Back to Journals » Eye and Brain » Volume 15
Neuro-Ophthalmological Manifestations of Horner’s Syndrome: Current Perspectives
Authors Maamouri R , Ferchichi M, Houmane Y, Gharbi Z, Cheour M
Received 1 April 2023
Accepted for publication 29 June 2023
Published 13 July 2023 Volume 2023:15 Pages 91—100
DOI https://doi.org/10.2147/EB.S389630
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Margaret Wong-Riley
Rym Maamouri, Molka Ferchichi, Yasmine Houmane, Zaineb Gharbi, Monia Cheour
Department of Ophthalmology, Habib Thameur Hospital, Tunis, Tunisia
Correspondence: Rym Maamouri, Department of Ophthalmology, Habib Thameur Hospital, 3, Rue Ali Ben Ayed, Montfleury, Tunis, 1089, Tunisia, Tel +21698314970, Email [email protected]
Abstract: Horner’s syndrome (HS) is caused by a damage to the oculosympathetic pathway. HS may be congenital, but it is usually acquired and may reveal a life-threatening condition. According to the anatomic location of the underlying pathologic process, HS is classified as central, pre- or postganglionic, when the lesion affects the first, second or third-order neuron, respectively. Pharmacological testing, if available, can be used to differentiate HS from « pseudo-HS » in patients with mild symptoms. Given the financial burden that imaging of the entire oculosympathetic pathway represents, a targeted imaging approach is advised. Although in the majority of cases, clinical examination may predict etiology, in other cases pharmacological testing can help in the localization process. We searched PubMed data base for papers published before December 2022 that concerned Horner’s syndrome, its neuro-ophthalmological manifestations and diagnosis. In this article, we describe the main neuro-ophthalmological manifestations of the three types of HS, the most common etiologies, and a targeted diagnostic strategy in each type.
Keywords: Horner’s syndrome, neuro-ophthalmology, pharmacological testing
Introduction
Although Horner’s syndrome (HS) has been discussed for over 150 years in the medical literature, it remains relevant to discuss this entity, especially from a diagnostic standpoint.
The classical presentation of HS consists of unilateral ptosis, miosis, and ipsilateral hemifacial anhidrosis.1 It is named after the Swiss ophthalmologist Johann Friedrich Horner who was widely believed to have discovered the phenomenon in 1869.2,3 However, other reports have predated Horner’s publication. François Pourfour du Petit and Claude Bernard have both described the results of animal studies in 1727 and 1853, respectively, characterizing the ocular effects of sympathetic nerve lesions in animal models.2 This explains why this condition is sometimes called Claude Bernard-Horner syndrome or Claude Bernard syndrome in French literature. Two previous observations have described miosis and ptosis in human years before Horner’s publication. The first was reported by Edward Selleck Hare in 1838 in a man with a brachial plexus tumor and the second by Silas Weir Mitchell in 1863 in a soldier suffering from a gunshot wound to the neck.4,5
To date, there is no exact number regarding the incidence and prevalence of HS in the general population. However, a few population-based studies have estimated the incidence and prevalence of HS. The incidence of pediatric HS in Olmsted County, Minnesota, was 1.42 per 100,000 patients younger than 19 years, with a birth prevalence of 1 in 6250 for those with a congenital onset.6 In South Korea, the annual incidence was 0.20 per 100,000 people and 0.39 per 100,000 people, in the pediatric and adult population, respectively.7
Even though the symptoms of HS are usually subtle and the visual function is typically preserved, its diagnosis has grave implications and should warn the clinician that the oculosympathetic pathway (OSP) has been damaged. The causes of HS vary with the age of the patient and site of the lesion. A thorough evaluation is necessary to detect and treat potentially life-threatening underlying pathology.1 This 3-neuron pathway follows a circuitous route from the hypothalamus to the eye passing through several vital areas susceptible to deadly injuries.8 Localizing the OSP lesion through clinical examination or pharmacological testing helps conduct anatomically focused imaging when needed.9
In this article, we have included a brief anatomical review of the OSP to explain the main neuro-ophthalmological manifestations of the three types of HS. The most common etiologies along with a targeted diagnostic strategy, in each type have also been discussed.
Anatomy of the Oculosympathetic Pathway
Comprehending the anatomy of the OSP is essential to understanding the clinical features of HS and can help in some cases in the localization of the level of nerve damage.
