Back to Journals » Clinical Ophthalmology » Volume 12
Neovascular age-related macular degeneration: intraocular inflammatory cytokines in the poor responder to ranibizumab treatment
Authors Pongsachareonnont P, Mak MYK, Hurst CP , Lam WC
Received 19 April 2018
Accepted for publication 28 June 2018
Published 26 September 2018 Volume 2018:12 Pages 1877—1885
DOI https://doi.org/10.2147/OPTH.S171636
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Pear Pongsachareonnont,1,2 Michael Ying Kit Mak,2 Cameron Paul Hurst,3 Wai-Ching Lam2,4
1Vitreo-Retinal Research Unit, Department of Ophthamology, Faculty of Medicine, Chulalongkorn University and King Chulalongkorn Memorial Hospital, Thai Red Cross Society, Bangkok, Thailand; 2Department of Ophthalmology and Vision Sciences, University of Toronto Faculty of Medicine, Toronto, ON, Canada; 3Biostatistics Center, Department of Research Affairs, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand; 4Department of Ophthalmology, University of Hong Kong, Hong Kong, China
Purpose: To determine the levels of interleukin (IL)-6, vascular endothelial growth factor-A, platelet-derived growth factor, placental growth factor (PLGF), and other cytokines in the aqueous fluid of patients with neovascular age-related macular degeneration who respond poorly to ranibizumab.
Patients and methods: This is an observational, prospective study. Thirty-two eyes from 30 patients were included in the study: 11 patients who responded poorly to ranibizumab and were switched to aflibercept (AF group), 8 patients who received ranibizumab and photodynamic therapy (PDT group), and 13 patients who responded to ranibizumab (control group). Aqueous fluid samples were collected for analysis of cytokine levels at baseline and after 1, 2, and 3 months of treatment. The effect of treatment on cytokine levels was compared between the study groups and between different time points using a linear mixed-effect regression model.
Results: In the AF group, there was an increase in vascular endothelial growth factor-C, IL-7, and angiopoeitin-2 levels (P=0.01) and a decrease in intercellular adhesion molecule and IL-17 levels (P=0.01) between baseline and 3 months. After adjustment for age, sex, race, and type of lesion at baseline, the PLGF level was higher (P=0.02) and the IL-7 level was lower (P=0.04) in the ranibizumab non-responder group than in the ranibizumab responder group.
Conclusion: Switching from ranibizumab to aflibercept did not reduce intraocular levels of angiogenesis cytokines, but resulted in improvement of central subfield thickness. PLGF levels were higher in poor responders to ranibizumab. The response of lesions to medication might be related to the stage of choroidal neovascularization.
Trial registration: www.ClinicalTrial.gov (NCT02218177c).
Keywords: neovascular age-related macular degeneration, choroidal neovascularization, anti-VEGF non-responder, poor responder, ranibizumab and AMD
Introduction
Wet age-related macular degeneration (AMD) is one of the leading causes of blindness in adults and its pathogenesis is characterized by choroidal neovascularization (CNV).1,2 Ranibizumab (Lucentis®; Genentech Inc./Novartis, San Francisco, CA, USA) has been approved for the treatment of AMD since 2006. It is a recombinant, humanized, monoclonal antibody antigen-binding fragment that inhibits active forms of vascular endothelial growth factor (VEGF)-A isoform and was shown to improve mean visual acuity (VA) in eyes with neovascular AMD in the MARINA and ANCHOR studies.3–5 Since then, ranibizumab has become one of the current standards for treatment of CNV secondary to AMD.
