Back to Journals » Research and Reports in Neonatology » Volume 9
Neonatal sepsis biomarkers: where are we now?
Authors Gilfillan M, Bhandari V
Received 28 November 2018
Accepted for publication 9 February 2019
Published 14 March 2019 Volume 2019:9 Pages 9—20
DOI https://doi.org/10.2147/RRN.S163082
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Robert Schelonka
Margaret Gilfillan, Vineet Bhandari
Section of Neonatal-Perinatal Medicine, St Christopher’s Hospital for Children, Department of Pediatrics, Drexel University College of Medicine, Philadelphia, PA, USA
Abstract: Given that neonatal sepsis remains an important factor contributing to mortality and morbidity in neonates, identification of accurate biomarkers to aid in its timely and accurate diagnosis is critical. In this review, we discuss the current evidence behind the use of biomarkers commonly used in clinical practice, as well as presenting recent developments in this area of research. Besides summarizing information regarding “traditional” biomarkers (eg, hematological indices, CRP, and other acute-phase reactants, cytokines), we provide the latest clinical status on some relatively “newer” biomarkers (eg, PCR- and “-omic” technology-based, biophysical biomarkers) in the diagnosis of neonatal sepsis. We believe that certain biophysical (RALIS, core–peripheral temperature differences) in combination with selective biochemical (procalcitonin, nCD64, presepsin) markers offer the best likelihood of being adopted for clinical use in the detection of neonatal sepsis in the near future. In addition, serial measurements of selective biochemical markers (procalcitonin, nCD64) offer promise in the decision to initiate and/or control the duration of antibiotic therapy. It is important to conduct adequately powered prospective multicenter studies to continue to establish the accuracy and safety of utilizing such biomarkers of neonatal sepsis so that appropriate and adequate therapy is tailored to each infant for optimal outcomes.
Keywords: infection, newborn, preterm infant, procalcitonin, neutrophil CD64
Introduction
Despite recent improvements in outcomes, neonatal sepsis (NS) remains a significant contributor to morbidity and mortality.1 Early-onset sepsis (EOS) refers to infection diagnosed in the first 72 hours of life with pathogens most likely acquired perinatally, in contrast to late-onset sepsis (LOS), which refers to infection diagnosed from day of life 4 onward, where the source is from either the community or hospital environment.2
For infants born at >34 weeks gestational age (GA), the incidence of EOS is 0.3–0.8/1,000 live births.3,4 Lower GA dramatically increases the risk for perinatal infection, as 4.8%–16.9% of preterm infants exposed to chorioamnionitis go on to develop culture-positive EOS, in contrast to only 0.47%–1.24% of similarly exposed infants born at ≥35 weeks GA.3,5 LOS has been shown to be associated with increased risk of bronchopulmonary dysplasia and neurological morbidities.6,7
The challenge of diagnosing NS is that both EOS and LOS often present with aspecific clinical signs that show significant overlap with common uninfectious conditions, such as transitional tachypnea and apnea of prematurity. While prompt aggressive treatment with antibiotics is critical, indiscriminate use of these medications is not without significant medical and financial costs. Use of broad-spectrum antibiotics has been shown to disrupt the microbiome of the neonatal gut and put the already-vulnerable preterm infant at greater risk of developing necrotizing enterocolitis (NEC)8 and invasive fungal infections.9 Treatment of the term baby with antibiotics usually requires some degree of separation from the mother during an important window for bonding,10 and recent data regarding early antibiotic use and risk of asthma,11,12 inflammatory bowel disease, and obesity13–15 strongly suggest that exposure in this age group is not without risk either.
The need for accurate biomarkers to aid in the timely and accurate diagnosis of NS thus remains as important as ever. The ideal biomarker should demonstrate a consistent and predictable pattern in both response to infection and to treatment. A high degree of sensitivity is essential to ensure that cases are not missed; however, specificity is also important to avoid exposing unaffected infants to unnecessary treatment. The perfect biomarker should also be able to give prognostic information, an indication of the identity of the pathogen, be easily measured with a technique that guarantees a fast turnaround, and if a serum sample is required, should only require a minimal volume of blood.16
There are many challenges. The normal range of the marker may be dependent on the GA or postnatal age and may also fluctuate in response to coexisting uninfectious disease processes. Although the presence of a positive blood culture has been the gold standard and has been shown to be sensitive in detecting relatively low bacterial loads,17 clinicians remain hesitant to reject the diagnosis of infection when presented with a sick infant and a negative blood-culture result.18 Variations in the definition of “culture-negative” or “clinical sepsis” between studies, in addition to differences in measurement technique, timing of sample collection, and choice of cutoff levels, also contribute to heterogeneity among studies, making it difficult to draw firm conclusions from meta-analyses.
In this review, we discuss the current evidence behind the use of biomarkers commonly used in clinical practice, as well as presenting recent developments in this area of research. This article focuses on developments that have occurred in the last 10 years, with an emphasis on the biomarkers that we consider are the most relevant to the practice of clinical neonatology.
Hematological indices
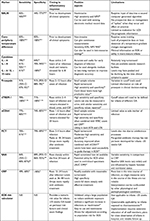
Newman et al noted that a low white blood cell (WBC) count and absolute neutrophil count (ANC) with a higher band count closely correlated with the presence of EOS.19 Diagnostic accuracy of WBC indices improved with advancing postnatal age, and was most useful after 4 hours of life. Scoring systems have been found to be more accurate when applied to preterm infants than term infants, and diagnostic accuracy found to improve with advancing postnatal age.20,21 Within the context of LOS, both high and low WBC counts, elevated ANC, elevated immature to total (I:T) neutrophil ratio >0.2, and thrombocytopenia have been noted to occur; however, none of these parameters shows a close-enough association to exclude LOS with appropriate sensitivity.22 Combining hematological indices with clinical findings and other biomarkers has been shown to improve diagnostic accuracy further, especially in cases of culture-negative sepsis.21,23,24 Table 1 shows selective studies that have used hematological indices to diagnose suspect/clinical sepsis.
Elevated red cell–distribution width (RDW) has been shown to be associated with increased mortality from sepsis in both adult and pediatric critical care populations.25,26 In the neonatal population, a normal RDW is reported to lie between 15.5% and 20%, although a value as high as 23% can be considered normal in the preterm population.27 RDW >16.35% was found to predict mortality with 70% sensitivity and 66.1% specificity in a population of 500 term infants diagnosed with EOS or LOS.28 These findings are consistent from those of another retrospective study investigating the utility of RDW as a prognostic marker in cases of both EOS and LOS,29 where RDW >14.5% was found to be associated with increased mortality (OR 1.31, 95% CI 1.241–1.399). RDW is a measurement already available on the standard complete blood count (CBC), but further work is needed to define cutoff values for infants of different chronological age and GA.
