Back to Journals » Journal of Experimental Pharmacology » Volume 16
Nephroprotective Effect of the Leaf Extract of Ajuga remota Benth Against Gentamicin-Induced Nephrotoxicity in Swiss Albino Mice
Authors Akinaw MA, P Nair SK, Usure RE, Leta B , Kedir A , Mamo SA, Waritu NC , Jemal M , Mulat BK
Received 18 January 2024
Accepted for publication 19 March 2024
Published 26 March 2024 Volume 2024:16 Pages 159—171
DOI https://doi.org/10.2147/JEP.S455226
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Metages Ayele Akinaw,1 Suresh Kumar P Nair,1 Rashed Edris Usure,2 Bati Leta,1 Abdo Kedir,3 Selam Ayele Mamo,4 Nuredin Chura Waritu,5 Mohammed Jemal,6 Berhane Kebede Mulat1
1Department of Biomedical Sciences, School of Medicine, Jimma University, Jimma, Ethiopia; 2Department of Pharmaceutical Chemistry, School of Pharmacy, Hawassa University, Hawassa, Ethiopia; 3Department of Pathology, School of Medicine, Jimma University, Jimma, Ethiopia; 4Department of Adult Health Nursing, School of Nursing, Jimma University, Jimma, Ethiopia; 5Department of Biomedical Sciences, School of Medicine, Wolaita Sodo University, Wolaita Sodo, Ethiopia; 6Department of Biomedical Sciences, School of Medicine, Debre Merkos University, Debre Merkos, Ethiopia
Correspondence: Nuredin Chura Waritu, Tel +251919617254, Email [email protected]
Background: Drug-induced kidney injury was among the most common renal damages, from which gentamicin occupies around 25% of this injury. Gentamicin-induced renal damage is caused by increased free radicals with subsequent amplified inflammation. Ajuga remota leaf extract has many phytochemicals with antioxidant activities, which may improve gentamicin-induced renal damage. Thus, we aimed to investigate the nephroprotective effect of Ajuga remota leaf methanolic extract on gentamicin-induced nephrotoxicity in Swiss Albino Mice.
Methods: An experimental study design was used on 30 experimental mice randomly allocated in six groups: Group I, II, II, IV, and VI, among which mice were given only distilled water, only gentamicin, 600 mg/kg Ajuga remota leaf extract only, gentamicin along with 200 mg/kg extract, gentamicin with 400 mg/kg extract and gentamicin with 600 mg/kg extract, respectively. At the end of the experiment, the mice were sacrificed after being anaesthetized, and blood samples were collected through a cardiac puncture for renal function tests while the kidneys were removed for histopathological evaluation. The data were entered into Epidata version 4.6 and exported to SPSS version 25 for further analysis using one-way analysis of variance. Statistical significance was set at p < 0.05.
Results: Group II mice had significantly higher levels of serum creatinine and blood urea levels compared to group I and III. The body weight of the mice in group V and group VI showed a significant increase compared with Group II. Serum creatinine and blood urea levels were reduced significantly in the Ajuga remota leaf extract administered group of mice compared to group II. Abnormal kidney architectural changes were seen among group II mice; however, those changes were improved after administration of Ajuga remota leaf methanolic extract.
Conclusion: Methanol extract of Ajuga remota leaf provided effective protection against gentamicin-induced oxidative renal damage through its antioxidant effects.
Keywords: Ajuga remota Benth, gentamicin, nephrotoxicity, methanol
Background
Kidney disease is a major health problem worldwide.1 One of the functions of the kidney is to eliminate toxic compounds from the body, which frequently expose the kidney to a number of poisons that culminate in the development of acute kidney injury (AKI), which in some cases progresses to chronic kidney disease (CKD).2 In 2019, over 850 million people were affected by kidney disease, among which 463 million were diabetic and more than twenty times that of HIV/AIDS-infected individuals (37.7 million in 2020).1,3–6 Elevated levels of serum biomarkers, such as creatinine and urea, indicate renal damage.7
Drug use was the factor that played the most significant role in renal damage, by which acetaminophen,8 aminoglycoside,9 angiotensin-converting enzyme inhibitors (ACEI),10 cisplatin,11 penicillin G,12 and ranitidine contributed the most to the development of AKI, possibly by inducing inflammation, acid–base disturbance, electrolyte abnormalities, intrarenal obstruction, and interstitial nephritis.13 Approximately 25% of AKI cases occur due to drug toxicity, with gentamicin being the most toxic.14–16 Nephrotoxicity occurs in 25% of the patients treated with gentamicin.17 Gentamicin is an aminoglycoside antibiotic widely used in the treatment of severe pneumonia in children, neonatal sepsis, and complicated acute malnutrition.18 Nevertheless, it is a major nephrotoxic drug that damages the renal tubules, deteriorates glomerular function, and increases vascular resistance, possibly through the production of reactive oxygen species, inducing inflammation within the renal tubules and activating renal cell apoptosis.19,20 Gentamicin disrupts the normal function of the kidney by accumulating in the cell membranes of the renal tubules, resulting in altered secretion and reabsorption of electrolytes.21 However, due to its low rate of resistance and low cost, patients still use gentamicin widely, regardless of its damaging effects on kidney function.9,14,22,23
Ajuga remota is a traditional medicinal plant belonging to the Lamiaceae family with more than 300 species grown in Ethiopia.24 The therapeutic uses of Ajuga remota extract claimed by multiple studies were its antihyperlipidemic,25 antimalarial,26,27 antidiarrheal,28,29 antidiabetic,30 and anti-inflammatory activities.31 In Ethiopia, this traditional medicinal plant is used to treat kidney disease, blood pressure, malaria, diarrhea, fever, stomach pain, hyperlipidemia, diabetes, and ascariasis.25,30,32–34 The leaves of ajuga remota also contain secondary metabolites such as flavonoids, alkaloids, terpenoids, phenols, and steroids.30 These active ingredients are thought to play a major role in the reduction of inflammation and oxidative stress-induced renal damage via detoxification of free radicals as well as boosting the body’s antioxidant scavenging system.35 Despite the fact that Ajuga remota is rich in phytochemicals with antioxidant properties that contributed to its ability to protect against oxidative-induced renal damage, no study has been conducted on its nephroprotective effect until now. Therefore, the present study aimed to investigate the nephroprotective effects of Ajuga remota methanol extract against gentamicin-induced nephrotoxicity in Swiss Albino Mice.
