Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Natural Topical Treatment Contributes to a Reduction of Dry Scalp Symptoms in Children
Authors Fithian E , Thivalapill N, Kosner J, Necheles J , Bilaver L
Received 29 June 2023
Accepted for publication 20 September 2023
Published 4 October 2023 Volume 2023:16 Pages 2757—2762
DOI https://doi.org/10.2147/CCID.S424077
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Eirene Fithian,1 Neil Thivalapill,1 John Kosner,1 Jonathan Necheles,2 Lucy Bilaver1
1Center for Food Allergy and Asthma Research, Northwestern University Feinberg School of Medicine, Chicago, IL, USA; 2Children’s Healthcare Associates, Chicago, IL, USA
Correspondence: Lucy Bilaver, Center for Food Allergy and Asthma Research, Northwestern University Feinberg School of Medicine, 750 N. Lake Shore Drive, 6th Floor, Chicago, IL, 60611, USA, Tel +1 312-503-5618, Email [email protected]
Background: Dry scalp conditions affect a significant portion of the population, including children. Emerging evidence indicates the potential for improvement of atopic symptoms through altering the skin microbiome. Therefore, a topical treatment consisting of probiotic extracts, honey, turmeric, and vitamin B12 was manufactured to improve dry scalp symptomology through sustained balance of the microbiome.
Purpose: This interventional clinical study aims to determine the safety and efficacy of the topical treatment in reducing dry scalp symptomology in children 1– 17 years old with dry scalp symptoms.
Methods: Participants applied the topical dry scalp treatment 2– 3 times per week for two weeks. Safety and efficacy of the topical treatment was determined through physician assessment using the validated Investigator’s Global Assessment (IGA) scale and the Total Severity Scale (TSS) during pre- and post-treatment clinic visits as well as parent reports at baseline, 1-week midpoint, and 2-week exit.
Results: Use of the topical treatment was associated with reduced symptoms of itchiness, dryness, irritation, and flakiness in children. The average IGA score was 3.0 at baseline and 2.0 after treatment, corresponding to a score difference of 1.0 (p < 0.001, 95% CI: 0.7, 1.2). The TSS score difference was 1.9 (p < 0.001, 95% CI: 1.4, 2.4). The total parent-reported scalp severity score decreased from 16.6 (95% CI: 14.8, 18.4) to 12.4 (p < 0.001, 95% CI: 11.0, 13.7) at 2-week exit.
Discussion: Study results mirror those reported in a study conducted in adults and point to the safety and efficacy of this natural topical treatment in reducing dry scalp symptomology in children. Based on our data, the combination of probiotic extracts and other anti-inflammatory ingredients appears to improve overall scalp health and appearance, though further studies will need to be conducted to further elucidate the link between clinical improvement and a balanced scalp microbiome.
Keywords: dry scalp, dandruff, seborrheic dermatitis, atopic disease, microbiome, probiotic
Background
Dry scalp conditions, such as dandruff, seborrheic dermatitis, and atopic dermatitis affect a significant portion of the population, including children.1 A study conducted in 2005 revealed that seborrheic dermatitis (SD) was prevalent in 6% of children aged 2 to 10 years.2 The impact of severe cases of SD on both parents and children often manifests as psychological distress and low self-esteem, indicating a considerable burden associated with this condition.3
In recent years, studies have highlighted the potential contribution of changes in the skin’s bacterial and fungal community, known as the skin microbiome, to the exacerbation of inflammation in atopic skin.4 Comparative analyses of the scalp microbiome in individuals with healthy scalps and those with dry scalp conditions have demonstrated significant differences in the relative abundance of Staphylococcus, Propionibacterium, and Malassezia.5–7 Moreover, emerging evidence has indicated the potential improvement of atopic symptoms through altering the skin microbiome.8
Considering the role the microbiome plays in atopic disease, our team developed a topical treatment primarily consisting of probiotic extracts (Lactobacillus spp.), honey, turmeric, and vitamin B12 to restore a healthy skin microbiome. Honey and turmeric were included in the product in conjunction with probiotic extracts for their prebiotic, antioxidant, and anti-inflammatory properties, which have been used to treat a plethora of dermatologic diseases.9–12 The preliminary phase of the clinical trial was conducted in adults 18 and older with dry scalp and dandruff symptoms.13 The study found that the topical dry scalp treatment reduced clinical symptoms and disease severity of the dry scalp condition in adults. Given the prevalence of dry scalp in the pediatric population and the results of the adult study, this study aimed to determine the safety and efficacy of the topical treatment in reducing dry scalp symptoms in children. We hypothesize that the use of the topical treatment will safely reduce clinical symptoms of dry scalp in a pediatric population.
