Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 15
Nebulized Therapies in COPD: Past, Present, and the Future
Authors Barjaktarevic IZ , Milstone AP
Received 28 March 2020
Accepted for publication 18 June 2020
Published 12 July 2020 Volume 2020:15 Pages 1665—1677
DOI https://doi.org/10.2147/COPD.S252435
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Richard Russell
Igor Z Barjaktarevic,1 Aaron P Milstone2
1Division of Pulmonary and Critical Care Medicine, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA; 2Williamson Medical Center, Franklin, TN, USA
Correspondence: Aaron P Milstone
Williamson Medical Center, 4323 Carothers Parkway, Suite 605, Franklin, TN 37067, USA
Tel +1 615-790-4159
Fax +1 615-790-4158
Email [email protected]
Abstract: Current guidelines recommend inhalation therapy as the preferred route of drug administration for treating patients with chronic obstructive pulmonary disease (COPD). Inhalation devices consist of nebulizers and handheld inhalers, such as dry-powder inhalers (DPIs), pressurized metered-dose inhalers (pMDIs), and soft mist inhalers (SMIs). Although pMDIs, DPIs and SMIs may be appropriate for most patients with COPD, certain patient populations may have challenges with these devices. Patients who have cognitive, neuromuscular, or ventilatory impairments (and receive limited assistance from caregivers), as well as those with suboptimal peak inspiratory flow may not derive the full benefit from handheld inhalers. A considerable number of patients are not capable of producing a peak inspiratory flow rate to overcome the internal resistance of DPIs. Furthermore, patients may have difficulty coordinating inhalation with device actuation, which is required for pMDIs and SMIs. However, inhalation devices such as spacers and valved holding chambers can be used with pMDIs to increase the efficiency of aerosol delivery. Nebulized treatment provides patients with COPD an alternative administration route that avoids the need for inspiratory flow, manual dexterity, or complex hand-breath coordination. The recent approval of two nebulized long-acting muscarinic antagonists has added to the extensive range of nebulized therapies in COPD. Furthermore, with the availability of quieter and more portable nebulizer devices, nebulization may be a useful treatment option in the management of certain patient populations with COPD. The aim of this narrative review was to highlight recent updates and the treatment landscape in nebulized therapy and COPD. We first discuss the pathophysiology of patients with COPD and inhalation device considerations. Second, we review the updates on recently approved and newly marketed nebulized treatments, nebulized treatments currently in development, and technological advances in nebulizer devices. Finally, we discuss the current applications of nebulized therapy in patients with COPD.
Keywords: COPD, inhaler, nebulizer
Introduction
Chronic obstructive pulmonary disease (COPD) is a common, treatable, and preventable disorder that is a significant cause of chronic morbidity and mortality. COPD is currently ranked as the fourth leading cause of death in the US and is predicted to become the third leading cause of death worldwide by 2030.1–4 More than 16.4 million people in the US have been diagnosed with COPD, but it is estimated that millions more have yet to be diagnosed.4 The global COPD burden is projected to increase5 because of persistent exposure to COPD risk factors, such as tobacco smoke and air pollution.6
Inhalation is the preferred administration route for COPD therapy due to the high drug concentration that can be achieved locally within the lungs, leading to increased efficacy and decreased systemic adverse events (AEs) versus other administration routes (eg, oral or intravenous).7 Bronchodilation with muscarinic antagonists, β-agonists, and inhaled corticosteroids (ICS) are the foundation of pharmacological treatment in patients with COPD.6 These agents are commonly delivered through nebulizers and handheld inhalers, which include dry-powder inhalers (DPIs), pressurized metered-dose inhalers (pMDIs), and soft mist inhalers (SMIs).
Although Tashkin8 previously discussed innovations in nebulized drug therapy and the role of nebulized therapy in patients with COPD, there have been new important developments since his review was published. With the recent approval of the nebulized long-acting muscarinic antagonists (LAMAs) glycopyrrolate9 and revefenacin,10 as well as the current development of the first nebulized dual phosphodiesterase 3/4 inhibitor RPL554,11 treatment via nebulization represents an increasingly promising alternative to handheld inhalers.
Thus, the aim of this narrative review was to highlight the recent updates and treatment landscape in nebulized therapy and COPD. We first discuss the pathophysiology of COPD and inhalation device considerations. Second, we review the updates on recently approved and newly marketed nebulized treatments, nebulized treatments currently in development, and technological advances in nebulizer devices. Finally, we discuss the current applications of nebulized therapy in patients with COPD.
Selection of Articles for Review
In this narrative review, a PubMed search (prior to May 12, 2020) was conducted using numerous primary topic headings combined with appropriate terms for each section of the article (eg, COPD + nebulizers or chronic obstructive pulmonary disease + nebulizers). The results of the PubMed search were supplemented by relevant papers from reference lists of published articles. Relevant ongoing and unpublished trials linked to nebulized treatments were identified in the clinicaltrials.gov database.
Pathophysiology of COPD and Inhalation Device Considerations
Small airways disease is one of the key features of COPD. The narrowing and destruction of small airways (<2 mm in diameter) characterizes early COPD and precedes the development of emphysema.12 Anatomical changes in these airways include structural abnormalities of the conducting airways (eg, peribronchiolar fibrosis, mucus plugging) and loss of alveolar attachments because of emphysema, resulting in destabilization of these airways related to decreased elastic recoil.12 Abnormal small airways represent the main site of airflow resistance in COPD,5 and pharmacological targeting of the small airways remains one of the primary goals in the management of COPD.