Methods and Materials
Study Area and Study Period
Phytochemical screening and extraction of Ajuga remota leaves were performed at the Jimma University Biochemistry Laboratory, Organic and Inorganic Chemistry Postgraduate Laboratory. Biochemical and histopathological analyses were performed at the Jimma University Medical Center. The extracted plant leaves were administered to mice to investigate their nephroprotective effects from December 25, 2021, to January 05, 2022, at the Tropical and Infectious Disease Research Center, Jimma University (TIDRC-JU), Sekoru district, Jimma, Ethiopia.
Study Design
A post-test control group experimental study design has been conducted on a mouse model to evaluate the nephroprotective effect of the leaf extract of Ajuga remota.
Reagents and Chemicals
In this study, multiple reagents and chemicals were used, including absolute methanol (Assay (GC) >99.9%), 10% formalin (Brand-Microxpress 500mL, Histological fixative), ketamine/xylazine intramuscular (50mg/mL) for anesthesia, acetone (ACA 280223), gentamicin (Brand name-Ruili, Model number-80mg/2mL) for induction, chloroform (EMSURE© ASC ISO), sulfuric acid (Laboratory use 98%), Mayer’s reagent, ninhydrin solution, and hydrochloric acid (Grade AR).
Equipment
Eppendorf tube (L-510-GRD, Thermo Fisher Scientific KK, 1.5mL), volumetric flasks (0.25L, 0.5L, and 2L), beakers (0.08L, 0.1L, 0.5L, and 1L), funnel (75mm and 100mm), Erlenmeyer flask (conical flask), measuring cylinder (2.5mL, 5 mL, 10mL, 25mL, 50mL and 100mL), glass rood (GL160-0200), spatula (steel, 0–10mm and 6inch), automatic pipette (Brand-hangxin, Model-A3001-A3030), gavage (oral feeding syringe), syringe (3cc), metal heater, refrigerator, Whatman filter paper number-1, digital/electric balance (Wensar, Mettler, and Aczet), water bath (Doubled walled construction with regular and GMP models), metallic stirrer, automated chemistry analyzer (analyzer URIT-810 URIT), rotary evaporator (Model-Laborota 4000; Brand-Heidolph), and plastic cage for experimental animals grouping.
Plant Collection and Authentication
Fresh leaves of Ajuga remota were collected from around the Jiren Mountain, Jimma town, in September 2021. The plant was identified and authenticated by a botanist, Mr Melaku Wondafrash, from the National Herbarium (ETH) of Addis Ababa University, and voucher number MA001 was given and deposited in the institute for further reference.
Preparation of Plant Crude Extract
The dried leaves of Ajuga remota were ground into a coarse powder using a mechanical grinder and weighed to approximately 300g using a digital balance. Two hundred and fifty gram coarse powders was measured and soaked in 2.5 L absolute methanol (Assay (GC): >99.9%) in 1:10 ratio in a flask for 72 h with periodic mechanical agitation in Jimma University Biochemistry Laboratory, Organic and Inorganic Chemistry Postgraduate Laboratory. The extracts were filtered through Whatman filter paper No. 1 and the solvent from the mixture was evaporated using a rotary evaporator (Model-Laborota 4000 and Brand-Heidolph) at 35°C. The final products were dried in a heat chamber at 25°C until all liquids were removed from the extract.
Phytochemical Analysis
The phytochemical analyses were performed using the following standard methods:
Test for alkaloid (Mayer’s test): The plant extract was mixed with a few drops of Mayer’s reagent and looked for white creamy precipitation, which showed the presence of alkaloids.36–39
Test for amino acids (Ninhydrin’s test): 100mg of the plant extract was mixed well with 10mL of distilled water. The mixture was filtered using Whatman no-1 filter paper, and 2mL of the filtrate was mixed with two drops of ninhydrin solution and appeared purple.36–39
Test for carbohydrate (Benedict’s test): each, a half milliliter of plant extract filtrate and Benedict’s reagent were mixed and heated for two minutes in a boiling water bath. The formation of a colored precipitate indicated the presence of carbohydrates (sugars).37–40
Test for fats and oil (spot test/stain test): A small quantity of plant extract was placed on a filter paper, pressed using a second filter paper and looking for an oil stain on the paper.37,40
Test for anthraquinones (Borntrager’s test): 1g of plant powder was dissolved in 20mL of chloroform in a test tube, and the mixture was placed in a steam bath for 5minutes. The mixture was then filtered (while hot) and allowed to cool. Equal parts of the filtrate and 10% ammonia solution were mixed and shaken well, and the presence of a pink color on the upper parts of the aqueous layer indicated anthraquinones.41
Test for phenolic compounds (Ferric chloride test): 50 mg of plant extract was dissolved in 5 mL of distilled water, a few drops of ferric chloride solution (5% neutral) were added, and the dark green color product was examined.37–39,42
Test for tannins: half a gram of plant extract and bromine water (10mL) were mixed, and the decolorization of bromine water indicated the presence of tannins.37,43
Test for proteins (Millon's test): 10mL of distilled water and 100mg of plant extract were mixed and filtered using Whatman no-1 filter paper. Then, 2mL of the filtrate was mixed with 2–3 drops of Million Reagent. The formation of a white precipitate indicated the presence of proteins.37–39
Test for saponins: 50 mg of the plant extract was dissolved in 20mL of distilled water. The mixture was shaken for 15 minutes in a cylinder, and the presence of saponins was indicated by the formation of a 2cm layer of foam.37–40
Test for flavonoids: The Shinoda test or alkaline reagents test was used to detect the presence of flavonoids (Mg2+ ribbon + concentrated HCL + crude extract → pink color or 2% of NaOH + crude extract → gives a deep yellow color + 2 drops of diluted acid → colorless).44,45
Salkowski’s test was used to check for the presence of glycoside components (2mL of H2SO4 + plant crude extract → reddish-brown color).44
Test for terpenoids: The presence of terpenoids was detected using chloroform and sulfuric acid (2mL of chloroform, 5 mL of crude extract, and 3 mL of H2SO4 → gray color).46
Test for steroids: The presence of steroids was determined using chloroform and concentrated sulfuric acid (H2SO4) (2mL of chloroform + H2SO4 + 5 mL crude extract → red colour layer).38,39,43
Study Variables
Independent variable: Leaf extract of Ajuga remota
Dependent variables: Renal function tests, serum electrolyte level, histopathological analysis
Sample Size Determination
Sample size determination was based on Federer calculation formula, which is (t – 1) (n – 1) ≥ 15; where “t” is the number of the groups and “n” is the experimental animal per group. (5–1) (n – 1) ≥ 15 → n ≥ 5. According to this calculation, the minimum sample size was five experimental animals in each treatment and control group. In this study, five experimental animals were included in each treatment group and a control group, which gives a total of 30 mice used for this experimental study.