Intraocular growth factors and cytokines, such as VEGF, pigment epithelium-derived factor, and interleukin (IL)-6, have been found to play an important role in the development of CNV. VEGF and pigment epithelium-derived factor have been reported to be increased in patients with CNV secondary to AMD; reduction of these cytokines is correlated with anatomic improvement in the macula.6–8 IL-6 is a proinflammatory cytokine that was found to be increased in the serum and aqueous humor of patients with neovascular AMD.9 Placental growth factor (PLGF) is a member of the VEGF family, which has been found to be induced during the early stages of angiogenesis in vivo and in vitro. Aflibercept (VEGF Trap; Bayer AG, Leverkusen, Germany; Regeneron, Tarrytown, NY, USA) is a recombinant soluble VEGF receptor protein that has a high affinity for all VEGF isoforms and for PLGF.10 The VIEW 1 and VIEW 2 studies showed non-inferiority and clinical equivalence of aflibercept to monthly ranibizumab in the treatment of wet AMD,11 with an average of five fewer injections at 96 weeks of treatment.12 Aflibercept has been reported to be a treatment option for patients who are refractory to ranibizumab and bevacizumab.13–16
Historically, photodynamic therapy (PDT) with verteporfin (Visudyne; Novartis International AG, Basel, Switzerland) was the standard treatment for neovascular AMD before the approval of anti-VEGF.17 The VIP study reported that PDT significantly reduced the risk of moderate and severe visual loss in classic CNV and recommended PDT for patients with AMD and subfoveal lesions.18 Combination therapy of PDT and ranibizumab improved VA, reduced central retinal thickness, and decreased CNV leakage on fluorescein angiography.19–21 This combination treatment has also been used as a rescue therapy in patients with AMD who fail anti-VEGF monotherapy.22
Approximately 1%–10% of patients with CNV secondary to AMD have a poor response to ranibizumab.23,24 The mechanisms of resistance to intravitreal ranibizumab can be categorized as non-response, development of tolerance, or tachyphylaxis. Poor response to treatment with intravitreal ranibizumab can also stem from misdiagnosis of the disease or its complications, such as tears of the retinal pigment epithelium, retinal detachment, and macular scarring. Changes in the choroidal neovascular membrane, such as increased fibrosis, changes in the type of CNV lesion, chronic changes in vessel permeability, and changes in the photoreceptors and retinal pigment epithelium are possible mechanisms for a reduced drug response in AMD.24 Moreover, increased expression of VEGF because of increased number of macrophages in CNV25 or change in signal transduction, such as polymorphism of the VEGF receptor (VEGF2/KDR) gene, has been reported to influence the response of neovascular AMD to treatment with ranibizumab.26
The objective of this study was to determine the levels of IL-6, VEGF, PLGF, and other cytokines in the aqueous humor of patients with AMD who are resistant or poor responders to treatment with ranibizumab.
Patients and methods
This prospective study was conducted at the Toronto Western Hospital, University Health Network, Toronto, ON, Canada, according to the tenets of the Declaration of Helsinki. The study protocol was approved by the University Health Network Research Ethics Board (approval number 13-6849-AE). The study was also registered on www.ClinicalTrial.gov (NCT02218177c). Written informed consent was obtained from all patients who participated in the study.
Inclusion/exclusion criteria
Patients were included in the study if they had a diagnosis of neovascular AMD, were over the age of 50 years, had previously been treated with ranibizumab, and could be classified as responders or poor responders.
Poor responders were defined as patients who received ranibizumab and had one or more of the following clinical features after at least six consecutive monthly 0.05 mg/0.05 mL ranibizumab injections:
- Increased central subfield thickness (CST) or persistent intraretinal/subretinal fluid documented by spectral-domain optical coherence tomography (OCT)
- Evidence of persistent leakage on fluorescein angiography
- Worsening of best-corrected VA of more than 5 Early Treatment Diabetic Retinopathy Study letters.
Responders were defined as patients who were treated with monthly ranibizumab with at least three consecutive 0.05 mg/0.05 mL ranibizumab injections and had the following:
- An increase or maintenance of VA during follow-up; evidence of improvement in CST or a reduction in intraretinal/subretinal fluid on spectral-domain OCT
- No improvement in VA, but otherwise remaining stable or losing not more than 5 Early Treatment Diabetic Retinopathy Study letters with evidence of improvement in CST or a reduction in intraretinal/subretinal fluid on spectral-domain OCT
- Improved leakage on fluorescein angiography.
Patients were excluded if they were uncooperative with intravitreal treatment, had received PDT treatment in the previous 6 months, had clinically active ocular inflammation, had received ocular treatment with an immunosuppressive agent within the previous 3 months, or were currently receiving systemic anti-VEGF medication or immunosuppressive agents.
Study groups
There were three treatment groups in the study. The first group consisted of poor responders to ranibizumab who switched to a combination of ranibizumab and PDT (PDT group; Supplementary material). An aqueous tap was performed before injection of dexamethasone and before the second, third, and fourth ranibizumab injections. The second group consisted of non-responders to ranibizumab who switched to monthly intravitreal aflibercept (AF group). Aqueous samples were collected by anterior chamber paracentesis before each injection of aflibercept. The third group consisted of controls who were classified as ranibizumab responder. The responder group was treated with a treat-and-extend protocol. Samples of aqueous humor were taken before each intravitreal ranibizumab injection at the baseline visit (after enrollment) and at visits 1, 2, and 3 months later. The aqueous sampling methods are shown in Supplementary material.
