Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Natural Plant Extract – Loganin: A Hypothesis for Psoriasis Treatment Through Inhibiting Oxidative Stress and Equilibrating Immunity via Regulation of Macrophage Polarization
Authors Chen X, Deng Q, Li X, Xian L , Xian D, Zhong J
Received 4 November 2022
Accepted for publication 12 January 2023
Published 13 February 2023 Volume 2023:16 Pages 407—417
DOI https://doi.org/10.2147/CCID.S396173
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Xiaofeng Chen,1,* Qiyan Deng,1,* Xiaolong Li,1,* Li Xian,2,* Dehai Xian,3 Jianqiao Zhong1
1Department of Dermatology, The Affiliated Hospital of Southwest Medical University, Luzhou, 646000, People’s Republic of China; 2Department of Emergency, The Affiliated Hospital of Southwest Medical University, Luzhou, 646000, People’s Republic of China; 3Department of Anatomy, Southwest Medical University, Luzhou, 646000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jianqiao Zhong, Email [email protected]; Dehai Xian, Email [email protected]
Abstract: Psoriasis, a chronic immune-mediated inflammatory skin disease, influences approximately 2– 3% of the world’s population. At present, the etiology of psoriasis remains unclear and there is still no causal treatment available. Recent studies indicate that oxidative stress (OS) and T cells dysregulation may participate in the pathogenesis of psoriasis, among which M1-dominant macrophage polarization is a crucial contributor. Macrophages mainly polarize into two different subsets, ie, classically activated macrophage (M1) and alternatively activated macrophage (M2). M1 polarization tends to exacerbate psoriasis via producing substantial reactive oxygen species (ROS) and inflammatory mediators, to encourage OS invasion and T cells dysregulation. Thus, targeting M1 polarization can be a possible therapeutic alternative for psoriasis. Loganin, belonging to iridoid glycosides, is a pharmaceutically active ingredient originated from Cornus officinalis, exerting multiple biological activities, eg, immunomodulation, antioxidation, anti-inflammation, etc. More importantly, it could effectively suppress M1 polarization, thereby arresting OS aggression and T cells’ dysregulation. Numerous studies have confirmed that loganin is quite reliable for diseases treatment via suppressing M1 polarization. Nevertheless, reports about loganin treating psoriasis have seldom appeared so far. Accordingly, we hold a hypothesis that loganin would availably manage psoriasis through preventing M1 polarization. Data from previous studies guarantee the potential of loganin in control of psoriasis.
Keywords: loganin, psoriasis, macrophage polarization, oxidative stress, T cells
Introduction
Psoriasis is a chronic and systemic, immune-mediated inflammatory disorder, histologically featuring as abnormal keratinocytes (KCs) proliferation/differentiation, angiogenesis, and immune/inflammatory cells’ infiltration1–3 It passively affects about 2–3% of the global individuals involving their mental and physical health, but few satisfactory treatments exist until now.4 Currently, therapeutic strategies for psoriasis are varied covering topical medications (emollients, topical corticosteroids, retinoid derivatives, synthetic vitamin D analogues, calcineurin inhibitors and keratolytics), systemic agents (methotrexate, cyclosporine, retinoids, and even the biological agents) and phototherapy; their application, however, unfolds a certain limitation due to their short efficacy, easy recurrence, serious side effects, and high costs5–7 Consequently, it would be helpful to develop a novel pharmaceutical product for psoriasis.
Since the specific pathogenesis of psoriasis remains somewhat unknown, oxidative stress (OS), T cells dysregulation, and M1 macrophage polarization could make a significant contribution to this part8,9 In general, macrophages roughly differentiate into two different subsets, namely classically activated macrophage (M1) and alternatively activated macrophage (M2)10; this is so-called macrophage polarization. Increasing evidence has confirmed that M1-dominated macrophage polarization positively works in the pathogenesis of psoriasis and the enhancement of M1 is proportional to the severity of psoriasis.11 Numerous studies, meanwhile, demonstrate that M1 polarization closely goes together with the aberrant expression of keratin in KCs and the formation of keratosis, erythema, and scales.12 Moreover, through generation of excessive reactive oxygen species (ROS) and inflammatory factors, M1 polarization facilitates OS occurrence and T cells dysregulation further to aggravate psoriasis. Therefore, it would be promising for psoriasis treatment by targeting macrophage polarization.
Loganin, a natural plant extract from Cornus officinalis, possesses immunomodulatory, antioxidant, anti-inflammatory, and anti-proliferative activities with few cytotoxic side effects,13,14 Growing data support that loganin is quite safe and effective in various diseases, such as inflammatory bowel disease (IBD), atopic dermatitis (AD), osteoarthritis, pancreatitis, neuroinflammation, diabetes, and so on, mainly by inhibiting M1 polarization.13–19 However, the effect of loganin on psoriasis has scarcely been reported up to now, so we propose the possibility of loganin in psoriasis treatment based on psoriasis pathogenesis and loganin’s function. Hence, we review the potential evidence of loganin in the management of psoriasis.