In terms of clinical utility, hematological indices are of limited efficacy in the early recognition of NS, as clinical signs or specific risk factors usually precede the decision to proceed with laboratory testing. Abnormal findings, such as neutropenia and elevated I:T ratio, may be helpful in confirming the decision to start antibiotics; however, normal values need to be viewed within the context of the clinical presentation of the patient. While reassuring CBC results can make the decision to stop antibiotics more straightforward, recent guidance from the American Academy of Pediatrics regarding the management of EOS recommended that antibiotics should be continued beyond 48 hours only in the presence of a positive blood culture or objective evidence of localized infection, rather than laboratory results.30
C-reactive protein (CRP)
CRP is an acute-phase protein, released by the liver in response to stimulation by proinflammatory cytokines. Elevation of CRP begins between 12 and 24 hours following the onset of infection, with levels peaking at 48 hours. Within the context of EOS, CRP has been shown to have a sensitivity of 9%–83%, with the majority of studies quoting values of 49%–68% when a cutoff value of 10 mg/L is used.31 Specificity values are higher, consistently reported as >90%, even though elevations of CRP can be caused by uninfectious inflammatory processes, such as meconium aspiration and prolonged transition.32 In both LOS and EOS, accuracy of CRP as a diagnostic marker improves with serial measurements.31,33 Using CRP in combination with other biomarkers, particularly those that rise early in the course of infection, such as nCD64, IL6, or IL8, has also been shown to be beneficial and can be useful in tracking response to treatment.34,35 Evidence is also available for the popular clinical practice of obtaining serial CRP values in combination with various WBC indices, such as I:T ratio, to “rule out” sepsis and limit the course of treatment.36 In summary, CRP remains a useful negative predictor of NS, and serial levels are valuable in helping clinicians monitor response to treatment. CRP as a late-rising diagnostic marker is of limited use in the diagnosis of NS and in the initial decision to start antibiotics, particularly if the result obtained is within the equivocal 5–10 mg/L range. Our recommendation is to use CRP alongside other biomarkers that rise earlier in the course of infection and to use serial measurements within the first 48 hours of a “sepsis rule out” to decide whether to continue antibiotics.
Procalcitonin
Procalcitonin (Pct) levels rise steadily 2–4 days after birth in physiological and pathophysiological states, with reference levels available for term and preterm infants.37–39 Bacterial or fungal infection results in a much more marked rise in Pct that can be detected within 6 hours of infection, peaking at 18–24 hours. Levels remain elevated for up to 48 hours, allowing for a relatively wide window for detection.40 In addition to the physiological rise following birth, a rise in Pct can also be triggered by other perinatal events, such as respiratory distress syndrome, intracranial hemorrhage, hypoxic ischemic encephalopathy, pneumothorax, and fetal distress.40 A meta-analysis that included a total of 1,959 neonates from 16 different studies evaluating the measurement of Pct prior to commencement of antibiotic therapy in both EOS and LOS demonstrated pooled sensitivity of 81% (95% CI 74%–87%) and pooled specificity of 79% (95% CI 69%–87%).41 Perhaps due to the multiple uninfectious conditions that can trigger a rise in Pct, this marker was found to perform better in the setting of LOS than in EOS, with area under the receiver-operating characteristic curve (AUC) values of 0.95 and 0.78, respectively. Another larger meta-analysis evaluating Pct in the cord blood of infants with suspected EOS suggested that earlier measurement may lead to increased diagnostic accuracy.42 Pooled sensitivity was 78% for studies investigating cord-blood Pct compared with a pooled value of 70% for studies measuring Pct at 24–48 hours. This meta-analysis quoted higher specificity of up to 89% when Pct was measured at 12 and 24 hours.43 Findings of both these meta-analyses are limited by significant heterogeneity in study design and cutoff values chosen; however, the overall trend is one of moderate–high sensitivity and specificity.
A study that investigated Pct in combination with nCD64, CRP, and WBC count showed that the combined measurement of Pct and nCD64 was the most effective, yielding an AUC of 0.922.35 Another study investigating the effect of combining Pct with IL6 or IL8 demonstrated similar sensitivity and specific values generated by Pct alone.44
Like CRP, Pct levels drop steadily in response to antibiotic therapy, and this strategy was recently tested in the setting of EOS by a large international multicenter (NeoPIns) trial.45 Pct-guided therapy was associated with a significant reduction in the duration of antibiotic therapy to 55.1 vs 65.0 hours (P<0.0001). When the per-protocol data were analyzed, this difference increased to 51.8 vs 64 hours (P<0.0001). No sepsis-related deaths were reported, and only nine (<1%) infants were suspected to have reinfection. Overall, this study provides an important potential framework for Pct-guided antibiotic stewardship within the context of EOS in a population with a low incidence of culture-proven infection. Further work is needed to evaluate how Pct-guided therapy can be applied to preterm and term neonatal populations with a higher risk profile for infection. Early results from at least one population with a higher incidence of infection suggest that this is still a promising strategy to conserve resources while maintaining patient safety.46
In summary, Pct as an intermediate- to early-rising inflammatory marker is of limited use in detection and early diagnosis of NS. Pct is not sufficiently sensitive to act as a sole indicator to initiate empiric antibiotic therapy but can be useful when combined with other biomarkers. Pct has been used to guide the duration of antibiotic therapy without adverse outcomes noted in a population with a low incidence of culture-positive EOS. It is within this area of antibiotic stewardship that Pct is likely to have the greatest utility.
Cytokines and chemokines
The proinflammatory cytokines IL6 and IL8 rise within 2–4 hours in response to detection of a pathogen, with levels peaking at 6–8 hours.47 Several studies have reported IL6 to have superior sensitivity to CRP in the detection of NS,23,48,49 with pooled sensitivity and specificity of 79%–84% noted in a meta-analysis.50 The utility of IL8 as an early marker of NS has also been evaluated in several studies, with sensitivity of 81%–92% and specificity of 70%–94% when cutoff values of 70–90 ng/mL were used.31,51 Studies that have combined the use of cytokines with later markers have demonstrated improved diagnostic accuracy when markers are combined.31,44,48
Imbalances in proinflammatory vs anti-inflammatory pathways have been investigated, with IL6:IL10 ratio >3.5 found to be more sensitive than CRP >10 mg/L (81.25% vs 62.5%), with identical specificity of 100%.49 Another study revealed NS to be associated with elevated levels of IL6, IL10, and GM-CSF, with low levels of IL4.52 This study also showed an association between increased mortality and low levels of anti-inflammatory cytokines, including IL4, IL12, and IFNγ. An earlier investigation had shown that the combination of elevated IL10 (>208 ng/L), IL6 (>168 ng/L), and decreased levels of RANTES (<3,110 ng/L) could be used to identify infected infants who went on to develop septic shock with disseminated intravascular coagulation.53
Although measurement of individual cytokines and profiling the inflammatory response has potential in the context of early diagnosis of infection, accurate measurement requires specialized equipment that is not yet available outside the research-laboratory setting. Presently, the turnaround for these tests is 1.5–6 hours, which is an unacceptable time frame for clinical decision-making.