Direct delivery of pharmacological therapy via inhalation is an attractive approach in pulmonary diseases because it promotes high bioavailability of the therapeutic agent (10–200 times greater than gastrointestinal delivery) and is independent of dietary variability, extracellular enzymes, and interpatient metabolic differences that can affect gastrointestinal absorption.13 Nevertheless, deposition of drug molecules into the lungs can be affected by particle and patient-related factors, such as airway geometry, airway humidity, particle size, pathological processes affecting lumen patency of the airways, breathing patterns, and lung clearance mechanisms.14 Consequently, these factors can influence the therapeutic effectiveness of inhaled therapies.14
Aerosol particle size is one of the most important determinants of drug deposition in the lungs.15 Each inhalation device has specificities on how to prepare the dose and deliver the drug into the airways, determining the consequent density and size of the particles generated (Figure 1).16 Aerosol particle size is usually described based on mass median aerodynamic diameter (MMAD), and the optimum MMAD range is 1–5 µm.17 Some studies have suggested that medium-sized particles (≈3 µm) may have higher efficacy for bronchodilation versus smaller particles.18 Inhalation devices with a higher proportion of aerosol particles >5 µm in size emit doses less effectively and are associated with more oropharyngeal deposition and decreased lung delivery versus those with a smaller aerosol particle size and more efficient emission.19 The patient’s peak inspiratory flow rate (PIFR) generally determines the velocity of the airborne particles, and this, in turn, also affects the probability of their impaction in the oropharynx and larynx.20 Therefore, optimizing drug delivery requires the use of fine aerosol particles inhaled at adequate flow rates, and a “one size fits all” approach may not be appropriate in the treatment of COPD.
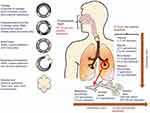 |
Figure 1 The adult lung with dimensions and generations of the airways with predicted aerosol deposition. Notes: Reprinted from European Journal of Pharmaceutical Sciences, Vol 49/edition number 5, Nahar K, Gupta N, Gauvin R, et al., In vitro, in vivo and ex vivo models for studying particle deposition and drug absorption of inhaled pharmaceuticals, Pages No.805–818, Copyright (2013), with permission from Elsevier.16Abbreviation: BSM, bronchial smooth muscle. |
Current Inhalation Delivery Systems
Although pMDIs, DPIs, and SMIs may be appropriate for some patients with COPD, certain patient populations may have challenges with these devices. Patients who have cognitive, neuromuscular, or ventilatory impairments (and receive limited assistance from caregivers), as well as those with suboptimal peak inspiratory flow, may not derive the full benefit of handheld inhalers.21,22 Regarding pMDIs, these patient populations may have difficulty coordinating inhaler activation with inspiration, struggle to produce a deep enough inhalation, inhale too quickly, and/or fail to hold their breath for long enough for effective drug delivery. To overcome some of the limitations associated with inadequate pMDI use, inhalation devices such as spacers and valved holding chambers can be used with pMDIs to increase the efficiency of aerosol delivery. Furthermore, breath-actuated pMDIs are useful for patients who struggle to time their inspiration correctly.19 However, breath-actuated pMDIs do not help patients who stop inhaling at the time of actuation, and they still require a minimum PIFR of 20–30 L/min.19 Similar to breath-actuated pMDIs, DPIs require a minimum PIFR of 20–50 L/min.23 Women with shorter heights, patients with lower percent predicted forced vital capacity (FVC), and those with reduced inspiratory muscle strength are among the main patient groups that have suboptimal PIFR.24,25 Furthermore, generating a PIFR is dependent on the patient’s respiratory muscle strength and level of effort, which may be compromised in patients with COPD as a result of an acute exacerbation, lung hyperinflation, hypoxemia, and/or muscle wasting.26
For SMIs, coordination between patient actuation and inspiratory effort is reduced (but not completely eliminated). Scintigraphic studies have shown that when compared to a CFC-based pMDI, lung deposition with a soft mist inhaler is up to 50% higher, and oropharyngeal deposition is lower.27,28 However, a recent meta-analysis has reported that device use errors similar to those with pMDIs occurred in approximately 60% of patients who used soft mist inhalers. The most common errors were breathing errors, hand-breath coordination, and difficulties with priming the inhalation device.29
Nebulized Drug Therapy
A considerable number of patients with COPD who remain breathless on high-dose pMDIs and DPIs derive benefits from nebulized treatment.30 Nebulizers are an appealing alternative to handheld inhalers for providing inhaled therapy and have been the foundation of inhalation therapy in acute and critical care settings.31 Nebulizers are now also widely used in clinics, outpatient settings, and the home environment. Current evidence suggests that the efficacy of treatments administered to patients with COPD via nebulizers is similar to that observed in patients who used pMDIs and DPIs with proper technique.32,33 Since nebulizers do not require patient coordination between inhalation and actuation or any special breathing technique (eg, a full exhalation followed by a full inhalation with a several-second breath hold near total lung capacity), these devices are particularly beneficial in patients with cognitive, neuromuscular, or ventilatory impairments and receive limited assistance from caregivers, as well as those with suboptimal PIFR.21,22 More than 50% of patients who use nebulizers instead of other devices do so because of physical or cognitive impairments.34 In terms of cost, reports on the use of nebulizers versus inhalers has shown varied financial impacts for hospitals. A recent US retrospective analysis evaluated respiratory drug costs at 28 hospitals in the health system after a phased implementation of the inhaler to nebulization protocol. Compared with pre-implementation, system-wide drug expenditures declined by approximately 40% in post-implementation years 1 and 2.35 On the other hand, a cohort study at a single US hospital showed that reducing nebulizer use, and implementing MDIs in the hospital resulted in significant savings annually. However, some limitations of this study included a lack of control group and possible overestimation of cost-savings since some of the costs were semifixed.36 Taking into consideration the effectiveness in relation to the cost of therapy, a personalized approach should be undertaken by healthcare personnel when treating their patients with COPD.