The OSP consists of three different sets of neurons. The first-order neuron (FON) is located in the posterolateral hypothalamus. From there, sympathetic fibers descend through the midbrain passing near the nucleus of the trochlear nerve then through the pons, the anterior part of the medulla, followed by the cervical and the upper thoracic spinal cord before synapsing onto the second-order neuron at the level C8-T2 in the ciliospinal center of Budge-Waller.10 The second-order neuron (SON) exits the Budge-Waller center through the ventral roots of the spinal cord to enter the cervical sympathetic chain. It ascends in the thorax, passing under the aorta, traveling by the lung apex and the subclavian vessels, to finally synapse onto the superior cervical ganglion at the level of C2-C3, near the bifurcation of the common carotid artery. For every SON that enters the superior cervical ganglion, around 15 third-order neurons (TON) are transmitted.11 The majority of the sudomotor and vasomotor fibers of the face follow the external carotid artery, while the remaining fibers that supply the skin of the midforehead follow the TON’s fibers and ascend in the adventitia of the internal carotid artery along the periarterial carotid plexus. At the level of the cavernous sinus, the TON travels with the abducens nerve, then the ophthalmic division of the trigeminal nerve before entering the orbit with its nasociliary branch through the superior orbital fissure.12 These fibers will then innervate the pupillary dilator muscle and the accessory smooth muscle retractors of the upper and lower eyelids via the long ciliary nerves.13
Neuro-Ophthalmological Manifestations and Diagnosis of Horner Syndrome
Clinical Signs
Horner syndrome typically presents with a mild upper eyelid ptosis and an anisocoria with miosis on the affected side. Ipsilateral anhidrosis of the face or the forehead is a less frequent finding. A retrospective case series including 450 patients with HS found that 98% had detectable miosis, 88% had evident ptosis, and 4% had anhidrosis.11 The authors admitted that anhidrosis could have been overlooked as a symptom in some cases because it was not actively looked for.
Pupillary Signs
The size of the pupil is usually determined by the balance between two opposing systems: the pupillary dilator muscle innervated by the sympathetic fibers, and the pupillary sphincter controlled by the parasympathetic autonomic system. Usually, the autonomic innervation in both eyes is equal leading to more or less symmetric pupils. The unilateral interruption of OSP leads to the loss of the sympathetic tone of the pupillary dilator muscle on the affected side. The unopposed parasympathetic action of the pupillary sphincter produces a smaller but normally reactive pupil. This results in an anisocoria most apparent in darkness since the sympathetic drive is maximal and the parasympathetic drive is minimal when the lights are turned off revealing any asymmetry between the two pupils. The degree of anisocoria depends on the operator, the brightness of the room, the concentration of adrenergic substances in the blood, the resting size of the pupils, and the completeness of the injury. Paresis of the pupillary dilator muscle impairs pupillary movement during dilation. This phenomenon is called dilation lag and is best evaluated with pupillography.14 The evaluation starts in bright light, then the lights are turned off suddenly. The affected pupil stays small for the first 5 seconds while the contralateral pupil reacts normally to darkness, making the anisocoria more apparent (Figure 1). Afterwards, the structural forces of the pupillary dilator muscle, because of the reduction of the parasympathetic tone to their counterpart, tend to slowly passively dilate the pupil in the affected side. The maximum degree of dilation (minimum degree of anisocoria) is reached after 15 seconds in the darkness. Comparing photographs taken at 5 seconds and 15 seconds will help assess the dilation lag. Although very specific of HS, this phenomenon is not always present. Recently, digital pupillometry proved to be an objective, fast, and reliable tool that can be used to quantify baseline inter-eye difference in pupil size as well as dilation lag in patients with HS.15
Ptosis
Muller’s muscle is a sympathetically innervated smooth muscle, that is only responsible for 2–3 mm of the upper eyelid elevation.16 When it is denervated, the ipsilateral upper eyelid appears slightly droopy compared with the unaffected side. This ptosis is usually subtle, and may be unnoticed. Severe ptosis cannot be explained by OSP paresis alone.17 The lower eyelid retractors also have some degree of sympathetic supply which is why patients with HS may have slightly elevated lower eyelids. This appearance has been named “upside down ptosis”.18 When combined, ptosis and lower eyelid elevation can mimic enophthalmos. It is important to note that true enophthalmos is absent in HS.19
A study published in 2022 suggested the use of facial imaging for the automatic detection of HS using an image dataset. Although their methods lacked sensitivity, they were highly specific. They explained this finding by the absence of ptosis in a significant proportion of HS patients and the physiological asymmetry found in normal individuals. They concluded that computers could be useful as a second advisor to clinicians on the diagnosis of HS.20 Further studies will be conducted with larger samples.