CD64
Increased expression of CD64 can be detected within 1–6 hours of bacterial invasion, and levels remain elevated for >24 hours.54 This relatively early response to infection in combination with the ability to quantify CD64 on specimens of as little as 50 µL within an hour makes CD64 a promising target for early detection of NS. A meta-analysis investigating the use of nCD64 as a biomarker of NS that included 17 studies with 3,478 participants revealed only modest pooled sensitivity of 0.77 (95% CI 0.74–0.79), specificity of 0.74 (95% CI 0.72–0.75), and AUC of 0.87.55 Subgroup analysis showed improved diagnostic accuracy in term infants when compared to preterm infants and in culture-positive sepsis in comparison to clinical sepsis. A more recent meta-analysis containing fewer subjects offered similar but slightly improved results, with pooled sensitivity of 80% (95% CI 69%–88%), specificity of 83% (95% CI 71%–90%), and AUC of 0.88 (95% CI 0.85–0.91).56 The authors noted that the heterogeneity of the studies was high, making application of the results to clinical practice challenging.
In a prospective observational cohort study with data from 1,156 sepsis evaluations performed on 684 infants, combining nCD64 expression with specific WBC indices was found to improve sensitivity for NS detection from 78% to 91% and specificity from 59% to 93%.24 nCD64 expression has also been evaluated in combination with CRP, yielding sensitivity of 77%–94% and specificity of 78%–86% in the context of EOS57 and sensitivity of 97% and specificity of 89% when CD64 count at the onset of infection was combined with CRP measured at 24 hours in the workup of LOS.58 In summary, nCD64 is of limited use as a sole marker of NS, but when combined with CRP can be useful in guiding the decision to continue antibiotics beyond 36–48 hours.
sTREM1
sTREM1 has been identified as a potential biomarker for NS, with sensitivity of 70%–100% and specificity 71%–100%.31 A recent meta-analysis containing data from 667 neonates reported similarly high sensitivity for the summary point as 0.95 (95% CI 0.81–0.99), with specificity of 0.87 (95% CI 0.56–0.97).59 A pilot study has suggested that sTREM1 can also be used as a prognostic marker, with higher levels being strongly predictive of septic shock and death.60 Conserving blood volume is often a concern in the vulnerable preterm population, and another recent study suggests that urinary sTREM1 levels can also be used to predict NS, with sensitivity and specificity of 90% and 78%, respectively, when a cutoff value of 78.5 pg/mL was used.61 Further work needs to be performed to better characterize appropriate cutoff values for infants of different GA and postnatal ages. At present, this biomarker is not yet ready for use outside the experimental setting.
Serum amyloid A
Elevated SAA levels have been reported in the presence of normal CRP, particularly in the early course of an infection.62,63 Several studies have demonstrated improved sensitivity relative to CRP in both EOS and LOS,63,64 where sensitivity of SAA was up to 90% compared to 30% for CRP at the point of initial clinical suspicion. However, data from a population with a higher incidence of culture-proven sepsis showed only a trend toward improved sensitivity at the initiation of antibiotic therapy.65 Pooled data from a meta-analysis (n=823) revealed pooled sensitivity of 84% (95% CI 80%–87%), pooled specificity of 89% (95% CI 86%–92%) and AUC of 0.96.66 A recent paper suggested efficacy in measuring SAA in cord-blood samples, reporting an AUC of 0.99 for culture-proven sepsis.67 One of the advantages of SAA over other early markers, such as inflammatory cytokines, is the availability of an automated rapid test for this marker.68 More widespread availability of this test kit may make SAA a more widely used marker for the diagnosis of NS. SAA may be of use in the early diagnosis of EOS when measured in cord-blood samples, and with further study could potentially help guide initiation of antibiotic therapy. Further studies are required before SAA measurement can be incorporated into guidance on clinical management of NS.
Presepsin
Presepsin has recently been recognized as a potentially important biomarker for sepsis.69 Data from a recent meta-analysis that included 783 infants from eleven studies revealed both high sensitivity of 91% (95% CI 87%–93%) and high specificity of 91% (95% CI 88%–94%).59 Several studies have demonstrated improved performance of presepsin, with a pooled AUC of 0.975 compared with 0.858 for CRP and 0.78 for Pct.59 A cord-blood presepsin level >1,370 pg/mL was found to be an independent predictor of EOS in the setting of preterm rupture of membranes, with an OR of 12.6 (95% CI 2.5–28.1, P=0.000).70 Normal levels of presepsin in both healthy term and preterm infants have been measured.71 The cutoff values used in various studies looking at the diagnostic capability of presepsin have varied widely: from as low as 539 pg/mL to 1,800 ng/mL. One of the advantages of presepsin as a diagnostic marker is the availability of automated testing using chemiluminescence that can provide results in as little as 15 minutes on blood specimens of only 100 µL.71 Although presepsin shows great promise, identification of reliable cutoff levels and testing of its utility in clinical decision-making is required before use becomes more widespread.
PCR-based technology
Positive blood culture remains the gold standard but is not without limitations.72 The long turnaround of blood cultures is also a concern, as at least 50% of deaths from infection occur within 3 days of the test being obtained.73 Prokaryotic organisms, such as bacteria, fungi, and viruses, differ from eukaryotes in that they carry a 16S ribosomal subunit, rather than an 18S subunit, and PCR-based technologies have evolved to specifically identify this.74 When 16S RNA–subunit PCR was evaluated alongside blood culture in a prospective clinical trial involving 706 infants with suspected sepsis, sensitivity, specificity, negative predictive value (NPV), and positive predictive value of 100%, 95.4%, 77.2%, and 100%, respectively, were reported. In contrast to blood cultures that take up to 5 days to grow, results from 16S rRNA–subunit PCR were available within hours. The authors did however emphasize that blood cultures are not currently replaceable by this technology, due to the need for pure isolates for antibiotic-susceptibility testing.43
One group developed a multiplex PCR assay (NeoSep-ID) tailored to detect the eight most common pathogens.75 Validation of the assay using 20 samples revealed 80% concordance of the two tests, with the NeoSep-ID failing to detect three of 12 blood cultures. A larger study evaluating the clinical efficacy of the multiplex PCR approach revealed sensitivity of 77%, specificity of 81%, positive predictive value of 87%, and NPV of 68% compared with blood culture.76 One of the greatest advantages of multiplex PCR detection is the possibility of obtaining meaningful results in as little as 4 hours. The Roche LightCycler SeptiFast Mgrade system requires 100 µL blood and can detect 20 different pathogens. When the SeptiFast system was evaluated alongside conventional blood cultures in a neonatal population, sensitivity of 90.5% (95% CI 86.2%–94.2%) and specificity of 72.9% (95% CI67.0%–79.0%) were noted.77 Although PCR-based technology is not yet ready to replace conventional blood cultures, with further development this system could provide a rapid method to help focus treatment on infected infants with a narrower spectrum of antibiotic coverage. If sufficiently sensitive, PCR-based technology could also be used as a tool to help stop antibiotics at an earlier time point in circumstances where the level of clinical suspicion is lower. At present, PCR-based technology remains in an experimental phase and is not yet suitable for clinical use.
