Back to Journals » Clinical Interventions in Aging » Volume 18
Neuro-Navigated rTMS Improves Sleep and Cognitive Impairment via Regulating Sleep-Related Networks’ Spontaneous Activity in AD Spectrum Patients
Authors You S, Lv T, Qin R , Hu Z, Ke Z, Yao W, Zhao H, Bai F
Received 13 April 2023
Accepted for publication 3 August 2023
Published 15 August 2023 Volume 2023:18 Pages 1333—1349
DOI https://doi.org/10.2147/CIA.S416992
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Maddalena Illario
Shengqi You,1 Tingyu Lv,1 Ruomeng Qin,2 Zheqi Hu,2 Zhihong Ke,2 Weina Yao,1 Hui Zhao,2 Feng Bai2,3
1Department of Neurology, Nanjing Drum Tower Hospital Clinical College of Traditional Chinese and Western Medicine, Nanjing University of Chinese Medicine, Nanjing, 210008, People’s Republic of China; 2Department of Neurology, Nanjing Drum Tower Hospital, Affiliated Hospital of Medical School, Nanjing University, Nanjing, 210008, People’s Republic of China; 3Geriatric Medicine Center, Taikang Xianlin Drum Tower Hospital, Affiliated Hospital of Medical School, Nanjing University, Nanjing, 210008, People’s Republic of China
Correspondence: Feng Bai, Department of Neurology, Nanjing Drum Tower Hospital, Affiliated Hospital of Medical School, Nanjing University, 321 Zhongshan Road, Nanjing, 210009, Jiangsu Province, People’s Republic of China, Tel +86-25-83105960, Email [email protected]
Study Objectives: By examining spontaneous activity changes of sleep-related networks in patients with the Alzheimer’s disease (AD) spectrum with or without insomnia disorder (ID) over time via neuro-navigated repetitive transcranial magnetic stimulation (rTMS), we revealed the effect and mechanism of rTMS targeting the left-angular gyrus in improving the comorbidity symptoms of the AD spectrum with ID.
Methods: A total of 34 AD spectrum patients were recruited in this study, including 18 patients with ID and the remaining 16 patients without ID. All of them were measured for cognitive function and sleep by using the cognitive and sleep subscales of the neuropsychiatric inventory. The amplitude of low-frequency fluctuation changes in sleep-related networks was revealed before and after neuro-navigated rTMS treatment between these two groups, and the behavioral significance was further explored.
Results: Affective auditory processing and sensory-motor collaborative sleep-related networks with hypo-spontaneous activity were observed at baseline in the AD spectrum with ID group, while substantial increases in activity were evident at follow-up in these subjects. In addition, longitudinal affective auditory processing, sensory-motor and default mode collaborative sleep-related networks with hyper-spontaneous activity were also revealed at follow-up in the AD spectrum with ID group. In particular, longitudinal changes in sleep-related networks were associated with improvements in sleep quality and episodic memory scores in AD spectrum with ID patients.
Conclusion: We speculated that left angular gyrus-navigated rTMS therapy may enhance the memory function of AD spectrum patients by regulating the spontaneous activity of sleep-related networks, and it was associated with memory consolidation in the hippocampus-cortical circuit during sleep.
Clinical Trial Registration: The study was registered at the Chinese Clinical Trial Registry, registration ID: ChiCTR2100050496, China.
Keywords: Alzheimer’s disease, insomnia disorder, sleep-related functional connectivity network, neuro-navigated repetitive transcranial magnetic stimulation, spontaneous activity
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disease characterized by memory loss and multidomain cognitive impairment, with a long course and progressive aggravation.1 In the treatment of Alzheimer’s disease, in addition to the improvement of cognitive impairment, it is also particularly important to understand and control the risk factors in the occurrence and development of the disease.2 Insomnia disorder (ID) often exists as concomitant symptoms in patients with AD, including difficulty in sleep maintenance, difficulty in falling asleep, early awakening, circadian rhythm disorders, drowsiness, etc. Previous studies have reported that insomnia disorder increases the risk of cognitive impairment in different stages of the continuum of AD we called the AD spectrum, including subjective cognitive impairment (SCD),3 mild cognitive impairment (MCI)4,5 and AD dementia6 and is highly correlated with pathological factors in AD,7 such as the deposition of Aβ, an increase in inflammatory factors, and damage to neuroplasticity.8 Therefore, understanding the interaction mechanism between AD spectrum-correlated cognitive decline and sleep is helpful to improve and may delay the condition of AD spectrum patients on the basis of controlling risk factors.
At present, the clinical treatment of AD and insomnia disorder mainly includes pharmacological and nonpharmacological pathways.9 As a kind of external intervention therapy, repetitive transcranial magnetic stimulation (rTMS) is painless, noninvasive, has strong manoeuvrability, wide applicability and high safety.10 Its treatment principle lies in the use of a pulsed magnetic field, which passes through the scalp and skull to produce a reverse induced current in the cerebral cortex, changing the cell membrane potential to excite or inhibit nerve cells and causing physiological changes.11 An increasing number of studies on the effective intervention of rTMS to improve cognitive function and sleep have been reported.12 In particular, our previous study showed that high-frequency rTMS in the left angular gyrus (MNI: −45, −67, 38) had a significant effect on improving episodic memory in AD spectrum patients,13 which was set in a cortical point with the strongest connectivity function to the left hippocampus in AD spectrum patients. Some studies also proved that high-frequency rTMS is applicable to improve cognitive impairment caused by sleep deprivation.14,15 Due to the close relationship between the two and the increasing trend of comorbidity symptoms, based on our previous research, we proposed a hypothesis that sleep and sleep-related networks may play a role in rTMS intervention in the AD spectrum to improve cognition. We try to measure the efficacy of rTMS treatment in AD spectrum patients with different sleep status levels and evaluate whether it makes common improvement and how it works in functional neuroimaging changes. Notably, due to the sensitivity and variability of sleep status, the previous neuroimaging mechanism analysis of insomnia disorder does not show consistent spatial coordinate markers,16 and the functional connectivity networks related to insomnia disorder also showed polymorphism,17 but based on the systematic review, we found that the amygdala, thalamus, and insular lobe are always regarded as regions of interest (ROI) related to insomnia disorder. It actually reflects that insomnia disorder is highly consistent with emotional disorders and cognitive states that are dominated by rumination meditation and increased consciousness.18 Therefore, in view of the difficulty of providing a univocal framework of insomnia disorder and its correlation with cognition, our study by way of observing the function of sleep-related ROI-based networks and their activity intensity changes after rTMS intervention in improving sleep and cognition of AD spectrum patients would make more sense to understanding the interaction mechanism of sleep and cognition.