While some of the older nebulizer devices had some limitations (lack of portability, long administration times), advances in technology have led to the recent development of novel nebulizers devices (breath-enhanced jet nebulizer, breath-actuated jet nebulizer, and vibrating mesh nebulizers) that reduce drug wastage and improve delivery efficiency (Figure 2). The key characteristics, advantages, and disadvantages of these novel nebulizers’ devices are described in Table 1.37,38
 |
Table 1 Characteristics, Advantages, and Disadvantages of Nebulizers with Novel Technologies |
 |
Figure 2 Examples of novel marketed nebulizers. Examples of the different types of commercially available nebulizers that incorporate newer aerosol generating technologies. PARI LC® Sprint ( PARI, USA83); SideStream Plus® (Philips, USA84); AeroEclipse® II (Monaghan Medical Corporation, USA85); Micro Air® NE-U22 ( Omron Healthcare, USA86); AKITA2® APIXNEB (PARI Pharma GmbH, Germany87). |
Breath-enhanced jet nebulizers (PARI LC® Sprint [PARI Respiratory Equipment, Midlothian, VA], NebuTech HDN® [Salter Labs, Arvin, CA], and SideStream Plus® [Philips, Murrysville, PA]) are designed to increase aerosol drug delivery only during active inspiration and to expel the expired air outside of the device.37 Similarly, breath-actuated jet nebulizers, like the AeroEclipse® II BAN (Monaghan Medical Corporation, Plattsburgh, NY), also deliver aerosol only on inspiration and tend to decrease drug wastage during aerosol therapy.39 Vibrating mesh nebulizers, such as eFlow®rapid (PARI Pharma GmbH, Stranberg, Germany) and Micro Air® NE-U22 (Omron Healthcare, Bannockburn, IL), use micropump technology for aerosol production and can produce aerosols with a fine-particle fraction, resulting in more efficient drug delivery when compared with conventional jet nebulizers.40,41 The AKITA2® APIXNEB (PARI Pharma GmbH, Gräfelfing, Germany) mesh nebulizer uses an adaptive aerosol delivery technology that coordinates drug delivery with the patient’s breathing pattern.42
Nebulizers are a form of aerosol generation and can be used at any age or COPD stage. With recent technological advances, nebulizers will continue to play an important role in the management of COPD.
Overview of Nebulized Pharmacological Therapy
A variety of nebulized short-acting and long-acting bronchodilators are available for the treatment of COPD. Overall, these nebulized therapies have demonstrated significant improvements in lung function and reduction in rescue medication use.
Nebulized Short-Acting β-Agonists (SABAs) and Short-Acting Muscarinic Antagonists (SAMAs)
Nebulized short-acting bronchodilators are widely used for the management of patients with acute COPD exacerbations in the hospital setting.43 Clinical studies of the nebulized SABAs albuterol sulfate and levalbuterol hydrochloride have demonstrated improvements in forced expiratory volume in 1 second (FEV1) when compared with placebo.44,45 No significant differences were observed between these two treatments in terms of efficacy, cost, occurrence of AEs, or hospitalizations.46 With regard to nebulized SAMAs, nebulized ipratropium demonstrated significant improvements in FEV1 within 15–30 minutes, which persisted for 4–5 hours.47 Furthermore, clinical studies of dual ipratropium-albuterol have demonstrated improvements in FEV1 versus both albuterol or ipratropium alone. Ipratropium-albuterol also demonstrated a mean time to peak FEV1 of 1.5 hours, and the effect persisted for approximately 4 hours.48
Nebulized Long-Acting β-Agonists (LABAs)
Nebulized arformoterol tartrate and formoterol are twice-daily LABAs indicated for the maintenance treatment of patients with COPD. Arformoterol demonstrated significant improvements in mean percentage change FEV1 over 12 weeks when compared with placebo and was well tolerated.49 A 12-month, Phase IV study demonstrated no increased risk of respiratory death or hospitalization related to COPD exacerbations.50 Nebulized formoterol significantly increased trough FEV1 versus placebo over 12 weeks and had efficacy and safety profiles similar to formoterol administered via a DPI.51
Nebulized LAMAs
Glycopyrrolate bromide is a twice-daily inhalation solution that is administered via mesh nebulizer using the eFlow® CS nebulizer (PARI Pharma GmbH, Stranberg, Germany) and was approved in 2017 by the US Food and Drug Administration (FDA) for the maintenance treatment of COPD.9 The MMAD of glycopyrrolate/eFlow CS is 3.7 µm, which is optimal for bronchodilation.18,52 Overall, Phase III trials demonstrated that glycopyrrolate significantly improves lung function and has an acceptable safety profile in patients with moderate to severe COPD (Table 2).33,53-58
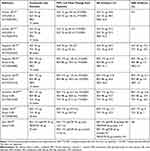 |
Table 2 Efficacy and Safety of Nebulized LAMAs – Glycopyrrolate and Revefenacin |
In two 12-week Phase III trials (GOLDEN 3 [NCT02347761] and GOLDEN 4 [NCT02347774]), glycopyrrolate significantly improved FEV1 compared with placebo, and the incidence of AEs was lowest among patients treated with glycopyrrolate 25 µg twice daily in both Phase 3 trials.53 Discontinuations due to AEs were more common with placebo versus glycopyrrolate, and the incidences of cardiovascular AEs and major adverse cardiovascular events (MACEs) were low in both trials. In a 48-week safety study (GOLDEN 5 [NCT02276222]), the incidences of overall and serious AEs were similar among patients treated with glycopyrrolate or tiotropium (active control); however, fewer MACEs were reported in patients who received glycopyrrolate.54
Revefenacin is a once-daily inhalation solution that is administered via standard jet nebulizer using the PARI LC® Sprint nebulizer (PARI Pharma GmbH, Starnberg, Germany) with a mouthpiece and the PARI Trek® S compressor (PARI Respiratory Equipment, Midlothian, VA, USA).10 The reported MMAD for the PARI LC® Sprint nebulizer/PARI Trek® S compressor is 3.8 µm, which is the optimal particle size for bronchodilation.18,52 Revefenacin was approved by the FDA for the maintenance treatment of COPD in 2018.10 Overall, Phase III trials demonstrated that revefenacin significantly improves lung function and has an acceptable safety profile in patients with moderate to very severe COPD (Table 2).33,55-58