Cytokine assays
The analysis was done at the Princess Margaret Genomics Center, Toronto, ON, Canada. Inflammatory cytokines were analyzed using Luminex xMAP technology (Bio-Rad Laboratories Inc., Hercules, CA, USA) with Milliplex® MAP Human cytokine/Angiogenesis/Growth factor and a Cardiovascular Magnetic Bead Panel Immunology Multiplex assay (EMD Millipore, Billerica, MA, USA). The cytokines analyzed included IL-1A, IL-1B, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL12p40, IL12p70, IL-13, IL-17A, platelet-derived growth factor-AA, tumor necrosis factor alpha (TNF-α), TNF-β, monocyte chemoattractant protein (MCP)-1, MCP-3, intercellular adhesion molecule (ICAM)-1, PLGF, VEGF-A, VEGF-C, VEGF-D, angiopoeitin-2, bone morphogenetic protein-9, epidermal growth factor (EGF), endothelin, fibroblast growth factor (FGF)-1, FGF-2, follistatin, granulocyte colony-stimulating factor, heparin-binding EGF-like growth factor, hepatocyte growth factor (HGF), and leptin.
Estimation of limits of detection
Several of the cytokines had lower limits of detection (LODs). A literature search identified a number of simple methods that involve replacing the missing LOD data with constants, such as structural zeros (cytokine absent) or LOD/2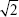 . However, imputation of values exceeding the LODs (in our case, lower than the lower LOD) is likely to result in artificially reduced SDs and increase the chance of type one error. Therefore, we developed an approach to estimate the values censored by the LODs that uses gamma distributions, the parameters (shape and scale) of which were estimated based on data at hand27 (values above the lower LOD), and then using Monte Carlo simulation28 to sample values that were lower than the lower LOD. Figure 1 illustrates this process for the VEGF-C cytokine. The gray region on the histogram of VEGF-C represents values below the LOD. By sampling the gamma distribution in this range, a more realistic sample of VEGF-C values below the LOD can be obtained.
. However, imputation of values exceeding the LODs (in our case, lower than the lower LOD) is likely to result in artificially reduced SDs and increase the chance of type one error. Therefore, we developed an approach to estimate the values censored by the LODs that uses gamma distributions, the parameters (shape and scale) of which were estimated based on data at hand27 (values above the lower LOD), and then using Monte Carlo simulation28 to sample values that were lower than the lower LOD. Figure 1 illustrates this process for the VEGF-C cytokine. The gray region on the histogram of VEGF-C represents values below the LOD. By sampling the gamma distribution in this range, a more realistic sample of VEGF-C values below the LOD can be obtained.
  | Figure 1 Histogram of gamma distribution range and prediction values below LOD of VEGF-C. |
Statistical analysis
The baseline data are shown as the mean and SD for continuous variables and as the count and percentage for categorical variables. Before formal modeling of each cytokine, we used propensity score adjustment of the baseline characteristics in an attempt to balance potentially confounding effects between the control, PDT, and AF groups. Given that there were three study groups, we could not use a standard approach for propensity score adjustment and instead used the propensity score adjustment method devised by McCaffrey et al.29 This method uses boosted logistic regression to generate propensity scores across three or more groups. After obtaining propensity scores, within- and between-subject effects were modeled using linear mixed-effect regression. Finally, we investigated the potential utility of levels of the cytokines of interest to identify patients who are not responding to therapy. First, we used a random forests feature selection process to identify the best subset of cytokines for discriminating between responders and poor responders. The analysis was performed using the R statistical package (version 3.2.4, R: A language and environment for statistical computing, 2016; R Foundation for Statistical Computing, Vienna, Austria), propensity score adjustment was performed using the R library twang,30 and variable selection was performed using the R library varSelRF.31 A P-value of 0.05 was considered statistically significant.
Results
Thirty-two eyes from 30 patients were included in the study from June 2014 to October 2015. The control group, combination ranibizumab/PDT group, and AF group contained 13, 8, and 11 eyes, respectively. The baseline demographic data are shown in Table 1. One Asian patient in the AF group was diagnosed with polypoidal choroidal vasculopathy that was confirmed on indocyanine green angiography.
  | Table 1 Baseline demographic data |
Thirty-seven cytokines was analyzed, of which only PLGF, VEGF-A, VEGF-C, angiopoeitin-2, EGF, endothelin-1, follistatin, granulocyte colony-stimulating factor, heparin-binding EGF-like growth factor, HGF, leptin, ICAM-1, IL-3, IL-6, IL-7, IL-8, IL-17, MCP-1, TNF-α, and platelet-derived growth factor were included in the statistical analysis and interpretation; the other cytokines could not be interpreted because of their extremely low LOD. Mean cytokine levels at baseline are shown in Table S1.