We used the method of constructing sleep-related functional connectivity networks in the resting state amplitude of low-frequency fluctuations (ALFF) to reflect the strength of intrinsic brain activity, the mechanism of which is based on blood oxygenation level-dependent changes in brain tissue in the resting state.19,20 The rTMS stimulation parameters and methods used in this study are consistent with our previous work.
Materials and Methods
Participants
The participants were enrolled by the Department of Neurology of the Nanjing Drum Tower Hospital of Nanjing University Medical School or recruited by advertising from April 2019 to November 2021. Based on the US National Institute on Aging-Alzheimer’s Association (NIA-AA) criteria,21 SCD is proposed for preclinical AD and diagnosed with the Jessen et al criteria22 in this study. Amnestic mild cognitive impairment (aMCI) is one type of MCI, often considered the prodromal phase of AD. aMCI and AD participants were enrolled based on the recommendations of our previous study,13 which followed the NIA-AA criteria.23,24 The staging of dementia severity in these patients was determined through a comprehensive evaluation of Mini-Mental State Examination (MMSE), Montreal Cognitive Assessment (MoCA) score and diagnosis by experienced neurologists (AD dementia was defined with education adjusted values by MMSE: 0 year ≤ 22, 1–6 years ≤ 23, 7–12 years ≤ 24, more than 12 years ≤ 26; MCI: MoCA ≤ 25). Ultimately, forty-six AD spectrum patients of different stages were included. After careful data analysis and screening, 12 subjects who did not complete the testing procedures dropped out of the study (for details, see the following). According to the differences in sleep quality, thirty-four patients were separated into two groups: 18 patients with ID (including 5 SCD, 10 aMCI and 3 AD) and the remaining 16 patients without ID (2 SCD, 11 aMCI and 3 AD) underwent the whole experimental procedure, and written informed consent was obtained from each subject prior to participation. This study was conducted in accordance with the Declaration of Helsinki standards and was approved by the ethics committees of the Nanjing Drum Tower Hospital of Nanjing University Medical School. The exclusion criteria were as follows: (i) other disease that may cause memory decline, such as cerebrovascular disease, epilepsy, Parkinson’s disease, brain tumor, traumatic brain injury, etc.; (ii) severe depression (Hamilton Depression Rating Scale, HAMD ≥ 17), anxiety (Hamilton Anxiety Rating Scale, HAMA ≥ 21), schizophrenia or other mental illness; (iii) severe systemic diseases such as heart failure, etc.; (iv) other contraindications for MRI or rTMS; and (v) other primary diseases that cause insomnia, such as apnea syndrome and restless leg syndrome.
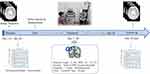
Experimental Procedure
The experimental procedure is shown in Figure 1. One week before the formal intervention, each patient underwent a comprehensive neuropsychological test (including cognitive assessment and sleep status evaluation) and 3.0-T whole brain functional and structural scanning. Based on the patient’s initial sleep assessment score, this study included participants with Pittsburgh Sleep Quality Index (PSQI) > 5 into the AD spectrum with ID group, which was consistent with the diagnosis of insomnia disorder in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5),25 and participants with PSQI ≤ 5 were classified into the AD spectrum without ID group. All subjects carried out a unified intervention process. The stimulation took place in a quiet and light-attenuated room, and participants were seated in a comfortable and adjustable height chair during the whole treatment, which lasted 20 minutes every day for a fixed time. During the treatment, 3 patients developed occasional dizziness at the time of initial treatment but spontaneously self-recovered later. We considered this to be caused by differences in individual sensitivity and adaptability. After four weeks of treatment, the patients received fMRI scanning and a comprehensive neuropsychological evaluation again.
Neuropsychological Assessment
Each patient underwent a series of neuropsychological assessments to evaluate their cognitive level and sleep status. The cognitive functions of all the subjects were evaluated by an experienced neuropsychologist, including general cognitive function assessment, the Chinese version of the Mini-Mental State Examination (MMSE)26 and the Beijing version of the Montreal Cognitive Assessment (MoCA);27 the auditory verbal learning test Huashan version (AVLT)28 was used as a verbal memory function assessment, the Wechsler Memory Scale-Visual Reproduction (WMS-VR) was used to measure visual memory, and they were jointly used to test the level of episodic memory of participants; HAMD and HAMA were used to test the basic emotional status.
The sleep status of participants was self-assessed using the PSQI to evaluate different dimensions of sleep quality and quantity in the last month; the Insomnia Severity Index (ISI) was used to evaluate the severity of daytime and nighttime insomnia disorder in the last week; the Self-Rating Scale of Sleep (SRSS) was used to evaluate general subjective severity of sleep in the last month; the Epworth Sleepiness Scale (ESS) was used to assess the general level of sleepiness during the day; and the patients were also required to report the average total sleep duration and subjective general sleep quality in the last month (evaluated on a Likert scale, ranging from 1 to 5). Seven patients were excluded from the study due to missing scale data.
Left Angular Gyrus Neuronavigated rTMS Protocol
rTMS was applied using a YIRUIDE YRD CCY-I Stimulator (YIRUIDE Company, WuHan, China) with a focal 70 mm figure-eight-shaped air-cooled coil. Before the treatment, we first measured motor threshold (MT) by applying single-pulse rTMS to the left motor cortex. This process was guided by registration of subjects’ T1-weighted image with actual brain location with an optical navigator. The MT was determined using the minimum stimulus intensity that would elicit an overt motor response in the right abductor pollicis brevis (APB) (5 of 10 times with amplitude of 50 μV), and the final stimulation intensity was set at 100% of each patient’s motor threshold. All participants were seated in a chair with a coil placed over the left angular gyrus (MNI: x, y, z = −45, −67, 38), which had proven significantly effective in improving AD spectrum patients’ memory based on our previous study. The target location was defined as a sphere of 6-mm radius by using an inverse matrix produced during T1 segmentation in the Statistical Parametric Mapping analysis package (SPM12, http://www.fil.ion.ucl.ac.uk/spm/software/spm12/) and then individually transformed into each participant’s T1 space by presenting and registering with the patients’ current head position according to the neuro-navigation Neuroaim system. The stimulation parameters were as follows: stimulation frequency (20 Hz), 1600 stimuli per session (40 trains with 40 stimuli per train), single-train duration (2 s), intertrain interval (28 s), and stimulation period (a session per day, five days per week for four weeks).
