Back to Journals » Neuropsychiatric Disease and Treatment » Volume 11
Neurological soft signs might be endophenotype candidates for patients with deficit syndrome schizophrenia
Authors Albayrak Y , Akyol E, Beyazyüz M, Baykal S, Kuloglu M
Received 25 June 2015
Accepted for publication 7 October 2015
Published 29 October 2015 Volume 2015:11 Pages 2825—2831
DOI https://doi.org/10.2147/NDT.S91170
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Yakup Albayrak,1 Esra Soydaş Akyol,2 Murat Beyazyüz,1 Saliha Baykal,1 Murat Kuloglu3
1Department of Psychiatry, Faculty of Medicine, Namik Kemal University, Tekirdag, 2Department of Psychiatry, Yenimahalle Education and Research Hospital, Ankara, 3Department of Psychiatry, Faculty of Medicine, Akdeniz University, Antalya, Turkey
Background: Schizophrenia is a chronic, disabling, disorder that affects approximately 1% of the population. The nature of schizophrenia is heterogeneous, and unsuccessful efforts to subtype this disorder have been made. Deficit syndrome schizophrenia (DS) is a clinical diagnosis that has not been placed in main diagnostic manuals. In this study, we aimed to investigate and compare neurological soft signs (NSS) in DS patients, non-deficit schizophrenia (NDS) patients, and healthy controls (HCs). We suggest that NSS might be an endophenotype candidate for DS patients.
Methods: Sixty-six patients with schizophrenia and 30 HCs were enrolled in accordance with our inclusion and exclusion criteria. The patients were sub-typed as DS (n=24) and NDS (n=42) according to the Schedule for the Deficit Syndrome. The three groups were compared in terms of sociodemographic and clinical variables and total scores and subscores on the Physical and Neurological Examination for Soft Signs (PANESS). Following the comparison, a regression analysis was performed for predictability of total PANESS score and its subscales in the diagnosis of DS and NDS.
Results: The groups were similar in terms of age, sex, and smoking status. The results of our study indicated that the total PANESS score was significantly higher in the DS group compared to the NDS and HC groups, and all PANESS subscales were significantly higher in the DS group than in the HC group. The diagnosis of DS was predicted significantly by total PANESS score (P<0.001, odds ratio =9.48, 95% confidence interval: 0.00–4.56); the synergy, graphesthesia, stereognosis, motor tasks, and ability to maintain posture subscales were found to be significant predictors.
Conclusion: This study confirms that NSS were higher in patients with DS. In addition, we suggest that our results might support the notion of DS as a different and distinct type of schizophrenia. NSS might also be a promising candidate as an endophenotype for DS. However, large sampled, multicentric studies are needed to clarify the place of NSS as an endophenotype in DS.
Keywords: biomarker, psychosis, sign, clinical subtypes of schizophrenia
Introduction
Schizophrenia is a chronic and disabling disorder that is estimated to affect 1% of the general population.1 Since its classification, there have been efforts to sub-classify schizophrenia, due to the heterogeneity of its symptoms and progression of illness. However, it can be said that current efforts to achieve this have not proven much more successful than efforts in the past. Specifically, during the revision of the Diagnostic and Statistical Manual of Mental Disorders (DSM)–IV Text Revision2 to DSM-V,3 the subtypes of schizophrenia, such as paranoid, disorganized, catatonic, undifferentiated, and residual, were eliminated because of their diagnostic validities. Currently, it may be considered that clinicians and researchers need to determine the subtypes of schizophrenia in a different manner.
Although the term deficit schizophrenia still does not have a place in diagnostic manuals of psychiatry, it has been accepted as a clinical diagnosis by both researchers and clinicians. Since Carpenter et al’s4 definition of deficit syndrome schizophrenia (DS), this subtype has been reported to be valid and it has been characterized by primary and continuous negative symptoms.5
Neurological soft signs (NSS) are the cluster of symptoms that aim to describe neurological abnormalities in such categories as motor coordination, sensory integration, and sequencing of complex motor activities.6–8 NSS have been well investigated in patients with schizophrenia, and it has been accepted that NSS are higher in these patients in comparison to healthy subjects.9 Furthermore, NSS were demonstrated to be higher in drug-naïve, first-episode schizophrenia patients, and thus, NSS have been suggested as an intrinsic feature of schizophrenia. There has also been evidence indicating that NSS can predict a poor prognosis in schizophrenia. In contrast, some studies have found that NSS are lower in correlation with psychopathology.10
There has been a limited number of studies that have compared NSS in DS and non-deficit schizophrenia (NDS) patients. Thus, the aim of this study was to compare NSS among NDS, DS, and healthy control (HC) groups. Our premise was that there would be significant differences in NSS among the groups.