The mean changes in aqueous cytokine levels from baseline to month 3 in the control group, PDT group, and AF group are shown in Table 2.
Clinical responses to switching treatment
The VA in each visit is shown in Table 3. In the control group, the mean improvement in VA from baseline to month 2 was 0.15 logMAR (P=0.02, 95% CI 0.01–0.29) and from baseline to month 3 was 0.14 logMAR (95% CI 0.01–0.28). The mean improvement in VA from baseline to month 3 was 0.01 logMAR (95% CI −0.24 to 0.21) and 0.03 logMAR (95% CI −0.24 to 0.17) in the PDT/ranibizumab group and aflibercept group, respectively. There was no statistically significant reduction in CST from baseline to month 3 in the control group (−2.36 μm; 95% CI −43.49 to 38.75) or in the aflibercept group (−43.4 μm, 95% CI −104.4 to 17.5). There was a significant mean change in CST from baseline to month 3 in the PDT group of −77.5 μm (95% CI −148 to −7.0). After adding PDT to ranibizumab, six of eight patients (75%) in the PDT group showed stabilization or improvement in AMD activity, while two patients showed persistence of subretinal fluid and a reduction in VA from baseline to month 3 (0.45 and 0.16 logMAR), but no new hemorrhage or signs of new choroidal neovascular membrane (CNVM). In the aflibercept group, eight of eleven patients (72%) showed stabilization and improvement of VA and anatomic outcomes from baseline to month 3. Three patients showed a reduction in VA (0.12, 0.16, and 0.1 logMAR), but a nonsignificant reduction of CST of 269 μm at month 2 (no OCT data were available at month 3) and 53 and 0.9 μm at month 3. None of these patients showed an increase in CST. One patient showed a marked reduction in CST (–266 μm) with improvement of VA of 0.3 logMAR after three injections.
  | Table 3 Mean visual acuity of patient in each study group |
Responders vs poor responders
After adjustment for age, sex, race, and type of lesion at the baseline visit, the PLGF level was higher in the non-responder group than in the responder group, with a mean difference of 474.30 pg/dL (95% CI 54.11–894.4, P=0.02). In contrast, the IL-7 level was lower in the non-responder group, with a mean difference of 0.8 pg/dL (95% CI -1.58 to −0.03, P=0.04). There was no statistically significant difference in the levels of the other cytokines between the responder group and the non-responder group.
Discussion
In this study, we found that levels of PLGF were significantly higher in patients who responded poorly to ranibizumab than in those who responded to ranibizumab. PLGF levels were also lower than the levels in the group that received PDT and AF. PLGF, flt-1, and the VEGFR-1 receptor have been found to be expressed on the human neovascular membrane. PLGF mRNA expression is also upregulated during development of CNV.32 Neutralization of the PLGF receptor can reduce the formation of CNV.32 Ranibizumab is an affinity-matured antigen-binding fragment (Fab) with high affinity for VEGF-A and neutralizes all human VEGF-A isoforms, except for PLGF.33,34 Our finding of an increase in the PLGF level in patients who did not respond to ranibizumab monotherapy is consistent with the evidence of multiple cytokines being involved in the development of CNV.
The current study also found that the levels of VEGF-A and PLGF, which play an important role in angiogenesis, were reduced after switching treatment to a combination of PDT and AF, albeit not significantly so. The present findings are consistent with the previous report of an additional effect of anti-PLGF in the reduction of subfoveal thickness on OCT and fluid activity after switching treatment from ranibizumab/bevacizumab to aflibercept in persistent fluid of exudative AMD.13,14 However, similar to other studies, the VA did not improve after switching treatment. In this study, there was a reduction of CST in the AF group, but this was not statistically significant. There was also no significant change in VA in three patients in the AF group, despite anatomic improvement; their VA was reduced. According to the Macular Photocoagulation Study Group, repeat measurement of VA in patients with AMD and poor VA (<20/100) has low reliability when compared with that in patients with better VA. The reduction in VA despite anatomic improvement in this study could reflect the large variation in VA measurements in poor vision patient.35 The chronicity of CNV activity has also been reported to result in a poor gain in VA despite successful anatomic treatment after switching from ranibizumab to aflibercept in refractory cases.36,37 Funk et al studied the change in aqueous VEGF of ranibizumab in AMD from baseline to 12 months follow-up; the level of VEGF was markedly reduced after the first treatment injection and was below the level of detection after repeated treatment.7 In our study, the control group was treated with ranibizumab and demonstrated favorable response. The lower levels of VEGF and lower than LODs might also mask the change in aqueous VEGF in the control group.
