression of AMPA-type glutamate receptors, thus altering synaptic efficacy by altering the number of glutamate receptors postsynaptically.51
RA receptors typically regulate protein expression at the genomic level, but here it is a non-genomic, direct effect altering translation and the expression of AMPA receptor protein. RA levels in the hippocampus appear to be regulated by synaptic activity, just as light regulates RA levels in the retina, and in this way synaptic efficacy is modulated. The hippocampus is known to be involved in memory consolidation, and it has been shown that vitamin A deprivation and RAR-alpha deletion in mice result in learning changes in animals confirming the significance of this synaptic modulation.52,53 Similarly, RA has recently been found to upregulate synaptic transmission and induce structural plasticity in human cortical synapses.54 Thus, RA is being explored as a potential treatment for neuropsychiatric and memory disorders such as Alzheimer’s disease.
RA signaling is involved at a number of levels in retinal degeneration and therefore has potential clinical application to retinal degenerative disorders as well. RA is a critical driver of chronic ganglion cell hyperactivity in photoreceptor-degenerate retinas, which complicates strategies to restore vision and drives further degradation of visual function downstream in visual circuits in the brain.55,56 Synaptic remodeling and altered gap junctional coupling are key contributors to circuit hyperactivity. Could the non-transcriptional actions of RA first described in horizontal cells play a role? This has yet to be explored.
Other Potential Modulators
Neuropeptide, vasoactive intestinal peptide (VIP), also has interesting effects on horizontal cells.57 First, it substantially depolarizes horizontal cells with a decrease in membrane resistance, suggesting it directly opens membrane channels as do classic excitatory neurotransmitters. However, VIP also strongly activates adenylate cyclase, producing substantial amounts of cAMP in the cells. As yet, we do not know what the effect of VIP has on the coupling of horizontal cells or their response to glutamate. VIP is found in amacrine cells in the inner plexiform layer, but no VIP containing processes have been observed in the outer retina, suggesting that the VIP is released some distance away from the horizontal cells but can diffuse to them to cause effects, something other peptides have been shown to do. Indeed, topically applied VIP/PACAP eye-drops have been shown to attenuate ischemic retinal degeneration in rodent models.58
Another potential modulator is zinc (Zn 2+) which is found in photoreceptor terminals co-localized with glutamate in synaptic vesicles.59 In the retina, zinc has been shown to modulate gamma-aminobutyric acid (GABA) receptors, AMPA-type glutamate receptors, K+ channels, and hemi-gap junction channels on horizontal cells; GABA receptors on bipolar cells; and NMDA and glycine receptors on ganglion cells.59–65 Zinc modulation of AMPA and NMDAR is widespread throughout the rest of the brain where it contributes to synaptic plasticity underpinning fear conditioning (AMPAR),66 and potentially contributing to major depression (NMDAR).67 In addition, in the retina, zinc is critical for neuroprotection. There is prominent zinc mobilization from amacrine to ganglion cells following optic nerve crush, the prevention of which with zinc chelators greatly enhances ganglion cell survival and axonal regeneration.68
Conclusions
We describe here a simple circuit involved in establishing center-surround receptive field organization in retinal bipolar cells. What is astonishing in our view is how modifiable the circuit is, by so many neuroactive substances and in so many ways. We understand a number of the mechanisms underlying the modulation, but almost certainly not all, and we have some notion of why it is important to modulate the circuit - to accommodate the visual environment. But the richness of the modulation, the number of substances and mechanisms involved is such that it is likely much more is going on than what we have described here.
Indeed, as noted above, there are structural changes that occur in fish horizontal cells that relate to light and dark and which appear to be partially modulated by DA and RA69,70 In the light, the horizontal cell processes making contact with the photoreceptor terminals from which they receive synaptic input extend small extensions, called spinules, deeper into the terminals. These spinules disappear in the dark and both DA and RA can induce the reappearance of spindles in the horizontal cells. However, neither substance appears to completely mimic light adaptation nor are horizontal cell spinules observed in other species, and their function even in fish is not understood. Thus, we have not emphasized them here.