Image Acquisition and Data Preprocessing
All of the participants were examined on a 3.0T MRI scanner (Philips Medical Systems), and they were asked to lie quietly and not think of anything specific or fall asleep while inside the scanner. The resting-state fMRI scan images were collected with echo-planar imaging (EPI) sequences covering the entire brain with the following parameters: repetition time (TR) = 2000 ms; echo time (TE) = 30 ms; slice number = 35; slice thickness = 4.0 mm; flip angle (FA) = 90◦; acquisition matrix = 64 × 64; spatial resolution = 3 × 3 × 3 mm3, FOV = 240 × 240 mm2. The high-resolution T1-weighted imaging parameters were TE = 4.6 ms, TR = 9.8 ms, slice number = 192, slice thickness = 1.0 mm, FA = 8◦, FOV = 250 × 250 mm2, acquisition matrix = 256 × 256.
The preprocessing of EPI data was carried out on the Resting State fMRI Data Analysis Toolkit plus (RESTplus, http://restfmri.net/forum/restplus) software. To reduce the signal interference when the equipment started, we removed the first 20 time points of the data, uniformly corrected the scanning time and head motion, removed the images whose head motion was more than 3 mm, registered each image to a unified Montreal Neurological Institute (MNI) standard space, Gaussian random field smoothing, FMHM = 6 mm, and removed linear offset. Head movement, white matter and cerebrospinal fluid were extracted as covariates, and the image frequency was limited to 0.01–0.08 Hz to reduce noise. Of all our enrolled subject data, 5 patients were excluded because of excessive head motion, and the flow of enrollment is presented in Figure 2.
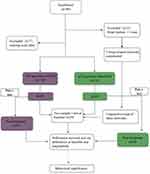 |
Figure 2 Flowchart of data analysis. Abbreviation: ALFF, amplitude of low-frequency fluctuations. |
Principal Component Analysis (PCA)
To reduce the number of sleep variables and make our sleep score more representative, we performed a principal component analysis (PCA; index of factorability KMO = 0.880) on the overall patients (N = 34) to generate new simplified factors that reflected the communalities between the various sleep quality measures. Principal components were generated from a correlation matrix of normalized original sleep variables (ie, ISI, AIS, PSQI, SRSS and ESS, sleep duration, sleep quality). To maximize the explanatory power and make it more interpretable to the original variables, we required that each principal component extracted explain at least 5% of the data variation and then employed a varimax rotation.29 Attributed to it, two factors were ultimately generated and cumulatively explained 91.5% of the total variance. ISI, AIS, PSQI, SRSS, sleep duration, and sleep quality loaded onto the first factor, and ESS loaded into the second factor. Table 1 shows the rotated factor matrix. Based on the proportion of each component, we used linear regression to calculate a combined sleep score for all patients, referred to as “subjective sleep quality”.
 |
Table 1 Varimax Rotated Factor Matrix |
Construction of Sleep-Related Networks
To date, multiple studies have suggested that insomnia disorder symptoms are associated with principal resting-state networks,17 such as the salience network (SN),30 sensory-motor network (SMN),31 auditory network (AN)32 and default mode network (DMN).33 Given the complexity and inconsistency of the sleep-related networks, we speculated that exploring intrinsic activity in these sleep-related collaborative networks was meaningful. Then, we selected 3 spatial coordinates in the literature, which proved that functional connectivity networks were correlated with insomnia disorder and were separately located in the thalamus, amygdala and insula.
First, 3 coordinates were located in the left thalamus (MNI: −4 −11 −6),34 right amygdala (MNI: 17.7 −1.3 −21.9),35 and left insular lobe (MNI: −42 −8 −6),36 centered at these MNI coordinates, sphere of 5-mm radius as the region of interest (ROI), and the average signal change of each ROI at baseline was calculated with 41 patients (after preprocessing of 46 included patients’ image data, 5 were excluded due to excessive head motion). We took it as a time-processed reference, analysed the cross-correlation between it and the time-series signal of each voxel in the whole brain, and finally obtained the spatial maps of the correlation coefficient, that is, the functional connectivity maps. We normalized these functional connectivity values by Fisher r-to-z transformation, and entire brain z value maps were created. Then, we performed a one-sample t test in each of the 3 ROI-based z value maps. The corrected network maps are separately shown in Figure 3 (voxel-wise p < 0.001, FWHM = 6 mm, cluster size > 351mm3; determined by Monte Carlo simulation), and we further calculated the conjunction of three corrected networks (Figure 3). The final conjunction maps comprised extensive fronto-temporal-parietal cortical and subcortical regions and nearly covered most key regions of the above insomnia disorder-related principal resting-state networks.
Second, to compare the spontaneous activity in this conjunction map before and after rTMS treatment in these two groups, whole brain ALFF was calculated to measure it; that is, the time series were converted to the frequency domain by fast Fourier transform on each voxel, and the square root of the power spectrum was calculated and averaged. For standardization purposes, the individual ALFF map was divided by the global mean ALFF within the mask, and we obtained zALFF maps. These processes were implemented in RESTplus software.
Statistical Analysis
(i) When judging if there were differences in gender, age, and education level between the AD spectrum with ID and without ID groups, we performed a chi-square test to compare the differences in gender, and a two-sample t test and Mann‒Whitney U-test were used based on whether the data satisfied variance homogeneity and normality to compare age and education level. Cognition and sleep level differences between two groups at baseline were performed by two-sample t test and Mann‒Whitney U-test; paired sample t test and the Wilcoxon test were used to compare the differences in neuropsychological scores before and after treatment within two groups. The thresholds were set at p < 0.05 using SPSS version 26.0 software (SPSS, Inc., Chicago, IL, USA).
