Back to Journals » Eye and Brain » Volume 12
Neuro-ophthalmic Complications of Immune Checkpoint Inhibitors: A Systematic Review
Authors Yu CW , Yau M, Mezey N , Joarder I , Micieli JA
Received 20 August 2020
Accepted for publication 24 September 2020
Published 3 November 2020 Volume 2020:12 Pages 139—167
DOI https://doi.org/10.2147/EB.S277760
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Margaret Wong-Riley
Caberry W Yu,1 Matthew Yau,2 Natalie Mezey,1 Ishraq Joarder,3 Jonathan A Micieli4,5
1Faculty of Medicine, Queen’s University, Kingston, Canada; 2Faculty of Medicine, University of Toronto, Toronto, Canada; 3Faculty of Science, University of Toronto, Scarborough, Ontario, Canada; 4Department of Ophthalmology and Vision Sciences and Division of Neurology, Department of Medicine, University of Toronto, Toronto, Canada; 5Kensington Vision and Research Centre, Toronto, Canada
Correspondence: Jonathan A Micieli
Kensington Vision and Research Centre, 340 College Street, Suite 501, Toronto, Ontario M5T 3A9, Canada
Tel +1(416) 928-1335
Fax +1(416) 928-5075
Email [email protected]
Objective: Immune checkpoint inhibitors (ICIs) are novel cancer therapies that may be associated with immune-related adverse events (IRAEs) and come to the attention of neuro-ophthalmologists. This systematic review aims to synthesize the reported ICI-associated IRAEs relevant to neuro-ophthalmologists to help in the diagnosis and management of these conditions.
Methods: A systematic review of the literature indexed by MEDLINE, Embase, CENTRAL, and Web of Science databases was searched from inception to May 2020. Reporting followed the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines. Primary studies on ICIs and neuro-ophthalmic complications were included. Outcomes included number of cases and incidence of neuro-ophthalmic IRAEs.
Results: Neuro-ophthalmic complications of ICIs occurred in 0.46% of patients undergoing ICI and may affect the afferent and efferent visual systems. Afferent complications include optic neuritis (12.8%), neuroretinitis (0.9%), and giant cell arteritis (3.7%). Efferent complications include myasthenia gravis (MG) (45.0%), thyroid-like eye disease (11.9%), orbital myositis (13.8%), general myositis with ptosis (7.3%), internuclear ophthalmoplegia (0.9%), opsoclonus-myoclonus-ataxia syndrome (0.9%), and oculomotor nerve palsy (0.9%). Pembrolizumab was the most common causative agent for neuro-ophthalmic complications (32.1%). Mortality was highest for MG (19.8%). Most patients (79.8%) experienced improvement or complete resolution of neuro-ophthalmic symptoms due to cessation of ICI and immunosuppression with systemic corticosteroids.
Conclusion: While incidence of neuro-ophthalmic IRAEs is low, clinicians involved in the care of cancer patients must be aware of their presentation to facilitate prompt recognition and management. Collaboration between oncology and neuro-ophthalmology teams is required to effectively manage patients and reduce morbidity and mortality.
Keywords: immune checkpoint inhibitors, cancer immunotherapy, CTLA-4 inhibitors, PD-1 inhibitors, PD-L1 inhibitors
Introduction
Immune checkpoint inhibitors (ICIs) are novel immunologic monoclonal antibodies that block inhibitory receptors of the immune system, such as cytotoxic T-lymphocyte associated antigen-4 (CTLA-4), programmed death-1 receptor (PD-1), and programmed death ligand-1 (PD-L1).1 They are increasingly used as cancer therapies for cancers such as melanoma due to their activation of specific antitumor T-cell immune responses.2 These immune checkpoint molecules maintain immune homeostasis and prevent autoimmunity, but are also used by cancers to suppress normal antitumor immune responses.1,2 CTLA-4, located on T-cells, regulates T-cell activity in the priming phase by preferentially binding to B7 on antigen-presenting cells. CTLA-4 inhibitors decrease the preferential binding between CTLA-4 and cluster of differentiation 28 (CD28) to allow binding of CD28 to B7 to occur and activate T-cells, thereby enhancing antitumor activity.3 PD-1 and PD-L1 inhibitors work by inhibiting the PD-1 (expressed on T or B cells) and the PD-L1 (expressed on cells like tumor cells) interaction that dampens immune response.4 There are currently seven ICIs approved by the US Food and Drug Administration, ipilimumab, pembrolizumab, nivolumab, cemiplimab, atezolizumab, avelumab, and durvalumab.
Due to their efficacious antitumor responses in advanced malignancies, ICIs are increasingly used, and potential new ICIs are investigated in clinical trials. However, there are frequent toxicities associated with their use that can lead to their discontinuation. The toxicities that occur due to immune system activation are termed immune-related adverse events (IRAEs), which can occur in 70–90% of patients and affect any organ system.5,6 The skin and gastrointestinal systems are most affected by ICIs and usually involve low-grade IRAEs such as rashes, diarrhea, and nausea.7,8 ICIs have also been associated with de novo endocrinopathies or exacerbations of existing ones.9 Ophthalmic IRAEs have been reported in less than 1% of patients, common examples include anterior uveitis and dry eye.10–12
Neuro-ophthalmic complications warrant their own investigation and can present with higher morbidity and mortality than IRAEs of other systems.7 Currently, established guidelines for the management of IRAEs contain very few neuro-ophthalmic conditions (eg myasthenia gravis (MG), general myositis and thyroid eye disease) and have been nonspecific in describing the unique complications in neuro-ophthalmology.13 While there have been systematic reviews on ophthalmic10,14 and neurologic15–17 complications alone, they focus on complications like uveitis or central nervous system disorders that may not involve the visual pathways. No systematic reviews exist on neuro-ophthalmic IRAEs specifically. Thus, the present review was conducted to investigate the neuro-ophthalmic IRAEs of ICIs to collate information on presentation, treatment, and outcome to guide diagnosis and management.
Methods
This systematic review and meta-analysis were performed in accordance with the Cochrane Handbook for Systematic Reviews of Interventions18 and the reporting followed the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines.19
Search Methods
MEDLINE, Embase, CENTRAL, and Web of Science databases were comprehensively searched from inception to May 8, 2020 (complete search strategy available in Table S1). Articles were limited to English language with no year restrictions. A manual search of references in original studies and reviews and editorials was also conducted. When full-texts were unavailable, library copies were requested. Covidence was used to manage records identified by the literature search.20
Eligibility Criteria and Study Selection
All published articles on ICIs and neuro-ophthalmic outcomes were considered for inclusion. Reviews were used to identify potential eligible articles, but excluded from final analysis. The primary outcomes of the review were the number of cases and incidence of neuro-ophthalmic IRAEs. These included complications of the afferent visual system (eg, optic neuritis; giant cell arteritis, GCA; neuroretinitis), efferent visual system (eg, MG, thyroid-like eye disease, orbital myositis, orbital apex syndrome, oculomotor nerve palsies), and other disorders (eg Tolosa–Hunt Syndrome, neuromyelitis optica). Neurological conditions such as MG were only included if ocular symptoms were involved.