Genomics, proteomics, and metabolomics
A study that employed genome-wide expression analysis on blood samples from both infected and healthy preterm infants revealed differential regulation of 292 genes, many of which were involved in either neutrophil function or T-cell activation.78 Differential expression of genes regulating natural killer–cell function was found to be present, indicating that this may be an interesting future target for research.78
Using surface-enhanced laser desorption/ionization time-of-flight mass spectrometry (MS) on cord-blood samples, one group of investigators were able to identify a precocious haptoglobin “switch-on” pattern (Hp&HpRp) that allowed for development of a “mass restricted score” for the diagnosis of EOS.79 The significance of the Hp&HpRp pattern was evaluated in a prospective cohort of 174 preterm newborns, and by combining this result with IL6 levels and hematological indices, investigators were able to identify infants who were most at risk of EOS.
Within the setting of LOS, a recent pilot study has identified how MS can identify combinations of proteins that can then be used to distinguish infected infants from controls and infants with sepsis from those with NEC.80 A panel characterized by high levels of CRP alongside low levels of both CETP and ApoA4 was found to be the most sensitive and specific for LOS, with an AUC of 0.99.80 In contrast, a panel for detection of NEC included ApoA4 at higher levels alongside ApoC1 and LCAT. This study offered interesting insights into how the associated proteome of these disease states changes over time; however, validation of these findings with larger prospective cohorts is required.
Metabolomics is the systematic study of changes in cell metabolism during physiological and pathophysiological conditions with the hypothesis that distinct signature changes in energy usage can be used for diagnostic purposes.81 MS and nuclear magnetic-resonance techniques were able to identify increased levels of acetate, glucose, and lactate and decreased levels of byproducts of the pentose-phosphate pathway in urine samples from infants with NS compared to controls.82 Another recent study also used nuclear magnetic resonance and targeted liquid-chromatography MS to analyze samples from infants with both confirmed and suspected NS, and revealed other distinctive changes in urinary metabolites.83 These changes relative to age-matched controls were most distinctive at the first suspicion of infection, with differences becoming insignificant by day 10 of the illness. This study also noted an increase in urinary lactate; however, significant differences were also noted in 16 other metabolites, including taurine and hypotaurine, which were postulated to be part of a protective anti-inflammatory response.83
Obtaining and interpreting “-omic” signatures still requires technological expertise and specialized equipment. Although it is possible to envision a future where the “-omic” profile of a sample of cord blood or urine sample may be able to give even more precise diagnostic and prognostic information, these methods are still very far from playing a role in routine clinical management.
Biophysical markers
Recently, heart rate-variability monitoring has been suggested as a more sensitive method to alert the clinician to the possibility of NS.84 In a large multicenter trial, having the HeRO score (a measure obtained from continuous analysis of electrocardiography for changes in heart-rate variability) visible to clinicians was associated with a statistically significant decrease in mortality; however, this change was also associated with more blood cultures being drawn and increased use of antibiotics.85 Spikes in the HeRO score have been found to be associated with multiple other conditions, such as surgery, non-infection-related deteriorations in respiratory status, and other unknown causes.86 Recently published data from one other large center also suggest a limited role for the HeRO score in the detection of NS.87 At present, the HeRO score remains a marker for a clinician to consider NS, rather than an absolute indication to start treatment.
The RALIS is a computerized-algorithm device that relies on the clinician to enter various data on a frequent basis, including heart rate, respiratory rate, core temperature, body weight, number of desaturation events (<85%), and number of bradycardiac events documented (heart rate <100 beats per minute). The initial pilot study that involved data input every 2 hours demonstrated that the RALIS was able to detect LOS with sensitivity of 95.8% and specificity of 77.3%.88 The RALIS algorithm was subsequently revised and then applied to a larger cohort of infants <33 weeks GA in a nested case–control study where data for input into the RALIS were obtained retrospectively from the electronic medical record.89 This study showed elevated RALIS score (>5) accuracy in detecting early inflammatory changes associated with both NS and NEC, with sensitivity of 83% and a high NPV of 93%.89 Changes in the RALIS score were noted on average 33 hours before the onset of clinical symptoms, prompting laboratory investigations suggesting potential for earlier diagnosis and treatment. The high NPV of the RALIS score may offer an opportunity for clinicians to withhold or shorten the duration of antibiotic therapy with greater confidence; however, information from larger prospective trials is required before the RALIS score can be used to guide routine practice.
The concept of measuring the difference between core and peripheral temperatures was first suggested by a small study that demonstrated a significant widening in both rectal–sole and axillary–sole temps in normothermic preterm infants diagnosed with NS that was not noted in either healthy preterm infants or term infants.90 Recently, a prospective observational cohort study that employed continuous measurement of both axillary and sole temperatures revealed that a temperature gradient >2°C sustained for at ≥4 hours was able to predict NS with 83% sensitivity and a NPV of 94%.91 The sustained change in temperature gradient was often the earliest indicator of infection, with initial CRP <1.5 mg/dL in 64% of cases and initial Pct <2 ng/mL in 36% of cases. Although these findings indicate great potential, particularly for the early detection of NS, validation of the core–periphery temperature gradient as a feasible biophysical marker is required for this strategy to enter common usage.