(ii) We compared the differences in spontaneous activity in brain regions between and within the two groups before and after treatment. A two-sample t test was performed on the maps of zALFF, which was limited to the conjunction region between two groups, and a paired t test was used to compare differences before and after treatment within groups. zALFF values were extracted from the changed regions within each subject. After controlling for the covariates, Pearson correlations were calculated to measure the association between the longitudinal changes in neuropsychological tests and spontaneous activity (Figure 2).
Results
Demographic and Clinical Characteristics
As shown in Table 2, we compared the differences in clinical data between groups both at baseline and follow-up neuro-navigated rTMS treatment. Differences in age, gender, and education level were excluded between the AD spectrum with ID group and without ID group, and there were no significant differences in cognitive domains at baseline between the two groups. For sleep assessments, there were significant differences in poor sleep quality at baseline in the AD spectrum with ID group compared to the control group (ISI, AIS, PSQI, SRSS, sleep duration and sleep quality, p < 0.001). However, four weeks of neuro-navigated rTMS treatment had a significant effect on general cognition (ie, MMSE, MoCA, p < 0.05), episodic memory (ie, AVLT-IDR, AVLT-LDR, WMS-VR, p < 0.05), and sleep improvement (ie, ISI, AIS, PSQI, SRSS, ESS, sleep duration and sleep quality, p < 0.05) in the AD spectrum with ID group, and cognitive function and sleep were significantly improved compared with the baseline stage. Conversely, the cognitive dimensions of the AD spectrum without ID group were also significantly improved, and the sleep status was basically the same as that at baseline.
 |
Table 2 Demographic and Neuropsychological Data Between and Within Groups |
Baseline Differences in Spontaneous Activity and Its Behavioral Significance
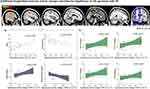
By comparing the spontaneous activity difference of sleep-related conjunction networks between the AD spectrum with ID and without ID groups at baseline, two-sample t tests were performed after adjusting for gender, age, education, HAMD and HAMA levels (voxel-wise p < 0.05, FWHM = 6 mm, cluster size >5292 mm3, determined by Monte Carlo simulation, see programme AlphaSim by D. Ward, and http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf). The main sleep-related networks were identified within the set of significant regional group effects in their spatial distribution (Figure 4). The AD spectrum with ID patients exhibited a significant decrease in a collaborative network at baseline, which comprised core regions of the affective auditory processing network, left amygdala and left middle temporal gyrus (LMTG), left superior temporal gyrus (LSTG), left putamen, left insula, and main regions of the sensory-motor network, left postcentral gyrus (LPoCG), and left precentral gyrus (LPreCG) (Table 3). The Pearson correlation further showed a significantly negative correlation between baseline collaborative network activity and subjective sleep quality score after adjusting for differences in gender, age, educational level, HAMD and HAMA (ie, sleep PCA scores, r = −0.497, p = 0.006) in all AD spectrum patients, but nothing with cognitive domains. However, there was no significant correlation in the AD spectrum with or without ID group.
 |
Table 3 Spontaneous Activity Difference of Baseline Between Groups and Longitudinal Changes in AD Spectrum with ID Group |
Neuro-Navigated rTMS Induced Longitudinal Activity Changes and Their Behavioural Significance
(i) Longitudinal changes in the baseline affective auditory processing and sensory-motor collaborative network difference between before and after rTMS treatment: Within-group comparisons (ie, posttreatment vs pretreatment, AD spectrum with and without ID group, respectively, Figure 5a), the AD spectrum with ID showed significantly increased spontaneous activity compared to these baseline collaborative network differences (corrected p < 0.01 by paired t test), while a decreasing trend but no significant changes were observed in the AD spectrum without ID (corrected p > 0.05 by paired t test). Between-group comparisons (ie, delta AD spectrum with ID vs delta AD spectrum without ID, Figure 5a) and delta AD spectrum with ID were associated with significantly increased collaborative network spontaneous activity (corrected p < 0.01, by two sample t test), and the follow-up activity on the collaborative network between two groups had no significant differences (corrected p > 0.05, by two sample t test). Furthermore, within the AD spectrum with ID group, increased spontaneous activity of the baseline difference collaborative network showed a significant negative correlation with sleep quality improvement (ie, PSQI changes, r = 0.661, p = 0.003; sleep duration changes, r = −0.567, p = 0.014, Figure 5b). After adjusting for gender, age, education, HAMD and HAMA level differentiation, it also showed a significant positive correlation with verbal memory improvement (ie, AVLT-S changes, r = 0.603, p = 0.029; AVLT-L changes, r = 0.679, p = 0.011, Figure 5c).
(ii) Additional longitudinal affective auditory processing, sensory-motor and default mode collaborative network activity changes and their behavioral significance. Except for the original difference of the baseline, AD spectrum with ID group also performed additional longitudinal collaborative network activity changes after four-weeks neuro-navigated rTMS treatment (voxel-wise p < 0.05, FWHM = 6 mm, cluster size > 5292 mm3, determined by Monte Carlo simulation, Table 3 and Figure 6), it showed significantly activity enhanced, including left auditory cortex (LMTG, LSTG) which was core region in affective auditory processing network, sensory-motor network (LPoCG, RPoCG, left paracentral lobule, LPreCG), default mode network (bilateral precuneus), but not in AD spectrum without ID group. Furthermore, the activity changes in the collaborative network in the AD spectrum with ID group were extracted, and the longitudinal difference in collaborative network activity at baseline showed a significant correlation with sleep status (ie, PSQI, r = 0.753, p = 0.003; sleep duration, r = −0.658, p = 0.014, Figure 6a), while no correlation was associated with cognitive domains. After rTMS treatment, the enhanced spontaneous activity of the longitudinal difference collaborative network was negatively correlated with sleep quality improvement (ie, PSQI changes, r = 0.556, p = 0.017; sleep duration changes, r = −0.520, p = 0.027, Figure 6a). Gender, age, education, HAMD and HAMA level were employed as covariates, and the longitudinal changes in collaborative network activity were significantly positively correlated with general cognitive improvement (MoCA score changes, r = 0.604, p = 0.029) and episodic memory improvement (ie, AVLT-S changes, r = 0.655, p = 0.015; AVLT-L changes, r = 0.597, p = 0.031; WMS score changes, r = 0.599, p = 0.031) in the AD spectrum with ID group (Figure 6b).