In two 12-week Phase III trials (studies 0126 [NCT02459080] and 0127 [NCT02512510]), revefenacin significantly improved trough FEV1 from baseline when compared with placebo, and the overall incidences of AEs and serious AEs were similar in the revefenacin and placebo groups.55 The incidences of cardiovascular AEs and MACEs were low.59 In a 52-week Phase III safety trial (study 0128 [NCT02518139]), revefenacin demonstrated significant improvement from baseline in trough FEV1, which was comparable with the improvement seen with tiotropium (active control).56 The effect of revefenacin on trough FEV1 in patients taking concomitant LABA ± ICS was comparable with that in patients who were not taking these medications.56 AEs and serious AEs were comparable across all treatment groups.57 The incidences of cardiovascular AEs and MACEs were low for all treatment groups, with only one MACE (atrial fibrillation) considered possibly/probably related to revefenacin 175 µg.59 In a 28-day Phase IIIb trial (study 0149 [NCT03095456]), revefenacin and tiotropium (active control) effectively improved trough FEV1 and FVC from baseline with improvement numerically favoring revefenacin versus tiotropium.33 In a prespecified subgroup analysis, revefenacin significantly improved trough FEV1 and FVC from baseline compared with tiotropium in patients with severe to very severe COPD (ie, FEV1 <50% of predicted) who accounted for 80% of enrolled patients. Very few AEs were reported for revefenacin or tiotropium, and only one serious AE (COPD exacerbation) was reported for tiotropium.33 In a 42-day Phase IIIb trial (study 0167 [NCT03573817]), the sequential and combination administration of revefenacin/formoterol via a standard jet nebulizer was well tolerated versus placebo/formoterol, with fewer AEs associated with revefenacin/formoterol.58 Revefenacin/formoterol (via sequential or combination administration) demonstrated statistically significant improvements from baseline in trough FEV1 when compared with placebo/formoterol.58
Nebulized ICS
GOLD recommends a LABA/ICS combination for initial treatment in patients with frequent exacerbations and an eosinophil count >300 cells/µL or those with a history of asthma and COPD.6 Furthermore, patients who develop exacerbations while on LAMA/LABA therapy may be escalated to LABA/LAMA/ICS therapy. A recent report indicated the benefits of triple inhaler therapy in COPD. Triple therapy with fluticasone furoate, umeclidinium, and vilanterol resulted in decreased moderate or severe COPD exacerbations and hospitalizations versus fluticasone furoate/vilanterol or umeclidinium/vilanterol in patients with COPD.60 To date, few studies have been conducted for nebulized ICS treatment in patients with COPD. A meta-analysis indicated that high-dose nebulized budesonide 4–8 mg/day was noninferior to systemic corticosteroids on the change in FEV1 from baseline to end of treatment. Hyperglycemia was less frequent with nebulized budesonide than systemic corticosteroids.61
Nebulized Antibiotics
Some patients with COPD who have chronic bronchial infection may have an infective phenotype, and chronic infections are associated with exacerbations.62,63 Recent studies have shown that regular use of some antibiotics may reduce exacerbations.64–66 However, very little research has been done to date on nebulized antibiotics in the treatment of COPD, with only four reports investigating the efficacy of nebulized antibiotics in patients with COPD.67–70 Dal Negro and colleagues evaluated the effect of nebulized tobramycin (300 mg twice daily for 2 weeks) on the incidence of exacerbations and proinflammatory markers in patients with severe to very severe COPD who were colonized with Pseudomonas aeruginosa.68 Tobramycin decreased the incidence of exacerbations by 42% when compared with the prior 6 months, and proinflammatory markers were significantly reduced after 2 weeks of tobramycin. Soltaninejad and colleagues evaluated the effect of nebulized gentamycin (80 mg twice daily for 5 days) versus placebo on lung function given in patients with acute exacerbations of COPD.70 Treatment with gentamicin resulted in significant improvements in FVC and FEV1 versus placebo. Bruguera-Avila and colleagues evaluated the effect of nebulized colistin solution (80 mg twice daily for 1 year) on the number of severe exacerbations requiring hospitalizations and on the length of hospitalizations in patients with COPD who were colonized with P. aeruginosa.67 Colistin decreased the number of hospitalizations from 2.0 to 0.9 per individual year, and hospitalizations were shorter (23.3 vs 10.9 days). These studies together suggest a potential therapeutic role for nebulized antibiotics in patients with COPD who are colonized with resistant pathogens. However, a Phase II study evaluating the efficacy of nebulized levofloxacin (240 mg twice daily for 5 days every 28 days for 9–12 cycles) in patients with COPD at high risk for exacerbations showed no significant decrease in the exacerbation rate or an increase in the time to the next exacerbation versus placebo.69 It was suspected that the “pulsed” treatment regimen may have been suboptimal.69 However, the impact of pulsed antibiotics remains uncertain and requires further research.
Nebulized Therapy in Development
Rpl554
RPL554 is a dual inhibitor of the phosphodiesterase 3 (PD3) and PD4 enzymes that is currently being developed in a nebulized formulation for maintenance treatment of COPD and the treatment of acute exacerbations of COPD in the hospital setting. In four proof-of-concept clinical studies, RPL554 demonstrated bronchodilator and anti-inflammatory effects and was well tolerated.11
In a single-dose, placebo-controlled, six-way crossover Phase IIa study, nebulized RPL554 (6 mg) in addition to standard doses of short-acting bronchodilators (salbutamol, ipratropium) produced significant and clinically meaningful additive bronchodilation (>60%; P<0.001) and was well tolerated, with no increase in AEs versus placebo.71 In a 3-day, randomized, placebo-controlled Phase IIa study, RPL554 (1.5 mg or 6 mg) in addition to tiotropium 18 µg produced a statistically significant peak FEV1 (1.5 mg, 104 mL, P=0.002; 6 mg, 127 mL, P<0.0001), and RPL554 was well tolerated as add-on treatment to tiotropium.72 In a 4-week, placebo-controlled Phase IIb study, RPL554 demonstrated significant improvements in lung function (>200 mL; P<0.001) and COPD symptoms (P≤0.002) and was shown to be well tolerated at all four doses (0.75 mg, 1.5 mg, 3 mg, or 6 mg) when compared with placebo.73
Together these studies demonstrate that RPL554 is a promising treatment in COPD; however, further research is required to determine its ability to elicit anti-inflammatory activity in patients with COPD.74 RPL554 is currently in Phase IIb development with Phase III trials planned in 2020.
Discussion
Important factors to consider when evaluating inhalation device options for patients with COPD include patient characteristics, drug combinations, and patient preference and satisfaction. While inhalers pose various challenges regarding effective delivery of therapies, nebulizers provide patients with COPD an alternative administration route that avoids the need for high inspiratory flow rates, manual dexterity, or complex hand-breath coordination.