Each study was reviewed by two reviewers, independently and in duplicate, by title and abstract, and subsequently by full text, with discrepancies resolved by an independent third reviewer. During abstract screening, all clinical trials, cohort studies, and case series on side effects not specific to neuro-ophthalmology with ICIs were included for full-text review to ensure that papers that only mentioned neuro-ophthalmic outcomes in the full-text were included.
Data Collection and Synthesis
Data extraction occurred for each study using predefined data abstraction forms in accordance with PRISMA. Extracted data included study characteristics (eg, author, publication year, country, study design), patient demographics (eg, age, sex, cancer type), intervention (eg ICI name, cycles and duration prior to onset), and outcome (eg, neuro-ophthalmic diagnosis, presentation, treatment, and final outcome). Prevalence was also collected for observational studies and clinical trials. Risk of bias was not assessed due to the higher number of case reports and series included. Qualitative analysis was carried out for each neuro-ophthalmic diagnosis reported. Quantitative analysis was performed using Microsoft Excel to calculate mean incidence or mortality of diagnoses when more than one pharmacovigilance or clinical trial reported such data. Overall prevalence of neuro-ophthalmic complications was calculated by dividing the number of cases of neuro-ophthalmic complications in included clinical trials and observational studies by the total number of patients who received ICIs in these studies.
Results
From 3507 abstracts obtained from the search strategy, 2469 abstracts were screened after de-duplication, and 394 full texts were reviewed. Of these, 115 papers met our inclusion criteria. Figure 1 depicts a PRISMA flow diagram.
Study Characteristics
Of the 115 included studies, 98 were case reports or series and 17 were retrospective chart reviews or clinical trials that reported incidence of neuro-ophthalmic complications. Table 1 provides a summary of these observational or pharmacovigilance studies.21–38 Tables 2–6 detail 109 individual cases (including cases described in observational studies) of optic neuritis/neuroretinitis, neuromuscular disorders, orbital disorders, GCA, and other diseases. A breakdown of the diagnoses can be found in Table 7. Of the cases, 31.2% of all patients with a neuro-ophthalmic complication were female. The mean (range) age at presentation was 66.5 (9–87) years. Cutaneous melanoma was the most common indication for ICI treatment for (48/109, 44.0%), followed by non-squamous cell lung cancer (NSCLC) (19/109, 17.4%). Pembrolizumab was the most common causative agent for neuro-ophthalmic complications (35/109, 32.1%), followed by nivolumab (27/109, 24.8%), ipilimumab (23/109, 21.1%), combination of ICIs (17/109, 15.6%), and atezolizumab (4/109, 3.7%). One case was reported for each of tremelimumab, durvalumab, and sintilimab. There were no reports on neuro-ophthalmic IRAEs for dostarlimab.
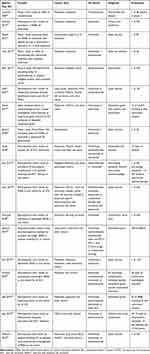 |
Table 1 Summary of Observational Studies or Clinical Trials |
 |
Table 2 Summary of Cases—Optic Neuritis or Neuroretinitis |
 |  |  |  |  |  |  |
Table 3 Summary of Cases – Neuromuscular |
 |  |  |  |
Table 4 Summary of Cases— Orbit |
 |
Table 5 Summary of Cases— Giant Cell Arteritis |
 |
Table 6 Summary of Cases— Other |
 |
Table 7 Breakdown of Neuro-ophthalmic Diagnoses. Excludes Pharmacovigilance or Observational Trials That Do Not Include Details of the Patients |
The overall incidence of neuro-ophthalmic outcomes following ICI therapy was 0.46%. The median time to symptom onset was two cycles and ranged from one to 51 doses. ICIs were terminated in most patients following neuro-ophthalmic complication (67/109, 61.5%). They were held in 12 patients (11.0%) and continued in 13 patients (11.9%). Death occurred in 20 of 109 patients (18.3%) due to various causes, including worsening symptoms or other causes prior to improvement of neuro-ophthalmic symptoms. Improvement in neuro-ophthalmic symptoms with persistent deficits (eg, ptosis, diplopia) at last follow-up was seen in 45 of 109 (41.3%) patients, while 42 of 109 (38.5%) patients experienced complete resolution of neuro-ophthalmic symptoms. Outcome was not reported in two patients.
Optic Neuritis
Optic neuritis11,23,26,30,31,34,36,39–47 (n=12 case reports, n=9 in larger studies) and neuroretinitis48 (n=1) have been associated with various ICIs, most commonly with ipilimumab (60%). Pharmacovigilance studies showed a combined incidence of 9/2094 (0.43%) for ICI-associated optic neuritis. Of cases that reported laterality, optic neuritis was bilateral in most cases (9/12, 75.0%). While corticosteroids form the mainstay of the treatment, four of 14 patients (28.6%) required additional interventions: intravenous immunoglobulin (IVIg), plasma exchange (PLEX), infliximab, and/or mycophenolate mofetil. All cases experienced resolution (4/14) or improvement with residual symptoms or signs (eg, visual defects, disc pallor) (10/14). Hahn and Pepple reported a patient with neuroretinitis, which involved optic disc and macular edema that resolved with topical and systemic corticosteroids.48
Patients were only confirmed to have optic neuritis if abnormalities in optic nerve enhancement were shown on MRI and clinical presentation was consistent with optic neuritis as highlighted in a previous paper.49 This could not be confirmed for several cases.26,41,44,45
Neuromuscular Disorders
The most common disorder of neuromuscular transmission reported with ICI was MG. Only one case of Lambert–Eaton Myasthenic Syndrome (LEMS) occurred with nivolumab, with symptoms improving with amifampridine.50 Forty-nine case reports of MG were found in the literature.51–96 Pharmacovigilance studies suggested that ICI-associated MG has a combined incidence of 303/64,828 (0.47%).25,28,35 However, milder cases of MG may be underdiagnosed due to nonspecific symptoms such as weakness and fatigue. While few cases involved exacerbation of underlying MG (4/49, 8.2%), the rest were de novo (45/49, 91.8%).