Discussion
Our study aimed to explore the functional mechanism of neuro-navigation guided rTMS in improving sleep and cognition in patients with the AD spectrum. The cognitive function of the two groups of AD spectrum patients with different sleep statuses was significantly improved after 4 weeks of rTMS treatment, mainly in episodic memory. In patients with the AD spectrum with ID, we found that the improvement in cognitive level was closely related to the change in sleep-related network activity.
Effects of Spontaneous Activity of Affective Auditory Processing and Sensory-Motor Collaborative Network on Sleep and Cognitive
In sleep-related integrated networks, we compared the differences in spontaneous activity between AD patients with and without ID and presented a cooperative network spontaneous activity change that collaborated with affective auditory processing and the sensory-motor network. In this collaborative network, the spontaneous activity of AD spectrum with ID patients was significantly lower than that of AD spectrum patients without ID at baseline, and it was significantly correlated with their corresponding subjective sleep quality in all AD spectrum patients. The affective processing network includes a core network composed of three brain systems: the amygdala-auditory cortex system,37,38 fronto-insular system, and basal ganglia (BG)-cerebellum system,39,40 which deal with auditory sensory information induced by internal and external stimuli in different stages in a complementary way; the sensory-motor network comprises motor (ie, PreCG) and somatosensory (ie, PoCG) regions and the supplementary motor area (SMA), playing a role in the sustained sensory processes of environmental stimuli and proprioceptive information.41 In the neurophysiological response to lexical tones, it has been found that the sensory-motor network is involved in the process of the auditory cortex42,43 mapping sound sensory signals to abstract language objects in lexical tone processing; that is, the processing of tonal vocabulary involves a cooperative cortical network, which promotes the acquisition of sound information and the processing of high-level semantic perception.44,45 However, we already know that the auditory cortex plays an important role in verbal working memory capacity.46 The L-amygdala, L-insula, and L-putamen, as the other core brain regions that form the affective auditory processing network, showed significant sensitivity to the acoustic features related to auditory emotions.47 Therefore, it can be speculated that the collaborative network we obtained jointly represents the abnormality of the auditory sensory-emotional processing circuit caused by insomnia disorder in AD spectrum patients with ID. Because auditory sensation still retains a certain degree of sensitivity, especially with emotionally relevant inputs during sleep,45 patients’ anxiety and fear of poor sleep further strengthened their sleep awakening,48 and the lowering of the threshold of arousal disturbed the continuity and sensitivity of brain activities during sleep, such as sensory-motor and sound perception processing, which caused the spontaneous activity of the affective auditory processing and sensory-motor collaborative network to decrease, which was also consistent with previous research results on insomnia.49,50 However, the group effects of the sleep-related network obtained by us were all located in the left hemisphere, which may be related to previous studies’ conclusion that the left hemisphere plays a dominant role in speech processing51 and is involved in tasks that require conscious emotional processing.52
rTMS was considered to be effective in improving sleep and cognition through different stimulation targets and different intensities.53,54 The stimulation target left angular gyrus in our study was identified in the cerebral cortex of AD spectrum patients with the strongest functional connectivity to the left hippocampus, which was directly related to the known role of hippocampus function in memory55 and the memory consolidation circuits in sleep.56 After treatment, the spontaneous activity of the baseline differential collaborative network was significantly enhanced in AD spectrum with ID patients, and the enhanced activity intensity was consistent with the improvement in clinical sleep quality and memory in patients with ID. The difference was that the greater the activity of the baseline differential collaborative network increased, the smaller the improvement in sleep quality and the greater the improvement in memory level. This result seemed to reveal that the affective auditory processing and sensory-motor collaborative network activity related to sleep quality was not proportionally enhanced to the improvement of subjective sleep quality in AD spectrum with ID patients, but a state in which brain spontaneous activity reached a balance to some extent, that is, the collaborative network activity regulation was spontaneously coordinated. Moreover, the improvement in verbal memory in AD spectrum with ID patients was consistent with the enhancement of the activity of the collaborative network, indicating that the regulation of spontaneous activity of collaborative networks related to general subjective sleep quality improved the verbal memory of AD spectrum with ID patients. Compared with patients with the same cognitive level on the AD spectrum without ID, insomnia disorder seemed to confirm the hypothesis that insomnia disorder may precede memory loss and was a risk factor for memory decline. The improvement in sleep quality will help to delay further memory loss.
Longitudinal Changes in Sleep-Related Network Activity and the Effect on Sleep and Memory in AD Spectrum with ID Patients
After rTMS treatment within the AD spectrum with ID group, longitudinal differences in collaborative network spontaneous activity were enhanced, including core regions of the affective auditory processing network, auditory cortex (LMTG, LSTG), sensory-motor network (LPoCG, RPoCG, left paracentral lobule, LPreCG), and key node of the default mode network (left precuneus, right precuneus). The baseline spontaneous activity of this longitudinal difference collaborative network was related to PSQI and sleep duration within group, but nothing correlated with cognitive, that is, the stronger the brain spontaneous activity of the collaborative network, the worse the sleep quality represented by PSQI and the shorter the sleep duration, while the AD spectrum without ID patients performed no activity changes in sleep-related network. Compared with affective auditory processing and sensory-motor network activity decreased in AD spectrum patients due to insomnia disorder at baseline, AD spectrum with ID patients’ longitudinal network activity changes comprised the bilateral precuneus. It was known that the precuneus was the key node of the default mode network, which participated in the retrieval of visuospatial imagery and episodic memory,57 and was also the key brain region of cognitive decline in patients with AD spectrum, while the precuneus participates in the characterization of longitudinal changes in sleep quality in patients with AD spectrum with ID, we speculated that it may be contributed to the fact that the precuneus was involved in perceptual feature screening, when the activity of sensory-motor network and auditory processing center was impaired, more cognitive resources were needed to participate in the processing of auditory sensory information.58 As a result, the information exchange between the precuneus and the auditory cortex increased, and their network activity jointly characterized the sleep status of the AD spectrum with ID, which may also be an imaging feature that gradually leads to the decline of cognitive function after insomnia disorders.