While the data we have reviewed here are primarily from teleost fish horizontal cells, there is evidence that similar processes and mechanisms occur in other vertebrate species, including mammals. We do know, however, that horizontal cells differ somewhat in different species, including even among fish species, but what does this mean? We do not know. Fish are particularly rich in terms of color vision,71 and perhaps some of this modulation relates to this. We have here only reported on research related to cone horizontal cells, and for the most part not on horizontal cells that show differential color responses.
In addition to its effects on horizontal cells, dopamine has now been shown to have effects on every retinal neuron in vertebrate retinas. A number of these effects are indicated in Figure 6. Many of these effects relate to modulation of electrical coupling between neurons (of which there is a substantial amount in the retina), but also there are effects on both ligand and ionic channels. Thus, the extent of modulation of circuitry in the retina by just dopamine is surprisingly extensive.
This review then makes it clear that not only can there be a substantial amount of neuromodulatory effects that can occur within a simple circuit but it further emphasizes that how to identify and understand these interactions is an enormous challenge. We can, of course, identify excitatory and inhibitory synapses anatomically, especially by electron microscopy. However, some neuroactive substances when released from a neuron, especially neuropeptides, can have effects on neurons many micrometers away, and so such interactions cannot be visualized. It is also the case that many inhibitory neurons using GABA as their transmitter also contain and release neuropeptides so that the effects of the interactions exerted by such neurons can be much more than simple inhibition. Further, substances not thought to be neurotransmitters or neuromodulators, such as retinoic acid, released from unknown cells, can also induce neuromodulatory effects on neurons. Finally, substances like NO, synthesized in a neuron, can have effects on that same neuron, regulating perhaps how that neuron responds to synaptic substances impinging on that cell, setting perhaps baseline activity levels for that neuron.
In summary, as we attempt to understand neural circuitry in the brain, we must keep in mind the numerous ways neuronal basal activity, neuronal responses to stimuli, and synaptic effectiveness for both chemical and electrical synapses can be modified. What we have discussed in this review, what we have learned thus far about neuromodulation from the study of the first simple circuit in the visual system, is a beginning to what will ultimately be a fuller story. We have much work to do, and far to go, to understand complex neural circuits and the brain of any species.
Acknowledgments
The authors gratefully acknowledge the assistance of Dr. Manuel Giannnoni-Guzmán with figures and the support of NIH R01 GM 117650 to DGM.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Dowling JE, Wald G. The biological function of vitamin A acid. Proc Natl Acad Sci USA. 1960;46(5):587–608. doi:10.1073/pnas.46.5.587
2. Dowling JE. Vitamin A: its many roles-from vision and synaptic plasticity to infant mortality. J Comp Physiol a Neuroethol Sens Neural Behav Physiol. 2020;206(3):389–399. doi:10.1007/s00359-020-01403-z
3. Dowling JE. The Retina: An Approachable Part of the Brain. Belknap Press of Harvard University Press; 2012.
4. Barlow HB, Fitzhugh R, Kuffler SW. Change of organization in the receptive fields of the cat’s retina during dark adaptation. J Physiol. 1957;137(3):338–354. doi:10.1113/jphysiol.1957.sp005817
5. Zucker CL, Dowling JE. Centrifugal fibres synapse on dopaminergic interplexiform cells in the teleost retina. Nature. 1987;330(6144):166–168. doi:10.1038/330166a0
6. Lasater EM, Dowling JE. Carp horizontal cells in culture respond selectively to L-glutamate and its agonists. Proc Natl Acad Sci USA. 1982;79(3):936–940. doi:10.1073/pnas.79.3.936
7. Chapot CA, Euler T, Schubert T. How do horizontal cells ‘talk’ to cone photoreceptors? Different levels of complexity at the cone-horizontal cell synapse. J Physiol. 2017;595(16):5495–5506. doi:10.1113/JP274177
8. Barnes S, Grove JCR, McHugh CF, Hirano AA, Brecha NC. Horizontal cell feedback to cone photoreceptors in mammalian retina: novel insights from the GABA-pH hybrid model. Front Cell Neurosci. 2020;14:14. doi:10.3389/fncel.2020.00014
9. Thoreson WB, Mangel SC. Lateral interactions in the outer retina. Prog Retin Eye Res. 2012;31(5):407–441. doi:10.1016/j.preteyeres.2012.04.003
10. Carlsson A, Lindqvist M, Magnusson T. 3,4-Dihydroxyphenylalanine and 5-hydroxytryptophan as reserpine antagonists. Nature. 1957;180(4596):1200. doi:10.1038/1801200a0
11. Massale C, Nash SR, Robinson SW, Jabern M, Caron M. Dopamine receptors: From Structure to function. Physiological Reviews. 1998;78(1):139–225.