Among the 49 cases, most (38/49, 77.6%) occurred with anti-PD-1 (eg, pembrolizumab, nivolumab). Pharmacovigilance studies also supported that MG occurred more commonly in anti-PD-1 (eg, pembrolizumab or nivolumab) or anti-PD-L1 (eg, atezolizumab) compared to anti-CTLA-4 (eg, ipilimumab) therapy (ROR: 3.9, 95%CI: 2.3–6.8).28 Suzuki et al found no cases of MG with ipilimumab.35
There was a shorter time to onset (median 29 days) for ICI-associated MG compared to other neurologic IRAEs (61–80 days).25 The median number of cycles prior to symptom onset amongst all cases was two cycles and ranged from three days after the first dose to three months after the sixth dose. The neuro-ophthalmic symptoms of MG included ptosis and diplopia. In comparison to idiopathic MG, ICI-associated MG patients were more likely to experience bulbar symptoms, specifically dysphagia, dysarthria, and dyspnea, as well as myasthenic crisis.35 ICI-associated MG was also more frequently associated with undetectable or lower acetylcholine receptor antibodies compared to idiopathic MG.28,97,98
ICI-associated MG overlapped with myositis (myalgia and/or elevated creatine kinase, CK) in 24 of 49 cases (49.0%). These results were lower than findings in larger studies—MG was associated with myositis in 85% and myocarditis in 8% of patients.28 There may be an underdiagnosis of concurrent myositis as many cases with elevated CK levels were not formally assessed. Few studies performed skeletal muscle biopsy, but five of seven tested patients had inflammatory infiltrates.28
ICI-associated MG presented with a more common life-threatening fulminant presentation than idiopathic MG.28 Myasthenic crisis had a weighted incidence of 35/78 (46.7%) in larger studies.28,35 In contrast, idiopathic MG has around 15% to 20% lifetime risk of myasthenic crisis in the literature.99 Median onset from presentation to respiratory failure requiring intubation was one week for ICI-associated MG.28
A wide spectrum of clinical severity existed for MG, however, aggressive treatment led to improvement of symptoms in 55.5% and complete remission in 18.9% of patients in larger studies.28,35 While corticosteroids are appropriate for MG, IVIg or PLEX used as a first-line therapy for patients presenting with severe respiratory or bulbar symptoms showed better MG outcomes compared to those who received steroids alone (95% vs 63% symptom improvement).28 However, none of those who had respiratory failure following first-line corticosteroids showed clinical improvement with secondary IVIg or PLEX, unlike patients with idiopathic MG.28
A fatality rate of 19.8% (70/354) was found in case reports and larger studies.25,28,35 Outcomes were worse in patients with concurrent myositis and/or myocarditis, with highest mortality in patients with both (5/8, 62.5%) compared with MG alone (29/179, 16.2%), or with myositis only (6/29; 20.7%).25,28 Overall, complete recovery of MG symptoms occurred in (28/123, 22.8%) of patients. Most patients were maintained on prolonged steroid tapers and showed improvement (63/123, 51.2%).
Orbital Disorders
ICIs were associated with both thyroid-like eye disease (TED)100–108 (n=10) and idiopathic orbital myositis103,109–119 (n=16). TED may develop in patients on ipilimumab, nivolumab, pembrolizumab, or tremelimumab, even in the absence of existing thyroid dysfunction. In TED, patients generally presented with proptosis, chemosis, and thickening of extra-ocular muscles. They were associated with Graves' disease in 6/10 (60%) of case reports. Labs usually showed abnormal thyroid function, but up to 5% of patients with TED can be euthyroid or hypothyroid.107 Orbital myositis occurred in 15 patients, either from pembrolizumab or ipilimumab with or without nivolumab therapy. The median number of cycles prior to onset of symptoms for TED and myositis was three doses and ranged from one to 51 doses. In TED, orbital imaging showed thickening and enlargement of extraocular muscles without involvement of tendons, while in orbital myositis, tendons were involved. For TED, 9/10 patients (90%) showed improvement or resolution of TED with systemic corticosteroids, while one patient required canthotomy/cantholysis.100 Outcomes were worse for orbital myositis, with nine of 16 patients requiring additional therapy beyond systemic steroids (IVIg, methotrexate, PLEX, mycophenolate mofetil). Thirteen of 16 patients improved or experienced resolution, while two patients died from respiratory failure111,114 and one did not experience improvement before dying from unknown causes.116
In addition, one case each of orbital apex syndrome120 and Tolosa–Hunt syndrome22 occurred with ipilimumab. The former presented with painless vision loss, ptosis, ophthalmoplegia as a result of simultaneous dysfunction of the optic nerve and cranial nerves, and showed improvement on systemic steroids, albeit with persistent esotropia.120 The latter presented with severe unilateral periorbital pain and ophthalmoplegia, which improved with systemic steroids and local radiotherapy.22
Giant Cell Arteritis (GCA)
Five cases of GCA were reported following nivolumab, ipilimumab, combination of both, or pembrolizumab with one to 30 cycles of therapy.121–125 Patients presented similar to idiopathic GCA with blurry vision, diplopia, transient vision loss, along with headache, scalp tenderness, and jaw claudication. One patient presented with sudden onset loss of vision alone, while another had no visual symptoms.124,125 Three of five cases also had polymyalgia rheumatica.121,123,124 Most cases resolved with discontinuation of ICI and high-dose corticosteroids between two and four days, while one case persisted with low-grade symptoms and worsened upon starting another ICI.124 No larger trials existed to evaluate incidence of ICI-associated GCA.
Other Neuro-ophthalmic Disorders
Eight cases of generalized myositis with ptosis were reported in literature, most commonly following pembrolizumab therapy (3/8, 37.5%).126–133 A high rate of mortality was seen with general myositis (4/8, 50%). The remaining cases improved or resolved with corticosteroids alone or with IVIg. Other neuro-ophthalmic disorders included one case of oculomotor nerve palsy in 526 patients receiving nivolumab.24 This followed three cycles of nivolumab and resolved with a prednisone taper. Another study showed one case of bilateral internuclear ophthalmoplegia following nivolumab of 347 patients, which also improved with corticosteroids.38 One case of opsoclonus-myoclonus-ataxia syndrome was reported following ipilimumab and nivolumab therapy, which resolved with systemic corticosteroids and IVIg.134
Discussion
Neuro-ophthalmic complications may occur in patients being treated with ICIs. These include afferent disorders including optic neuritis, neuroretinitis, and GCA, and efferent disorders such as TED, MG, LEMS, orbital apex syndrome, oculomotor nerve palsy, orbital myositis, myositis with ptosis, Tolosa–Hunt Syndrome, and bilateral internuclear ophthalmoplegia. In general, ICIs may be held or discontinued for neuro-ophthalmic IRAEs, this decision should be made in consultation with the oncology team and appropriate guidelines, in particular, for more common IRAEs such as myasthenia gravis and myositis.13,135 Almost all patients require initial therapy with high-dose corticosteroids and may require other immunomodulatory therapy. Most afferent visual disorders (12/20, 60.0%) were treated with intravenous corticosteroids while others were treated orally. Out of all the afferent and efferent complications, four of 20 (20.0%) and 40 of 89 (44.9%), respectively, required additional immunomodulatory therapy, most commonly single therapy of IVIg. ICI re-challenge can be considered in cases of mild symptoms that resolve. In our review, 19 cases had either continued or held and then were re-challenged with ICI. Of these cases, four had recurrence or worsening of the same IRAE.84,106,124,136 In cases refractory to corticosteroids and recurrence of IRAE occurs to tapering of corticosteroids, IVIg and plasma exchange have been useful in the acute setting. Given the severity of symptoms and concern for new neuro-ophthalmic symptoms in patients with cancer, hospitalization is often necessary in patients with severe symptoms and multidisciplinary care involving oncology, ophthalmology, neurology, and neuro-ophthalmology is often required.
While ICIs are highly effective in stimulating the immune system to lead to a robust antitumor response, our study supports existing literature that ICIs have significant IRAEs that must be properly managed. In the literature, combination therapies have been discontinued more frequently than monotherapy.137 The mechanisms of induction of IRAEs is not fully elucidated, but are hypothesized to involve decreased peripheral tolerance and induction of organ-specific inflammatory processes.