Methods
To examine the efficacy of the topical treatment in children, our study was conducted on children ages 1–17 with dry scalp and dandruff symptoms as determined by a trained research physician. The interventional study was conducted as an Investigational New Drug with the FDA, approval from our Institutional Review Board (IRB), and registered as a clinical trial. Participants diagnosed with other scalp diseases such as psoriasis, tinea capitis, and pediculosis capitis, who had used systemic steroids or oral antibiotics in the past two months, or who had an allergy to any of the preparation components were excluded. Forty-two children with dry scalp were recruited to participate in the study for two weeks between April 2022 and April 2023.
During the study, participants completed a baseline clinic visit and post-treatment clinic visit where a trained research physician assessed the severity of dry scalp using validated scales, as described below. Between these visits, participants were instructed to liberally apply the topical treatment to the scalp, leave it on for five minutes, and rinse it off in the shower, repeating this process 2–3 times a week for two weeks. Participants also completed surveys at three time points of the study: baseline pre-treatment, 1-week midpoint, and 2-week exit. These surveys gathered information on participant demographics, parent-reported scalp condition history and current symptomology, as well as the efficacy and safety of the topical treatment.
To measure the effectiveness of the active topical treatment in improving clinical disease severity, a trained research physician employed the Investigator’s Global Assessment (IGA) scale and the Total Severity Scale (TSS) during pre- and post-treatment clinic visits. The IGA scale is a five-point assessment ranging from clear (0) to severe disease (4) to assess the overall severity of dry scalp.14 The TSS assessment averages severity scores for erythema (redness), scaling, and pruritus (itching) using a four-point scale ranging from none (0) to severe (3). Efficacy was also measured by parent-reported severity of itchiness, redness, sensitivity, dryness, irritation, and flakiness at baseline, 1-week midpoint, and 2-week exit on a scale of very mild (1) to very severe (5). To determine the safety of the active topical treatment, participants were asked to report if they experienced irritation or adverse effects at the midway point and the endpoint of the study.
Characteristics including demographics, socioeconomic status, and validated Scalpdex15 quality-of-life instrument scores were reported using simple frequencies for categorical data and means and standard deviations for continuous data. IGA, total TSS, and TSS sub-component scores were compared between baseline and after treatment with paired t-tests and a pre-determined Type I error rate of 0.05.16 Parent-reported scalp severity scores were compared between baseline, 1-week midpoint, and 2-week exit with paired t-tests between baseline and 2-week exit time points. All data analysis was completed with Stata 18.17
Results
In total, 42 participants were included in the analysis, of which 27 (64.3%) were female and 15 (35.7%) were male. All recruited participants completed the full duration of the study. The average age was 11.4 (SD: 4.0). Approximately 47.6% of the participants identified as Non-Hispanic White (NHW), 26.2% identified as Hispanic/Latino (H/L), 7.1% identified as Non-Hispanic Asian (NHA), and 4.8% identified as Non-Hispanic Black (NHB). Regarding socioeconomic status, 13 (31.0%) of participants had missing household income, however, among those that reported income, participants were relatively equally distributed among income strata. The average total Scalpdex score was 44.2 (SD: 21.1), with similar scores for the symptom subscore (44.5, SD: 17.8), emotional subscore (45.0, SD: 32.1), and functional subscore (41.2, SD: 20.8) (Table 1).
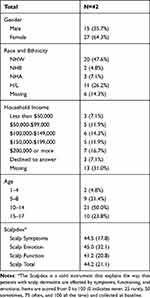 |
Table 1 Demographic Characteristics of Cohort |
The average IGA score at baseline was 3.0 (95% CI: 2.8, 3.3) and after the treatment, the average IGA score was 2.0 (95% CI: 1.8, 2.2), corresponding to a score difference of 1.0 (95% CI: 0.7, 1.2). The total TSS score difference comparing baseline to post-treatment was 1.9 (95% CI: 1.4, 2.4). With respect to the sub-components, similar changes were seen before and after treatment between the scaling and pruritis sub-components (score difference 0.7, 95% CI: 0.5, 1.0). A difference of 0.4 (95% CI: 0.2, 0.7) was observed for the change in erythema. All differences in IGA scores, TSS, and TSS sub-component scores were significantly different (p < 0.001) (Table 2).