In addition, within the AD spectrum with ID group, the stronger the baseline spontaneous activity of the longitudinally changed collaborative network was, the worse the overall sleep quality was. After rTMS treatment, the improvement in sleep was still negatively correlated with the enhanced spontaneous activity of the collaborative network, which seemed to further confirm that good sleep quality was a state in which network activity reached stability, and the sleep quality of patients was not linearly related to their cognitive level. This was consistent with previous studies that subjective sleep quality and SCD, MCI, and AD, which represented different degrees of cognitive impairment, generally showed nonlinear or no direct correlation.7,50 Compared with the improvement in verbal memory after the increase in collaborative network activity at baseline, the rTMS treatment to longitudinally differentiate sleep-related collaborative network activity changes in patients with the AD spectrum with ID improved the general cognitive level (ie, MoCA), verbal memory (ie, AVLT-S and AVLT-L) and visual memory (ie, WMS-VR). We speculated that the stability of intrinsic activity in the sleep-related network made corresponding memories better consolidated in specific cortices.
Previous studies on sleep consolidation memory have shown that declarative/episodic memory depends on novel cross-cortical connections,59 and the cross-cortical plasticity of multiple cortical regions and other brain structures is the key to memory consolidation. This gradual consolidation process depends on hippocampal-neocortical connectivity. N2 and slow wave sleep in sleep were the longest and deepest sleep cycle in humans, and hippocampal reactivation during these periods guided the process of cortical network changes, which is considered to be the most important mechanism of memory stability and integration. Its physiological basis lies in the sharp-wave/ripple complexes (SWRs) in the hippocampus, thalamo-cortical sleep spindles and cortical slow oscillations (SOs) interactively nested during sleep;60 then, the cortico-cortical connections will be enhanced by the interaction between the hippocampal network and cerebral cortical network, and the declarative memory trace will be gradually solidified. According to this process of sleep consolidation of memory, we speculated that sleep interruption, lack of deep sleep, difficulty falling asleep induced by habitual or emotional disorders, decrease in awakening threshold, etc., may disturb the process of hippocampal reactivation during sleep, decrease the functional activity of the cortex, and limit the consolidation of memory through the hippocampal-neocortical network in sleep, resulting in memory and cognitive decline. rTMS stimulation of the corresponding cerebral cortex effectively regulated this network circuit. In addition, the baseline and longitudinal sleep-related collaborative network we obtained actually comprised meta-analyses of reported brain regions,61 which were referred to as potential targets by noninvasive brain stimulation (NIBS) (eg, TMS) to intervene in insomnia disorder (eg, SMA, MTG). It also further proved that our rTMS target was effective and appropriate to improve both cognition and sleep.
Conclusion
Therefore, in our study of patients with and without ID in the AD spectrum, the affective auditory processing network and sensorimotor network seemed to be the first to decline in functional activity induced by insomnia disorder, followed by a cortical activity abnormality coordinated by the default mode network (ie, bilateral precuneus). The left angular gyrus-navigated rTMS acts on the intervention process of the cortex, that is, modulating the spontaneous activity in sleep-related networks, which may act on the top-down activity regulation of the cortical-hippocampus by stabilizing the memory consolidation pathway through changes in sleep-related network activity. Importantly, it provides a new effective clinical treatment paradigm of rTMS to AD spectrum with ID patients. Some limitations still existed in this study. First, at present, our sample size is still small, and we should further expand the sample size of patients with different stages of the AD spectrum, including SCD, MCI, and AD, and explore the possible network activity interactions between different cognitive impairment stages and sleep. Second, our sleep measurement was limited to subjective sleep quality assessment in the form of a questionnaire, and objective sleep monitoring and sleep EEG records should be included in the future to study the changes in brain functional activity in different sleep stages combined with fMRI. Third, our rTMS intervention study is not double-blind, the placebo effect of sleep quality is still strong, and the sham stimulation group needs to be included to more accurately study the therapeutic effects of rTMS.
Date Sharing Statement
Clinical initial data are accessible on request via e-mail to the corresponding author. Demographic characteristics, neuroimaging data, neuropsychological tests (including cognitive assessment and sleep status evaluation), and individualized rTMS treatment parameters were included. The study protocol, statistical analysis plan and informed consent are also available.
Ethics Statement
This study was conducted in accordance with the Declaration of Helsinki standards and was approved by the ethics committees of the Nanjing Drum Tower Hospital of Nanjing University Medical School. All participants provided informed consent prior to the experiment.
Acknowledgments
We are grateful to all the participants who took part in this study.
Funding
This work was supported partly by grants from the National Natural Science Foundation of China (No. 82071186), Clinical Trials from the Affiliated Drum Tower Hospital, Medical School of Nanjing University (No. 2022-LCYG-MS-05), National Key Research and Development Program of China (No. 2022YFA1105300) and Jiangsu Province Senior Health Project (No. LKZ2023014).
Disclosure
The authors declare no financial conflicts of interest in this paper.
References
1. Joe E, Ringman JM. Cognitive symptoms of Alzheimer’s disease: clinical management and prevention. BMJ. 2019;367:l6217. doi:10.1136/bmj.l6217
2. Irwin MR, Vitiello MV. Implications of sleep disturbance and inflammation for Alzheimer’s disease dementia. Lancet Neurol. 2019;18(3):296–306. doi:10.1016/S1474-4422(18)30450-2
3. Lauriola M, Esposito R, Delli Pizzi S, et al. Sleep changes without medial temporal lobe or brain cortical changes in community-dwelling individuals with subjective cognitive decline. Alzheimers Dement. 2017;13(7):783–791. doi:10.1016/j.jalz.2016.11.006
4. da Silva RAPC. Sleep disturbances and mild cognitive impairment: a review. Sleep Sci. 2015;8(1):36–41. doi:10.1016/j.slsci.2015.02.001
5. Hita-Yañez E, Atienza M, Cantero JL. Polysomnographic and subjective sleep markers of mild cognitive impairment. Sleep. 2013;36(9):1327–1334. doi:10.5665/sleep.2956
6. Bliwise DL. Sleep in normal aging and dementia. Sleep. 1993;16(1):40–81. doi:10.1093/sleep/16.1.40
7. Yaffe K, Falvey CM, Hoang T. Connections between sleep and cognition in older adults. Lancet Neurol. 2014;13(10):1017–1028. doi:10.1016/S1474-4422(14)70172-3
8. Sadeghmousavi S, Eskian M, Rahmani F, Rezaei N. The effect of insomnia on development of Alzheimer’s disease. J Neuroinflammation. 2020;17(1):289. doi:10.1186/s12974-020-01960-9
9. Scheltens P, De Strooper B, Kivipelto M, et al. Alzheimer’s disease. Lancet. 2021;397(10284):1577–1590. doi:10.1016/S0140-6736(20)32205-4
10. Lin Y, Jin J, Lv R, et al. Repetitive transcranial magnetic stimulation increases the brain’s drainage efficiency in a mouse model of Alzheimer’s disease. Acta Neuropathologica Commun. 2021;9(1):102 doi:10.1186/s40478-021-01198-3.