In our review, several IRAEs occurred with a long duration after ICI administration and occurred with various doses and cycles of ICI. The earliest complication was TED, which arose after one dose (three days) of ipilimumab and pembrolizumab combination therapy,106 while the latest complication of orbital myositis arose after 51 doses (three years) of ipilimumab.115 Thus, the potential dose effects of ICIs on toxicity is difficult to determine at this time. There are key differences between neuro-ophthalmic and ophthalmic complications, our study found that neuro-ophthalmic ones were more likely to be associated with pembrolizumab, while ocular side effects were more common with ipilimumab in literature, likely due to differences in reporting adverse events between the two ICIs.14 In addition, the mean age of patients with neuro-ophthalmic complications was 66.5 years, higher than the mean age of 54 years for ophthalmic complications like uveitis.46 Ophthalmic complications generally had more favorable clinical outcomes compared to neuro-ophthalmic complications such as MG, which had a fatality rate of 19.8%.46
It is also unknown currently if neuro-ophthalmic IRAE severity can be used to predict treatment efficacy. An early study suggested that IRAE such as enterocolitis could signify response to treatment for metastatic melanoma;138 however, other studies have shown that occurrence of IRAE did not correlate with survival outcome or ICI treatment failure.139,140 Horvat et al also reported that the use of corticosteroids for IRAEs (primarily diarrhea, hepatitis, and dermatitis) did not impair overall survival in patients receiving ipilimumab for melanoma.140 This finding was supported by a recent systematic review of nine studies.141 There are currently a large number of novel ICIs, including two anti-CTLA-4, nine anti-PD-1, and four anti-PD-L1 currently in late-stage clinical studies for cancer indications.142
There are several limitations in this review. Data largely consisted of case series, which do not prove cause and effect between ICI and neuro-ophthalmic IRAEs. This link has not been firmly established, especially for conditions such as GCA where prevalence is higher in the patient demographics reported. We have reviewed each case to ensure that other common entities have been excluded in diagnostic consideration. Epidemiologic data on ICI complications were limited by the few number of studies that had neuro-ophthalmic complications. Some conditions may be under-reported due to nonspecific symptoms. While some diseases render a resemblance to established disorders, such as TED, it is possible that ICI-associated disorders (eg, inflammatory orbitopathy) is a distinct clinical syndrome. Future studies should aim to evaluate syndromes consistently (eg, tested for thyroid receptor antibody, thyroglobulin antibody, and thyroid peroxidase antibody). We have described misdiagnosis in previous reports of optic neuritis and emphasized the importance of proper investigations to confirm the diagnosis (eg, use of orbital MRI to detect optic nerve enhancement postgadolinium administration for optic neuritis, anti-aquaporin-4 antibodies for neuromyelitis optica).49 Efforts must be made to exclude common entities.
With the growing number of ICIs and increasing number of indications, it is important for neuro-ophthalmologists to be aware of potential adverse events. Future directions will include identifying minimum active doses for ICIs to achieve antitumor responses while minimizing IRAEs. The rapid identification and initiation of immunosuppression can improve patient outcomes. A collaborative approach and open communication with oncology is necessary in management of these IRAEs.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sharma P, Wagner K, Wolchok JD, Allison JP. Novel cancer immunotherapy agents with survival benefit: recent successes and next steps. Nat Rev Cancer. 2011;11:805–812.
2. Garrett MD, Collins I. Anticancer therapy with checkpoint inhibitors: what, where and when? Trends Pharmacol Sci. 2011;32:308–316. doi:10.1016/j.tips.2011.02.014
3. Buchbinder E, Stephen Hodi F. Cytotoxic T lymphocyte antigen-4 and immune checkpoint blockade. J Clin Invest. 2015;125:3377–3383. doi:10.1172/JCI80012
4. Ohaegbulam KC, Assal A, Lazar-Molnar E, et al. Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends Mol Med. 2015;21:24–33. doi:10.1016/j.molmed.2014.10.009
5. Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139–148. doi:10.1016/j.ejca.2015.11.016
6. Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev. 2016;44:51–60. doi:10.1016/j.ctrv.2016.02.001
7. Cousin S, Italiano A. Molecular pathways: immune checkpoint antibodies and their toxicities. Clin Cancer Res. 2016;22:4550–4555. doi:10.1158/1078-0432.CCR-15-2569
8. Bertrand A, Kostine M, Barnetche T, et al. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med. 2015;13. doi:10.1186/s12916-015-0455-8
9. Puwanant A, Isfort M, Lacomis D, Živković SA. Clinical spectrum of neuromuscular complications after immune checkpoint inhibition. Neuromuscul Disord. 2019;29:127–133. doi:10.1016/j.nmd.2018.11.012
10. Antoun J, Titah C, Cochereau I. Ocular and orbital side-effects of checkpoint inhibitors: a review article. Curr Opin Oncol. 2016;28:288–294. doi:10.1097/CCO.0000000000000296
11. Noble CW, Gangaputra SS, Thompson IA, et al. Ocular adverse events following use of immune checkpoint inhibitors for metastatic malignancies. Ocul Immunol Inflamm. 2020;28(6):854–859. doi:10.1080/09273948.2019.1583347
12. Cunningham ET, Moorthy RS, Zierhut M. Immune checkpoint inhibitor-induced uveitis. Ocul Immunol Inflamm. 2020;28:847–849. doi:10.1080/09273948.2020.1801286
13. Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: american society of clinical oncology clinical practice guideline. J Clin Oncol. 2018;36:1714–1768. doi:10.1200/JCO.2017.77.6385
14. Dalvin LA, Shields CL, Orloff M, et al. Checkpoint inhibitor immune therapy. Retina. 2018;38:1063–1078. doi:10.1097/IAE.0000000000002181
15. Pan PCW, Haggiagi A. Neurologic immune-related adverse events associated with immune checkpoint inhibition. Curr Oncol Rep. 2019;21. doi:10.1007/s11912-019-0859-2
16. Möhn N, Beutel G, Gutzmer R, et al. Neurological immune related adverse events associated with nivolumab, ipilimumab, and pembrolizumab therapy—review of the literature and future outlook. J Clin Med. 2019;8:1777. doi:10.3390/jcm8111777
17. Haugh AM, Probasco JC, Johnson DB. Neurologic complications of immune checkpoint inhibitors. Expert Opin Drug Saf. 2020;19:479–488. doi:10.1080/14740338.2020.1738382
18. Higgins JPT GS, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Cochrane Collab; 2011.
19. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700–b2700. doi:10.1136/bmj.b2700
20. Veritas Health Innovation. Covidence systematic review software. 2019. Available from: www.covidence.org.
21. Camacho LH, Antonia S, Sosman J, et al. Phase I/II trial of tremelimumab in patients with metastatic melanoma. J Clin Oncol. 2009;27:1075–1081. doi:10.1200/JCO.2008.19.2435
22. Voskens CJ, Goldinger SM, Loquai C, et al. The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS One. 2013;8:e53745. doi:10.1371/journal.pone.0053745
23. Kaur A, Doberstein T, Amberker RR, et al. Immune-related adverse events in cancer patients treated with immune checkpoint inhibitors: a single-center experience. Med. 2019;98.