 |
Table 2 IGA and TSS Scores Physician Assessments Before and After Treatment |
The total parent-reported scalp severity score decreased from 16.6 (95% CI: 14.8, 18.4) at baseline to 12.4 (95% CI: 11.0, 13.7) at the 2-week exit. The subcomponents for which the baseline and 2-week exit survey score showed significant difference were itchiness (0.8, 95% CI: 0.3, 1.2), dryness (1.7, 95% CI: 1.3, 2.0), irritation (0.7, 95% CI: 0.1, 1.2), and flakiness (1.3, 95% CI: 0.9, 1.8). No significant differences between baseline and post-treatment exit surveys were observed for redness (−0.2, 95% CI: −0.7, 0.4) and sensitivity (0.4, 95% CI: −0.1, 1.0). The treatment was also well tolerated (Table 3). One participant reported the development of pimples on the scalp. However, all participants reported that the topical scalp treatment was gentle, without skin, scalp, or eye irritation.
 |
Table 3 Parent-Reported Dry Scalp Symptom Severity Scores Reported on a Scale of Very Mild (1) to Very Severe (5) |
Discussion
Data from the pediatric study of this novel topical treatment for dandruff and dry scalp demonstrates that the topical dry scalp product is associated with reduced clinical symptoms of dry scalp including erythema, scaling, and pruritis. Parent reports also indicate a significant decrease in itchiness, dryness, irritation, and flakiness post-treatment. While parents found that redness did not significantly improve between baseline and post-treatment, physician assessment through TSS score did show significant difference in redness post-treatment, with a similar reduction previously reported in our study conducted with adult participants. This may be attributed to specialized physician training to observe redness on different skin tones as well as on skin that may be covered by hair. IGA scores in children at baseline were higher than what was previously reported in the adult cohort, however the change in IGA and TSS scores post-treatment were similar in both groups.13 There were no reports of skin, scalp, or eye irritation. The results of the study point to the safety and efficacy of the topical treatment to relieve dry scalp symptomology in children.
Studies have shown dry scalp symptoms may be related to an imbalance of the skin’s microbiome.7 Based on the anti-inflammatory and probiotic properties of the honey, turmeric, and probiotic extracts in the topical scalp treatment, we hypothesize that improvement to dry scalp symptoms may be related to a shift and rebalancing of the skin’s microbiome. However, further research is required to assess the relationship between a balanced skin microbiome and the observed improvement in dry scalp symptoms. One limitation of the study is that while participants were recruited widely through a hospital base network, they were all seen at one clinic, limiting the geographic diversity of our sample. A more diverse participant population from multiple clinics or regions would provide a broader perspective and enhance the generalizability of the findings. Another limitation of the pre/post study design is that all participants received the topical ointment treatment. Although outside the scope of the current study, a randomized, double-blind, control trial would be more effective in reducing patient and physician bias for dry scalp symptom improvement and inferring causality. Additional randomized-control studies would be beneficial in associating causality, however, the primary aim of this study, to demonstrate the safety and efficacy of our topical treatment for children, was met.
Conclusion
The present study measures the safety and efficacy of a novel topical treatment in reducing dry scalp symptoms in children. We found that the combination of probiotic extracts, honey, turmeric, and vitamin B12 as a natural topical application improves overall scalp health and appearance in children experiencing dry scalp symptoms. While overall conditions for scalp health and appearance improved through topical treatment use, further studies will aid in determining the association between clinical improvement and alteration in the scalp microbiome.
IRB Approval
Ann and Robert H. Lurie Children’s Hospital of Chicago, IL. IRB 2019–2309.
Data Sharing Statement
Upon publication of study findings, deidentified data presented in the manuscript and data collection instruments will be made available to interested parties by request. Validated tools used for assessment may also be shared. Data will be retained for 10 years. Please Contact Lucy Bilaver: [email protected] for data requests.
Ethical Considerations
The protocol for this study was approved by the Institutional Review Board of Northwestern University and Lurie Children’s Hospital of Chicago and complies with the Declaration of Helsinki. Written informed consent was obtained from all participants, including parental/legal guardians. Clinical trial registration number: NCT03830177.