Iris Hypochromia
Sympathetic innervation is required for the synthesis of melanin by the melanocytes of the iris stroma. The denervation of the sympathetic flow can lead to iris hypochromia on the affected side. Typically seen in congenital HS (Figure 2), it is occasionally noted in patients with chronic, acquired HS, but never in patients with an acute or recently acquired HS.21
Anhidrosis
We have established earlier that the face is innervated by sympathetic sudomotor fibers that branch off when exiting the superior cervical ganglion. The majority follows the path of the external carotid artery supplying a large portion of the face, whereas the fibers that travel with the internal carotid artery supply a small area of the forehead above the eyebrow. This anatomical distribution results in ipsilateral facial anhidrosis with lesions involving the FON and SON, and anhidrosis of a small patch of skin on the forehead when the lesions are distal along the TON.1
Anhidrosis is rarely reported by patients especially in temperature-controlled environments. One way to actively search for it in a clinical setting, is by passing a metallic spoon over each side of the patient’s face. Because of the lack of perspiration in the affected side, the spoon will not slide as smoothly on the skin by comparison to the other side.
The starch-iodine test is another simple alternative to detect anhidrosis. Iodine is applied on a dried skin then covered with starch. After leaving the patient in a hot room, or asking him to exercise, the unaffected side acquires a dark blue coloration when in contact with sweat, while color of the affected side remains unchanged.22
Temperature Changes
In some cases, immediately after an injury to the OSP, the sympathetic denervation of the vessels of the ipsilateral hemiface leads to their dilation causing the skin temperature to rise. For this same reason, cases of transient conjunctival hyperemia (Figure 3), facial flushing, and nasal stuffiness have been reported in the acute stage of HS.8 However, when time passes, the cutaneous vessels become supersensitive to the adrenergic substances circulating in the blood. Consequently, the skin of the face ipsilateral to the lesion becomes cooler and paler in comparison with the contralateral side. Clinical evaluation of temperature changes in patients with HS is challenging. Some authors have suggested the use of thermal imaging as an objective screening tool for patients with HS.23,24 This imaging technique could also be useful during the follow-up of these patients.24
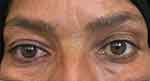 |
Figure 3 A 42-year-old woman presented with a 6-month history of persistent headache associated with a posterior neck pain that recently worsened. Right Horner’s syndrome was found on examination with right ptosis, anisocoria and conjunctival hyperemia (right eye on the left). CT-scan revealed the presence of bilateral Eagle’s syndrome, which was more severe on the right side. CBH occurrence is explained by a compression along the oculosympathetic pathway by the abnormal elongation of the styloid process. An informed written consent was obtained from the patient. Reprinted from Maamouri R, Ouederni M, Oueslati Y, Mbarek C, Chammakhi C and Cheour M. Acute Painful Horner’s Syndrome Revealing Eagle’s Syndrome: A Report of Two Cases. Neuro-Ophthalmology. 2022;46(4), 244–247.25 |
Rare cases of paradoxical sweating and flushing in patients who underwent cervical sympathectomy have been reported. This finding can be explained by the existence of anomalous vagal connections with TON in the superior cervical ganglion.17
Pharmacological Diagnostic Tests
In HS, miosis and ptosis are usually mild. It is also sometimes difficult to differentiate true HS from « pseudo-HS ». For this reason, pharmacological testing can be useful.
Apraclonidine
Apraclonidine has been used as a pressure lowering agent because of its α2 agonist activity. However, it also has weak α1 agonist properties that can dilate the pupil. In patients without OSP paresis, the effect of apraclonidine on the pupil size is unremarkable. On the other hand, in HS syndrome, an upregulation of the α1 receptors on the iris dilator muscle occurs and the pupil becomes very sensitive to this drug. This test involved the instillation of topical 1% Apraclonidine eye drops in both eyes and waiting for 30 to 45 minutes.26 However, more recent studies have recommended the use of topical 0.5% Apraclonidine eye drops because it was associated with fewer side effects while maintaining the same diagnostic efficacy.27–30 At the end of that time period, patients with HS develop « reversed anisocoria », with the pathological pupil becoming more dilated than the normal one. This upregulation also happens at the level of Muller’s muscle causing the eyelid to elevate in the affected side.27 But since eyelid elevation is also present to some degree in normal control eyes, it could not be used as a diagnostic tool.28 Several case series have reported a high sensitivity of the apraclonidine test in patients with different causes of HS.26,28–30 However, there is no consensus on the time needed for sympathetic super sensitivity to develop and for apraclonidine testing to have accurate results. Another uncertainty is whether an incomplete interruption of the OSP is sufficient to produce a positive response.31
While there have been published cases describing the use of apraclonidine to diagnose HS in the acute stage with a positive test after a delay as short as 36 hours from the onset, others have reported false-negative tests 16 days to 3 years after.32,33 This suggests that in case of a negative apraclonidine test, if HS is highly suspected, further investigations should be pursued because of the high implications of a false-negative test.