EOS-risk calculator
The development of the EOS-risk calculator has led to an increasing number of centers using this algorithm to guide patient care.10,92–95 Application of the EOS-risk calculator (n=204, 485 infants ≥35 GA) was associated with threefold decrease in blood-culture investigations and a decrease in empiric antibiotic use in the first 24 hours of life: from 5% to 2.6%. These improvements were not associated with any significant increases in adverse outcomes, such as mortality from infection or readmission to hospital. The incidence of culture-proven EOS was within the lower reported range of 0.3–0.8/1,000 cases.96 Overall, this study showed potential for application of the EOS-risk calculator to reduce the use of antibiotics without compromising safety in a population with a relatively low incidence of culture-proven EOS and good access to follow-up care.97 Given that up to 95% of hospitals still require some period of mother–infant separation for the administration of antibiotics and up to 44% of institutions may require this for the entire antibiotic course, widespread adoption of the EOS-risk calculator could have a significant positive impact on mother–infant bonding and breastfeeding.10 Of note, across all three eras of the study, it was found that approximately half the infants who went on to develop culture-proven EOS were asymptomatic at birth. This finding underlines the critical importance of adequate observation and reliable interpretation of the risk calculator. The authors made it clear that “no antibiotic treatment is not the same as no care”, and that closer clinical observation with more frequent monitoring of vital signs and thorough parental education may be required to maintain safety.97
Application of the EOS-risk calculator specifically to infants ≥35 weeks GA born to mothers diagnosed with chorioamnionitis has been a focus of recent interest, with several groups retrospectively analyzing data to see if this approach would safely reduce antibiotic use in this group of infants.98,99 Although results predict that antibiotic use could be decreased significantly, concern has arisen that widespread adoption of the risk calculator both generally and to this specific population may result in delay of care and adverse outcomes in infants who would qualify for early empiric treatment under 2010 Centers for Disease Control and Prevention guidelines.98,100,101 A reappraisal of the guidelines for management of infants with EOS advocates for close observation of well-appearing infants of ≥35 weeks GA born to mothers diagnosed with chorioamnionitis, rather than empiric antibiotic treatment.3 A pathway where infants with exposure to chorioamnionitis can be observed in a transitional neonatal intensive-care unit was shown to decrease antibiotic usage in one recent study102 and has been proposed as a counterargument to the concern that application of the EOS-risk calculator may result in adverse outcomes for the small number of infants that are affected.103 We would advocate a measured approach to adoption of the risk calculator, especially in populations at higher risk of infection who have less reliable access to follow-up.
Summary
In the field of NS, trends seem to be moving away from reliance on conventional markers, such as CBC and CRP, and more toward advanced monitoring algorithms and population-based risk calculators. Early cord-blood markers, such as presepsin, could be considered in parallel with the EOS-risk calculator in late-preterm and term infants. The idea that clinicians should rely more on correctly drawn blood cultures to confirm infection, rather than decide to continue treatment according to the results of inflammatory markers, has also gained traction, with many advocating a reduction in the diagnosis of culture-negative sepsis.18,104,105 In our opinion, there are many interesting strategies that require further investigation (see Table 2). With established evidence for the use of Pct-guided antibiotic stewardship,45 this strategy should be considered to limit the ongoing treatment of culture-negative sepsis. There is no single biomarker that can confirm or refute NS with complete certainty, so we must continue to evaluate accessible markers alongside the clinical history and examination findings to determine the optimum course of action.
Disclosure
The authors report no conflicts of interest in this work.
References
Stoll BJ, Hansen NI, Bell EF, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA. 2015;314(10):1039–1051. | ||
Lever A, Mackenzie I. Sepsis: definition, epidemiology, and diagnosis. BMJ. 2007;335(7625):879–883. | ||
Benitz WE, Wynn JL, Polin RA. Reappraisal of guidelines for management of neonates with suspected early-onset sepsis. J Pediatr. 2015;166(4):1070–1074. | ||
van Herk W, Stocker M, van Rossum AM. Recognising early onset neonatal sepsis: an essential step in appropriate antimicrobial use. J Infect. 2016;72(Suppl):S77–S82. | ||
Polin RA, Watterberg K, Benitz W, Eichenwald E. The conundrum of early-onset sepsis. Pediatrics. 2014;133(6):1122–1123. | ||
Bassler D, Stoll BJ, Schmidt B, et al. Using a count of neonatal morbidities to predict poor outcome in extremely low birth weight infants: added role of neonatal infection. Pediatrics. 2009;123(1):313–318. | ||
Haller S, Deindl P, Cassini A, et al. Neurological sequelae of healthcare-associated sepsis in very-low-birthweight infants: umbrella review and evidence-based outcome tree. Euro Surveill. 2016;21(8). | ||
Neu J. Preterm infant nutrition, gut bacteria, and necrotizing enterocolitis. Curr Opin Clin Nutr Metab Care. 2015;18(3):285–288. | ||
Greenberg RG, Benjamin DK. Neonatal candidiasis: diagnosis, prevention, and treatment. J Infect. 2014;69(Suppl 1):S19–S22. | ||
Mukhopadhyay S, Taylor JA, von Kohorn I, et al. Variation in sepsis evaluation across a national network of nurseries. Pediatrics. 2017;139(3):e20162845. | ||
Ahmadizar F, Vijverberg SJ, Arets HG, et al. Early life antibiotic use and the risk of asthma and asthma exacerbations in children. Pediatr Allergy Immunol. 2017;28(5):430–437. | ||