Experimental Animals
For this study, a total of thirty-five (30 males and 5 females for this experimental study and oral toxicity test, respectively) Swiss albino mice aged 6–8 weeks old (weighing 28–32 grams) and physically normal were selected from the Tropical and Infectious Diseases Research Center, Jimma University (TIDR-JU), Sekoru district, Jimma. The experimental mice were placed in plastic cages with stainless-steel covers and housed in an animal laboratory, provided with pellets and water ad libitum. The mice were maintained under natural light for 24 hrs (12-hour dark–light cycle) at a standard room temperature of 25°C and relative humidity of approximately 50% before the experimental procedures. During acclimatization for one week, the mice were randomly divided into six groups, with five experimental animals in each group. They were handled in accordance with the internationally accepted guidelines.47–49
Oral Acute Toxicity Test
An acute oral toxicity test was performed to determine the median lethal dose (LD50) for the leaf extract of Ajuga remota in female Swiss albino mice, according to the OECD 425 guidelines. Prior to testing, five female Swiss albino mice weighing 28–32 grams and aged 6–8 weeks were selected randomly and caged for six days in the laboratory to become familiar with the conditions of the laboratory. The animals have an unlimited supply of drinking water. Following the measurement of the body weight of the mice after fasting for 3 hours, one mouse was selected randomly, and a 2000 mg/kg methanolic extract of Ajuga remota leaf was administered via stainless-steel oral gavage. The mouse was frequently observed for behavioral changes and common toxicity signs after dosing for the first 24 hours, with special consideration being given during the first 4 hours. The mouse did not develop any signs of toxicity for 24 and 14 days of observation. The other four Swiss albino mice were given comparable dose and were monitored for 14 days.50,51
Experimental Induction of Nephrotoxicity in Mice
Nephrotoxicity was induced by intraperitoneal (IP) injection of gentamicin at 80 mg/kg per day for consecutive 8 days. This dose caused nephrotoxicity when administered for more than 5 days based on a previous study by Huang et al, 2020.52,53
Experimental Design and Treatment Protocol
The six groups of experimental mice were assigned accordingly:
Group I (normal control) received distilled water (1mL) via IP for 8 days.
Group II (Gentamicin control) received gentamicin-80 mg/kg body weight via IP for 8 days.52
Group III (methanol extract Ajuga remota (MEAR) control) received 600 mg/kg per body weight of MEAR alone for 8 days, orally;
Group IV (200 MEAR) received gentamicin-80 mg/kg for 8 days and MEAR (200 mg/kg per body weight) orally for 10 days (started two days before co-administration of gentamicin).
Group V (400 MEAR) received gentamicin-80 mg/kg for 8 days and MEAR (400 mg/kg per body weight) orally for 10 days (started two days before co-administration of gentamicin).
Group VI (600 MEAR) received gentamicin-80 mg/kg for 8 days and MEAR (600 mg/kg per body weight) orally for 10 days (started two days before co-administration of gentamicin).25
Determination of Body Weight and Kidney Weight of Mice
The weights of the mice were measured at the beginning, middle, and at the end of the experiment before sacrifice. After scarifying by cervical dislocation, both the right and left kidneys were removed, the right kidney was weighed to obtain the absolute kidney weight of the mice, and the left kidney was placed in a screwed cup container with 10% formalin (in 1 to 10 ratios) for histopathological examination.
Sample Collection
Blood samples were collected 12 h after the last treatment by anesthetizing the mice with ketamine/xylazine (intramuscular, 500 mg/10mL (50 mg/mL)). The sample was collected via cardiac puncture (diaphragmatic approach), which was performed by inserting a 1 inch 22-gauge needle attached to a 3 mL (3cc) syringe through either notch. The collected blood samples were kept in an Eppendorf tube for approximately 30 minutes at room temperature to separate the serum from the formed elements and centrifuged at 3000 rpm for 10 min to completely separate the serum from the formed element. The serum was stored in a refrigerator (4–8°C) for blood urea, creatinine, sodium, potassium, and chloride analysis.54,55
Histopathological Examination of the Kidney
The animals were sacrificed using cervical dislocation techniques before awakening from anesthesia. The abdominal cavity was opened using a surgical blade, and both kidneys were removed and processed for histological examination. Kidney weight was measured using a digital/electronic balance. The kidneys were fixed in 10% formal saline 1to10 ratio for histopathological examinations.56,57 Tissue processing involves dehydration, clearing, and paraffin impregnation. Dehydration is the process of water removal by immersing the tissue in ascending concentrations of alcohol until water is removed. Next, the alcohol was cleared using an organic solvent, xylene, and paraffin impregnation, followed by immersion of the tissue in molten wax at 60°C. Embedding was then performed using melted paraffin and a metal mold, which was achieved by assembling the metal mold and lubricating it using liquid paraffin, after which the tissue was carefully placed at the bottom of the metallic mold. The melted paraffin was allowed to harden at room temperature, after which the metallic mold was removed and the blocks were trimmed and placed in a refrigerator before sectioning. A microtome was used to cut tissue sections (4–5μm thick) with one tissue block at a time. The cut sections were floated and flattened in lukewarm water in a water bath (50–55°C), placed on a clean slide, and left to dry. The dried slides were labeled and stained in multiple steps using hematoxylin and eosin and examined by a pathologist who was blinded to the experiment via light microscopy at a magnification of 400x to assessment of the kidney sections.58,59
Data Analysis
Data were collected and entered into Epidata version 4.6 and analyzed using SPSS version 25. The results of various biochemical parameters were expressed as mean ± SEM and compared between the groups using analysis of variance (ANOVA) followed by post hoc Tukey’s multiple comparisons to determine the particular group showing a significant difference at p < 0.05. Histopathological examinations were performed under a microscope by a senior pathologist and presented using photomicrographs.