The use of apraclonidine in young children below the age of 6 months has raised concerns because of the immaturity of the blood–brain barrier at that age. There have been reports of lethargy, bradycardia, and low respiratory rate after the use of apraclonidine eye drops in this population.34 A more recent retrospective study including 46 pediatric patients, 17 of whom were aged under 6 months, who were all tested with topical 0.5% apraclonidine to confirm HS, reported the absence of any documented adverse effects.35 Nonetheless, it is advisable to avoid this test in young infants. When used, the patients should be observed for at least 2 hours after instillation.34
Cocaine
Topical cocaine 2–10% was the first drug used to confirm the diagnosis of HS before the introduction of apraclonidine as a possible alternative. Cocaine acts by blocking the reuptake of norepinephrine by the presynaptic ends of the sympathetic nerves causing it to cumulate in the synaptic cleft. In normal pupils, placing a cocaine drop in the eye results in pupil dilation because of the accumulation of norepinephrine at the level of the receptors of the effective cells. However, in HS pupils, because of sympathetic denervation, the amount of epinephrine produced by the presynaptic end is negligeable. Thus, these pupils respond poorly to cocaine eyedrops. The test is performed by placing cocaine eyedrops in both eyes. After 45 minutes, if the miotic pupil does not dilate more than 2 mm and the difference between the smaller pupil and the normal one is of at least 0.8 mm, the test is considered positive.17,36 In other words, the absence of response in the pathological side is what defines a positive test and is in favor of HS. However, the use of cocaine has three major drawbacks. The first being that it is a controlled drug, hard to obtain especially in outpatient settings. The second limitation is that there are other factors that may interfere with pupillary dilation such as posterior synechiae or iris atrophy, resulting in a false-positive test.17 Patients with dark irises also seem to have a slow response to cocaine eyedrops and 3 hours may be needed before concluding to a positive test. And finally, an incomplete interruption of the OSP can lead to a false-negative test.17 Nonetheless, it remains, to some physicians, the drug of choice in young children since apraclonidine use is controversial in this population.
Etiology: Clinical and Pharmacological Orientation
In a series where 270 patients had HS with an identifiable etiology, 13% had a lesion in the first-order neuron, 44% in the second-order neuron, and 43% in the third-order neuron.11
Etiologies of HS regarding the site of the neuronal order are summarized in Table 1.
 |
Table 1 Etiologies of Horner’s Syndrome According to the Site of the Lesion |
Clinical Orientation
Central (FON) Horner’s Syndrome
It is usually ipsilateral to the lesion and rarely isolated because of the proximity of the FON fibers to important neurological structures.10 Associated symptoms help localize the lesion. Damage to the thalamus/hypothalamus results in a central HS with contralateral hemiparesis and hypoesthesia. A midbrain lesion can associate an ipsilateral HS with a contralateral trochlear nerve palsy. In the presence of an ipsilateral abducens palsy, it is safe to think of a cavernous sinus lesion associated with a postganglionic HS because of the trajectory of the TON. However, one case of a hemorrhagic pontine lesion with bilateral abducens nerve palsy and central HS has been reported.37
Lateral medullary syndrome whether from vessel infarcts, arterial dissection, cardiac embolism, or less commonly from demyelinating diseases, is the most common cause of central HS. It occurs with several other neurological deficits forming a triad known as the Wallenberg syndrome: ipsilateral HS, ipsilateral ataxia, and contralateral hypoalgesia. Nystagmus, vertigo, dysphagia, ipsilateral facial hypoalgesia, and facial weakness can also be found. In a series including 33 patients with lateral medullary infarction, ipsilateral HS was the most frequent sign found in 91% of the cases.38 A larger series of 130 patients with lateral medullary infarction showed that HS was present in 88% of the patients.39 Lower cervical or upper thoracic spinal cord lesions can occasionally cause isolated central HS but are more often associated with other signs. Brown-Séquard syndrome can be associated with central HS ipsilateral to the side with the loss of motor function and light touch. Patients with lower cervical or upper thoracic cord lesions can develop on very rare occasions an alternating HS.40,41
Preganglionic (SON) Horner’s Syndrome
When faced with a preganglionic HS, after ruling out iatrogenic causes and trauma, malignancies should always be considered since they are the cause of up to 25% of preganglionic HS.17
Lesions of the upper chest cavity, the cervical sympathetic chain, or the neck can result in preganglionic HS. When associated with ipsilateral shoulder pain, arm paresthesia, hand muscle atrophy, an underlying malignancy of the pulmonary apex or the brachial plexus should be suspected. This combination of symptoms is called Pancoast syndrome.17
Postganglionic (TON) Horner’s Syndrome
In postganglionic HS (unlike central and preganglionic HS), because of the anatomical distribution of the sudomotor fibers, hemifacial anhidrosis is absent except for a small patch of skin above the eyebrow. This made some authors call it an incomplete HS.17
Even though postganglionic HS is often idiopathic, carotid artery dissection should always be eliminated. Unilateral HS is the third most common symptom present in up to 58% of carotid artery dissection following pain and focal cerebral ischemic symptoms.42
Lesions of the cavernous sinus will often associate with deficits of cranial nerves III, IV, V and VI.