Yamamoto-Hanada K, Yang L, Narita M, Saito H, Ohya Y. Influence of antibiotic use in early childhood on asthma and allergic diseases at age 5. Ann Allergy, Asthma Immunol. 2017;119(1):54–58. | ||
Bailey LC, Forrest CB, Zhang P, Richards TM, Livshits A, Derusso PA. Association of antibiotics in infancy with early childhood obesity. JAMA Pediatr. 2014;168(11):1063–1069. | ||
Saari A, Virta LJ, Sankilampi U, Dunkel L, Saxen H. Antibiotic exposure in infancy and risk of being overweight in the first 24 months of life. Pediatrics. 2015;135(4):617–626. | ||
Ajslev TA, Andersen CS, Gamborg M, Sørensen TI, Jess T. Childhood overweight after establishment of the gut microbiota: the role of delivery mode, pre-pregnancy weight and early administration of antibiotics. Int J Obes. 2011;35(4):522–529. | ||
Bhandari V. Effective biomarkers for diagnosis of neonatal sepsis. J Pediatric Infect Dis Soc. 2014;3(3):234–245. | ||
Schelonka RL, Chai MK, Yoder BA, Hensley D, Brockett RM, Ascher DP. Volume of blood required to detect common neonatal pathogens. J Pediatr. 1996;129(2):275–278. | ||
Klingenberg C, Kornelisse RF, Buonocore G, Maier RF, Stocker M. Culture-negative early-onset neonatal sepsis — at the crossroad between efficient sepsis care and antimicrobial stewardship. Front. Pediatr. 2018;6:285. | ||
Newman TB, Puopolo KM, Wi S, Draper D, Escobar GJ. Interpreting complete blood counts soon after birth in newborns at risk for sepsis. Pediatrics. 2010;126(5):903–909. | ||
Rodwell RL, Leslie AL, Tudehope DI. Early diagnosis of neonatal sepsis using a hematologic scoring system. J Pediatr. 1988;112(5):761–767. | ||
Makkar M, Gupta C, Pathak R, Garg S, Mahajan NC. Performance evaluation of hematologic scoring system in early diagnosis of neonatal sepsis. J Clin Neonatol. 2013;2(1):25–29. | ||
Hornik CP, Benjamin DK, Becker KC, et al. Use of the complete blood cell count in early-onset neonatal sepsis. Pediatr Infect Dis J. 2012;31(8):799–802. | ||
Gonzalez BE, Mercado CK, Johnson L, Brodsky NL, Bhandari V. Early markers of late-onset sepsis in premature neonates: clinical, hematological and cytokine profile. J Perinat Med. 2003;31(1):60–68. | ||
Streimish I, Bizzarro M, Northrup V, et al. Neutrophil CD64 with hematologic criteria for diagnosis of neonatal sepsis. Am J Perinatol. 2014;31(01):021–030. | ||
Han YQ, Zhang L, Yan L, et al. Red blood cell distribution width predicts long-term outcomes in sepsis patients admitted to the intensive care unit. Clin Chim Acta. 2018;487:112–116. | ||
Ramby AL, Goodman DM, Wald EL, Weiss SL. Red blood cell distribution width as a pragmatic marker for outcome in pediatric critical illness. Plos One. 2015;10(6):e0129258. | ||
Christensen RD, Yaish HM, Henry E, Bennett ST. Red blood cell distribution width: reference intervals for neonates. J Matern Fetal Neonatal Med. 2015;28(8):883–888. | ||
Martin SL, Desai S, Nanavati R, Colah RB, Ghosh K, Mukherjee MB. Red cell distribution width and its association with mortality in neonatal sepsis. J Matern Fetal Neonatal Med. 2018;133:1–6. | ||
Ellahony DM, El-Mekkawy MS, Farag MM. A study of red cell distribution width in neonatal sepsis. Pediatr Emerg Care. 2017:1. | ||
Puopolo KM, Benitz WE, Zaoutis TE, NEWBORN COFA, DISEASES COI. Management of neonates born at ≤34 6/7 weeks’ gestation with suspected or proven early-onset bacterial sepsis. Pediatrics. 2018;142(6). | ||
Delanghe JR, Speeckaert MM. Translational research and biomarkers in neonatal sepsis. Clin Chim Acta. 2015;451(Pt A):46–64. | ||
Perrone S, Lotti F, Longini M, et al. C reactive protein in healthy term newborns during the first 48 hours of life. Arch Dis Child Fetal Neonatal Ed. 2018;103(2):F163–F166. | ||
Hofer N, Zacharias E, Müller W, Resch B. An update on the use of C-reactive protein in early-onset neonatal sepsis: current insights and new tasks. Neonatology. 2012;102(1):25–36. | ||
Franz AR, Bauer K, Schalk A, et al. Measurement of interleukin 8 in combination with C-reactive protein reduced unnecessary antibiotic therapy in newborn infants: a multicenter, randomized, controlled trial. Pediatrics. 2004;114(1):1–8. | ||
Yang AP, Liu J, Yue LH, Wang HQ, Yang WJ, Yang GH. Neutrophil CD64 combined with Pct, CRP and WBC improves the sensitivity for the early diagnosis of neonatal sepsis. Clin Chem Lab Med. 2016;54(2):345–351. | ||
Clyne B, Olshaker JS. The C-reactive protein. J Emerg Med. 1999;17(6):1019–1025. | ||
Turner D, Hammerman C, Rudensky B, Schlesinger Y, Goia C, Schimmel MS. Procalcitonin in preterm infants during the first few days of life: introducing an age related nomogram. Arch Dis Child Fetal Neonatal Ed. 2006;91(4):F283–F286. | ||
Stocker M, Hop WC, van Rossum AM. Neonatal procalcitonin intervention study (NeoPInS): effect of Procalcitonin-guided decision making on duration of antibiotic therapy in suspected neonatal early-onset sepsis: a multi-centre randomized superiority and non-inferiority intervention study. BMC Pediatr. 2010;10(1):89. | ||
Hahn WH, Song JH, Park IS, Kim H, Park S, Oh MH. Reference intervals of serum procalcitonin are affected by postnatal age in very low birth weight infants during the first 60 days after birth. Neonatology. 2015;108(1):60–64. | ||
Altunhan H, Annagür A, Örs R, Mehmetoğlu I. Procalcitonin measurement at 24 hours of age may be helpful in the prompt diagnosis of early-onset neonatal sepsis. Int J Infect Dis. 2011;15(12):e854–e858. | ||
Vouloumanou EK, Plessa E, Karageorgopoulos DE, Mantadakis E, Falagas ME. Serum procalcitonin as a diagnostic marker for neonatal sepsis: a systematic review and meta-analysis. Intensive Care Med. 2011;37(5):747–762. | ||
Yu Z, Liu J, Sun Q, Qiu Y, Han S, Guo X. The accuracy of the procalcitonin test for the diagnosis of neonatal sepsis: a meta-analysis. Scand J Infect Dis. 2010;42(10):723–733. | ||
Liu D, du L, Yu J, et al. 16S rDNA PCR-DGGE and sequencing in the diagnosis of neonatal late-onset septicemia. Mol Med Rep. 2015;12(4):6346–6352. | ||