In our study, IL-7 level was lower in the poor responder group. IL-7 stimulates the proliferation of B-cell progenitors, mature T-cells, and thymocytes via the high-affinity hematopoietin receptor (IL-7R).38 IL-7 also has a role in the enhancement of homeostatic proliferation and prolonged survival of naïve T-cells,39 and inhibition of IL-7 is used in the treatment of patients with lymphopenia.40 IL-7 was found to support both T-cells and B-cells in exacerbating the non-T/non-B-cell–mediated chronic inflammatory response.41 Upregulation of ICAM-1 can also lead to development of CNV.42 The presence of surface expression levels of ICAM-1 is associated with increased levels of IL-1 and IL-7.43 Fauser et al studied cytokine levels in the aqueous humor of eyes with naïve AMD that underwent cataract surgery or treatment with ranibizumab and reported elevation of MCP-1 and VCAM in the patients with neovascular AMD when compared with controls.44 IL-8 and MCP-1 have been found to promote angiogenesis via accumulation of macrophages that release angiogenic promoters and degrade Bruch’s membrane.45 Grossniklaus et al studied surgically excised subfoveal CNV specimens from an eye bank and found that VEGF tissue factor and MCP-1 expression levels were higher in specimens with “inflammatory active” CNV than in those with fibrotic “inflammatory inactive” CNV. The MCP-1 and VEGF are high during the active stage of CNV and minimal in fibrotic matured CNV.25 The reduction in IL-7 in our non-responder group might reflect a fibrotic stage of CNV. In our study, the significant reduction of ICAM-1 in the AF group likely reflected the decreased activity of CNV after switching medication.
In this study, we found a significant decrease in leptin and IL-6 levels after PDT. This can be explained by the regression of CNV that occurs in response to PDT.46,47 Leptin has been found to regulate the tyrosine kinase-dependent intracellular pathway and to stimulate angiogenesis via the leptin receptor (Ob-R).48,49 Immunohistochemical analysis of CNV membrane tissue revealed leptin staining and absence of leptin staining in normal posterior segment tissue.50 IL-6 is a pleiotropic cytokine involved in the acute inflammatory phase of wound healing, fibrogenesis, angiogenesis, and is associated with CNV activity.51–53 Anti-VEGF monotherapy limits leakage and inhibits vessel growth without complete and sustained resolution of CNV. In the Focus54 and CLOVER55 studies, the combination of therapy of ranibizumab and PDT improved VA, reduced angiogenesis, and extended the fluid-free interval. In our study, we also showed adding PDT to treatment in non-responders helped in the regression of CNV and in downregulation of the cytokines involved in angiogenesis.
In our study, VEGF-C, angiopoeitin-2, and HGF levels were elevated in the AF group. The angiopoeitin-2 in the CNV membrane stimulates the initial angiogenic response of blood vessels. Angiopoeitin-1 and angiopoeitin-2 were found to be promoters of the VEGF gene and to regulate expression of VEGF through the MAPK pathway. Hera et al reported that in 18 CNV membranes secondary from AMD showed low expression of VEGF-A and that expression of this cytokine correlated with the activity of CNV. HGF has been reported to be increased in retinopathy of prematurity56 and retinal detachment,57 and is induced by FGF-2.58 The possible role of HGF in angiogenesis via expression of urokinase has been studied in a mouse model.58 HGF has been identified as an angiogenic factor found in corneal wound healing, tear, aqueous humor, and vitreous humor and found to increase in proliferative diabetic retinopathy.59 Therefore, the elevation of HGF levels from baseline in the AF group in our study was likely related to the persistent activity of the CNV.
One strength of our study is its prospective design, whereby aqueous humor was collected at the time of the intraocular injections. Analysis of the results for cytokines involved in angiogenesis and inflammation will aid our understanding of the pathogenesis of neovascular AMD in patients who respond poorly to treatment. The finding of elevated angiopoeitin-2 levels in non-responders in our AF group suggests a potential benefit from using anti-angiopoietin medication in current clinical studies. IL-17 might be important in the pharmacologic treatment of neovascular AMD.
There are some limitations to this study, including the cytokine analysis being performed in patients who had previously received treatment, which may have altered the baseline cytokine profile. In addition, this study had a relatively small sample size, so we were unable to test the external validity of our prediction model of response to therapy based on cytokine levels. The follow-up visit in ranibizumab responders utilizes a treat-and-extend protocol which might influence the cytokine activity.