Methods
Participants
The study was conducted at the Department of Psychiatry, Yenimahalle Education and Research Hospital, Ankara, Turkey, between January 2015 and June 2015. Patients diagnosed with schizophrenia according to DSM-V and who were under treatment at the Department of Psychiatry, Yenimahalle Education and Research Hospital, were included in the study. The diagnosis of schizophrenia was also confirmed by a senior psychiatrist (ESA) according to the Structured Clinical Interview for DSM-IV Axis I (SCID-I), before patients were included in the study. The inclusion criteria were as follows: diagnosis of schizophrenia, age 18–60 years, and willing to participate in the research after a detailed explanation of the study procedure. The exclusion criteria were as follows: current or history of, a neurological disease, diagnosed with mental retardation or dementia or other cognitive deteriorating disorders, history of alcohol or substance dependence, any recent or previous chronic illness, history of major head trauma, extrapyramidal symptoms due to medication, unwillingness to participate in the study, and being in an exacerbation phase of schizophrenia. After applying the inclusion and exclusion criteria, 66 patients were included in the study. All of the patients were under treatment as well as being treated with atypical antipsychotics. Twenty-two patients were treated with olanzapine, 12 patients were treated with risperidone, ten patients were treated with aripiprazole, and 22 patients were treated with a combination of atypical antipsychotics (olanzapine, quetiapine, risperidone, paliperidone, aripiprazole). Thirty healthy individuals selected from the hospital staff were included as the HC group. Detailed psychiatric and medical family histories were obtained from the HC group, and none of them had a family history of schizophrenia.
The local ethics committee of Yenimahalle Education and Research Hospital approved the present study. All of the participants reported that they understood the written informed consent and subsequently provided this. A clear explanation was also provided to the families of the patients included in the study and they accepted our study protocol and design, as well as the method of obtaining the informed consent.
Instruments
SCID-I
SCID-I is a semi-structured tool for diagnostic use according to Diagnostic and Statistical Manual of Mental Disorders, 4th edition, text revised Axis I. It can be administered by a psychiatrist or anyone who has been trained in terms of SCID. This tool was developed by First et al,11 and it was reported to be reliable and validated in the Turkish language.12
Scale for the Assessment of Negative Symptoms
The Scale for the Assessment of Negative Symptoms (SANS) was created to measure negative symptoms such as affective blunting, alogia (impoverished thinking), avolition/apathy, anhedonia/asociality, and disturbance of attention in patients with schizophrenia. It was developed by Andreasen,13 and reported to be reliable and validated in the Turkish language.14
Brief Psychiatric Rating Scale
The Brief Psychiatric Rating Scale (BPRS) was created to measure psychiatric symptoms, such as negative symptoms, positive symptoms, disorganized behavior and speech, anxiety, and depression. The scores range from 1 to 7, and there are two versions, with 18 to 24 symptoms. BPRS was created by Overall and Gorham, and its Turkish translation is available and widely used in clinical practice.15
Scale for the Assessment of Positive Symptoms
The Scale for the Assessment of Positive Symptoms (SAPS) was produced by Andreasen16 to evaluate positive symptoms such as hallucinations, delusions, bizarre behavior, and positive formal thought disorder in patients with schizophrenia. The Turkish translation has been reported to be validated and reliable.17
Schedule for the Deficit Syndrome
The patients were sub-typed as either being with or without deficit by using the Schedule for the Deficit Syndrome.18 This semi-structured interviewing tool describes DS as the existence of at least two of six continuous negative symptoms of schizophrenia – restricted affect, diminished emotional range, speech poverty, diminished interests, diminished sense of aim, and diminished social drive – of at least moderate severity. The present negative symptoms must have existed for a minimum of 12 months, even during periods of clinical stability. Confounding factors, such as anxiety, medication effect, positive symptoms, mental retardation, and depression, must be excluded. Family members and associated clinicians also confirm the deficit state. This schedule has been reported to be validated and reliable in the Turkish language.19
Physical and Neurological Examination for Soft Signs