Neuroblastoma and Horner’s Syndrome in the Pediatric Population
Neuroblastoma has been reported to be the most common occult malignancy to be associated with pediatric HS.43,44 Despite this association of HS and neuroblastoma in the literature, Musarella et al and Jaffe et al have concluded that as few as 3.5% to 13% of children with neuroblastoma have associated Horner syndrome, while in only 2.2%, Horner syndrome is the initial symptom.45,46 Extrapolating from the incidence of neuroblastoma in patients younger than 5 years, Smith et al found that neuroblastoma concurrent with HS would have a projected incidence of 1 in 54,000 to 200,000 pediatric patients, while 1 in 318,200 would initially manifest HS.6 Mahoney et al recommended that children with suspected HS undergo a general physical examination and palpation of the neck, axilla, and abdomen. If HS is then clinically or pharmacologically confirmed, magnetic resonance imaging (MRI) of the head, neck, and chest with and without contrast, in addition to urine catecholamine testing should be ordered.43 However, Smith et al suggested that the current recommendation be reconsidered, in view of the extremely low extrapolated incidence of concurrent Horner syndrome and neuroblastoma.6
Pharmacological Localization
Hydroxyamphetamine acts by releasing the norepinephrine stored at the presynaptic ends of the postganglionic nerve fibers. In central and preganglionic HS, postganglionic neurons remain intact. Thus, the use of 0.5% hydroxyamphetamine eyedrops induces pupillary dilation in the affected eye. On the other hand, in case of damage at the postganglionic level of the OSP, the norepinephrine storage at the end of the fibers dissipates. Thus, the use of hydroxyamphetamine produces a greater dilation response in the normal pupil compared to Horner’s pupil.47 However, since there is usually a certain delay between the onset of postganglionic HS and the total depletion of norepinephrine from the nerve endings, false-negative results remain possible in acute HS, especially within the first 2–3 weeks.48 This test has also some limitations in infants since TON are usually damaged in congenital HS or early acquired HS (during the first year of life) even if it is spared by the causative lesion. Also, hydroxyamphetamine eyedrop testing is not reliable when performed within 48 hours from the use of apraclonidine or cocaine testing, since cocaine inhibits the uptake of hydroxyamphetamine by the presynaptic nerve endings.
Pholedrine is a derivative of hydroxyamphetamine. In one study, 1% pholedrine proved to be as effective as 0.5% hydroxyamphetamine in distinguishing postganglionic HS from central and preganglionic HS.49
Unfortunately, both hydroxyamphetamine and pholedrine are not available commercially and are therefore hard to obtain.
Phenylephrine
Some authors believe that Phenylephrine 1% could be an alternative to hydroxyamphetamine as a localizing substance. One study showed that phenylephrine when diluted to 1% concentration and used as eyedrops in the eyes of patients with HS, had 81% sensitivity and 100% specificity in determining postganglionic lesions from central or preganglionic lesions.50
Thompson and Mensher, using a 10% concentration, which dilates the normal pupil, determined that the affected pupil of the three patients with post-ganglionic lesions dilated sooner and more vigorously than the unaffected pupil.51 Danesh-Meyer et al’s study showed that phenylephrine 1% dilates the post-ganglionic Horner’s pupil, but not the non-post-ganglionic or normal pupil. The affected pupil did not dilate in patients with central HS, dilated minimally in cases of preganglionic HS, and showed noticeable dilation in patients with postganglionic HS. The authors suggest that denervation hypersensitivity of the dilator muscle’s cells is superior in case of postganglionic lesions.50
One of the limitations of the 1% concentration is that it does not dilate the normal pupil (contrary to the 10% concentration). This implies that if neither pupil dilates with phenylephrine 1% it could be either because the lesion is non-post-ganglionic or the drops are ineffective.50
Imaging Strategies
Patients with HS can have associated symptoms that help localize the defect and strategically use targeted imaging evaluation. However, cases of isolated HS represent a clinical challenge.