The results of this study will aid in our understanding of the role of cytokines in the response of patients to treatment with ranibizumab. In the future, larger studies examining changes in cytokine levels may help to clarify whether some cytokines can be biomarkers that identify poor responders and guide the selection of treatment for these patients.
Data sharing statement
The datasets generated and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
Acknowledgments
This study was funded by the Global Ophthalmology Award Program (Bayer Pharmaceuticals Inc.). The funding organization did not have any role in the design or conduct of this research.
This study was presented as a poster at the ARVO 2017 annual meeting, Baltimore, USA.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106–e116. | ||
Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96(5):614–618. | ||
Rofagha S, Bhisitkul RB, Boyer DS, et al. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP). Ophthalmology. 2013;120(11):2292–2299. | ||
Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. | ||
Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432–1444. | ||
Muether PS, Hermann MM, Viebahn U, Kirchhof B, Fauser S. Vascular endothelial growth factor in patients with exudative age-related macular degeneration treated with ranibizumab. Ophthalmology. 2012;119(10):2082–2086. | ||
Funk M, Karl D, Georgopoulos M, et al. Neovascular age-related macular degeneration: intraocular cytokines and growth factors and the influence of therapy with ranibizumab. Ophthalmology. 2009;116(12):2393–2399. | ||
Ahn JK, Moon HJ. Changes in aqueous vascular endothelial growth factor and pigment epithelium-derived factor after ranibizumab alone or combined with verteporfin for exudative age-related macular degeneration. Am J Ophthalmol. 2009;148(5):e711:718–724. | ||
Izumi-Nagai K, Nagai N, Ozawa Y, et al. Interleukin-6 receptor-mediated activation of signal transducer and activator of transcription-3 (STAT3) promotes choroidal neovascularization. Am J Pathol. 2007;170(6):2149–2158. | ||
Holash J, Davis S, Papadopoulos N, et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A. 2002;99(17):11393–11398. | ||
Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537–2548. | ||
Schmidt-Erfurth U, Kaiser PK, Korobelnik JF, et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology. 2014;121(1):193–201. | ||
Cho H, Shah CP, Weber M, Heier JS. Aflibercept for exudative AMD with persistent fluid on ranibizumab and/or bevacizumab. Br J Ophthalmol. 2013;97(8):1032–1035. | ||
Kumar N, Marsiglia M, Mrejen S, et al. Visual and anatomical outcomes of intravitreal aflibercept in eyes with persistent subfoveal fluid despite previous treatments with ranibizumab in patients with neovascular age-related macular degeneration. Retina. 2013;33(8):1605–1612. | ||
Bakall B, Folk JC, Boldt HC, et al. Aflibercept therapy for exudative age-related macular degeneration resistant to bevacizumab and ranibizumab. Am J Ophthalmol. 2013;156(1):15–22. | ||
Wykoff CC, Brown DM, Maldonado ME, Croft DE. Aflibercept treatment for patients with exudative age-related macular degeneration who were incomplete responders to multiple ranibizumab injections (TURF trial). Br J Ophthalmol. 2014;98(7):951–955. | ||
Miller JW, Schmidt-Erfurth U, Sickenberg M, et al. Photodynamic therapy with verteporfin for choroidal neovascularization caused by age-related macular degeneration: results of a single treatment in a Phase 1 and 2 study. Arch Ophthalmol. 1999;117(9):1161–1173. | ||
Verteporfin in Photodynamic Therapy Study Group. Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization – verteporfin in photodynamic therapy report 2. Am J Ophthalmol. 2001;131(5):541–560. | ||
Wolf-Schnurrbusch UE, Brinkmann CK, Berger L, Wolf S. Effects of combination therapy with verteporfin photodynamic therapy and ranibizumab in patients with age-related macular degeneration. Acta Ophthalmol. 2011;89(6):585–590. | ||
Spielberg L, Leys A. Treatment of neovascular age-related macular degeneration with a variable ranibizumab dosing regimen and one-time reduced-fluence photodynamic therapy: the TORPEDO trial at 2 years. Graefes Arch Clin Exp Ophthalmol. 2010;248(7):943–956. | ||