Results
Acute Oral Toxicity Test
An oral acute toxicity test indicated that the crude extract of Ajuga remota leaf was safe when administered orally at a dose of 2000 mg/kg. After 24 hours and 14 days of observation, the first tested mouse as well as the remaining four mice did not show any signs of toxicity, which was evidenced by the absence of central nervous system signs such as tremors, convulsions, salivation, lethargy, and coma. In addition, none of the MEAR administered mice died. Based on these oral toxicity test results, the LD50 for MEAR leaf was considered to be greater than 2000 mg/kg body weight. This encouraged us to use 200, 400, and 600 mg/kg of MEAR leaf dosages for our current experimental study.
Phytochemical Screening Tests
Phytochemical screening of MEAR (Table 1) revealed the presence of glycosides, terpenoids, steroids, phenolic compounds, flavonoids, tannins, fats, oils, and saponins.
 |
Table 1 Results of Phytochemical Screening Test of MEAR Leaf |
Effects of Ajuga remota Extract on Mouse Body Weight
As shown in Table 2, the body weights of the mice did not show a statistically significant difference among the groups at the beginning (1st day) and 5th day of the experiment. However, on the 10th day of the experiment, the body weights of mice in all groups showed a statistically significant difference. Compared to the normal control, the mean body weights of all mice in groups IV (p = 0.001, 17.57%), V (p = 0.049, 9.1%), and VI (p = 0.018, 10.52%) were significantly reduced. This study also reported that the mean body weights of mice in groups V (p = 0.001, 18.8%) and VI (p = 0.003, 16.94%) were significantly higher than those in groups II. In addition, a significant increase in the mean body weight was observed in groups I and III (30.69% and 19.29%, respectively) compared to that in groups II (p = 0.01). In general, there were reductions in body weight in all groups compared with the 1st day of body weight measurement of each group, except for the normal control group (GI).
 |
Table 2 Mean Body Weight of Swiss Albino Mice from the Beginning to the End of the Experimental Day |
Effects of Ajuga remota Extract on Serum Urea and Creatinine Level
As shown in Table 3, groups IV and V (p < 0.001) showed a statistically significant increase in serum urea levels compared to those of group I and III, while group VI (p > 0.05) did not show a significant difference but showed significant reductions when compared to group IV and II (p < 0.001, 194.55%, and 200%, respectively). This study also determined lower urea levels in groups IV, V, and VI (p < 0.001, 21.3%, 38.58%, and 53.09%, respectively) than in group II. A higher mean serum creatinine level was observed in group IV (p = 0.008, 78.46%) than in group I. The results of this study also revealed that mice in group IV (p = 0.002, 90.2%) and V (p = 0.037, 65.6%) showed a significant increase in serum creatinine levels compared with those in group III. Moreover, decreased serum creatinine levels were observed in group V (p = 0.027, 30.34%) and VI (p < 0.001, 47.59%) mice compared with group II mice. In contrast, lower mean serum creatinine levels were observed in groups I and III than in the distilled water control group.
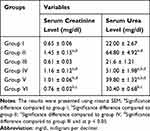 |
Table 3 Effects of Methanol Extract of Ajuga Remota on Serum Creatinine and Urea Level in Gentamicin-Induced Nephrotoxicity |
Effects of Ajuga remota Extract on Serum Electrolyte Level
In this study, as shown in Table 4, there was no significant difference in serum electrolyte levels among all groups; however, there was a slight non-significant increase in serum sodium, potassium, and chloride levels among the groups and a slight non-significant decrease in serum calcium levels compared with groups I and III.
 |
Table 4 Effects of Methanol Extract of Ajuga Remota on Serum Electrolyte Level on Gentamicin-Induced Nephrotoxicity in Mice |
Effects of Ajuga remota Extract on Relative Kidney Weight of the Mice
At the end of this experiment (Table 5), the relative kidney weights of mice in group IV (p = 0.016, 8.83%), V (p = 0.007, 9.89%), and VI (p = 0.006, 10.25%) were significantly lower than those of group II. In contrast, there were no significant differences among groups I, III, IV, V, and VI. However, there was a significant increase in the mean kidney weight of mice in groups I and III (13.65% and 12.3%, respectively) compared with that of mice in group II (p < 0.02).
 |
Table 5 Effects of Methanol Extract of Ajuga Remota on Kidney Weight in Gentamicin-Induced Nephrotoxicity |
Effects of Ajuga remota Extract on Kidney Histopathological Changes
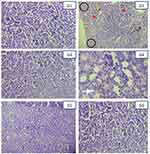
Microscopic examinations of the renal tissue of the mice in groups I and III showed normal glomerular structure and renal tubular interstitium with no evidence of protein casts, inflammatory infiltration, necrosis, and vacuolization. In contrast, kidney sections of the mice induced with only gentamicin (Group II) showed multiple renal histopathological abnormalities with renal tubular necrosis, inflammatory infiltration, protein casts, diminished glomeruli, and dilated as well as degenerated renal tubules. However, Group IV (200 mg/kg), group V (400 mg/kg), and group VI (600 mg/kg) of mice showed improved renal histopathological abnormalities as evidenced by reduced renal tubular necrosis, inflammatory infiltration, and diminished glomeruli compared with those of group II mice. In a dose-dependent manner, 600 mg/kg was observed to have better nephroprotective effects (see Figure 1 for further detail).