The Physical and Neurological Examination scale was reported to be valid and reliable to evaluate physical and neurological soft signs.20 The PANESS consists of two parts – physical and neurological. The neurological part consists of 43 items: 1) coordination (items 1–8; eg, finger to nose test; range 8–32); 2) graphesthesia (items 9–16; eg, identifying drawn figures on palms; range 8–32); 3) stereognosis (items 17–20; eg, identifying objects put on palm; range 4–16); 4) motor tasks (items 21–29; eg, heel walking, face-hand and face-noise tests, two-point discrimination); 5) ability to maintain posture (items 30–36; eg, Romberg; range 7–28); 6) ability to tap in a smooth rhythm (items 37–42; eg, number of taps in a 5-second period and quality of tapping at a speed of 4 beats/second); and 7) string test (item 43; optokinetic test; range 0–4).20
Statistical methods
The data was analyzed by Statistical Package for the Social Sciences, PC version 17.0 (SPSS Inc., Chicago, IL, USA). A CI of 95% and a two-tailed P<0.05 were accepted to be statistically significant for all of the analyses. All numeric variables were tested with the Levene test for homogeneity of variance and the Kolmogorov–Smirnov test was used for analyzing the normality of distribution. The differences between age and total score and subscores of PANESS were assessed using a series of one-way analyses of variance. Tukey’s honest significance test was applied to determine the differences between the groups in detail. Additionally, a general linear model was used to compare total PANESS score and its subscores between DS and NDS groups (age of onset and duration of illness as covariates). The categorical variables were assessed using a χ2 test. The Student’s t-test was used to evaluate the differences between DS and NDS groups in terms of the scores on the SANS, SAPS, and BPRS, age of onset and the duration of illness. The numerical variants are expressed as the mean ± standard deviation values. A linear regression model was constructed to investigate the association between NSS and having a diagnosis of DS and NDS.
Results
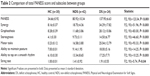
Sixty-six patients who were diagnosed with schizophrenia and 30 HCs were included in this study. After evaluating patients according to the Schedule for the Deficit Syndrome, 42 patients were sub-typed as NDS (43.8%) and 24 patients were grouped as DS (25%). There was not a significant difference between groups in terms of age (F[2, 93]=1.26, P=0.59). The female–male ratio was found to be similar between groups (χ2:1.49, P=0.47). There was no significant difference between groups in terms of smoking status, and history of psychiatric disorders in the family (respectively: χ2=0.89, P=0.77; χ2=6.92, P=0.14). The duration of illness was significantly longer in DS group compared with NDS group (t[63]=−2.56, P=0.013). The age of onset of illness was found to be significantly earlier in the DS group (t[63]=2.19, P=0.032). The mean score of BPRS in NDS group and DS groups was found to be similar (t[63]=−1.41, P=0.16). The mean scores of SANS was significantly higher in NDS (t[63]=−4.85, P<0.001); whereas the mean score of SAPS in the DS group was significantly higher compared to NDS group (t[63]=−2.84, P=0.006) (Table 1).
There was a significant difference between groups in terms of PANESS scores (F[2, 93]=123.34, P<0.001). In post hoc analyses, it has been found that the total score of PANESS was significantly higher in the DS group compared to HC and NDS groups (respectively; P<0.001, P<0.001). Total PANESS score was also significantly higher in the NDS group compared with the HC group (P<0.001). There was a significant difference between the groups in terms of subscale of synergy (F[2, 93]=51.96, P<0.001). In post hoc analyses it has been found that the subscale of synergy was also significantly higher in the DS group compared to the HC and NDS groups (P<0.001, P<0.001, respectively). The subscale score of synergy was also significantly higher in the NDS group compared to the HC group (P<0.001). There was a significant difference between the groups in terms of subscale of graphesthesia (F[2, 93]=83.00, P<0.001). In post hoc analyses it has been found that the subscale of graphesthesia was significantly higher in the DS group compared to the HC and NDS groups (P<0.001, P<0.001, respectively). Subscale score of graphesthesia was also significantly higher in the NDS group compared with the HC group (P=0.04). There was a significant difference between the groups in terms of subscale of stereognosis (F[2, 93]=46.60, P<0.001). It has been found that the subscale of stereognosis was significantly higher in the DS group compared to the HC and NDS groups (P<0.001, P<0.001, respectively). Subscale score of stereognosis was also significantly higher in the NDS group compared with the HC group (P<0.001). There was a significant difference between the groups in terms of subscale of motor tasks (F[2, 93]=93.33, P<0.001). The