Schmidt-Erfurth U, Wolf S; PROTECT Study Group. Same-day administration of verteporfin and ranibizumab 0.5 mg in patients with choroidal neovascularisation due to age-related macular degeneration. Br J Ophthalmol. 2008;92(12):1628–1635. | ||
Tozer KR, Chong L, Sadda SR. Rescue Therapy With Combined Anti-vegf And Pdt For Refractory AMD. Investigative Ophthalmology & Visual Science. 2011;52(14):1671. | ||
Eghøj MS, Sørensen TL. Tachyphylaxis during treatment of exudative age-related macular degeneration with ranibizumab. Br J Ophthalmol. 2012;96(1):21–23. | ||
Binder S. Loss of reactivity in intravitreal anti-VEGF therapy: tachyphylaxis or tolerance? 2012:1–2. | ||
Grossniklaus HE, Ling JX, Wallace TM, et al. Macrophage and retinal pigment epithelium expression of angiogenic cytokines in choroidal neovascularization. Mol Vis. 2002;8:119–126. | ||
Hermann MM, van Asten F, Muether PS, et al. Polymorphisms in vascular endothelial growth factor receptor 2 are associated with better response rates to ranibizumab treatment in age-related macular degeneration. Ophthalmology. 2014;121(4):905–910. | ||
Cox NJ, Warburton J, Armstrong A, Holliday VJ, et al. Fitting concentration and load rating curves with generalized linear models. Earth Surf Process Landf. 2008;33(1):25–39. | ||
Robert CP. Monte Carlo Methods. Wiley Online Library; 2004. | ||
Mccaffrey DF, Ridgeway G, Morral AR. Propensity score estimation with boosted regression for evaluating causal effects in observational studies. Psychol Methods. 2004;9(4):403–425. | ||
Burgette L, Griffin BA, Mccaffrey D. Propensity scores for multiple treatments: a tutorial for the mnps function in the twang package. R Package Rand Corporation; 2016. | ||
Díaz-Uriarte R, Alvarez de Andrés S. Gene selection and classification of microarray data using random forest. BMC Bioinformatics. 2006;7:3. | ||
Rakic JM, Lambert V, Devy L, et al. Placental growth factor, a member of the VEGF family, contributes to the development of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44(7):3186–3193. | ||
Ferrara N, Damico L, Shams N, Lowman H, Kim R. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina. 2006;26(8):859–870. | ||
Chen Y, Wiesmann C, Fuh G, et al. Selection and analysis of an optimized anti-VEGF antibody: crystal structure of an affinity-matured Fab in complex with antigen. J Mol Biol. 1999;293(4):865–881. | ||
Blackhurst DW, Maguire MG. Reproducibility of refraction and visual acuity measurement under a standard protocol. The Macular Photocoagulation Study Group. Retina. 1989;9(3):163–169. | ||
Muether PS, Hoerster R, Hermann MM, Kirchhof B, Fauser S. Long-term effects of ranibizumab treatment delay in neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2013;251(2):453–458. | ||
de Massougnes S, Dirani A, Ambresin A, et al. Pigment epithelial detachment response to aflibercept in neovascular age-related macular degeneration refractory to ranibizumab: time course and drug effects. Retina. 2016;36(5):881–888. | ||
Peschon JJ, Morrissey PJ, Grabstein KH, et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med. 1994;180(5):1955–1960. | ||
Tan JT, Dudl E, LeRoy E, et al. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci U S A. 2001;98(15):8732–8737. | ||
Rosenberg SA, Sportès C, Ahmadzadeh M, et al. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. J Immunother. 2006;29(3):313–319. | ||
von Freeden-Jeffry U, Davidson N, Wiler R, Fort M, Burdach S, Murray R. IL-7 deficiency prevents development of a non-T cell non-B cell-mediated colitis. J Immunol. 1998;161(10):5673–5680. | ||
Sakurai E, Taguchi H, Anand A, et al. Targeted disruption of the CD18 or ICAM-1 gene inhibits choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44(6):2743–2749. | ||
Dennig D, Lacerda J, Yan Y, Gasparetto C, O’Reilly RJ. ICAM-1 (CD54) expression on B lymphocytes is associated with their costimulatory function and can be increased by coactivation with IL-1 and IL-7. Cell Immunol. 1994;156(2):414–423. | ||
Fauser S, Viebahn U, Muether PS. Intraocular and systemic inflammation-related cytokines during one year of ranibizumab treatment for neovascular age-related macular degeneration. Acta Ophthalmol. 2015;93(8):734–738. | ||