Patients who present with central HS should be imaged with MRI from the brain to the upper thoracic spinal cord. In preganglionic and postganglionic HS, computed tomography (CT)-angiography from the orbits to T4-T5 is advised. Patients identified as having isolated HS are those who have no history of malignancy and whose clinical evaluation does not reveal any localizing signs.9
In one study including 88 patients with isolated HS who underwent radiological evaluation, 18 (20%) had an identifiable cause with internal carotid dissection being the leading etiology present in 7/88. However, in this study, patients with painful HS were not excluded and pain was in fact present in 6/7 of the patients with an internal carotid dissection. Thus, the authors suggest that urgent imaging after the onset of HS is only recommended in case of associated pain.52
The cause of the damage to OSP is usually known before the diagnosis of HS is made. In the majority of cases where the diagnosis of HS required pharmacological testing, no etiology was found. The syndrome was considered as « idiopathic ».52
The diagnosis of carotid artery dissection should be suspected in all patients with HS and headache or neck pain, especially in the presence of focal neurological signs. However, in very rare cases, it may be responsible for HS even in the absence of neck or face pain.
In adults with longstanding HS, it is possible to differ further explorations.
Conclusion
Horner’s syndrome results from a lesion anywhere along the oculosympathetic pathway. Etiologies range from benign to serious with some being life-threatening. Three types of HS are distinguished according to the location of the lesion. Understanding the clinical features of the three types of HS and their implications assists in localization of the underlying pathologic process. The diagnosis of HS requires a methodologic approach based on a complete physical examination and/or ancillary pharmacological testing. In most cases, targeted imaging of the sympathetic pathway according to the symptoms and/or pharmacological tests allows one to identify the etiology of HS.
Digital Pupillometry
Using digital pupillometry to measure baseline inter-eye difference in maximal pupil size and dilation lag proved to be an objective, fast, and reliable tool, for the diagnosis of HS.15
Facial Imaging
In 2022, a group of experts published a paper suggesting the use of a computer to automatically detect patients with HS through facial imaging. They concluded that their methods were accurate enough to be used as a second advisor to clinicians before diagnosing HS. They plan on conducting future studies with larger samples and to investigate the possible use of video detection.20
Disclosure
The authors report no conflicts of interest in this work.
References
1. Martin TJ. Horner syndrome: a clinical review. ACS Chem Neurosci. 2018;9(2):177–186. doi:10.1021/acschemneuro.7b00405
2. Dafereras M, Sapouridis H, Laios K, Chrysikos D, Mavrommatis E, Troupis T. The pioneer ophthalmologist Johann Friedrich Horner (1831–1886) and the clinical anatomy of the homonymous syndrome. Acta Chir Belg. 2020;120(5):363–365. doi:10.1080/00015458.2020.1746528
3. Van der Wiel HL. Johann Friedrich Horner (1831–1886). J Neurol. 2002;49:636–637. doi:10.1007/s004150200079
4. Roper-Hall G. Historical vignette: Johann Friedrich Horner (1831–1886): Swiss ophthalmologist, scientific contributor, and accomplished academician. Am Orthopt J. 2016;66(1):126–134. doi:10.3368/aoj.66.1.126
5. Abbas A, Manjila S, Singh M, Belle V, Chandar K, Miller JP. Johann Friedrich Horner and the repeated discovery of oculosympathoparesis: whose syndrome is it? Neurosurgery. 2015;77(3):486–491. doi:10.1227/NEU.0000000000000832
6. Smith SJ, Diehl N, Leavitt JA, Mohney BG. Incidence of pediatric Horner syndrome and the risk of neuroblastoma: a population-based study. Arch Ophthalmol. 2010;128(3):324–329. doi:10.1001/archophthalmol.2010.6
7. Han J, Park SY, Lee JY. Nationwide population-based incidence and etiologies of pediatric and adult Horner syndrome. J Neurol. 2021;268(4):1276–1283. doi:10.1007/s00415-020-10270-2
8. Smith PG, Dyches TJ, Burde RM. Topographic analysis of Horner’s syndrome. Otolaryngol Head Neck Surg. 1986;94(4):451–457. doi:10.1177/019459988609400409
9. Davagnanam I, Fraser CL, Miszkiel K, Daniel CS, Plant GT. Adult Horner’s syndrome: a combined clinical, pharmacological, and imaging algorithm. Eye. 2013;27(3):291–298. doi:10.1038/eye.2012.281
10. Nagy AN, Hayman LA, Diaz-Marchan PJ, Lee AG. Horner’s syndrome due to first-order neuron lesions of the oculosympathetic pathway. Am J Roentgenol. 1997;169(2):581–584.