With the availability of quieter and more portable nebulizer devices, patients should be able to administer nebulized treatment with minimum inconvenience. Despite some steps that are generally involved with nebulizers (eg, assembly of device, insertion of vial into device, and cleaning, which, in the case of the vibrating mesh nebulizer, requires disassembly of the device), patients are generally satisfied with nebulizers and consider these devices to be easy and convenient to use, as well as fast acting.75 A suboptimal PIFR (<60 L/min) can identify patients who are more likely to have a less than favorable response to a DPI versus those with an optimal PIFR (≥60 L/min).76 Suboptimal PIFR has been demonstrated in 19–78% of outpatients and 32–52% of inpatients before discharge from the hospital after treatment.23,77-79 Two randomized controlled trials showed that patients with severe to very severe COPD and a suboptimal PIFR had greater improvements in lung function with a nebulized bronchodilator versus a DPI.33,80
For elderly patients and patients with arthritis, musculoskeletal, or neurological conditions, dexterity and grip strength should be considered when prescribing an inhalation device. DPIs could be unsuitable for patients with tremors, as shaking or instability of the inhaler may lead to loss of the dose.81 Patients with reduced dexterity and weak grip strength may find it difficult to actuate a pMDI device.21 Furthermore, coordination between inhalation and actuation is a common problem among these patients. Nebulizers can overcome these concerns, and therefore, may be suitable devices in these patient populations.
As stated by GOLD,6 LAMAs have a greater effect on the reduction of exacerbations and hospitalizations versus LABAs. Before revefenacin and glycopyrrolate were approved, a nebulized LAMA was not available for maintenance treatment of COPD to provide an alternative to inhalers. Revefenacin and glycopyrrolate demonstrated significant improvements in lung function and have an acceptable safety profile.33,53-59 Combination therapy with a LABA and a LAMA is recommended for patients with very severe COPD who are highly symptomatic.6 No nebulized fixed-dose LAMA/LABA combination is currently on the market; however, a recent pilot study demonstrated that the administration of revefenacin/formoterol via standard jet nebulizer was well tolerated compared with placebo/formoterol.58 Furthermore, a recent study demonstrated that revefenacin was stable for at least 60 minutes at room temperature when combined with either albuterol, arformoterol, or budesonide.82 Further research and development into a nebulized dual bronchodilator may be beneficial from a patient compliance standpoint. In addition, the benefits of triple therapy (LABA/LAMA/ICS) have been demonstrated. Triple therapy has the potential to further decrease COPD exacerbations and hospitalizations versus dual bronchodilator therapy (LABA/LAMA).60 The availability of these bronchodilators via nebulization could certainly allow for concomitant delivery. The development of the first nebulized PD3/4 inhibitor may provide another treatment option for patients who develop further exacerbations on LABA/LAMA or LABA/LAMA/ICS.
In conclusion, consideration of patient characteristics, drug combinations, and patient preference and satisfaction, is important when recommending and prescribing an inhalation device to patients with COPD. With the evolution of more sophisticated nebulizer devices and the recent availability of nebulized LAMAs, treatment via nebulization could be a suitable alternative to handheld inhalation devices, particularly in patients who have cognitive, neuromuscular, or ventilatory impairments, and receive limited assistance from caregivers, as well in those with suboptimal PIFR. When compared with inhalers, nebulizers offer ease of use with no requirements for forceful inspiratory maneuvers or complex hand-breath coordination. Considering that COPD is a significant cause of chronic morbidity and mortality and is predicted to become the third leading cause of death worldwide by 2030, the role of nebulizers in the management of patients with COPD is likely to become more significant in the near future.
Acknowledgments
Medical writing support was funded by Theravance Biopharma US, Inc. (South San Francisco, CA, USA) and Mylan Inc. (Canonsburg, PA, USA). The authors acknowledge Gráinne Faherty, MPharm, for medical writing and Mary Kacillas for editorial assistance (both from Ashfield Healthcare Communications) in the preparation of the manuscript. The authors would like to thank Dr. Donald P. Tashkin, MD for his critical review and contributions to this manuscript.
Disclosure
Dr Igor Z Barjaktarevic has no relevant conflicts related to the subject of this review. He has consulted with Astra Zeneca, Boehringer Ingelheim, CSL Behring, GE Healthcare, GlaxoSmithKline, Grifols, Mylan, Theravance Biopharma, and Verona Pharma. He has also received research grants from AMGEN, GE Healthcare, Mylan and Theravance Biopharma.
Dr Aaron P Milstone has received speaking and consulting fees from Boehringer Ingelheim, GlaxoSmithKline, Grifols, Insmed, Mylan, and Theravance Biopharma.
References
1. Centers for Disease Control and Prevention. COPD. Available from: https://www.cdc.gov/dotw/copd/index.html.
2. Centers for Disease Control and Prevention. National vital statistics reports. Final data for 2017. Available from: https://www.cdc.gov/nchs/data/nvsr/nvsr68/nvsr68_09-508.pdf.
3. COPD predicted to be third leading cause of death in 2030. World health stat; 2008. Available from: https://www.who.int/respiratory/copd/World_Health_Statistics_2008/en/.
4. American Lung Association. How serious is COPD; 2019. Available from: https://www.lung.org/lung-health-and-diseases/lung-disease-lookup/copd/learn-about-copd/how-serious-is-copd.html.
5. Mathers C, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. doi:10.1371/journal.pmed.0030442
6. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease; 2020. Available from: https://goldcopd.org/wp-content/uploads/2019/11/GOLD-2020-POCKET-GUIDE-FINAL-pgsized-wms.pdf.
7. Borghardt J, Kloft C, Sharma A. Inhaled therapy in respiratory disease: the complex interplay of pulmonary kinetic processes. Can Respir J. 2018;2018:2732017. doi:10.1155/2018/2732017
8. Tashkin DP. A review of nebulized drug delivery in COPD. Int J Chron Obstruct Pulmon Dis. 2016;18(11):2585–2596. doi:10.2147/COPD.S114034
9. Lonhala® Magnair® (glycopyrrolate) inhalation solution [highlights of prescribing information]. Marlborough, MA: Sunovion Respiratory Development Inc; 2019. Available from: https://www.lonhalamagnair.com/LonhalaMagnair-Prescribing-Information.pdf.
10. YUPELRI® (revefenacin) inhalation solution [highlights of prescribing information]. Morgantown, WV: Mylan Specialty L.P; 2019. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/210598s001lbl.pdf.