Higgins GT, Wang JH, Dockery P, Cleary PE, Redmond HP. Induction of angiogenic cytokine expression in cultured RPE by ingestion of oxidized photoreceptor outer segments. Invest Ophthalmol Vis Sci. 2003;44(4):1775–1782. | ||
Schlötzer-Schrehardt U, Viestenz A, Naumann GO, Laqua H, Michels S, Schmidt-Erfurth U. Dose-related structural effects of photodynamic therapy on choroidal and retinal structures of human eyes. Graefes Arch Clin Exp Ophthalmol. 2002;240(9):748–757. | ||
Bressler NM; Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: two-year results of 2 randomized clinical trials-tap report 2. Arch Ophthalmol. 2001;119(2):198–207. | ||
Park HY, Kwon HM, Lim HJ, et al. Potential role of leptin in angiogenesis: leptin induces endothelial cell proliferation and expression of matrix metalloproteinases in vivo and in vitro. Exp Mol Med. 2001;33(2):95–102. | ||
Bouloumié A, Drexler HC, Lafontan M, Busse R. Leptin, the product of Ob gene, promotes angiogenesis. Circ Res. 1998;83(10):1059–1066. | ||
Cui JZ, Hornan D, Potter MJ, et al. The role of leptin in choroidal neovascularization. Am J Ophthalmol. 2001;132(5):792–794. | ||
Barnes TC, Anderson ME, Moots RJ. The many faces of interleukin-6: the role of IL-6 in inflammation, vasculopathy, and fibrosis in systemic sclerosis. Int J Rheumatol. 2011;2011:721608–6. | ||
Roh MI, Kim HS, Song JH, Lim JB, Koh HJ, Kwon OW. Concentration of cytokines in the aqueous humor of patients with naive, recurrent and regressed CNV associated with and after bevacizumab treatment. Retina. 2009;29(4):523–529. | ||
Izumi-Nagai K, Nagai N, Ozawa Y, et al. Interleukin-6 receptor-mediated activation of signal transducer and activator of transcription-3 (STAT3) promotes choroidal neovascularization. Am J Pathol. 2007;170(6):2149–2158. | ||
Lanzetta P, Group FS. Combination intravitreal ranibizumab 0.5 mg and verteporfin PDT versus verteporfin PDT alone in the FOCUS study of neovascular age-related macular degeneration (AMD). Invest Ophthalmol Vis Sci. 2007;48:2869. | ||
Larsen B, Davis M, Mudvari S. Combination Lucentis and Ocular Photodynamic Therapy with Visudyne, with Evaluation-Based Retreatments (CLOVER) Trial. Invest Ophthalmol Vis Sci. 2010;51:902. | ||
Lashkari K, Hirose T, Yazdany J, McMeel JW, Kazlauskas A, Rahimi N. Vascular endothelial growth factor and hepatocyte growth factor levels are differentially elevated in patients with advanced retinopathy of prematurity. Am J Pathol. 2000;156(4):1337–1344. | ||
Jin M, Chen Y, He S, Ryan SJ, Hinton DR. Hepatocyte growth factor and its role in the pathogenesis of retinal detachment. Invest Ophthalmol Vis Sci. 2004;45(1):323–329. | ||
Naldini L, Vigna E, Narsimhan RP, et al. Hepatocyte growth factor (HGF) stimulates the tyrosine kinase activity of the receptor encoded by the proto-oncogene c-MET. Oncogene. 1991;6(4):501–504. | ||
Grierson I, Heathcote L, Hiscott P, et al. Hepatocyte growth factor/scatter factor in the eye. Prog Retin Eye Res. 2000;19(6):779–802. |
Supplementary material
Photodynamic therapy procedure
Verteporfin photodynamic therapy (PDT) was administered to the leakage area in fluorescein angiography with a full fluence (50 J/cm2) and a light delivery time for 83 seconds. At ~1 hour after PDT, dexamethasone 200 μg/0.05 mL was injected intravitreally, and 1 week later, the patient received intravitreal ranibizumab 0.5 mg/0.05 mL. Subsequently, the patient received monthly injections of ranibizumab.
Aqueous sampling through anterior chamber paracentesis
Topical 0.5% tetracaine hydrochloride was applied before the procedure and a wire speculum was used. A sterile cotton tip soaked in 10% povidone iodine was applied at the sites of the aqueous tap and the intravitreal injection. A sterile unplugged 0.1 mL syringe with a 30 G needle was placed at the limbus parallel to the iris plane. Aqueous fluid (0.05 mL) was removed by passive pressure. Intravitreous injection of either ranibizumab or aflibercept was administered promptly afterward. The aqueous fluid was placed in 0.2 mL PCR tubes and stored at −80°C. All aqueous fluid samples were analyzed at the end of the study.
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