11. Klomjai W, Katz R, Lackmy-Vallee A. Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS). Ann Phys Rehabil Med. 2015;58(4):208–213. doi:10.1016/j.rehab.2015.05.005
12. Iglesias AH. Transcranial Magnetic Stimulation as Treatment in Multiple Neurologic Conditions. Curr Neurol Neurosci Rep. 2020;20(1):1. doi:10.1007/s11910-020-1021-0
13. Yang Z, Sheng X, Qin R, et al. Cognitive Improvement via Left Angular Gyrus-Navigated Repetitive Transcranial Magnetic Stimulation Inducing the Neuroplasticity of Thalamic System in Amnesic Mild Cognitive Impairment Patients. J Alzheimer’s Dis. 2022;86(2):537–551. doi:10.3233/JAD-215390
14. Martinez-Cancino DP, Azpiroz-Leehan J, Jimenez-Angeles L, Garcia-Quintanar A, Santana-Miranda R Effects of high frequency rTMS on sleep deprivation: a pilot study.
15. Guo Z, Jiang Z, Jiang B, McClure MA, Mu Q. High-Frequency Repetitive Transcranial Magnetic Stimulation Could Improve Impaired Working Memory Induced by Sleep Deprivation. Neural Plast. 2019;2019:7030286. doi:10.1155/2019/7030286
16. Tahmasian M, Noori K, Samea F, et al. A lack of consistent brain alterations in insomnia disorder: an activation likelihood estimation meta-analysis. Sleep Med Rev. 2018;42:111–118. doi:10.1016/j.smrv.2018.07.004
17. Fasiello E, Gorgoni M, Scarpelli S, Alfonsi V, Ferini Strambi L, De Gennaro L. Functional connectivity changes in insomnia disorder: a systematic review. Sleep Med Rev. 2022;61:101569. doi:10.1016/j.smrv.2021.101569
18. Fernández-Mendoza J, Vela-Bueno A, Vgontzas AN, et al. Cognitive-emotional hyperarousal as a premorbid characteristic of individuals vulnerable to insomnia. Psychosom Med. 2010;72(4):397–403. doi:10.1097/PSY.0b013e3181d75319
19. Chen JE, Glover GH. Functional Magnetic Resonance Imaging Methods. Neuropsychol Rev. 2015;25(3):289–313. doi:10.1007/s11065-015-9294-9
20. Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26(1):15–29. doi:10.1002/hbm.20113
21. Jack CR, Albert MS, Knopman DS, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dementia. 2011;7(3):257–262. doi:10.1016/j.jalz.2011.03.004
22. Jessen F, Amariglio RE, van Boxtel M, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement. 2014;10(6):844–852. doi:10.1016/j.jalz.2014.01.001
23. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dementia. 2011;7(3):263–269. doi:10.1016/j.jalz.2011.03.005
24. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–279. doi:10.1016/j.jalz.2011.03.008
25. Seow LSE, Verma SK, Mok YM, et al. Evaluating DSM-5 Insomnia Disorder and the Treatment of Sleep Problems in a Psychiatric Population. J Clin Sleep Med Feb. 2018;14(2):237–244. doi:10.5664/jcsm.6942
26. Katzman R, Zhang M, Wang Z, et al. A Chinese version of the Mini-Mental State Examination; impact of illiteracy in a Shanghai dementia survey. J Clin Epidemiol. 1988;41(10):971–978. doi:10.1016/0895-4356(88)90034-0
27. Lu J, Li D, Li F, et al. Montreal cognitive assessment in detecting cognitive impairment in Chinese elderly individuals: a population-based study. J Geriatr Psychiatry Neurol. 2011;24(4):184–190. doi:10.1177/0891988711422528
28. Zhao Q, Guo Q, Liang X, et al. Auditory verbal learning test is superior to Rey-Osterrieth complex figure memory for predicting mild cognitive impairment to Alzheimer’s disease. Curr Alzheimer Res. 2015;12(6):520–526. doi:10.2174/1567205012666150530202729
29. Osborne J, Costello A, Kellow J. Best Practices in Exploratory Factor Analysis. Louisville, KY: CreateSpace Independent Publishing Platform; 2014:86–99.