Bender L, Thaarup J, Varming K, Krarup H, Ellermann-Eriksen S, Ebbesen F. Early and late markers for the detection of early-onset neonatal sepsis. Dan Med Bull. 2008;55(4):219–223. | ||
Stocker M, van Herk W, El Helou S, et al. Procalcitonin-guided decision making for duration of antibiotic therapy in neonates with suspected early-onset sepsis: a multicentre, randomised controlled trial (NeoPIns). Lancet. 2017;390(10097):871–881. | ||
Mathur NB, Behera B. Blood procalcitonin levels and duration of antibiotics in neonatal sepsis. J Trop Pediatr. 2018;10. | ||
Chiesa C, Pacifico L, Natale F, Hofer N, Osborn JF, Resch B. Fetal and early neonatal interleukin-6 response. Cytokine. 2015;76(1):1–12. | ||
Ganesan P, Shanmugam P, Sattar SB, Shankar SL. Evaluation of IL-6, CRP and hs-CRP as early markers of neonatal sepsis. J Clin Diagn Res. 2016;10(5):DC13–17. | ||
Ye Q, Du LZ, Shao WX, Shang SQ. Utility of cytokines to predict neonatal sepsis. Pediatr Res. 2017;81(4):616–621. | ||
Shahkar L, Keshtkar A, Mirfazeli A, Ahani A, Roshandel G. The role of IL-6 for predicting neonatal sepsis: a systematic review and meta-analysis. Iran J Pediatr. 2011;21(4):411–417. | ||
Zhou M, Cheng S, Yu J, Lu Q. Interleukin-8 for diagnosis of neonatal sepsis: a meta-analysis. Plos One. 2015;10(5):e0127170. | ||
Leal YA, Álvarez-Nemegyei J, Lavadores-May AI, Girón-Carrillo JL, Cedillo-Rivera R, Velazquez JR. Cytokine profile as diagnostic and prognostic factor in neonatal sepsis. J Matern Fetal Neonatal Med. 2018;30(5):1–7. | ||
Ng PC, Li K, Leung TF, et al. Early prediction of sepsis-induced disseminated intravascular coagulation with interleukin-10, interleukin-6, and RANTES in preterm infants. Clin Chem. 2006;52(6):1181–1189. | ||
Choo YK, Cho HS, Seo IB, Lee HS. Comparison of the accuracy of neutrophil CD64 and C-reactive protein as a single test for the early detection of neonatal sepsis. Korean J Pediatr. 2012;55(1):11–17. | ||
Shi J, Tang J, Chen D. Meta-analysis of diagnostic accuracy of neutrophil CD64 for neonatal sepsis. Ital J Pediatr. 2016;42(1):57. | ||
Dai J, Jiang W, Min Z, et al. Neutrophil CD64 as a diagnostic marker for neonatal sepsis: meta-analysis. Adv Clin Exp Med. 2017;26(2):327–332. | ||
Ng PC, Li G, Chui KM, et al. Neutrophil CD64 is a sensitive diagnostic marker for early-onset neonatal infection. Pediatr Res. 2004;56(5):796–803. | ||
Ng PC, Li K, Wong RP, Chui KM, Wong E, Fok TF. Neutrophil CD64 expression: a sensitive diagnostic marker for late-onset nosocomial infection in very low birthweight infants. Pediatr Res. 2002;51(3):296–303. | ||
Bellos I, Fitrou G, Pergialiotis V, Thomakos N, Perrea DN, Daskalakis G. The diagnostic accuracy of presepsin in neonatal sepsis: a meta-analysis. Eur J Pediatr. 2018;177(5):625–632. | ||
Arízaga-Ballesteros V, Alcorta-García MR, Lázaro-Martínez LC, et al. Can sTREM-1 predict septic shock & death in late-onset neonatal sepsis? A pilot study. Int J Infect Dis. 2015;30:27–32. | ||
Ozdemir SA, Ozer EA, Ilhan O, Sutcuoglu S, Tatlı M. Diagnostic value of urine soluble triggering receptor expressed on myeloid cells (sTREM-1) for late-onset neonatal sepsis in infected preterm neonates. J Int Med Res. 2018;46(4):1606–1616. | ||
Pizzini C, Mussap M, Plebani M, Fanos V. C-reactive protein and serum amyloid A protein in neonatal infections. Scand J Infect Dis. 2000;32(3):229–235. | ||
Arnon S, Litmanovitz I, Regev RH, Bauer S, Shainkin-Kestenbaum R, Dolfin T. Serum amyloid A: an early and accurate marker of neonatal early-onset sepsis. J Perinatol. 2007;27(5):297–302. | ||
Arnon S, Litmanovitz I, Regev R, et al. Serum amyloid A protein is a useful inflammatory marker during late-onset sepsis in preterm infants. Biol Neonate. 2005;87(2):105–110. | ||
Çetinkaya M, Özkan H, Köksal N, Çelebi S, Hacimustafaoğlu M. Comparison of serum amyloid A concentrations with those of C-reactive protein and procalcitonin in diagnosis and follow-up of neonatal sepsis in premature infants. J Perinatol. 2009;29(3):225–231. | ||
Yuan H, Huang J, Lv B, et al. Diagnosis value of the serum amyloid A test in neonatal sepsis: a meta-analysis. Biomed Res Int. 2013;2013(1):1–9. | ||
Mithal LB, Palac HL, Yogev R, Ernst LM, Mestan KK. Cord blood acute phase reactants predict early onset neonatal sepsis in preterm infants. Plos One. 2017;12(1):e0168677. | ||
Arnon S, Litmanovitz I, Regev R, Lis M, Shainkin-Kestenbaum R, Dolfin T. Serum amyloid A protein in the early detection of late-onset bacterial sepsis in preterm infants. J Perinat Med. 2002;30(4):329–332. | ||
Chenevier-Gobeaux C, Borderie D, Weiss N, Mallet-Coste T, Claessens Y-E, Presepsin CYE. Presepsin (sCD14-ST), an innate immune response marker in sepsis. Clin Chim Acta. 2015;450:97–103. | ||
Seliem W, Sultan AM. Presepsin as predictor of EONS in umbilical cord blood of premature with PROM. Pediatr Int. 2018;60(5):428–432. | ||
Pugni L, Pietrasanta C, Milani S, et al. Presepsin (soluble CD14 subtype): reference ranges of a new sepsis marker in term and preterm neonates. PLoS One. 2015;10(12):e0146020. | ||
Ottolini MC, Lundgren K, Mirkinson LJ, Cason S, Ottolini MG. Utility of complete blood count and blood culture screening to diagnose neonatal sepsis in the asymptomatic at risk newborn. Pediatr Infect Dis J. 2003;22(5):430–434. | ||
Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research network. Pediatrics. 2002;110(2):285–291. | ||
Chan KY, Lam HS, Cheung HM, et al. Rapid identification and differentiation of gram-negative and Gram-positive bacterial bloodstream infections by quantitative polymerase chain reaction in preterm infants*. Crit Care Med. 2009;37(8):2441–2447. | ||
van den Brand M, Peters RP, Catsburg A, et al. Development of a multiplex real-time PCR assay for the rapid diagnosis of neonatal late onset sepsis. J Microbiol Methods. 2014;106:8–15. | ||
van den Brand M, van den Dungen FA, Bos MP, et al. Evaluation of a real-time PCR assay for detection and quantification of bacterial DNA directly in blood of preterm neonates with suspected late-onset sepsis. Crit Care. 2018;22(1):105. | ||