subscale of motor tasks was also significantly higher in the DS group compared to the HC and NDS groups (P<0.001, P<0.001, respectively). Subscale score of motor tasks was also significantly higher in the NDS group compared with the HC group (P<0.001). There was a significant difference between the groups in terms of subscale of ability to maintain posture (F[2, 93]=14.25, P<0.001). The subscale of ability to maintain posture was significantly higher in the DS group compared to the HC and NDS groups (P<0.001, P<0.001, respectively). Subscale score of ability to maintain posture was also significantly higher in the NDS group compared with the HC group (P=0.007). There was a significant difference between the groups in terms of subscale of ability to tap on a smooth rhythm (F[2, 93]=10.25, P<0.001). In post hoc analyses it has been found that the subscale of ability to tap on a smooth rhythm was significantly higher in the DS group compared to the HC group (P<0.001). Subscale score of ability to tap on a smooth rhythm was also significantly higher in the NDS group compared with the HC group (P=0.005). The score of ability to tap on a smooth rhythm was similar between DS and NDS groups. There was a significant difference between the groups in terms of subscale of string test (F[2, 93]=5.74, P=0.04). It has been found that the subscale of string test was significantly higher in the DS group compared to the HC group (P<0.03). There was no significant difference between the HC and NDS, and DS and NDS groups (P=0.19, P=0.12, respectively). The summary of these results are presented in Table 2.
In the general linear model, total PANESS score and its subscores were regarded as dependent variables and age of onset and duration of illness were included to model as covariates. Because HC group did not have variants of age of onset and duration of illness, total PANESS score and its subscores were compared between the DS and NDS groups. Total PANESS score, score of synergy, score of graphesthesia, score of stereognosis, score of motor tasks, and score of ability to maintain posture were found to be significantly higher in the DS group compared with the NDS group (F[1, 61]=96.97, P<0.001; F[1, 61]=23.03, P<0.001; F[1, 61]=68.53, P<0.001; F[1, 61]=96.97, P<0.001; F[1, 61]=16.01, P<0.001; F[1, 61]=96.97, P<0.001, respectively). The scores of ability to tap on a smooth rhythm and string test were similar between the DS and NDS groups (F[1, 61]=0.84, P<0.36; F[1, 61]=1.79, P<0.18, respectively). The results of the comparison of PANESS scores between the NDS and DS did not change after age of onset and duration of illness were regarded as covariates.
In performing the regression analysis, diagnosis of NDS and diagnosis of DS were set as dependent variables and total PANESS score and its subscales were determined as co-variated. Diagnosis of DS was predicted significantly by total PANESS score and the synergy, graphesthesia, stereognosis, motor tasks, and ability to maintain posture subscales were found to be significant predictors (Table 3).
Discussion
The results of our study indicated that total PANESS score was significantly higher in the DS group compared to the NDS and HC groups. In addition, the scores of all PANESS subscales were significantly higher in the DS group than in the HC group. The NDS group also had a higher total PANESS score than the HC group, and higher scores in all subscales except the string test subscale. In performing the regression analysis, total PANESS score and most of the subscale scores were found to be significant predictors for DS.
NSS are considered to be state and trait characteristics of patients with schizophrenia.21–23 Previous studies have investigated NSS in drug-naïve, first-episode schizophrenia patients and chronic, medicated schizophrenic patients. According to a recent meta-analysis by Bachmann et al, NSS may be used to monitor disease progression or to identify subjects with an increased tendency toward schizophrenia in general.10
Although NSS have been well established in patients with schizophrenia, the number of studies that have compared NSS between DS and NDS patients is limited. Most of the studies concluded that total and subscale PANESS scores were higher in patients with DS.24–28 However, the studies mentioned conducted comparisons in chronic and medicated patients, and most of them did not include HCs. To our knowledge, there is only one study that has researched NSS in drug-naïve, first-episode DS and NDS patients. In that study, Peralta et al reported that drug-naïve, first-episode deficit patients had more NSS compared with NDS patients.29 To date, only one Turkish study has researched and compared NSS in DS and NDS patients. In that study, Turkish DS patients were reported to have higher scores in terms of NSS.28 However, HCs were not included in the study.