12. Perreault ML, Hasbi A, O’Dowd BF, George SR. Heteromeric dopamine receptor signaling complexes: emerging neurobiology and disease relevance. Neuropsychopharmacology. 2014;39(1):156–168. doi:10.1038/npp.2013.148
13. Dowling JE, Ehinger B. Synaptic organization of the amine-containing interplexiform cells of the goldfish and Cebus monkey retinas. Science. 1975;188(4185):270–273. doi:10.1126/science.804181
14. Boycott BB, Dowling JE, Fisher SK, Kolb H, Laties AM. Interplexiform cells of the mammalian retina and their comparison with catecholamine-containing retinal cells. Proc R Soc Lond B Biol Sci. 1975;191(1104):353–368. doi:10.1098/RSPB.1975.0133
15. Frazão R, McMahon DG, Schunack W, Datta P, Heidelberger R, Marshak DW. Histamine elevates free intracellular calcium in mouse retinal dopaminergic cells via H1-receptors. Invest Ophthalmol Vis Sci. 2011;52(6):3083–3088. doi:10.1167/iovs.10-6160
16. Zhang DQ, Wong KY, Sollars PJ, Berson DM, Pickard GE, McMahon DG. Intraretinal signaling by ganglion cell photoreceptors to dopaminergic amacrine neurons. Proc Nat Acad Sci. 2008;105(37):14181–14186. doi:10.1073/pnas.0803893105
17. Zhang DQ, Belenky MA, Sollars PJ, Pickard GE, McMahon DG. Melanopsin mediates retrograde visual signaling in the retina. PLoS One. 2012;7(8):e42647. doi:10.1371/journal.pone.0042647
18. Prigge CL, Yeh PT, Liou NF, et al. M1 ipRGCs influence visual function through retrograde signaling in the retina. J Neurosci. 2016;36(27):7184–7197. doi:10.1523/JNEUROSCI.3500-15.2016
19. Vuong HE, Hardi CN, Barnes S, Brecha NC. Parallel inhibition of dopamine Amacrine cells and intrinsically photosensitive retinal ganglion cells in a non-image-forming visual circuit of the mouse retina. J Neurosci. 2015;35(48):15955–15970. doi:10.1523/JNEUROSCI.3382-15.2015
20. Boatright JH, Gordon JR, Michael Iuvone P. Inhibition of endogenous dopamine release in amphibian retina by L-2-amino-4-phosphonobutyric acid (L-AP4) and trans-2-aminocyclopentane-1,3-dicarboxylate (ACPD). Brain Res. 1994;649(1–2):339–342. doi:10.1016/0006-8993(94)91084-7
21. Boelen MK, Boelen MG, Marshak DW. Light-stimulated release of dopamine from the primate retina is blocked by 1-2-amino-4-phosphonobutyric acid (APB). Vis Neurosci. 1998;15(1):97–103. doi:10.1017/S0952523898151040
22. Pérez-Fernández V, Milosavljevic N, Allen AE, et al. Rod photoreceptor activation alone defines the release of dopamine in the retina. Curr Biol. 2019;29(5):763–774.e5. doi:10.1016/j.cub.2019.01.042
23. Lucas RJ. Mammalian inner retinal photoreception. Curr Biol. 2013;23(3):R125–R133. doi:10.1016/j.cub.2012.12.029
24. Hedden WL, Dowling JE. The interplexiform cell system. II. Effects of dopamine on goldfish retinal neurones. Proc R Soc Lond B Biol Sci. 1978;201(1142):27–55.