Straub J, Paula H, Mayr M, et al. Diagnostic accuracy of the Roche SeptiFast PCR system for the rapid detection of blood pathogens in neonatal sepsis—A prospective clinical trial. PLoS One. 2017;12(11):e0187688. | ||
Hilgendorff A, Windhorst A, Klein M, et al. Gene expression profiling at birth characterizing the preterm infant with early onset infection. J Mol Med. 2017;95(2):169–180. | ||
Buhimschi CS, Bhandari V, Dulay AT, et al. Proteomics Mapping of Cord Blood Identifies Haptoglobin “Switch-On” Pattern as Biomarker of Early-Onset Neonatal Sepsis in Preterm Newborns. PLoS ONE. 2011;6(10):e26111. | ||
Chatziioannou AC, Wolters JC, Sarafidis K, et al. Targeted LC-MS/MS for the evaluation of proteomics biomarkers in the blood of neonates with necrotizing enterocolitis and late-onset sepsis. Anal Bioanal Chem. 2018;410(27):7163–7175. | ||
Fanos V, van den Anker J, Noto A, Mussap M, Atzori L. Metabolomics in neonatology: fact or fiction? Semin Fetal Neonatal Med. 2013;18(1):3–12. | ||
Fanos V, Caboni P, Corsello G, et al. Urinary 1H-NMR and GC-MS metabolomics predicts early and late onset neonatal sepsis. Early Hum Dev. 2014;90(Suppl 1):S78–S83. | ||
Sarafidis K, Chatziioannou AC, Thomaidou A, et al. Urine metabolomics in neonates with late-onset sepsis in a case-control study. Sci Rep. 2017;7(1):45506. | ||
Fairchild KD. Predictive monitoring for early detection of sepsis in neonatal ICU patients. Curr Opin Pediatr. 2013;25(2):172–179. | ||
Moorman JR, Carlo WA, Kattwinkel J, et al. Mortality reduction by heart rate characteristic monitoring in very low birth weight neonates: a randomized trial. J Pediatr. 2011;159(6):e901:900–906. | ||
Sullivan BA, Grice SM, Lake DE, Moorman JR, Fairchild KD. Infection and other clinical correlates of abnormal heart rate characteristics in preterm infants. J Pediatr. 2014;164(4):775–780. | ||
Coggins SA, Weitkamp J-H, Grunwald L, et al. Heart rate characteristic index monitoring for bloodstream infection in an NICU: a 3-year experience. Arch Dis Child Fetal Neonatal Ed. 2016;101(4): F329–F332. | ||
Gur I, Markel G, Nave Y, Vainshtein I, Eisenkraft A, Riskin A. A mathematical algorithm for detection of late-onset sepsis in very-low birth weight infants: a preliminary diagnostic test evaluation. Indian Pediatr. 2014;51(8):647–650. | ||
Mithal LB, Yogev R, Palac H, Gur I, Mestan KK. Computerized vital signs analysis and late onset infections in extremely low gestational age infants. J Perinat Med. 2016;44(5):491–497. | ||
Bhandari V, Narang A. Thermoregulatory alterations as a marker for sepsis in normothermic premature neonates. Indian Pediatr. 1992;29(5):571–575. | ||
Leante-Castellanos JL, Martínez-Gimeno A, Cidrás-Pidré M, Martínez-Munar G, García-González A, Fuentes-Gutiérrez C. Central-peripheral temperature monitoring as a marker for diagnosing late-onset neonatal sepsis. Pediatr Infect Dis J. 2017;36(12):e293–e297. | ||
Kuzniewicz MW, Walsh EM, Li S, Fischer A, Escobar GJ. Development and implementation of an early-onset sepsis calculator to guide antibiotic management in late preterm and term neonates. Jt Comm J Qual Patient Saf. 2016;42(5):232–239. | ||
Beavers JB, Bai S, Perry J, Simpson J, Peeples S. Implementation and evaluation of the early-onset sepsis risk calculator in a high-risk university nursery. Clin Pediatr. 2017;9922817751337. | ||
Strunk T, Buchiboyina A, Sharp M, Nathan E, Doherty D, Patole S. Implementation of the neonatal sepsis calculator in an Australian tertiary perinatal centre. Neonatology. 2018;113(4):379–382. | ||
Ayrapetyan M, Carola D, Lakshminrusimha S, Bhandari V, Aghai Z. Infants born to mothers with clinical chorioamnionitis: a cross-sectional survey on the use of early-onset sepsis risk calculator and prolonged use of antibiotics. Am J Perinatol. 2018. | ||
Stoll BJ, Hansen NI, Sánchez PJ, et al. Early onset neonatal sepsis: the burden of group B streptococcal and E. coli disease continues. Pediatrics. 2011;127(5):817–826. | ||
Kuzniewicz MW, Puopolo KM, Fischer A, et al. A quantitative, Risk-Based approach to the management of neonatal early-onset sepsis. JAMA Pediatr. 2017;171(4):365–371. | ||
Carola D, Vasconcellos M, Sloane A, et al. Utility of early-onset sepsis risk calculator for neonates born to mothers with chorioamnionitis. J Pediatr. 2018;195:e41:48–52. | ||
Money N, Newman J, Demissie S, Roth P, Blau J. Anti-microbial stewardship: antibiotic use in well-appearing term neonates born to mothers with chorioamnionitis. J Perinatol. 2017;37(12):1304–1309. | ||
Aghai ZH. Is early-onset sepsis risk calculator safe for the management of neonates born to mothers with chorioamnionitis? J Perinatol. 2018;38(6):769–770. | ||
Kerste M, Corver J, Sonnevelt MC, et al. Application of sepsis calculator in newborns with suspected infection. J Matern Fetal Neonatal Med. 2016;29(23):3860–3865. | ||
Joshi NS, Gupta A, Allan JM, et al. Clinical monitoring of Well-Appearing infants born to mothers with chorioamnionitis. Pediatrics. 2018;141(4):e20172056. | ||
Money N, Roth P, Blau J. In response: is early onset sepsis risk calculator safe for the management of neonates born to mothers with chorioamnionitis? J Perinatol. 2018;38(6):771–772. | ||
Greenberg RG, Kandefer S, do BT, et al. Late-onset sepsis in extremely premature infants: 2000-2011. Pediatr Infect Dis J. 2017;36(8):774–779. | ||
Cantey JB, Baird SD. Ending the culture of culture-negative sepsis in the neonatal ICU. Pediatrics. 2017;140(4):e2017004419 09 2017. | ||
Bellos I, Fitrou G, Daskalakis G, Thomakos N, Papantoniou N, Pergialiotis V. Soluble TREM-1 as a predictive factor of neonatal sepsis: a meta-analysis. Inflamm Res. 2018;67(7):571–578. | ||
Kordek A, Torbé A, Tousty J, et al. The determination of procalcitonin concentration in early-onset neonatal infection: a valuable test regardless of prenatal antibiotic therapy. Clin Pediatr. 2017;56(4):333–340. | ||
Yuan Z, Syed MA, Panchal D, Rogers D, Joo M, Sadikot RT. Curcumin mediated epigenetic modulation inhibits TREM-1 expression in response to lipopolysaccharide. Int J Biochem Cell Biol. 2012;44(11):2032–2043. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.