11. Franciosi L, Diamant Z, Banner K, et al. Efficacy and safety of RPL554, a dual PDE3 and PDE4 inhibitor, in healthy volunteers and in patients with asthma or chronic obstructive pulmonary disease: findings from four clinical trials. Lancet Respir Med. 2013;1:714–727. doi:10.1016/S2213-2600(13)70187-5
12. Martin C, Frija J, Burgel P. Dysfunctional lung anatomy and small airways degeneration in COPD. Int J Chron Obstruct Pulmon Dis. 2013;8:7–13. doi:10.2147/COPD.S28290
13. Labiris N, Dolovich M. Pulmonary drug delivery. Part II: the role of inhalant delivery devices and drug formulations in therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol. 2003;56(6):600–612. doi:10.1046/j.1365-2125.2003.01893.x
14. Labiris N, Dolovich M. Pulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol. 2003;56(6):588–599. doi:10.1046/j.1365-2125.2003.01892.x
15. Pleasants R, Hess D. Aerosol delivery devices for obstructive lung diseases. Respir Care. 2018;63(6):708–733. doi:10.4187/respcare.06290
16. Nahar K, Gupta N, Gauvin R, et al. In vitro, in vivo and ex vivo models for studying particle deposition and drug absorption of inhaled pharmaceuticals. Eur J Pharm Sci. 2013;49(5):805–818. doi:10.1016/j.ejps.2013.06.004
17. Telko M, Hickey A. Dry powder inhaler formulation. Respir Care. 2005;50(9):1209–1227.
18. Usmani O, Biddiscombe M, Barnes P. Regional lung deposition and bronchodilator response as a function of beta2-agonist particle size. Am J Respir Crit Care Med. 2005;172(12):1497–1504. doi:10.1164/rccm.200410-1414OC
19. Laube B, Janssens H, de Jongh F, et al. What the pulmonary specialist should know about the new inhalation therapies. Eur Respir J. 2011;37(6):1308–1331. doi:10.1183/09031936.00166410
20. Dolovich M. Influence of inspiratory flow rate, particle size, and airway caliber on aerosolized drug delivery to the lung. Respir Care. 2000;45(6):597–608.
21. Taffet G, Donohue J, Altman P. Considerations for managing chronic obstructive pulmonary disease in the elderly. Clin Interv Aging. 2014;9:23–30. doi:10.2147/CIA.S52999
22. Yawn B, Colice G, Hodder R. Practical aspects of inhaler use in the management of chronic obstructive pulmonary disease in the primary care setting. Int J Chron Obstruct Pulmon Dis. 2012;7:495–502. doi:10.2147/COPD.S32674
23. Ghosh S, Ohar J, Drummond M. Peak inspiratory flow rate in chronic obstructive pulmonary disease: implications for dry powder inhalers. J Aerosol Med Pulm Drug Deliv. 2017;30(6):381–387. doi:10.1089/jamp.2017.1416
24. Duarte A, Tung L, Zhang W, Hsu E, Kuo Y, Sharma G. Spirometry measurement of peak inspiratory flow identifies suboptimal use of dry powder inhalers in ambulatory patients with COPD. Chronic Obstr Pulm Dis. 2019;6(3):246–255. doi:10.15326/jcopdf.6.3.2018.0163
25. Mahler D, Waterman L, Gifford A. Prevalence and COPD phenotype for a suboptimal peak inspiratory flow rate against the simulated resistance of the Diskus® dry powder inhaler. J Aerosol Med Pulm Drug Deliv. 2013;26(3):174–179. doi:10.1089/jamp.2012.0987
26. Mahler D. Peak inspiratory flow rate as a criterion for dry powder inhaler use in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2017;14(7):1103–1107. doi:10.1513/AnnalsATS.201702-156PS
27. Iwanaga T, Tohda Y, Nakamura S, Suga Y. The Respimat® soft mist inhaler: implications of drug delivery characteristics for patients. Clin Drug Investig. 2019;39(11):1021–1030. doi:10.1007/s40261-019-00835-z
28. Dalby R, Spallek M, Voshaar T. A review of the development of respimat soft mist inhaler. Int J Pharm. 2004;283:1–9. doi:10.1016/j.ijpharm.2004.06.018
29. Navaie M, Dembek C, Cho-Reyes S, Yeh K, Celli R. Device use errors with soft mist inhalers: a global systematic literature review and meta-analysis. Chron Respir Dis. 2020;17:1479973119901234. doi:10.1177/1479973119901234
30. O’Driscoll B, Kay E, Taylor R, Weatherby H, Chetty M, Bernstein A. A long-term prospective assessment of home nebulizer treatment. Respir Med. 1992;86(4):317–325. doi:10.1016/S0954-6111(06)80031-4
31. Gardenhire D, Burnett D, Strickland S, Myers T. A guide to aerosol delivery devices for respiratory therapists; 2017. Available from: https://www.aarc.org/wp-content/uploads/2015/04/aerosol_guide_rt.pdf.
32. Dolovich M, Ahrens R, Hess D, et al. Device selection and outcomes of aerosol therapy: evidence-based guidelines: American College of Chest Physicians/American College of Asthma, Allergy, and Immunology. Chest. 2005;127(1):335–371. doi:10.1378/chest.127.1.335
33. Mahler D, Ohar J, Barnes C, Moran E, Pendyala S, Crater G. Nebulized versus dry powder long-acting muscarinic antagonist bronchodilators in patients with COPD and suboptimal peak inspiratory flow rate. Chronic Obstr Pulm Dis. 2019;6:4.
34. Bowles S, Sketris I, Kephart G. Drug evaluation alliance of nova scotia. Use of wet nebulized inhaled respiratory medications under criteria-based reimbursement guidelines in a publicly funded seniors’ pharmacare program in Nova Scotia, Canada. Am J Geriatr Pharmacother. 2007;5(2):120–128. doi:10.1016/j.amjopharm.2007.05.003
35. Larson T. Economic impact and chronic obstructive pulmonary disease outcomes of a comprehensive inhaler to nebulization therapy protocol implementation in a large multi-state healthcare system. Curr Med Res Opin. 2019;35(10):1805–1817. doi:10.1080/03007995.2019.1628562
36. Moriates C, Novelero M, Quinn K, Khanna R, Mourad M. “Nebs no more after 24”: a pilot program to improve the use of appropriate respiratory therapies. JAMA Intern Med. 2013;173(17):1647–1648. doi:10.1001/jamainternmed.2013.9002
37. Ari A. Jet, ultrasonic, and mesh nebulizers: an evaluation of nebulizers for better clinical outcomes. Eurasian J Pulmonol. 2014;16:1–7. doi:10.5152/ejp.2014.00087
38. Ibrahim M, Verma R, Garcia-Contreras L. Inhalation drug delivery devices: technology update. Med Devices (Auckl). 2015;8:131–139. doi:10.2147/MDER.S48888
39. Rau J, Ari A, Restrepo R. Performance comparison of nebulizer designs: constant-output, breath-enhanced, and dosimetric. Respir Care. 2004;49:174–179.