The concept of endophenotype refers to a heritable and measurable component or trait that is assumed to be directly attributable to the underlying biology of a disorder and that can be accepted as a risk factor for it.30 Recent discussions about the concept have drawn a line between “risk indicator models” and “mediation models”.31 Mediation models assume that the causal pathway from genetic risk to explicit disorder passes exclusively through the endophenotype.31 Because we compared groups cross-sectionally in terms of NSS severity, it is plausible to assess the results of our study in the context of mediation models. As it is not a longitudinal observational study, the results cannot be interpreted in a manner that identifies NSS as a risk indicator.
Some criteria have been established for a biomarker to be accepted as an endophenotype.32 These criteria include segregation of the disorder in the population, heritability, independent nature of the state of the disorder, co-segregation of the disorder in families, high rate of prevalence in affected families, specificity for the disorder, and measurability.
Until recently, the most studied and strongest endophenotype candidates for schizophrenia were lack of sensory gating, decline in working memory, and prepulse inhibition. While they seem to be consistent findings in schizophrenic populations, they do not meet the criteria to be an exact phenotype.30
Our results are in line with those of studies that reported higher NSS in DS patients compared to NDS patients. Regarding the heterogeneity of schizophrenia and the variable results of the studies that have investigated NSS in patients with schizophrenia, it can be concluded that NSS might be a powerful candidate for an endophenotype for DS. Our study is neither detailed enough nor adequate for determining whether or not NSS meet all the criteria to be an endophenotype. Nevertheless, the criteria of measurability and segregation or increased coexistence with the disorder are met, while further research is needed for the other criteria.
Because the contemporary classification systems in psychiatry are more focused on clinical descriptions of disorders, neurobiological and genetic studies regarding psychiatric disorders remain inadequate. Endophenotype research may be beneficial to meeting the challenge of establishing a more biologically based classification system and understanding the variable symptoms in a more systematic manner.
The lack of a significant difference between the DS and NDS groups in terms of BPRS mean scores is also a remarkable result of our study. This finding implies that DS patients have been suffering from the disease as much as NDS patients. Having predominantly negative symptoms does not mean that the disease is less severe, and having more positive symptoms must not be assumed to be an indicator of disease severity. This finding also supports the idea that DS is not an amelioration state during the course of the illness, and that DS can be accepted as a subtype of schizophrenia.
Our study has some limitations. First, all of our patients were under treatment. We suggest that the best way to investigate an endophenotype is in drug-naïve, first-episode patients. Because of the strict inclusion and exclusion criteria, we could not include a large number of patients in the present study. We consider this issue to be another limitation.
In conclusion, we argue that NSS could be a good endophenotype candidate in patients with DS. However, multicenter studies with larger sample populations are needed to clarify whether NSS has a place as an endophenotype in DS.
Acknowledgments
This research was presented as a poster at the World Biological Psychiatry Congress, 2015, Athens. This study was not supported financially by any organization.
Disclosure
The authors report no conflicts of interest in this work.
References
Eaton WW, Martins SS, Nestadt G, Bienvenu OJ, Clarke D, Alexandre P. The burden of mental disorders. Epidemiol Rev. 2008;30:1–14. | ||
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington: DC: American Psychiatric Association; 2000. | ||
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington: DC: American Psychiatric Association; 2013. | ||
Carpenter WT Jr, Heinrichs DW, Wagman AM. Deficit and nondeficit forms of schizophrenia: the concept. Am J Psychiatry. 1988;145(5):578–583. | ||