24. Mancone S, Lycan T, Ahmed T, et al. Severe neurologic complications of immune checkpoint inhibitors: a single-center review. J Neurol. 2018;265:1636–1642. doi:10.1007/s00415-018-8890-z
25. Johnson DB, Manouchehri A, Haugh AM, et al. Neurologic toxicity associated with immune checkpoint inhibitors: a pharmacovigilance study. J Immunother Cancer. 2019;7:134. doi:10.1186/s40425-019-0617-x
26. Kim JM, Materin MA, Sznol M, et al. Ophthalmic immune-related adverse events of immunotherapy: a single-site case series. Ophthalmology. 2019;126:1058–1062. doi:10.1016/j.ophtha.2019.01.031
27. Moreira A, Loquai C, Pföhler C, et al. Myositis and neuromuscular side-effects induced by immune checkpoint inhibitors. Eur J Cancer. 2019;106:12–23. doi:10.1016/j.ejca.2018.09.033
28. Safa H, Johnson DH, Trinh VA, et al. Immune checkpoint inhibitor related myasthenia gravis: single center experience and systematic review of the literature. J Immunother Cancer. 2019;7:319. doi:10.1186/s40425-019-0774-y
29. Seki M, Uruha A, Ohnuki Y, et al. Inflammatory myopathy associated with PD-1 inhibitors. J Autoimmun. 2019;100:105–113. doi:10.1016/j.jaut.2019.03.005
30. Williams KJ, Grauer DW, Henry DW, Rockey ML. Corticosteroids for the management of immune-related adverse events in patients receiving checkpoint inhibitors. J Oncol Pharm Pract. 2019;25:544–550. doi:10.1177/1078155217744872
31. Weber JS, Kudchadkar RR, Yu B, et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J Clin Oncol. 2013;31:4311–4318. doi:10.1200/JCO.2013.51.4802
32. Hodi FS, Lawrence D, Lezcano C, et al. Bevacizumab plus ipilimumab in patients with metastatic melanoma. Cancer Immunol Res. 2014;2:632–642. doi:10.1158/2326-6066.CIR-14-0053
33. Balar AV, Castellano D, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18:1483–1492. doi:10.1016/S1470-2045(17)30616-2
34. Diehl A, Yarchoan M, Hopkins A, et al. Relationships between lymphocyte counts and treatmentrelated toxicities and clinical responses in patients with solid tumors treated with PD-1 checkpoint inhibitors. Oncotarget. 2017;8:114268–114280. doi:10.18632/oncotarget.23217
35. Suzuki S, Ishikawa N, Konoeda F, et al. Nivolumab-related myasthenia gravis with myositis and myocarditis in Japan. Neurology. 2017;89:1127–1134. doi:10.1212/WNL.0000000000004359
36. Omuro A, Vlahovic G, Lim M, et al. Nivolumab with or without ipilimumab in patients with recurrent glioblastoma: results from exploratory Phase I cohorts of CheckMate 143. Neuro Oncol. 2018;20:674–686. doi:10.1093/neuonc/nox208
37. Touat M, Maisonobe T, Knauss S, et al. Immune checkpoint inhibitor-related myositis and myocarditis in patients with cancer. Neurology. 2018;91:e985–e994. doi:10.1212/WNL.0000000000006124
38. Kao JC, Liao B, Markovic SN, et al. Neurological complications associated with anti–programmed death 1 (PD-1) antibodies. JAMA Neurol. 2017;74:1216–1222. doi:10.1001/jamaneurol.2017.1912
39. Boisseau W, Touat M, Berzero G, et al. Safety of treatment with nivolumab after ipilimumab-related meningoradiculitis and bilateral optic neuropathy. Eur J Cancer. 2017;83:28–31. doi:10.1016/j.ejca.2017.05.036
40. Francis JH, Jaben K, Santomasso BD, et al. Immune checkpoint inhibitor associated optic neuritis. Ophthalmology. 2020.
41. Yeh OL, Francis CE. Ipilimumab-associated bilateral optic neuropathy. J Neuro-Ophthalmology. 2015;35:144–147.
42. Kartal Ö, Ataş E. Bilateral optic neuritis secondary to nivolumab therapy: a case report. Med. 2018;54:82.
43. Mori S, Kurimoto T, Ueda K, et al. Optic neuritis possibly induced by anti-PD-L1 antibody treatment in a patient with non-small cell lung carcinoma. Case Rep Ophthalmol. 2018;9:348–356. doi:10.1159/000491075
44. Samanci NS, Ozan T, Çelik E, Demirelli FH. Optic neuritis related to atezolizumab treatment in a patient with metastatic non–small-cell lung cancer. JCO Oncol Pract. 2020;16:96–98. doi:10.1200/JOP.19.00438
45. Sun J, Schiffman J, Raghunath A, et al. Concurrent decrease in IL-10 with development of immune-related adverse events in a patient treated with anti-CTLA-4 therapy. Cancer Immun. 2008;8:9.
46. Sun MM, Levinson RD, Filipowicz A, et al. Uveitis in patients treated with CTLA-4 and PD-1 checkpoint blockade inhibition. Ocul Immunol Inflamm. 2020;28:217–227. doi:10.1080/09273948.2019.1577978
47. Wilson MA, Guld K, Galetta S, et al. Acute visual loss after ipilimumab treatment for metastatic melanoma. J Immunother Cancer. 2016;4. doi:10.1186/s40425-016-0170-9
48. Hahn L, Pepple KL. Bilateral neuroretinitis and anterior uveitis following ipilimumab treatment for metastatic melanoma. J Ophthalmic Inflamm Infect. 2016;6. doi:10.1186/s12348-016-0082-3
49. Micieli JA, Margolin E. Re: Francis et al.: immune checkpoint inhibitor associated optic neuritis (Ophthalmology. 2020 May 8 [Epub ahead of print]). Ophthalmology. 2020. doi:10.1016/j.ophtha.2020.05.043
50. Nakatani Y, Tanaka N, Enami T, et al. Lambert-Eaton Myasthenic syndrome caused by Nivolumab in a patient with squamous cell lung cancer. Case Rep Neurol. 2018;10:346–352. doi:10.1159/000494078
51. Algaeed M, Mukharesh L, Heinzelmann M, Kaminski HJ. Pearls & Oy-sters: pembrolizumab-induced myasthenia gravis. Neurology. 2018;91:E1365–E1367. doi:10.1212/WNL.0000000000006278
52. Alnahhas I, Wong J. A case of new-onset antibody-positive myasthenia gravis in a patient treated with pembrolizumab for melanoma. Muscle Nerve. 2017;55:E25–E26. doi:10.1002/mus.25496
53. Fazel M, Jedlowski PM. Severe myositis, myocarditis, and myasthenia gravis with elevated anti-striated muscle antibody following single dose of ipilimumab-nivolumab therapy in a patient with metastatic melanoma. Case Reports Immunol. 2019;2019:1–3. doi:10.1155/2019/2539493
54. Fellner A, Makranz C, Lotem M, et al. Neurologic complications of immune checkpoint inhibitors. J Neurooncol. 2018;137:601–609. doi:10.1007/s11060-018-2752-5
55. Fukasawa Y, Sasaki K, Natsume M, et al. Nivolumab-induced myocarditis concomitant with myasthenia gravis. Case Rep Oncol. 2017;10:809–812. doi:10.1159/000479958
56. Gonzalez NL, Puwanant A, Lu A, et al. Myasthenia triggered by immune checkpoint inhibitors: new case and literature review. Neuromuscul Disord. 2017;27:266–268. doi:10.1016/j.nmd.2017.01.002
57. Hasegawa Y, Kawai S, Ota T, et al. Myasthenia gravis induced by nivolumab in patients with non-small-cell lung cancer: a case report and literature review. Immunotherapy. 2017;9:701–707. doi:10.2217/imt-2017-0043
58. Hibino M, Maeda K, Horiuchi S, et al. Pembrolizumab-induced myasthenia gravis with myositis in a patient with lung cancer. Respirol Case Reports. 2018;6:e00355. doi:10.1002/rcr2.355
59. Huh SY, Shin SH, Kim MK, et al. Emergence of myasthenia gravis with myositis in a patient treated with pembrolizumab for thymic cancer. J Clin Neurol. 2018;14:115–117. doi:10.3988/jcn.2018.14.1.115
60. Johnson DB, Saranga-Perry V, Lavin PJM, et al. Myasthenia gravis induced by ipilimumab in patients with metastatic melanoma. J Clin Oncol. 2015;33:e122–e124. doi:10.1200/JCO.2013.51.1683
61. Kim JS, Nam TS, Kim J, et al. Myasthenia gravis and myopathy after nivolumab treatment for non-small cell lung carcinoma: a case report. Thorac Cancer. 2019;10:2045–2049.