40. Dhand R. Nebulizers that use a vibrating mesh or plate with multiple apertures to generate aerosol. Respir Care. 2002;47(12):1406–1416.
41. Skaria S, Smaldone G. Omron NE U22: comparison between vibrating mesh and jet nebulizer. J Aerosol Med Pulm Drug Deliv. 2010;23(3):173–180. doi:10.1089/jamp.2010.0817
42. Biddiscombe M, Usmani O. Is there room for further innovation in inhaled therapy for airways disease? Breathe. 2018;14:216–224. doi:10.1183/20734735.020318
43. Kopsaftis Z, Sulaiman N, Mountain O, Carson-Chahhoud K, Phillips P, Smith B. Short-acting bronchodilators for the management of acute exacerbations of chronic obstructive pulmonary disease in the hospital setting: systematic review. Syst Rev. 2018;7(1):213. doi:10.1186/s13643-018-0860-0
44. Datta D, Vitale A, Lahiri B, ZuWallack R. An evaluation of nebulized levalbuterol in stable COPD. Chest. 2003;124(3):844–849. doi:10.1016/S0012-3692(15)37638-8
45. Nair S, Thomas E, Pearson S, Henry M. A randomized controlled trial to assess the optimal dose and effect of nebulized albuterol in acute exacerbations of COPD. Chest. 2005;128(1):48–54. doi:10.1378/chest.128.1.48
46. Borkowski J, Crader M. Nebulized albuterol versus levalbuterol in pediatric and adult patients: a review. Formulary. 2009;44:108–118.
47. Ipratropium Bromide (ipratropium bromide solution) [prescribing information]. Bonita Springs, FL: Cobalt Laboratories Inc; 2007. Available from: https://dailymed.nlm.nih.gov/dailymed/getFile.cfm?setid=lf69f100f-c766-490f-9143-5e8e0c8cb7c9&type=pdf&name=lf69f100f-c766-490f-9143-5e8e0c8cb7c9.
48. DuoNeb® (Ipratropium Bromide 0.5 mg/Albuterol Sulfate 3.0 mg*) inhalation solution [prescribing information]. CA: Dey Pharma, L.P. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020950s037lbl.pdf.
49. Baumgartner R, Hanania N, Calhoun W, Sahn S, Sciarappa K, Hanrahan J. Nebulized arformoterol in patients with COPD: a 12-week, multicenter, randomized, double-blind, double-dummy, placebo- and active-controlled trial. Clin Ther. 2007;29(2):261–278. doi:10.1016/j.clinthera.2007.02.009
50. Donohue J, Hanania N, Make B, et al. One-year safety and efficacy study of arformoterol tartrate in patients with moderate to severe COPD. Chest. 2014;146(6):1531–1542. doi:10.1378/chest.14-0117
51. Gross N, Nelson H, Lapidus R, et al. Efficacy and safety of formoterol fumarate delivered by nebulization to COPD patients. Respir Med. 2008;102(2):189–197. doi:10.1016/j.rmed.2007.10.007
52. Zanen P, Go L, Lammers J. Optimal particle size for beta 2 agonist and anticholinergic aerosols in patients with severe airflow obstruction. Thorax. 1996;51(10):977–980. doi:10.1136/thx.51.10.977
53. Kerwin E, Donohue J, Goodin T, Tosiello R, Wheeler A, Ferguson G. Efficacy and safety of glycopyrrolate/eFlow® CS (nebulized glycopyrrolate) in moderate-to-very-severe COPD: results from the glycopyrrolate for obstructive lung disease via electronic nebulizer (GOLDEN) 3 and 4 randomized controlled trials. Respir Med. 2017;132:238–250. doi:10.1016/j.rmed.2017.07.011
54. Ferguson G, Goodin T, Tosiello R, Wheeler A, Kerwin E. Long-term safety of glycopyrrolate/eFlow® CS in moderate-to-very-severe COPD: results from the Glycopyrrolate for Obstructive Lung Disease via Electronic Nebulizer (GOLDEN) 5 randomized study. Respir Med. 2017;132:251–260. doi:10.1016/j.rmed.2017.08.020
55. Ferguson G, Feldman G, Pudi K, et al. Improvements in lung function with nebulized revefenacin in the treatment of patients with moderate to very severe COPD: results from two replicate phase III clinical trials. Chronic Obstr Pulm Dis. 2019;6(2):154–165. doi:10.15326/jcopdf.6.2.2018.0152
56. Donohue J, Kerwin E, Sethi S, et al. Maintained therapeutic effect of revefenacin over 52 weeks in moderate to very severe chronic obstructive pulmonary disease (COPD). Respir Res. 2019;20(1):241. doi:10.1186/s12931-019-1187-7
57. Donohue J, Kerwin E, Sethi S, et al. Revefenacin, a once-daily, lung-selective, long-acting muscarinic antagonist for nebulized therapy: safety and tolerability results of a 52-week phase 3 trial in moderate to very severe chronic obstructive pulmonary disease. Respir Med. 2019;153:38–43. doi:10.1016/j.rmed.2019.05.010
58. Siler TM, Moran EJ, Barnes CN, Crater GD. Safety and efficacy of revefenacin and formoterol in sequence and combination via a standard jet nebulizer in patients with chronic obstructive pulmonary disease: a phase 3b, randomized, 42-day study. Chronic Obstr Pulm Dis. 2020;7(2):99–106. doi:10.15326/jcopdf.7.2.2019.0154
59. Donohue J, Feldman G, Sethi S, et al. Cardiovascular safety of revefenacin, a once-daily, lung-selective, long-acting muscarinic antagonist for nebulized therapy of chronic obstructive pulmonary disease: evaluation in phase 3 clinical trials. Pulm Pharmacol Ther. 2019;57:101808. doi:10.1016/j.pupt.2019.101808
60. Lipson D, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671–1680. doi:10.1056/NEJMoa1713901
61. Pleasants R, Wang T, Xu X, et al. Nebulized corticosteroids in the treatment of COPD exacerbations: systematic review, meta-analysis, and clinical perspective. Respir Care. 2018;63(10):1302–1310. doi:10.4187/respcare.06384
62. Matkovic Z, Miravitlles M. Chronic bronchial infection in COPD. Is there an infective phenotype? Respir Med. 2013;107(1):10–22. doi:10.1016/j.rmed.2012.10.024
63. Miravitlles M, Anzueto A. Chronic respiratory infection in patients with chronic obstructive pulmonary disease: what is the role of antibiotics? Int J Mol Sci. 2017;18:7. doi:10.3390/ijms18071344
64. Albert R, Connett J, Bailey W, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365(8):689–698. doi:10.1056/NEJMoa1104623
65. Cui Y, Luo L, Li C, Chen P, Chen Y. Long-term macrolide treatment for the prevention of acute exacerbations in COPD: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2018;13:3813–3829. doi:10.2147/COPD.S181246
66. Herath S, Normansell R, Maisey S, Poole P. Prophylactic antibiotic therapy for chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2018;10:CD009764.