11. Maloney WF, Younge BR, Moyer NJ. Evaluation of the causes and accuracy of pharmacologic localization in Horner’s syndrome. Am J Ophthalmol. 1980;90(3):394–402. doi:10.1016/S0002-9394(14)74924-4
12. Parkinson D. Further observations on the sympathetic pathways to the pupil. Anat Rec. 1988;220(1):108–109. doi:10.1002/ar.1092200113
13. Reede DL, Garcon E, Smoker WRK, Kardon R. Horner’s Syndrome: clinical and radiographic evaluation. Neuroimaging Clin N Am. 2008;18(2):369–385. doi:10.1016/j.nic.2007.11.003
14. Pellegrini F, Capello G, Napoleone R. Dilation lag in Horner syndrome can be measured with a diagnostic imaging system. Neurology. 2018;90(13):618. doi:10.1212/WNL.0000000000005217
15. Yoo YJ, Yang HK, Hwang JM. Efficacy of digital pupillometry for diagnosis of Horner syndrome. Anderson A, editor. PLoS One. 2017;12(6):e0178361. doi:10.1371/journal.pone.0178361
16. Beard C. Muller’s superior tarsal muscle: anatomy, physiology, and clinical significance. Ann Plast Surg. 1985;14(4):324–333. doi:10.1097/00000637-198504000-00005
17. Kanagalingam S, Miller NR. Horner syndrome: clinical perspectives. Eye Brain. 2015;7:35–46. doi:10.2147/EB.S63633
18. Nielsen PJ. Upside down ptosis in patients with Horner’s syndrome. Acta Ophthalmol. 1983;61(5):952–957.
19. Van der Wiel HL, van Gijn J. No enophthalmos in Horner’s syndrome. J Neurol Neurosurg Psychiatry. 1987;50(4):498–499. doi:10.1136/jnnp.50.4.498
20. Fan J, Qin B, Gu F, et al. Automatic detection of Horner syndrome by using facial images. J Healthc Eng. 2022;2022:8670350. doi:10.1155/2022/8670350
21. Zweifel TJ, Thompson HS. Congenital Horner’s syndrome. Arch Ophthalmol. 1980;98(6):1074–1078. doi:10.1001/archopht.1980.01020031064011
22. Ribeiro L, Rocha R, Martins J, Monteiro A. Starch–iodine test: a diagnostic tool for Horner syndrome. BMJ Case Rep. 2020;13(9):e238541. doi:10.1136/bcr-2020-238541
23. Henderson AD, Ramulu PY, Lawler JF. Teaching neuroimages: thermal imaging in Horner syndrome. Neurology. 2019;93(13):e1324–e1325. doi:10.1212/WNL.0000000000008182
24. Kim MH. Effects of acupuncture on the symptoms and thermal imaging of idiopathic Horner’s syndrome: a case report. Acupunct Med. 2021;39(6):730–732. doi:10.1177/09645284211025987
25. Maamouri R, Ouederni M, Oueslati Y, Mbarek C, Chammakhi C, Cheour M. Acute Painful Horner’s Syndrome Revealing Eagle’s Syndrome: A Report of Two Cases. Neuro-Ophthalmology. 2022;46(4):244–247. doi:10.1080/01658107.2021.2003821
26. Morales J, Brown SM, Abdul-Rahim AS, Crosson CE. Ocular effects of apraclonidine in Horner syndrome. Arch Ophthalmol. 2000;118:951–954.
27. Garibaldi DC, Hindman HB, Grant MP, Lliff NT, Merbs SL. Effect of 0.5% apraclonidine on ptosis in Horner syndrome. Ophthal Plast Reconstr Surg. 2006;22:53–55. doi:10.1097/01.iop.0000196322.05586.6a
28. Koc F. The sensitivity and specificity of 0.5% apraclonidine in the diagnosis of oculosympathetic paresis. Br J Ophthalmol. 2005;89(11):1442–1444. doi:10.1136/bjo.2005.074492
29. Chen PL, Hsiao CH, Chen JT, Lu DW, Chen WY. Efficacy of apraclonidine 0.5% in the diagnosis of Horner syndrome in pediatric patients under low or high illumination. Am J Ophthalmol. 2006;142:469–472. doi:10.1016/j.ajo.2006.04.052
30. Brown SM, Aouchiche R, Freedman KA. The utility of 0.5% apraclonidine in the diagnosis of Horner syndrome. Arch Ophthalmol. 2003;121:1201–1203.