25. Werblin FS, Dowling JE. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969;32(3):339–355. doi:10.1152/jn.1969.32.3.339
26. Negishi K, Drujan BD. Reciprocal changes in center and surrounding S potentials of fish retina in response to dopamine. Neurochem Res. 1979;4(3):313–318. doi:10.1007/BF00963801
27. Lasater EM, Dowling JE. Dopamine decreases conductance of the electrical junctions between cultured retinal horizontal cells. Proc Natl Acad Sci USA. 1985;82(9):3025–3029. doi:10.1073/pnas.82.9.3025
28. McMahon DG, Knapp AG, Dowling JE. Horizontal cell gap junctions: single-channel conductance and modulation by dopamine. Proc Natl Acad Sci USA. 1989;86(19):7639–7643. doi:10.1073/pnas.86.19.7639
29. Van Buskirk R, Dowling JE. Isolated horizontal cells from carp retina demonstrate dopamine-dependent accumulation of cyclic AMP. Proc Natl Acad Sci USA. 1981;78(12):7825–7829. doi:10.1073/pnas.78.12.7825
30. Mangel SC, Dowling JE. Responsiveness and receptive field size of carp horizontal cells are reduced by prolonged darkness and dopamine. Science. 1985;229(4718):1107–1109. doi:10.1126/science.4035351
31. Knapp AG, Dowling JE. Dopamine enhances excitatory amino acid-gated conductances in cultured retinal horizontal cells. Nature. 1987;325(6103):437–439. doi:10.1038/325437a0
32. Liman ER, Knapp AG, Dowling JE. Enhancement of kainate-gated currents in retinal horizontal cells by cyclic AMP-dependent protein kinase. Brain Res. 1989;481(2):399–402. doi:10.1016/0006-8993(89)90822-6
33. Knapp AG, Schmidt KF, Dowling JE. Dopamine modulates the kinetics of ion channels gated by excitatory amino acids in retinal horizontal cells. Proc Natl Acad Sci USA. 1990;87(2):767–771. doi:10.1073/pnas.87.2.767
34. Kothmann WW, Massey SC, O’Brien J. Dopamine-stimulated dephosphorylation of connexin 36 mediates AII amacrine cell uncoupling. J Neurosci. 2009;29(47):14903–14911. doi:10.1523/JNEUROSCI.3436-09.2009
35. Chaffiol A, Ishii M, Cao Y, Mangel SC. Dopamine regulation of GABAA receptors contributes to light/dark modulation of the on-cone bipolar cell receptive field surround in the retina. Curr Biol. 2017;27(17):2600–2609.e4. doi:10.1016/j.cub.2017.07.063
36. DeVries SH, Schwartz EA Modulation of an electrical synapse between solitary pairs of catfish horizontal cells by dopamine and second messengers. J Physiol. 1989;414(1):351–375. doi:10.1113/JPHYSIOL.1989.SP017692
37. Lu C, McMahon DG. Modulation of hybrid bass retinal gap junction channel gating by nitric oxide. J Physiol. 1997;499(3):689–699.