62. Konstantina T, Konstantinos R, Anastasios K, et al. Fatal adverse events in two thymoma patients treated with anti-PD-1 immune check point inhibitor and literature review. Lung Cancer. 2019;135:29–32. doi:10.1016/j.lungcan.2019.06.015
63. Becquart O, Lacotte J, Malissart P, et al. Myasthenia gravis induced by immune checkpoint inhibitors. J Immunother. 2019;42:309–312. doi:10.1097/CJI.0000000000000278
64. Lara MS, Afify A, Ellis MP, et al. Immune checkpoint inhibitor-induced myasthenia gravis in a patient with advanced NSCLC and remote history of thymoma. Clin Lung Cancer. 2019;20:e489–e491. doi:10.1016/j.cllc.2019.04.007
65. Lau KHV, Kumar A, Yang IH, Nowak RJ. Exacerbation of myasthenia gravis in a patient with melanoma treated with pembrolizumab. Muscle Nerve. 2016;54:157–161.
66. Liao B, Shroff S, Kamiya-Matsuoka C, Tummala S. Atypical neurological complications of ipilimumab therapy in patients with metastatic melanoma. Neuro Oncol. 2014;16:589–593. doi:10.1093/neuonc/nou001
67. Liu Q, Ayyappan S, Broad A, Narita A. Pembrolizumab-associated ocular myasthenia gravis. Clin Experiment Ophthalmol. 2019;47:
68. Loochtan AI, Nickolich MS, Hobson-Webb LD. Myasthenia gravis associated with ipilimumab and nivolumab in the treatment of small cell lung cancer. Muscle Nerve. 2015;52:307–308. doi:10.1002/mus.24648
69. Maeda O, Yokota K, Atsuta N, et al. Nivolumab for the treatment of malignant melanoma in a patient with pre-existing myasthenia gravis. Nagoya J Med Sci. 2016;78:119–122.
70. Mancano MA, Von BJE, Ro M. ISMP adverse drug reactions: lithium-induced cardiomyopathy fixed drug eruption due to cetirizine, levocetirizine, and hydroxyzine nivolumab-induced myasthenia gravis nivolumab-induced cholangitic liver disease torsade de pointes caused by psychiatric polypharmacy trichotillomania associated with aripiprazole. Hosp Pharm. 2018;53:371–375.
71. March KL, Samarin MJ, Sodhi A, Owens RE. Pembrolizumab-induced myasthenia gravis: a fatal case report. J Oncol Pharm Pract. 2018;24:146–149. doi:10.1177/1078155216687389
72. Mitsune A, Yanagisawa S, Fukuhara T, et al. Relapsed myasthenia gravis after nivolumab treatment. Intern Med. 2018;57:1893–1897. doi:10.2169/internalmedicine.9153-17
73. Möhn N, Sühs KW, Gingele S, et al. Acute progressive neuropathy-myositis-myasthenia-like syndrome associated with immune-checkpoint inhibitor therapy in patients with metastatic melanoma. Melanoma Res. 2019;29:435–440.
74. Chang E, Sabichi AL, Sada YH. Myasthenia gravis after nivolumab therapy for squamous cell carcinoma of the bladder. J Immunother. 2017;40:114–116. doi:10.1097/CJI.0000000000000161
75. Montes V, Sousa S, Pita F, et al. Myasthenia gravis induced by ipilimumab in a patient with metastatic melanoma. Front Neurol. 2018;9:150. doi:10.3389/fneur.2018.00150
76. Nguyen BHV, Kuo J, Budiman A, et al. Two cases of clinical myasthenia gravis associated with pembrolizumab use in responding melanoma patients. Melanoma Res. 2017;27:152–154. doi:10.1097/CMR.0000000000000310
77. Onda A, Miyagawa S, Takahashi N, et al. Pembrolizumab-induced ocular Myasthenia Gravis with anti-titin antibody and necrotizing myopathy. Intern Med. 2019;58:1635–1638. doi:10.2169/internalmedicine.1956-18
78. Phua CS, Murad A, Fraser C, et al. Myasthenia gravis and concurrent myositis following PD-L1 checkpoint inhibitor for non-small cell lung cancer. BMJ Neurol Open. 2020;2:e000028. doi:10.1136/bmjno-2019-000028
79. Polat P, Donofrio PD. Myasthenia gravis induced by nivolumab therapy in a patient with non–small-cell lung cancer. Muscle Nerve. 2016;54:507. doi:10.1002/mus.25163
80. Sciacca G, Nicoletti A, Rampello L, et al. Benign form of myasthenia gravis after nivolumab treatment. Muscle Nerve. 2016;54:507–509. doi:10.1002/mus.25212
81. So H, Ikeguchi R, Kobayashi M, et al. PD-1 inhibitor-associated severe myasthenia gravis with necrotizing myopathy and myocarditis. J Neurol Sci. 2019;399:97–100.
82. Takai M, Kato D, Iinuma K, et al. Simultaneous pembrolizumab-induced myasthenia gravis and myocarditis in a patient with metastatic bladder cancer: a case report. Urol Case Reports. 2020;31:101145.