67. Bruguera-Avila N, Marin A, Garcia-Olive I, et al. Effectiveness of treatment with nebulized colistin in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:2909–2915. doi:10.2147/COPD.S138428
68. Dal Negro R, Micheletto C, Tognella S, Visconti M, Turati C. Tobramycin nebulizer solution in severe COPD patients colonized with Pseudomonas aeruginosa: effects on bronchial inflammation. Adv Ther. 2008;25(10):1019–1030. doi:10.1007/s12325-008-0105-2
69. Sethi S, Rennard S, Miravitlles M, et al. A Phase 2 study to evaluate the safety, tolerability and efficacy of levofloxacin inhalation solution (MP-376) administered for 5 days every 28 days to prevent acute exacerbations in high risk COPD patients. Am J Respir Crit Care Med. 2012;185:A3057.
70. Soltaninejad F, Kheiri S, Habibian R, Amra A, Asgari-Savadjani S. Evaluation effects of nebulized gentamicin in exacerbation of chronic obstructive lung disease. J Res Med Sci. 2016;21:56. doi:10.4103/1735-1995.187278
71. Singh D, Abbott-Banner K, Newman K. The novel inhaled dual PDE3/4 inhibitor RPL554 produces significant additional improvements in lung function when administered on top of existing standard of care in COPD patients. Eur Respir J. 2016;48:OA1970.
72. Newman K, Singh D. RPL554, a first-in-class dual PDE3/4 inhibitor, causes rapid additional bronchodilation when dosed with tiotropium in COPD patients. Am J Respir Crit Care Med. 2018;197:A4227.
73. Maurer B, Singh D, Martinez F, Newman K. RPL554, a first-in-class dual PDE3/4 inhibitor, causes significant bronchodilation and symptom relief; a Phase 2b COPD study. Eur Respir J. 2018;52:OA1940. doi:10.1183/13993003.01675-2018
74. Cazzola M, Calzetta L, Rogliani P, Matera M. Ensifentrine (RPL554): an investigational PDE3/4 inhibitor for the treatment of COPD. Expert Opin Investig Drugs. 2019;28(10):827–833. doi:10.1080/13543784.2019.1661990
75. Dhand R, Mahler D, Carlin B, et al. Results of a patient survey regarding COPD knowledge, treatment experiences, and practices with inhalation devices. Respir Care. 2018;63(7):833–839. doi:10.4187/respcare.05715
76. Mahler D. Peak inspiratory flow rate: an emerging biomarker in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2019;199(12):1577–1579. doi:10.1164/rccm.201901-0005LE
77. Janssens W, VandenBrande P, Hardeman E, et al. Inspiratory flow rates at different levels of resistance in elderly COPD patients. Eur Respir J. 2008;31:78–83. doi:10.1183/09031936.00024807
78. Loh C, Peters S, Lovings T, Ohar J. Suboptimal inspiratory flow rates are associated with chronic obstructive pulmonary disease and all-cause readmissions. Ann Am Thorac Soc. 2017;14(8):1305–1311. doi:10.1513/AnnalsATS.201611-903OC
79. Sharma G, Mahler D, Mayorga V, Deering K, Harshaw O, Ganapathy V. Prevalence of low peak inspiratory flow rate at discharge in patients hospitalized for COPD exacerbation. Chronic Obstr Pulm Dis. 2017;4(3):217–224. doi:10.15326/jcopdf.4.3.2017.0183
80. Mahler D, Waterman L, Ward J, Gifford A. Comparison of dry powder versus nebulized β-agonist in patients with COPD who have suboptimal peak inspiratory flow rate. J Aerosol Med Pulm Drug Deliv. 2014;27(2):103–109. doi:10.1089/jamp.2013.1038
81. Barrons R, Pegram A, Borries A. Inhaler device selection: special considerations in elderly patients with chronic obstructive pulmonary disease. Am J Health Syst Pharm. 2011;68(13):1221–1232. doi:10.2146/ajhp100452
82. Mazyck A, Szymborski S, Nhan E, Reilly J. Chemical and physical compatibility of revefenacin inhalation solution mixed with either albuterol sulfate, arformoterol tartrate, or budesonide. Am Soc Health System Pharmacists. 2019.
83. PARI. Available from: https://pariorder.com.
84. Philips. SideStream Plus: Reusable nebulizer. Available from: https://www.usa.philips.com/healthcare/product/HC0077830/sidestream-plus-reusable-nebulizer.
85. Monaghan. Aeroeclipse® XL ban with Ombra® Compressor. Available from: https://www.monaghanmed.com/Aeroeclipse-XL-Ban-with-Ombra-Compressor/#1540799872824-7b01c579-2e33628f-29b55344-99b1.
86. Omron. Portable MicroAir® Nebulizer. Available from: https://omronhealthcare.com/products/microair-nebulizer-neu22v.
87. Akita® Jet. Available from: http://www.akita-jet.com.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