Acknowledgments
The authors thank Jennifer Long and Dr. Waheeda Samady, without whose contributions to the present manuscript would not have been possible.
Funding
Funded by Yobee Care Inc.
Disclosure
Dr. Bilaver receives research support from the National Institutes of Health (NIH) (R21 ID # 1AI159562 AI135705, R01 ID # AI130348, U01 ID # AI138907), Food Allergy Research & Education (FARE), Thermo Fisher Scientific, and Genentech. She is currently employed by Northwestern University and is an Associate Professor of Pediatrics at Northwestern University Feinberg School of Medicine. The authors report no other conflicts of interest in this work.
References
1. Borda LJ, Wikramanayake TC. Seborrheic dermatitis and dandruff: a comprehensive review. J Clin Investig Dermatol. 2015;3(2). doi:10.13188/2373-1044.1000019
2. Williams JV, Eichenfield LF, Burke BL, Barnes-Eley M, Friedlander SF. Prevalence of scalp scaling in prepubertal children. Pediatrics. 2005;115(1):e1–6. doi:10.1542/peds.2004-1616
3. Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55(3):490–500. doi:10.1016/j.jaad.2006.05.048
4. Kim JE, Kim HS. Microbiome of the skin and gut in Atopic Dermatitis (AD): understanding the pathophysiology and finding novel management strategies. J Clin Med. 2019;8(4):444.
5. Clavaud C, Jourdain R, Bar-Hen A, et al. Dandruff is associated with disequilibrium in the proportion of the major bacterial and fungal populations colonizing the scalp. PLoS One. 2013;8(3):e58203. doi:10.1371/journal.pone.0058203
6. Nakatsuji T, Chen TH, Narala S, et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci Transl Med. 2017;9(378):eaah4680. doi:10.1126/scitranslmed.aah4680
7. Park T, Kim HJ, Myeong NR, et al. Collapse of human scalp microbiome network in dandruff and seborrhoeic dermatitis. Exp Dermatol. 2017;26(9):835–838. doi:10.1111/exd.13293
8. Myles IA, Earland NJ, Anderson ED, et al. First-in-human topical microbiome transplantation with Roseomonas mucosa for atopic dermatitis. JCI Insight. 2018;3(9). doi:10.1172/jci.insight.120608
9. Vaughn AR, Branum A, Sivamani RK. Effects of turmeric (curcuma longa) on skin health: a systematic review of the clinical evidence. Phytother Res. 2016;30(8):1243–1264. doi:10.1002/ptr.5640
10. Burlando B, Cornara L. Honey in dermatology and skin care: a review. J Cosmet Dermatol. 2013;12(4):306–313. doi:10.1111/jocd.12058
11. Schell KR, Fernandes KE, Shanahan E, et al. The potential of honey as a prebiotic food to re-engineer the gut microbiome toward a healthy state. Front Nutr. 2022;9:957932. doi:10.3389/fnut.2022.957932
12. Ghiamati Yazdi F, Soleimanian-Zad S, van den Worm E, Folkerts G. Turmeric extract: potential use as a prebiotic and anti-inflammatory compound? Plant Foods Hum Nutr. 2019;74(3):293–299. doi:10.1007/s11130-019-00733-x
13. Xiao A, Warren C, Samady W, Bilaver L. Novel topical treatment for dandruff & dry scalp through sustained balance in skin microbiome. Clin Cosmet Investig Dermatol. 2021;14:945–947. doi:10.2147/CCID.S321238
14. Langley RG, Feldman SR, Nyirady J, van de Kerkhof P, Papavassilis C. The 5-point Investigator’s Global Assessment (IGA) Scale: a modified tool for evaluating plaque psoriasis severity in clinical trials. J Dermatolog Treat. 2015;26(1):23–31. doi:10.3109/09546634.2013.865009
15. Chen SC, Yeung J, Chren -M-M. Scalpdex: a quality-of-life instrument for scalp dermatitis. Arch Dermatol. 2002;138(6):803–807. doi:10.1001/archderm.138.6.803
16. Lee SW. Methods for testing statistical differences between groups in medical research: statistical standard and guideline of life cycle committee. Life Cycle. 2022;2:e1. doi:10.54724/lc.2022.e1
17. StataCorp. STATA Statistical Software: Release 18. College Station, TX: StataCorp LLC; 2023.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