38. Mills SL, Massey SC. Differential properties of two gap junctional pathways made by AII amacrine cells. Nature. 1995;377(6551):734–737. doi:10.1038/377734a0
39. McMahon DG, Ponomareva LV. Nitric oxide and cGMP modulate retinal glutamate receptors. J Neurophysiol. 1996;76(4):2307–2315. doi:10.1152/jn.1996.76.4.2307
40. McMahon DG, Schmidt KF. Horizontal cell glutamate receptor modulation by NO: mechanisms and functional implications for the first visual synapse. Vis Neurosci. 1999;16(3):425–433. doi:10.1017/S0952523899163041
41. Erdinest N, London N, Ovadia H, Levinger N. Nitric oxide interaction with the eye. Vision. 2021;5(2):29. doi:10.3390/vision5020029
42. Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10(9):940–954. doi:10.1096/fasebj.10.9.8801176
43. Marsh-Armstrong N, Mccaffery P, Gilbert W, Dowling JE, Dräger UC. Retinoic acid is necessary for development of the ventral retina in zebrafish. Proc Natl Acad Sci USA. 1994;91(15):7286–7290. doi:10.1073/pnas.91.15.7286
44. Hyatt GA, Schmitt EA, Marsh-Armstrong N, McCaffery P, Dräger UC, Dowling JE. Retinoic acid establishes ventral retinal characteristics. Development. 1996;122(1):195–204. doi:10.1242/dev.122.1.195
45. Hyatt GA, Schmitt EA, Fadool JM, Dowling JE. Retinoic acid alters photoreceptor development in vivo. Proc Natl Acad Sci USA. 1996;93(23):13298–13303. doi:10.1073/pnas.93.23.13298
46. da Silva S, Cepko CL. Fgf8 Expression and degradation of retinoic acid are required for patterning a high-acuity area in the retina. Dev Cell. 2017;42(1):68–81.e6. doi:10.1016/j.devcel.2017.05.024
47. Weiler R, Pottek M, He S. Vaney DI. Modulation of coupling between retional horizontal cells by retinoic acid and endogenous dopamine. Brain Res Rev. 2000;93:121–129.
48. McCaffery P, Mey J, Dräger UC. Light-mediated retinoic acid production. Proc Natl Acad Sci USA. 1996;93(22):12570–12574. doi:10.1073/pnas.93.22.12570
49. Weiler R, Pottek M, Schultz K, Janssen-Bienhold U. Retinoic acid, a neuromodulator in the retina. In: Kolb H, Ripps H, Wu S, editors. Concepts and Challenges in Retinal Biology. Amsterdam: Elsevier; 2001:309–318.
50. Zhang D-Q, McMahon DG. Direct gating by retinoic acid of retinal electrical synapses. Proc Nat Acad Sci. 2000;97(26):14754–14759. doi:10.1073/pnas.010325897
51. Chen L, Lau AG, Sarti F. Synaptic retinoic acid signaling and homeostatic synaptic plasticity. Neuropharmacology. 2014;78(C):3–12. doi:10.1016/j.neuropharm.2012.12.004
52. Misner DL, Jacobs S, Shimizu Y, et al. Vitamin A deprivation results in reversible loss of hippocampal long-term synaptic plasticity. Proc Natl Acad Sci USA. 2001;98(20):11714–11719. doi:10.1073/pnas.191369798
53. Hsu YT, Li J, Wu D, Südhof TC, Chen L. Synaptic retinoic acid receptor signaling mediates mTOR-dependent metaplasticity that controls hippocampal learning. Proc Natl Acad Sci USA. 2019;116(14):7113–7122. doi:10.1073/pnas.1820690116
54. Lenz M, Kruse P, Eichler A, et al. All-trans retinoic acid induces synaptic plasticity in human cortical neurons. Elife. 2021;2021:10.
55. Telias M, Denlinger B, Helft Z, Thornton C, Beckwith-Cohen B, Kramer RH. Retinoic acid induces hyperactivity, and blocking its receptor unmasks light responses and augments vision in retinal degeneration. Neuron. 2019;102(3):574–586.e5. doi:10.1016/j.neuron.2019.02.015
56. Telias M, Sit KK, Frozenfar D, et al. Retinoic acid inhibitors mitigate vision loss in a mouse model of retinal degeneration. Sci Adv. 2022;8(11):eabm4643. doi:10.1126/sciadv.abm4643
57. Lasater EM, Watling KJ, Dowling JE. Vasoactive intestinal peptide alters membrane potential and cyclic nucleotide levels in retinal horizontal cells. Science. 1983;221(4615):1070–1072.