83. Tan RYC, Toh CK, Takano A. Continued response to one dose of nivolumab complicated by myasthenic crisis and myositis. J Thorac Oncol. 2017;12:e90–e91. doi:10.1016/j.jtho.2017.02.024
84. Tedbirt B, De Pontville M, Branger P, et al. Rechallenge of immune checkpoint inhibitor after pembrolizumab-induced myasthenia gravis. Eur J Cancer. 2019;113:72–74. doi:10.1016/j.ejca.2019.03.006
85. Chen JH, Lee KY, Hu CJ, Chung CC. Coexisting myasthenia gravis, myositis, and polyneuropathy induced by ipilimumab and nivolumab in a patient with non-small-cell lung cancer: a case report and literature review. Med. 2017;96.
86. Thakolwiboon S, Karukote A, Wilms H. De novo myasthenia gravis induced by atezolizumab in a patient with urothelial carcinoma. Ann Neurol. 2019;86:S109–S110.
87. Tozuka T, Sugano T, Noro R, et al. Pembrolizumab-induced agranulocytosis in a pulmonary pleomorphic carcinoma patient who developed interstitial lung disease and ocular myasthenia gravis. Oxford Med Case Reports. 2018;11:398–402.
88. Veccia A, Kinspergher S, Grego E, et al. Myositis and myasthenia during nivolumab administration for advanced lung cancer: a case report and review of the literature. Anticancer Drugs. 2020;31:540–544. doi:10.1097/CAD.0000000000000903
89. Werner J-M, Schweinsberg V, Schroeter M, et al. Successful treatment of myasthenia gravis following PD-1/CTLA-4 combination checkpoint blockade in a patient with metastatic melanoma. Front Oncol. 2019;9:84. doi:10.3389/fonc.2019.00084
90. Xing Q, Zhang Z-W, Lin Q-H, et al. Myositis-myasthenia gravis overlap syndrome complicated with myasthenia crisis and myocarditis associated with anti-programmed cell death-1 (sintilimab) therapy for lung adenocarcinoma. Ann Transl Med. 2020;8:250. doi:10.21037/atm.2020.01.79
91. Wilson R, Menassa DA, Davies AJ, et al. Seronegative antibody-mediated neurology after immune checkpoint inhibitors. Ann Clin Transl Neurol. 2018;5:640–645. doi:10.1002/acn3.547
92. Chen YH, Liu FC, Hsu CH, Chian CF. Nivolumab-induced myasthenia gravis in a patient with squamous cell lung carcinoma. Med. 2017;96.
93. Cooper DS, Meriggioli MN, Bonomi PD, Malik R. Severe exacerbation of myasthenia gravis associated with checkpoint inhibitor immunotherapy. J Neuromuscul Dis. 2017;4:169–173. doi:10.3233/JND-170219
94. Crusz SM, Radunovic A, Shepherd S, et al. Rituximab in the treatment of pembrolizumab-induced myasthenia gravis. Eur J Cancer. 2018;102:49–51. doi:10.1016/j.ejca.2018.07.125
95. Dhenin A, Samartzi V, Lejeune S, Seront E. Cascade of immunologic adverse events related to pembrolizumab treatment. BMJ Case Rep. 2019;12:e229149. doi:10.1136/bcr-2018-229149
96. Earl DE, Loochtan AI, Bedlack RS. Refractory myasthenia gravis exacerbation triggered by pembrolizumab. Muscle Nerve. 2018;57:E120–E121. doi:10.1002/mus.26021
97. Kolb NA, Trevino CR, Waheed W, et al. Neuromuscular complications of immune checkpoint inhibitor therapy. Muscle Nerve. 2018;58:10–22. doi:10.1002/mus.26070
98. Psimaras D, Velasco R, Birzu C, et al. Immune checkpoint inhibitors-induced neuromuscular toxicity: from pathogenesis to treatment. J Peripher Nerv Syst. 2019;24:S74–S85. doi:10.1111/jns.12339
99. Wendell LC, Levine JM. Myasthenic crisis. Neurohospitalist. 2011;1:16–22. doi:10.1177/1941875210382918
100. Borodic G, Hinkle DM, Cia Y. Drug-induced graves disease from CTLA-4 receptor suppression. Ophthalmic Plast Reconstr Surg. 2011;27:e87–e88. doi:10.1097/IOP.0b013e3181ef72a1
101. Campredon P, Imbert P, Mouly C, et al. Severe inflammatory ophthalmopathy in a euthyroid patient during nivolumab treatment. Eur Thyroid J. 2018;7:84–87. doi:10.1159/000485742
102. Ricciuti B, Metro G, Baglivo S, et al. Long-lasting response to nivolumab and immune-related adverse events in a nonsquamous metastatic non–small cell lung cancer patient. J Thorac Oncol. 2017;12:e51–e55. doi:10.1016/j.jtho.2016.12.027
103. Sagiv O, Kandl TJ, Thakar SD, et al. Extraocular muscle enlargement and thyroid eye disease-like orbital inflammation associated with immune checkpoint inhibitor therapy in cancer patients. Ophthal Plast Reconstr Surg. 2019;35:50–52. doi:10.1097/IOP.0000000000001161
104. McElnea E, Á NM, Moran S, et al. Thyroid-like ophthalmopathy in a euthyroid patient receiving Ipilimumab. Orbit. 2014;33:424–427. doi:10.3109/01676830.2014.949792
105. Min L, Vaidya A, Becker C. Thyroid autoimmunity and ophthalmopathy related to melanoma biological therapy. Eur J Endocrinol. 2011;164:303–307. doi:10.1530/EJE-10-0833
106. Rhea L, Yoon JW, Jang S. Rapid development of Graves’ ophthalmopathy after treatment with ipilimumab and recurrence with pembrolizumab in a patient with previously treated Graves’ disease. J Oncol Pract. 2018;14:747–749. doi:10.1200/JOP.18.00442
107. Park ESY, Rabinowits G, Hamnvik OPR, Dagi LR. A case of Graves’ ophthalmopathy associated with pembrolizumab (Keytruda) therapy. J AAPOS. 2018;22:310–312. doi:10.1016/j.jaapos.2018.01.006
108. Sabini E, Sframeli A, Marinò M. A case of drug-induced Graves’ Orbitopathy after combination therapy with Tremelimumab and Durvalumab. J Endocrinol Invest. 2018;41:877–878. doi:10.1007/s40618-018-0906-0
109. Bitton K, Michot JM, Barreau E, et al. Prevalence and Clinical patterns of ocular complications associated with anti-PD-1/PD-L1 anticancer immunotherapy. Am J Ophthalmol. 2019;202:109–117. doi:10.1016/j.ajo.2019.02.012
110. Patel D, Tremont A, Fuloria J. Orbital myositis secondary to cytotoxic T-lymphocyte-associated protein 4 antibody – a case report. Clin Oncol. 2016;1:1149.