Kirkpatrick B, Buchanan RW, Ross DE, Carpenter WT Jr. A separate disease within the syndrome of schizophrenia. Arch Gen Psychiatry. 2001;58(2):165–171. | ||
Heinrichs DW, Buchanan RW. Significance and meaning of neurological signs in schizophrenia. Am J Psychiatry. 1988;145(1):11–18. | ||
Schröder J, Niethammer R, Geider FJ, et al. Neurological soft signs in schizophrenia. Schizophr Res. 1991;6(1):25–30. | ||
Jahn T, Hubmann W, Karr M, et al. Motoric neurological soft signs and psychopathological symptoms in schizophrenic psychoses. Psychiatry Res. 2006;142(2–3):191–199. | ||
Hirjak D, Wolf RC, Koch SC, et al. Neurological abnormalities in recent-onset schizophrenia and Asperger-syndrome. Front Psychiatry. 2014;5:91. | ||
Bachmann S, Degen C, Geider FJ, Schröder J. Neurological soft signs in the clinical course of schizophrenia: results of a meta-analysis. Front Psychiatry. 2014;5:185. | ||
First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV). Washington: DC: American Psychiatric Press; 1996. | ||
Corapcioglu A, Aydemir O, Yildiz M. Structured Clinical Interview for DSM-IV (SCID-IV), Turkish Version (Turkish). Ankara: Turkey: Hekimler Yayin Birligi; 1999. | ||
Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS). Iowa City: IA: College of Medicine, The University of Iowa; 1984. | ||
Erkoç S, Arkonaç O, Atakli C, Özmen E. Negatif semptomlari değerlendirme ölçeğinin güvenirlirliği ve geçerliliği. [The reliability and validity of scale for the assessment of negative symptoms.] Düşünen Adam. 1991;4(2):16–19. | ||
Overall JE, Gorham DR. The brief psychiatric rating scale. Psychol Rep. 1962;10(3):779–781. | ||
Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS). Iowa City: IA: College of Medicine, The University of Iowa; 1984. | ||
Erkoç S, Arkonaç O, Atakli C, Özmen E. Pozitif semptomlari değerlendirme ölçeğinin güvenilirliği ve geçerliliği. [Reliability and validity of scale for the assessment of positive symptoms.] Düşünen Adam. 1991;4(2):20–24. Turkish. | ||
Kirkpatrick B, Buchanan RW, McKenney PD, Alphs LD, Carpenter WT Jr. The Schedule for the Deficit Syndrome: an instrument for research in schizophrenia. Psychiatry Res. 1989;30(2):119–123. | ||
Citak S, Oral ET, Aker AT, Senocak M. Reliability and validity of the schedule for deficit syndrome in schizophrenia. Turk Psikiyatri Derg. 2006;17(2):115–127. | ||
Werry JS, Aman MG. The reliability and diagnostic validity of the physical and neurological examination for soft signs (PANESS). J Autism Child Schizophr. 1976;6(3):253–262. | ||
Niethammer R, Weisbrod M, Schiesser S, et al. Genetic influence on laterality in schizophrenia? A twin study of neurological soft signs. Am J Psychiatry. 2000;157(2):272–274. | ||
Torrey EF, Taylor EH, Bracha HS, et al. Prenatal origin of schizophrenia in a subgroup of discordant monozygotic twins. Schizophr Bull. 1994;20(3):423–432. | ||
Rossi A, De Cataldo S, Di Michele V, et al. Neurological soft signs in schizophrenia. Br J Psychiatry. 1990;157(5):735–739. | ||
Buchanan RW, Kirkpatrick B, Heinrichs DW, Carpenter WT Jr. Clinical correlates of the deficit syndrome of schizophrenia. Am J Psychiatry. 1990;147(3):290–294. | ||
Galderisi S, Maj M, Mucci A, et al. Historical, psychopathological, neurological, and neuropsychological aspects of deficit schizophrenia: a multicenter study. Am J Psychiatry. 2002;159(6):983–990. | ||
McGlashan TH, Fenton WS. The positive-negative distinction in schizophrenia. Review of natural history validators. Arch Gen Psychiatry. 1992;49(1):63–72. | ||
Arango C, Kirkpatrick B, Buchanan RW. Neurological signs and the heterogeneity of schizophrenia. Am J Psychiatry. 2000;157(4):560–565. | ||
Tiryaki A, Yazici MK, Anil AE, Kabakçi E, Karaağaoğlu E, Göğüş A. Reexamination of the characteristics of the deficit schizophrenia patients. Eur Arch Psychiatry Clin Neurosci. 2003;253(5):221–227. | ||
Peralta V, Moreno-Izco L, Sanchez-Torres A, García de Jalón E, Campos MS, Cuesta MJ. Characterization of the deficit syndrome in drug-naive schizophrenia patients: the role of spontaneous movement disorders and neurological soft signs. Schizophr Bull. 2014;40(1):214–224. | ||
Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160(4):636–645. | ||
Kendler KS, Neale MC. Endophenotype: a conceptual analysis. Mol Psychiatry. 2010;15(8):789–797. | ||
Beauchaine TP. The role of biomarkers and endophenotypes in prevention and treatment of psychopathological disorders. Biomark Med. 2009;3(1):1–3. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.