58. Atlasz T, Werling D, Song S, et al. Retinoprotective effects of TAT-bound vasoactive intestinal peptide and pituitary adenylate cyclase activating polypeptide. J Mol Neurosci. 2019;68(3):397–407. doi:10.1007/s12031-018-1229-5
59. Wu SM, Xiaoxi Q, Noebels JL, Li Yang X. Localization and modulatory actions of zinc in vertebrate retina. Vision Res. 1993;33(18):2611–2616. doi:10.1016/0042-6989(93)90219-M
60. Gottesman J, Miller RF. Pharmacological properties of N-methyl-D-aspartate receptors on ganglion cells of an amphibian retina. J Neurophysiol. 1992;68(2):596–604. doi:10.1152/jn.1992.68.2.596
61. Dong CJ, Werblin FS. Use-dependent and use-independent blocking actions of picrotoxin and zinc at the GABAC receptor in retinal horizontal cells. Vision Res. 1996;36(24):3997–4005. doi:10.1016/S0042-6989(96)00141-1
62. Qian H, Li L, Chappell RL, Ripps H. GABA receptors of bipolar cells from the skate retina: actions of zinc on GABA-mediated membrane currents. J Neurophysiol. 1997;78(5):2402–2412. doi:10.1152/JN.1997.78.5.2402
63. Han Y, Wu SM. Modulation of glycine receptors in retinal ganglion cells by zinc. Proc Natl Acad Sci U S A. 1999;96(6):3234–3238. doi:10.1073/PNAS.96.6.3234
64. Zhang DQ, Ribelayga C, Mangel SC, McMahon DG. Suppression by zinc of AMPA receptor-mediated synaptic transmission in the retina. J Neurophysiol. 2002;88(3):1245–1251. doi:10.1152/JN.2002.88.3.1245
65. Sun Z, Zhang DQ, McMahon DG. Zinc modulation of hemi-gap-junction channel currents in retinal horizontal cells. J Neurophysiol. 2009;101(4):1774–1780. doi:10.1152/jn.90581.2008
66. Tedesco V, Roquet RF, DeMis J, Chiamulera C, Monfils MH. Extinction, applied after retrieval of auditory fear memory, selectively increases zinc-finger protein 268 and phosphorylated ribosomal protein S6 expression in prefrontal cortex and lateral amygdala. Neurobiol Learn Mem. 2014;115:78–85. doi:10.1016/J.NLM.2014.08.01
67. Petrilli MA, Kranz TM, Kleinhaus K, et al. The emerging role for zinc in depression and psychosis. Front Pharmacol. 2017;8(JUN). doi:10.3389/fphar.2017.00414
68. Li Y, Andereggen L, Yuki K, et al. Mobile zinc increases rapidly in the retina after optic nerve injury and regulates ganglion cell survival and optic nerve regeneration. Proc Nat Acad Sci. 2017;114(2):E209–E218.
69. Weiler R, Schultz K, Pottek M, Tieding S, Janssen-Bienhold U. Retinoic acid has light-adaptive effects on horizontal cells in the retina. Proc Natl Acad Sci USA. 1998;95(12):7139–7144. doi:10.1073/pnas.95.12.7139
70. Rodrigues PDS, Dowling JE. Dopamine induces neurite retraction in retinal horizontal cells via diacylglycerol and protein kinase C. Proc Natl Acad Sci USA. 1990;87(24):9693–9697. doi:10.1073/pnas.87.24.9693
71. Fadool JM, Dowling JE. Zebrafish: a model system for the study of eye genetics. Prog Retin Eye Res. 2008;27(1):89–110. doi:10.1016/j.preteyeres.2007.08.002
72. Dumitrescu ON, Pucci FG, Wong KY, Berson DM. Ectopic retinal ON bipolar cell synapses in the OFF inner plexiform layer: contacts with dopaminergic amacrine cells and melanopsin ganglion cells. J Compar Neurol. 2009;517(2):226–244. doi:10.1002/cne.22158
73. Hoshi H, Liu WL, Massey SC, Mills SL. ON Inputs to the OFF layer: bipolar cells that break the stratification rules of the retina. J Neurosci. 2009;29(28):8875–8883. doi:10.1523/JNEUROSCI.0912-09.2009
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.