31. Cooper-Knock J, Pepper I, Hodgson T, Sharrack B. Early diagnosis of Horner syndrome using topical apraclonidine. J Neuroophthalmol. 2011;31(3):214–216. doi:10.1097/WNO.0b013e31821a91fe
32. Lebas M, Seror J, Debroucker T. Positive apraclonidine test 36 hours after acute onset of Horner syndrome in dorsolateral pontomedullary stroke. J Neuroophthalmol. 2010;30:12–17. doi:10.1097/WNO.0b013e3181b1b41f
33. Kawasaki A, Borruat FX. False negative apraclonidine test in two patients with Horner syndrome. Klin Monbl Augenheilkd. 2008;225:520–522. doi:10.1055/s-2008-1027349
34. Watts P, Satterfield D, Lim MK. Adverse effects of apraclonidine used in the diagnosis of Horner syndrome in infants. J Am Assoc Pediatr Ophthalmol Strabismus. 2007;11(3):282–283. doi:10.1016/j.jaapos.2007.02.015
35. Eldib AA, Patil P, Nischal KK, Mitchell ER, Hiasat JG, Pihlblad MS. Safety of apraclonidine eye drops in diagnosis of Horner syndrome in an outpatient pediatric ophthalmology clinic. J Am Assoc Pediatr Ophthalmol Strabismus. 2021;25(6):336.e1–336.e4. doi:10.1016/j.jaapos.2021.07.011
36. Kardon RH, Denison CE, Brown CK, Thompson HS. Critical evaluation of the cocaine test in the diagnosis of Horner’s syndrome. Arch Ophthalmol. 1990;108(3):384–387. doi:10.1001/archopht.1990.01070050082036
37. Kellen RI, Burde RM, Hodges FJ 3rd, Roper-Hall G. Central bilateral sixth nerve palsy associated with a unilateral preganglionic Horner’s syndrome. J Clin Neuroophthalmol. 1988;8:179–184.
38. Sacco RL, Freddo L, Bello JA, Odel JG, Onesti ST, Mohr JP. Wallenberg’s lateral medullary syndrome. Clinical-magnetic resonance imaging correlations. Arch Neurol. 1993;50(6):609–614. doi:10.1001/archneur.1993.00540060049016
39. Kim JS. Pure lateral medullary infarction: clinical-radiological correlation of 130 acute, consecutive patients. Brain. 2003;126(Pt 8):1864–1872. doi:10.1093/brain/awg169
40. Tan E, Kansu T, Saygi S, Zileli T. Alternating Horner’s syndrome: a case report and review of the literature. Neuroophthalmology. 1990;10:19–22. doi:10.3109/01658109008997256
41. Furukawa T, Toyokura Y. Alternating Horner syndrome. Arch Neurol. 1974;30(4):311–313. doi:10.1001/archneur.1974.00490340039008
42. Anson J, Crowell RM. Cervicocranial arterial dissection. Neurosurgery. 1991;29:89–96. doi:10.1227/00006123-199107000-00015
43. Mahoney NR, Liu GT, Menacker SJ, Wilson MC, Hogarty MD, Maris JM. Pediatric Horner syndrome: etiologies and roles of imaging and urine studies to detect neuroblastoma and other responsible mass lesions. Am J Ophthalmol. 2006;142(4):651–659. doi:10.1016/j.ajo.2006.05.047
44. Zafeiriou DI, Economou M, Koliouskas D, Triantafyllou P, Kardaras P, Gombakis N. Congenital Horner’s syndrome associated with cervical neuroblastoma. Eur J Paediatr Neurol. 2006;10(2):90–92. doi:10.1016/j.ejpn.2006.01.004
45. Musarella MA, Chan HS, DeBoer G, Gallie BL. Ocular involvement in neuroblastoma: prognostic implications. Ophthalmology. 1984;91(8):936–940. doi:10.1016/S0161-6420(84)34211-7
46. Jaffe N, Cassady R, Petersen R, Traggis D. Heterochromia and Horner syndrome associated with cervical and mediastinal neuroblastoma. J Pediatr. 1975;87(1):75–77.
47. Cremer SA, Thompson HS, Digre KB, Kardon RH. Hydroxyamphetamine mydriasis in normal subjects. Am J Ophthalmol. 1990;110:66–70. doi:10.1016/S0002-9394(14)76940-5
48. Donahue SP, Lavin PJ, Digre K. False-negative hydroxyamphetamine (Paredrine) test in acute Horner’s syndrome. Am J Ophthalmol. 1996;122:900–901. doi:10.1016/S0002-9394(14)70395-2
49. Bates AT, Chamberlain S, Champion M, et al. Pholedrine: a substitute for hydroxyamphetamine as a diagnostic eyedrop test in Horner’s syndrome. J Neurol Neurosurg Psychiatry. 1995;58(2):215–217. doi:10.1136/jnnp.58.2.215
50. Danesh-Meyer HV, Savino P, Sergott R. The correlation of phenylephrine 1% with hydroxyamphetamine 1% in Horner’s syndrome. Br J Ophthalmol. 2004;88:592–593. doi:10.1136/bjo.2003.029371
51. Thompson HS, Mensher JH. Adrenergic mydriasis in Horner’s syndrome. Hydroxyamphetamine test for diagnosis of postganglionic defects. Am J Ophthalmol. 1971;72(2):472–480.
52. Beebe JD, Kardon RH, Thurtell MJ. The yield of diagnostic imaging in patients with isolated Horner syndrome. Neurol Clin. 2017;35(1):145–151. doi:10.1016/j.ncl.2016.08.005
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