Discussion
In the present study, the nephroprotective effects of Ajuga remota leaf extract were examined on gentamicin-induced nephrotoxicity in Swiss albino mice. According to the findings of this study, Ajuga remota leaf extract reduced the deleterious effects of gentamicin, such as loss of body weight, increased relative kidney weight, and increased blood urea and serum creatinine levels. Moreover, histopathological analysis of the kidney revealed a reduced loss of normal kidney structural changes induced by gentamicin after administration of MEAR.
Various drugs cause nephrotoxicity through multiple mechanisms, such as dose, route, and time of drug exposure to the kidney, higher concentration, and accumulation of some drugs in the renal cells.60 Gentamicin is a clinically effective drug against many severe infections caused by the gram-negative bacteria, Enterococcus and Staphylococcus.61 Gentamicin is a well-known nephrotoxic drug that causes tubular, glomerular, and blood vessel damage, which results from the production of reactive oxygen species.62
The results of this study indicate that the IP administration of gentamicin (80 mg/kg) reduces kidney function, which manifests in increased serum creatinine and blood urea levels. This finding is congruent with the results of a study conducted in Ethiopia on gentamicin-induced mice.63 Furthermore, the increased in serum creatinine and blood urea levels due to gentamicin injection was remarkably reduced by the administration of methanol leaf extract of Ajuga remota, which is in line with the results of previous studies of different medicinal plants performed on gentamicin-induced mice.64–66 The current study also found significantly decreased mean serum creatinine and blood urea levels in MEAR-treated mice. These effects are expected to be due to the presence of nephroprotective bioactive molecules in Ajuga species. Bioactive molecules such as phytoecdysteroids can activate gene transcription and increase biosynthesis of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx).35 In addition, flavonol and iridoid glycosides extracted from Ajuga remota have antioxidant activities, which may attenuate oxidative stress-induced renal damage.67,68
In the present study, a non-significant reduction in serum calcium levels was found in the gentamicin-treated groups, which is in agreement with previous study findings.69 The reduction in serum calcium levels occurs due to the stimulation of the entry of extracellular calcium and the inhibition of calcium ion transporter by gentamicin.20,70,71 Increased phosphorus retention due to gentamicin-induced tubular damage (necrosis), leading to increased urinary excretion of calcium, is another possible reason for the decreased serum calcium level.20,72
However, regarding serum sodium, potassium, and chloride levels, a non-significant increase was seen in gentamicin-induced mice. This finding is consistent with study finding reported by Bouhrim et al.69 The possible mechanisms for elevated levels of these electrolytes have been ascribed to the alteration of kidney function, or reduced glomerular filtration rate, and decreased reabsorption of water caused by gentamicin.73,74
Moreover, in the current study, an increase in kidney weight and a loss of body weight at the end of the experiment were observed in gentamicin-administered mice. This is in line with a previous study done on gentamicin-treated rats that reported a significant increase in kidney weight as a result of tubular necrosis, increased catabolism, and loss of appetite, which ultimately resulted in the loss of body weight.65,74 Loss of body weight in gentamicin-induced mice has been attributed to be due to increased catabolism and renal tubular damage, with reduced water reabsorption in the renal tubules, followed by dehydration.73,74
Administration of MEAR for ten consecutive days resulted in reduced morphological changes compared with the gentamicin control group, which was illustrated by the significant difference in relative kidney weight among the groups. In the experimental groups, 600mg/kg and 400mg/kg MEAR administered mice showed normal renal anatomy, whereas mild-to-moderate damage to the renal tubules, glomeruli, and renal tubular cells was observed in mice administered 200mg/kg MEAR. This might be due to the antioxidant and anti-inflammatory effects of the phytochemicals present in the extract, which activate the transcription of genes encoding antioxidant enzymes and improve immune function.35,75
Furthermore, the current study is also designed to assess the microscopic examination of the kidney sections of the mice. We observed necrosis, inflammatory infiltration, dilated and degenerated tubules, protein casts, and vacuolization in the gentamicin control (GII) group. This finding is in agreement with those of previous studies on gentamicin-induced nephrotoxicity in mice.20,21 A possible reason for this abnormal histopathological result in mice induced by gentamicin was gentamicin accumulation in the renal tubules (proximal convoluted tubules), which subsequently resulted in the loss of tubular integrity, necrosis, degeneration, and inflammatory infiltration within the renal cells and tissue.17 However, improved abnormal histopathological results were observed after mice were treated with ajuga remota leaf extract. This finding is in agreement with that of a study conducted in Ethiopia.63
Limitations
We were unable to include all parts (root, flower, seed, and stem) of Ajuga remota to compare their nephroprotective effects. The study was also unable to identify the standard marker compounds from the extract and the effects of the individual active ingredients of Ajuga remota on serum creatinine, blood urea nitrogen, and kidney histopathology.
Conclusion and Recommendations
This study finding indicates that 600 MEAR leaf extract of ajuga remota had nephroprotective effects relative to gentamicin-induced mice, as confirmed by decreased serum creatinine and blood urea levels and improved histopathological results after treatment with ajuga remota leaf extract. In general, mice treated with methanol extract of ajuga remota leaf exhibited nephroprotective effects against gentamicin-induced renal damage.
Further experimental research is needed to compare the effects of Ajuga remota leaf extract and standard drugs on gentamicin-induced nephrotoxicity. In addition, we would like to recommend the examination of various parts of the plant for their nephroprotective effects. Moreover, the antioxidant activities of the Ajuga remota leaf extract should also be examined in relation to its nephroprotective effects.
Abbreviations
ACEI, angiotensin-converting enzyme I, AKI; Acute kidney disease; CKD, chronic kidney disease; IP, intraperitoneal; MEAR, methanol extract of ajuga remota; TIDRC, Tropical Infectious Disease Research Center.