111. Haddox CL, Shenoy N, Shah KK, et al. Pembrolizumab induced bulbar myopathy and respiratory failure with necrotizing myositis of the diaphragm. Ann Oncol. 2017;28:673–675. doi:10.1093/annonc/mdw655
112. Henderson AD, Thomas DA. A case report of orbital inflammatory syndrome secondary to ipilimumab. Ophthal Plast Reconstr Surg. 2015;31:e68–e70. doi:10.1097/IOP.0000000000000081
113. Kamo H, Hatano T, Kanai K, et al. Pembrolizumab-related systemic myositis involving ocular and hindneck muscles resembling myasthenic gravis: A case report. BMC Neurol. 2019;19:184. doi:10.1186/s12883-019-1416-1
114. Liewluck T, Kao JC, Mauermann ML. PD-1 inhibitor-associated myopathies: emerging immune-mediated myopathies. J Immunother. 2018;41:208–211. doi:10.1097/CJI.0000000000000196
115. Nardin C, Borot S, Beaudoin MA, et al. Long-term adverse event: inflammatory orbitopathy induced by pembrolizumab in a patient with metastatic melanoma. Invest New Drugs. 2019;37:375–377. doi:10.1007/s10637-018-0659-9
116. Nasr F, El Rassy E, Maalouf G, et al. Severe ophthalmoplegia and myocarditis following the administration of pembrolizumab. Eur J Cancer. 2018;91:171–173. doi:10.1016/j.ejca.2017.11.026
117. Pushkarevskaya A, Neuberger U, Dimitrakopoulou-Strauss A, et al. Severe ocular myositis after ipilimumab treatment for melanoma: a report of 2 cases. J Immunother. 2017;40:282–285. doi:10.1097/CJI.0000000000000178
118. Valenti-Azcarate R, Esparragosa Vazquez I, Toledano Illan C, et al. Nivolumab and Ipilimumab-induced myositis and myocarditis mimicking a myasthenia gravis presentation. Neuromuscul Disord. 2020;30:67–69. doi:10.1016/j.nmd.2019.10.006
119. Williams KJ, Allen R. Immune checkpoint inhibitor–induced ptosis in a patient with prostate cancer. J Neuro-Ophthalmology. 2020. doi:10.1097/WNO.0000000000000927
120. Hassanzadeh B, DeSanto J, Kattah JC. Ipilimumab-induced adenohypophysitis and orbital apex syndrome: importance of early diagnosis and management. Neuro-Ophthalmology. 2018;42:176–181. doi:10.1080/01658107.2017.1368090
121. Betrains A, Blockmans DE. Immune checkpoint inhibitor-associated polymyalgia rheumatica/giant cell arteritis occurring in a patient after treatment with nivolumab. J Clin Rheumatol. 2019 Feb 19 [Epub ahead of print]. doi:10.1097/RHU.0000000000001012
122. Chow KL, Perju-Dumbrava L, Malhotra A, et al. Giant cell arteritis secondary to combined nivolumab and ipilimumab in metastatic pleural mesothelioma. Neurol Asia. 2020;25.
123. Goldstein BL, Gedmintas L, Todd DJ. Drug-associated polymyalgia rheumatica/giant cell arteritis occurring in two patients after treatment with ipilimumab, an antagonist of CTLA-4. Arthritis Rheumatol. 2014;66:768–769. doi:10.1002/art.38282
124. Hid Cadena R, Abdulahad WH, Hospers GAP, et al. Checks and balances in autoimmune vasculitis. Front Immunol. 2018;9:315. doi:10.3389/fimmu.2018.00315
125. Micaily I, Chernoff M. An unknown reaction to pembrolizumab: giant cell arteritis. Ann Oncol. 2017;28:2621–2622. doi:10.1093/annonc/mdx306
126. Bourgeois-Vionnet J, Joubert B, Bernard E, et al. Nivolumab-induced myositis: a case report and a literature review. J Neurol Sci. 2018;387:51–53. doi:10.1016/j.jns.2018.01.030
127. Hellman JB, Traynis I, Lin LK. Pembrolizumab and epacadostat induced fatal myocarditis and myositis presenting as a case of ptosis and ophthalmoplegia. Orbit. 2019;38:244–247. doi:10.1080/01676830.2018.1490439
128. Hamada S, Fuseya Y, Tsukino M. Pembrolizumab-induced rhabdomyolysis with myositis in a patient with lung adenocarcinoma. Arch Bronconeumol. 2018;54:346–348. doi:10.1016/j.arbres.2018.01.026
129. Diamantopoulos PT, Tsatsou K, Benopoulou O, et al. Inflammatory myopathy and axonal neuropathy in a patient with melanoma following pembrolizumab treatment. J Immunother. 2017;40:221–223. doi:10.1097/CJI.0000000000000172
130. Alnabulsi R, Hussain A, DeAngelis D. Complete ophthalmoplegia in Ipilmumab and Nivolumab combination treatment for metastatic melanoma. Orbit. 2018;37:381–384. doi:10.1080/01676830.2017.1423349
131. Carrera W, Baartman BJ, Kosmorsky G. A case report of drug-induced myopathy involving extraocular muscles after combination therapy with tremelimumab and durvalumab for non-small cell lung cancer. Neuro-Ophthalmology. 2017;41:140–143. doi:10.1080/01658107.2017.1291686
132. Khoo A, Zhuang YZ, Boundy K, Frasca J. Immune checkpoint inhibitor-related myositis associated with atezolizumab therapy. Neurol Clin Pract. 2019;9:E25–E26. doi:10.1212/CPJ.0000000000000597
133. Kang KH, Grubb W, Sawlani K, et al. Immune checkpoint-mediated myositis and myasthenia gravis: a case report and review of evaluation and management. Am J Otolaryngol - Head Neck Med Surg. 2018;39:642–645.
134. Maller B, Peguero E, Tanvetyanon T. Ipilimumab/nivolumab-related opsoclonus-myoclonus-ataxia syndrome variant in a patient with malignant pleural mesothelioma. J Immunother. 2018;41:411–412. doi:10.1097/CJI.0000000000000228
135. Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the society for immunotherapy of cancer (SITC) toxicity management working group. J Immunother Cancer. 2017;5:95. doi:10.1186/s40425-017-0300-z
136. Fazel M, Jedlowski PM. Severe myositis, myocarditis, and myasthenia gravis with elevated anti-striated muscle antibody following single dose of ipilimumab-nivolumab therapy in a patient with metastatic melanoma. Case Reports Immunol. 2019;2019:1–3.
137. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi:10.1056/NEJMoa1504030
138. Beck KE, Blansfield JA, Tran KQ, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24:2283–2289. doi:10.1200/JCO.2005.04.5716
139. Ascierto PA, Simeone E, Sileni VC, et al. Clinical experience with ipilimumab 3 mg/kg: real-world efficacy and safety data from an expanded access programme cohort. J Transl Med. 2014;12:116. doi:10.1186/1479-5876-12-116
140. Horvat TZ, Adel NG, Dang TO, et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at memorial sloan kettering cancer center. J Clin Oncol. 2015;33:3193–3198. doi:10.1200/JCO.2015.60.8448
141. Petrelli F, Signorelli D, Ghidini M, et al. Association of steroids use with survival in patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Cancers. 2020;12:546. doi:10.3390/cancers12030546
142. Kaplon H, Muralidharan M, Schneider Z, Reichert JM. Antibodies to watch in 2020. MAbs. 2020;12:1703531. doi:10.1080/19420862.2019.1703531
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