30. Wang T, Yan J, Li S, et al. Increased insular connectivity with emotional regions in primary insomnia patients: a resting-state fMRI study. Eur Radiol. 2017;27(9):3703–3709. doi:10.1007/s00330-016-4680-0
31. Bai Y, Tan J, Liu X, Cui X, Li D, Yin H. Resting-state functional connectivity of the sensory/somatomotor network associated with sleep quality: evidence from 202 young male samples. Brain Imaging Behav. 2022;16(4):1832–1841. doi:10.1007/s11682-022-00654-5
32. Fogel S, Ray L, Fang Z, Silverbrook M, Naci L, Owen AM. While you were sleeping: evidence for high-level executive processing of an auditory narrative during sleep. Conscious Cogn. 2022;100:103306. doi:10.1016/j.concog.2022.103306
33. Nie X, Shao Y, Liu SY, et al. Functional connectivity of paired default mode network subregions in primary insomnia. Neuropsychiatr Dis Treat. 2015;11:3085–3093. doi:10.2147/NDT.S95224
34. Zou G, Li Y, Liu J, et al. Altered thalamic connectivity in insomnia disorder during wakefulness and sleep. Hum Brain Mapp. 2021;42(1):259–270. doi:10.1002/hbm.25221
35. Baglioni C, Spiegelhalder K, Regen W, et al. Insomnia disorder is associated with increased amygdala reactivity to insomnia-related stimuli. Sleep. 2014;37(12):1907–1917. doi:10.5665/sleep.4240
36. Guadagni V, Burles F, Ferrara M, Iaria G. Sleep quality and its association with the insular cortex in emotional empathy. Eur J Neurosci. 2018;48(6):2288–2300. doi:10.1111/ejn.14124
37. Fruhholz S, Schlegel K, Grandjean D. Amygdala structure and core dimensions of the affective personality. Brain Struct Funct. 2017;222(9):3915–3925. doi:10.1007/s00429-017-1444-9
38. Pannese A, Grandjean D, Fruhholz S. Amygdala and auditory cortex exhibit distinct sensitivity to relevant acoustic features of auditory emotions. Cortex. 2016;85:116–125. doi:10.1016/j.cortex.2016.10.013
39. Trost W, Frühholz S, Schön D, et al. Getting the beat: entrainment of brain activity by musical rhythm and pleasantness. NeuroImage. 2014;103:55–64. doi:10.1016/j.neuroimage.2014.09.009
40. Trost W, Frühholz S, Cochrane T, Cojan Y, Vuilleumier P. Temporal dynamics of musical emotions examined through intersubject synchrony of brain activity. Soc Cogn Affect Neurosci. 2015;10(12):1705–1721. doi:10.1093/scan/nsv060
41. Yeo BT, Krienen FM, Sepulcre J, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;2:57 doi:10.1152/jn.00338.2011.
42. Londei A, D’Ausilio A, Basso D, et al. Sensory‐motor brain network connectivity for speech comprehension. Hum Brain Mapp. 2010;31(4):567–580. doi:10.1002/hbm.20888
43. Si X, Zhou W, Hong B. Cooperative cortical network for categorical processing of Chinese lexical tone. Proce National Acad Sci. 2017;114(46):12303–12308. doi:10.1073/pnas.1710752114
44. Behroozmand R, Shebek R, Hansen DR, et al. Sensory–motor networks involved in speech production and motor control: an fMRI study. Neuroimage. 2015;109:418–428. doi:10.1016/j.neuroimage.2015.01.040
45. Portas CM, Krakow K, Allen P, Josephs O, Armony JL, Frith CD. Auditory processing across the sleep-wake cycle: simultaneous EEG and fMRI monitoring in humans. Neuron. 2000;28(3):991–999. doi:10.1016/S0896-6273(00)00169-0
46. Bidelman GM, Brown JA, Bashivan P. Auditory cortex supports verbal working memory capacity. Neuroreport. 2021;32(2):163. doi:10.1097/WNR.0000000000001570
47. Pannese A, Grandjean D, Frühholz S. Subcortical processing in auditory communication. Hear Res. 2015;328:67–77. doi:10.1016/j.heares.2015.07.003
48. Schiel JE, Holub F, Petri R, et al. Affect and Arousal in Insomnia: through a Lens of Neuroimaging Studies. Curr Psychiatry Rep. 2020;22(9):44. doi:10.1007/s11920-020-01173-0
49. Liu Y-S, Wang Y-M, Zha D-J. Brain Functional and Structural Changes in Alzheimer’s Disease With Sleep Disorders: a Systematic Review. Front Psychiatry. 2021;12 doi:10.3389/fpsyt.2021.772068.
50. Li K, Luo X, Zeng Q, et al. Interactions between sleep disturbances and Alzheimer’s disease on brain function: a preliminary study combining the static and dynamic functional MRI. Sci Rep. 2019;9(1):19064. doi:10.1038/s41598-019-55452-9
51. Xu J, Dong H, Li N, et al. Weighted RSA: an Improved Framework on the Perception of Audio-visual Affective Speech in Left Insula and Superior Temporal Gyrus. Neuroscience. 2021;469:46–58. doi:10.1016/j.neuroscience.2021.06.002
52. Dyck M, Loughead J, Kellermann T, Boers F, Gur RC, Mathiak K. Cognitive versus automatic mechanisms of mood induction differentially activate left and right amygdala. NeuroImage. 2011;54(3):2503–2513. doi:10.1016/j.neuroimage.2010.10.013
53. Herrero Babiloni A, Bellemare A, Beetz G, et al. The effects of non-invasive brain stimulation on sleep disturbances among different neurological and neuropsychiatric conditions: a systematic review. Sleep Med Rev. 2021;55:101381. doi:10.1016/j.smrv.2020.101381
54. Lin Y, Jiang W-J, Shan P-Y, et al. The role of repetitive transcranial magnetic stimulation (rTMS) in the treatment of cognitive impairment in patients with Alzheimer’s disease: a systematic review and meta-analysis. J Neurol Sci. 2019;398:184–191. doi:10.1016/j.jns.2019.01.038
55. Wang JX, Rogers LM, Gross EZ, et al. Targeted enhancement of cortical-hippocampal brain networks and associative memory. Science. 2014;345(6200):1054–1057. doi:10.1126/science.1252900
56. Marshall L, Born J. The contribution of sleep to hippocampus-dependent memory consolidation. Trends Cogn Sci. 2007;11(10):442–450. doi:10.1016/j.tics.2007.09.001
57. Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564–583. doi:10.1093/brain/awl004
58. Zhang L, Huang Y, Zhang Y, Xin W, Shao Y, Yang Y. Enhanced high-frequency precuneus-cortical effective connectivity is associated with decreased sensory gating following total sleep deprivation. Neuroimage. 2019;197:255–263. doi:10.1016/j.neuroimage.2019.04.057
59. Paller KA. Cross-cortical consolidation as the core defect in amnesia: prospects for hypothesis-testing with neuropsychology and neuroimaging. Neuropsychol Memory. 2002;3:73–87.
60. Staresina BP, Bergmann TO, Bonnefond M, et al. Hierarchical nesting of slow oscillations, spindles and ripples in the human hippocampus during sleep. Nat Neurosci. 2015;18(11):1679–1686. doi:10.1038/nn.4119
61. Gong L, Xu R, Qin M, et al. New potential stimulation targets for noninvasive brain stimulation treatment of chronic insomnia. Sleep Med. 2020;75:380–387. doi:10.1016/j.sleep.2020.08.021
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.