Data Sharing Statements
The corresponding author can be contacted to get all the necessary data that support the finding of this study.
Ethical Approval
This study was conducted after obtaining a letter of ethical clearance from the Institutional Review Board of Jimma University with reference number of IHRPG1/9/21. All experimental activities were conducted following the ethical declaration of national and international standards for experimental animals to protect their rights of experimental animals with treating the animals to avoid pain, suffering, injury, hunger, and thirst.47,76,77
Acknowledgments
We want to be very grateful to the Biochemistry unit and Organic and Inorganic Chemistry Postgraduate Laboratory of Jimma University for providing their laboratory space to us as well as the data collectors at Jimma University and Tropical and Infectious Disease Research Center for their unreserved contributions.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No funding was received for this study.
Disclosure
The authors declare that they have no conflict of interest for this work
References
1. Jager KJ, Kovesdy C, Langham R, Rosenberg M, Jha V, Zoccali C. A single number for advocacy and communication—worldwide more than 850 million individuals have kidney diseases. Kidney Int. 2019;96(5):1048–1050. doi:10.1016/j.kint.2019.07.012
2. Pazhayattil GS, Shirali AC. Drug-induced impairment of renal function. Int J Nephrol Renovasc Dis. 2014;7:457–468. doi:10.2147/IJNRD.S39747
3. UNAIDS. FACT SHEET 2021 global HIV statistics. End AIDS Epidemic. 2021:1–3.
4. Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabet Res Clin Pract. 2019;157:107843. doi:10.1016/j.diabres.2019.107843
5. Bikbov B, Purcell CA, Levey AS, et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395(10225):709–733. doi:10.1016/S0140-6736(20)30045-3
6. Miyahara T, Assadi F. Drug-induced renal disorders. J Ren Inj Prev. 2015;4(3):57–60. doi:10.12861/jrip.2015.12
7. Salazar JH. Overview of urea and creatinine. Lab Med. 2014;45(1):e19–20. doi:10.1309/LM920SBNZPJRJGUT
8. Mazer M, Perrone J. Acetaminophen-induced nephrotoxicity: pathophysiology, clinical manifestations, and management. J Med Toxicol. 2008;4(1):2–6. doi:10.1007/BF03160941
9. Sandhu JS, Sehgal A, Gupta O, Singh A. Aminoglycoside nephrotoxicity revisited. J Indian Acad Clin Med. 2007;8(4):331–333.
10. Perazella MA, Navis G, Faber HJ, de Zeeuw D, de Jong PE. ACE inhibitors and the kidney. Drug Safety. 1996;15(3):200–211. doi:10.2165/00002018-199615030-00005
11. Fang CY, Lou DY, Zhou LQ, et al. Natural products: potential treatments for cisplatin-induced nephrotoxicity. Acta Pharmacol Sin. 2021;1–19.
12. Bryan CS, Stone WJ. “Comparably massive” penicillin G therapy in renal failure. Ann Intern Med. 1975;82(2):189–195. doi:10.7326/0003-4819-82-2-189
13. Emovon OE, King JAC, Holt CO, Browne BJ. Ranitidine-induced acute interstitial nephritis in a cadaveric renal allograft. Am J Kidney Dis. 2001;38(1):169–172. doi:10.1053/ajkd.2001.25211
14. Selby NM, Shaw S, Woodier N, Fluck RJ, Kolhe NV. Gentamicin-associated acute kidney injury. Qjm. 2009;102(12):873–880. doi:10.1093/qjmed/hcp143
15. Sales GTM, Foresto RD. Drug-induced nephrotoxicity. Rev Assoc Med Bras. 2020;66(Suppl 1):82–90. doi:10.1590/1806-9282.66.s1.82
16. Walther CP, Podoll AS, Finkel KW. Summary of clinical practice guidelines for acute kidney injury. Hosp Pract. 2014;42(1):7–14. doi:10.3810/hp.2014.02.1086
17. Lopez-Novoa JM, Quiros Y, Vicente L, Morales AI, Lopez-Hernandez FJ. New insights into the mechanism of aminoglycoside nephrotoxicity: an integrative point of view. Kidney Int. 2011;79(1):33–45. doi:10.1038/ki.2010.337
18. USAID. Module III gentamicin. Man Procure Supply Qual MNCH Commod. 2019;4(1):121–140.
19. Oliveira JFP, Silva CA, Barbieri CD, Oliveira GM, Zanetta DMT, Burdmann EA. Prevalence and risk factors for aminoglycoside nephrotoxicity in intensive care units. Antimicrob Agents Chemother. 2009;53(7):2887–2891. doi:10.1128/AAC.01430-08
20. Randjelović P, Veljković S, Stojiljković N, Sokolović D, Ilić I. Gentamicin nephrotoxicity in animals: current knowledge and future perspectives. EXCLI J. 2017;16:388–399. doi:10.17179/excli2017-165
21. Dhodi JB, Thanekar DR, Mestry SN, Juvekar AR. Carissa carandas Linn. fruit extract ameliorates gentamicin–induced nephrotoxicity in rats via attenuation of oxidative stress. J Acute Dis. 2015;4(2):135–140. doi:10.1016/S2221-6189(15)30023-8
22. Duffy CR, Huang Y, Andrikopoulou M, et al. Clindamycin, gentamicin, and risk of clostridium difficile infection and acute kidney injury during delivery hospitalizations. Obstet Gynecol. 2020;135(1):59–67. doi:10.1097/AOG.0000000000003568
23. National Institute for Health and Care Excellence. Clostridium difficile infection: risk with broad-spectrum antibiotics. NICE Guidel. 2015;3(March):1–30.
24. Seifu A. Bioprospecting Potential of Ajuga Integrifolia for Access and Benefit Sharing. Vol. 2017. Rome, Italy: FAO; 2017.
25. Freshet A, Daniel S, Eyasu M. Antihyperglycemic and antihyperlipidemic activities of ethanol extract of Ajuga remota Benth (Harmegusa) leaves in streptozotocin induced diabetic rats. Afr J Pharm Pharmacol. 2017;11(2):17–24. doi:10.5897/AJPP2014.4699
26. Cocquyt K, Cos P, Herdewijn P, et al. Ajuga remota Benth.: from ethnopharmacology to phytomedical perspective in the treatment of malaria. Phytomedicine. 2011;18(14):1229–1237. doi:10.1016/j.phymed.2011.08.063
27. Kuria KAM, De Coster S, Muriuki G, et al. Antimalarial activity of Ajuga remota Benth (Labiatae) and Caesalpinia volkensii Harms (Caesalpiniaceae): in vitro confirmation of ethnopharmacological use. J Ethnopharmacol. 2001;74(2):141–148. doi:10.1016/S0378-8741(00)00367-6
28. Yacob T, Shibeshi W, Nedi T. Antidiarrheal activity of 80 % methanol extract of the aerial part of Ajuga remota Benth (Lamiaceae) in mice. BMC Complement Altern Med. 2016;16(1):1–8. doi:10.1186/s12906-016-1277-8
29. Kuria KAM, Chepkwony H, Govaerts C, et al. The antiplasmodial activity of isolates from Ajuga remota. J Nat Prod. 2002;65(5):789–793. doi:10.1021/np0104626
30. Tafesse TB, Hymete A, Mekonnen Y, Tadesse M. Antidiabetic activity and phytochemical screening of extracts of the leaves of Ajuga remota Benth on alloxan-induced diabetic mice. BMC Complement Altern Med. 2017;17(1). doi:10.1186/s12906-017-1757-5
31. Ajuga L, Lamiaceae AL, Toiu A, et al. Comparative phytochemical profile, antioxidant, antimicrobial and in Vivo anti-inflammatory activity of different Extracts of traditionally used Romanian Ajuga genevensis L. and A. reptans L. (Lamiaceae). Molecules. 2019;24:1–21.
32. Giday M, Asfaw Z, Woldu Z. Medicinal plants of the Meinit ethnic group of Ethiopia: an ethnobotanical study. J Ethnopharmacol. 2009;124(3):513–521. doi:10.1016/j.jep.2009.05.009
33. Nardos A, Makonnen E. In vivo antiplasmodial activity and toxicological assessment of hydroethanolic crude extract of Ajuga remota. Malar J. 2017;16(1):1–8. doi:10.1186/s12936-017-1677-3
34. Ragunathan M, Abay SM. Ethnomedicinal survey of folk drugs used in Bahirdar Zuria district, Northwestern Ethiopia. Indian J Tradit Knowl. 2009;8(2):281–284.
35. Hamden K, Ayadi F, Jamoussi K, Masmoudi H, Elfeki A. Therapeutic effect of phytoecdysteroids rich extract from Ajuga iva on alloxan induced diabetic rats liver, kidney and pancreas. BioFactors. 2008;33(3):165–175. doi:10.1002/biof.5520330302
36. Subhashini SVSNJP. Pharmacognostic analysis and phytochemical screening of methanolic extract of ipomoea batata leaves; 2017:2016–2018.
37. Banu KS, Cathrine L. General techniques involved in phytochemical analysis. Int J Adv Res Chem Sci. 2015;2(4):25–32.
38. Ezeonu CS, Ejikeme CM. Qualitative and quantitative determination of phytochemical contents of indigenous Nigerian softwoods. New J Sci. 2016;2016:1–9. doi:10.1155/2016/5601327
39. Khalid S, Shahzad A, Basharat N, Abubakar M, Anwar P. Phytochemical screening and analysis of selected medicinal plants in Gujrat. J Phytochem Biochem. 2018;2(1):2–4.
40. Linn T, Convolvulaceae HF. Standardization of aerial parts of merremia; 2013:99–105.
41. Auwal MS, Saka S, Mairiga IA, Sanda KA, Shuaibu A, Ibrahim A. Preliminary phytochemical and elemental analysis of aqueous and fractionated pod extracts of Acacia nilotica (Thorn mimosa). Vet Res Forum. 2014;5(2):95–100.
42. Latifat AT, Teli PK, Tiwari S, Gupta VK. Quantitative and qualitative analysis of Eucalyptus grandis, Moringa oleifera Leaf, Punica granatum Pericarp and Syzygium aromaticum Dried Bud. J Emerg Technol Innov Res. 2018;5(8):551–560.
43. Gul R, Jan SU, Faridullah S, Sherani S, Jahan N. Preliminary Phytochemical screening, quantitative analysis of alkaloids, and antioxidant activity of crude plant extracts from Ephedra intermedia indigenous to Balochistan. Sci World J. 2017;2017:1–7. doi:10.1155/2017/5873648
44. Fitokimia P, Asai J, Flavonoid K, Ekstrak P, Tepal P. Phytochemical screening, total flavonoid and phenolic content assays of various solvent extracts of tepal of Musa paradisiaca. Malays J Anal Sci. 2016;20(5):1181–1190.
45. Benkova M, Soukup O, Marek J. Antimicrobial susceptibility testing: currently used methods and devices and the near future in clinical practice. J Appl Microbiol. 2020;129(4):806–822.
46. Zohra FT, Program B, Sciences N. Extraction of secondary metabolites, phytochemical screening and the analysis of antibacterial activity in Stevia rebaudiana; 2015.
47. National Research Council (US) Institute for Laboratory Animal Research. Guidance for the description of animal research in scientific publications. ILAR J. 2014;55(3):536–540. doi:10.1093/ilar/ilu070
48. Festing MFW, Altman DG. Guidelines for the design and statistical analysis of experiments using laboratory animals. ILAR J. 2002;43(4):244–257. doi:10.1093/ilar.43.4.244
49. Canadian Council on Animal Care. CCAC Guidelines: Mice. Canadian Council on Animal Welfare; 2019:1–127.
50. Document D, Of P, Test THE, On I. October 2001 OECD guideline for testing of chemicals; 2001:1–11.
51. Chinedu E, Arome D, Ameh FS. A new method for determining acute toxicity in animal models. Toxicol Int. 2013;20(3):224–226. doi:10.4103/0971-6580.121674
52. Huang H, Jin WW, Huang M, et al. Gentamicin-induced acute kidney injury in an animal model involves programmed necrosis of the collecting duct. J Am Soc Nephrol. 2020;31(9):2097–2115. doi:10.1681/ASN.2019020204
53. Ali BH. Effect of dimethyl sulfoxide on gentamicin-induced nephrotoxicity in rats. Hum Exp Toxicol. 2001;20(4):199–203. doi:10.1191/096032701678766859
54. Kitchens JL. The effects of blood storage time on the accuracy of comprehensive metabolic panel results. Maryv Coll. 2006;2006:1.
55. Stevens M, Oltean S. Assessment of kidney function in mouse models of glomerular disease. J Vis Exp. 2018;2018(136):1–10.
56. Shepherd C. Guidelines for collection of blood from laboratory animals; 2017:1–5.
57. Morales AI, Buitrago JM, Santiago JM, Fernández-Tagarro M, López-Novoa JM, Pérez-Barriocanal F. Protective effect of trans-resveratrol on gentamicin-induced nephrotoxicity. Antioxid Redox Signal. 2002;4(6):893–898. doi:10.1089/152308602762197434
58. Lau SK. Basic and advanced laboratory techniques in histopathology and cytology. J Histotechnol. 2019;42:52. doi:10.1080/01478885.2019.1559501
59. Slaoui M, Fiette L. Histopathology procedures: from tissue sampling to histopathological evaluation. Methods Mol Biol. 2011;691(January):69–82.
60. Perazella MA. Pharmacology behind common drug nephrotoxicities. Clin J Am Soc Nephrol. 2018;13(12):1897–1908. doi:10.2215/CJN.00150118
61. Tam VH, Kabbara S, Vo G, Schilling AN, Coyle EA. Comparative pharmacodynamics of gentamicin against Staphylococcus aureus and Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2006;50(8):2626–2631. doi:10.1128/AAC.01165-05
62. Abouzed TK, Sherif EAE, Barakat MES, et al. Assessment of gentamicin and cisplatin-induced kidney damage mediated via necrotic and apoptosis genes in albino rats. BMC Vet Res. 2021;17(1):1–9. doi:10.1186/s12917-021-03023-4
63. Kedir WM, Dubiwak AD, Ahmed ET. Nephroprotective effect of Asparagus africanus Lam. Root Extract against Gentamicin-Induced Nephrotoxicity in Swiss Albino Mice. J Toxicol. 2022;2022(4):8.
64. Karahan I, Ateşşahin A, Yilmaz S, Çeribaşi AO, Sakin F. Protective effect of lycopene on gentamicin-induced oxidative stress and nephrotoxicity in rats. Toxicology. 2005;215(3):198–204. doi:10.1016/j.tox.2005.07.007
65. Jeyanthi T, Subramanian P. Nephroprotective effect of withania somnifera: a dose-dependent study. Ren Fail. 2009;31(9):814–821. doi:10.3109/08860220903150320
66. Sadidi M, Bakhtiyari M, Alirezaei A. Effects of the Portulaca oleracea extract on gentamicin-induced nephrotoxicity in male rats. J Nuts. 2018;9(2):92–100.
67. Ansar S, Hamed S, AlGhosoon HT, AlSaedan RA, Iqbal M. The protective effect of rutin against renal toxicity induced by lead acetate. Toxin Rev. 2016;35(1–2):58–62. doi:10.3109/15569543.2016.1155623
68. Zhu W, Pang M, Dong L, Huang X, Wang S, Zhou L. Anti-inflammatory and immunomodulatory effects of iridoid glycosides from Paederia scandens (LOUR.) MERRILL (Rubiaceae) on uric acid nephropathy rats. Life Sci. 2012;91(11–12):369–376. doi:10.1016/j.lfs.2012.08.013
69. Bouhrim M, Bencheikh N, Imtara H, et al. Protective effect of Opuntia dillenii (Ker Gawl.) Haw. Seed Oil on gentamicin-induced nephrotoxicity: a biochemical and histological analysis. Sci World J. 2021;2021(2):1–45. doi:10.1155/2021/2173012
70. Raghavan V, Weisz OA. Discerning the role of mechanosensors in regulating proximal tubule function. Am J Physiol Ren Physiol. 2015;310(1):F1–F5. doi:10.1152/ajprenal.00373.2015
71. Rhee WJ, Lee SY, Lee JH, et al. The effect of high concentration of magnesium with ropivacaine, gentamicin, rocuronium, and their combination on neuromuscular blockade. Korean J Anesthesiol. 2015;3(1):12–56.
72. Kidney Health Australia. Fact sheet calcium and phosphate balance with kidney disease; 2017. Available from: www.kidney.org.au.
73. Ali BH, Gayoum AAA, Bashir AA. Gentamicin nephrotoxicity in rat: some biochemical correlates. Pharmacol Toxicol. 1992;70(6):419–423. doi:10.1111/j.1600-0773.1992.tb00500.x
74. Feyissa T, Asres K, Engidawork E. Renoprotective effects of the crude extract and solvent fractions of the leaves of Euclea divinorum Hierns against gentamicin-induced nephrotoxicity in rats. J Ethnopharmacol. 2013;145(3):758–766. doi:10.1016/j.jep.2012.12.006
75. Israili ZH, Lyoussi B. Ethnopharmacology of the plants of genus Ajuga. Pak J Pharm Sci. 2009;22:425–462.
76. Liu Y, Huang Y, Xiao Z, Ren X, Yang C. Guide for the care and use of laboratory animals Eighth Edition. In: Fenmo Yejin Cailiao Kexue Yu Gongcheng/Materials Science and Engineering of Powder Metallurgy. Vol. 21. national academies press; 2016:408–414.
77. Romare J. Principles and approaches in ethics assessment human subjects research; 2015:1–14.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.