Possible Pathogenesis of Psoriasis
As a multifactorial disease, psoriasis etiologically keeps complex and unclear, possibly involving genetic (eg, some histocompatibility complexes and susceptibility genes, like HLACw6, HLACw7, IL-12B, IL-23R, TNFAIP3, etc), environmental (bacterial/viral infections, trauma, stress, etc), and immunological factors.20 Studies in increasing numbers have verified that OS and T cells dysregulation are important players in the pathogenesis of psoriasis,8,9 whereas excessive recruitment and activation of M1 promote ROS accumulation, OS aggression and T cells imbalance, thereby aggravating the uncontrolled psoriatic lesions and inflammation21–23 Furthermore, the peripheral blood monocytes of psoriasis patients are more inclined to M1 polarization rather than M2 polarization, resulting in a higher proportion of M1/M2 in psoriasis individuals.11 The following section will focus on the relationship between macrophage polarization and OS as well T cells’ dysregulation in the pathogenesis of psoriasis.
Macrophage Polarization and Its Role in Psoriasis
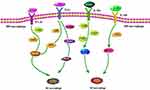
Macrophages, a group of innate immune cells derived from peripheral blood monocytes, functionally mediate in homeostasis maintenance, inflammatory response, angiogenesis, tissue repair, and so on.24 Under the stimulation of microenvironment signals, peripheral blood monocytes tend to differentiate into Naïve macrophages (Mφ, also called M0) and then polarize into two main subtypes: M1 and M2,25,26 M1, marked by CD86, CD40, and CD80, primarily serves inflammation and cell/tissue injury; while M2, featuring the expression of CD163, CD206, and CD209, has functions of anti-inflammation and tissue repair,27,28 M1 polarization is largely driven by T helper 1 (Th1) cell-oriented cytokines [especially interferon-γ (IFN-γ)] and microbial products [like lipopolysaccharide (LPS).29 IFN-γ and LPS first bind to the corresponding receptors on the surface of macrophages – IFN-γ receptor (IFN-γR) and Toll-like receptor-4 (TLR4). The combination of IFN-γ with IFN-γR inspires Janus kinase 1/2 (JAK1/2) and signal transducer and activator of transcription 1 (STAT1) phosphorylation, and further triggers M1 polarization.25,30 On the other side, LPS coupling TLR4 recruits the signals like TIR domain-containing adaptor protein inducing IFNβ (TRIF) and myeloid differentiation factor 88 (MyD88), subsequently exciting the downstream-related factors, ie, interferon regulatory factor 3 (IRF3), TNF receptor-associated factor 6 (TRAF-6), STAT1 and nuclear factor kappa B (NF-κB), ultimately initiating M1 polarization.25,31–36 Instead, M2 polarization occurs in the presence of Th2 cytokines (mostly IL-4/13 and IL-10).37 IL-4/13 and IL-10 in combination with their corresponding receptor (IL4Rα) mainly stimulate the activation of JAK1/2/3, STAT6, and STAT3, thus spurring macrophage polarization towards M2.38,39 The process of M1 and M2 macrophage polarization is clearly sketched in Figure 1.
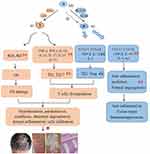
Numerous studies have revealed that M1-dominated polarization acts as a vital player in psoriasis.10,11 M1-polarized macrophages could generate excessive ROS and inflammatory factors in the persistence of psoriatic inflammatory stimulation.9,40 The activation of M1 provokes a variety of inflammatory mediators release, such as antigen-presenting molecules (MHC-II), chemokines (CXCL8-11, NO, ROS), and inflammatory cytokines (TNF-α, IFN-γ, IL-1β, IL-12, IL-17, IL-23, IL-27, etc).,41,42 further triggering OS occurrence and T cells dysregulation (Th1/Th17 cells overactivation) as well as Th1/Th17-derived cytokines hypersecretion (mainly IL-17, IFN-γ, TNF-α, IL-22, and IL-23).43 Moreover, substantial M1-produced ROS induces oxidative damage that aggravates psoriatic complaint.11,40 Through production of superfluous ROS, M1 could exacerbate OS damage and inflammatory response.44,45 As a result, aggressive OS and activated Th1/Th17 cells encourage the initiation and progression of skin inflammation via secreting the above inflammatory cytokines, thus accelerating the tissue damage in psoriasis patients8,9,46,47 Along with the relative inhibition of M2 in psoriasis, on the contrary, M2-secreted chemokines (such as CCL17, CCL18, CCL22, and CCL24) and anti-inflammatory cytokines [like transforming growth factor-β (TGF-β), IL-10 and IL-4] decrease remarkably, which are unfavorable for tissue repair and immunosuppression.34,48,49 At present, numerous studies have demonstrated that macrophage polarization critically serves in immune, inflammatory or tumor diseases, eg, psoriasis, IBD, AD, Behcet’s disease (BD), atherosclerosis (AS), systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), systemic sclerosis (SS), infantile hemangioma (IH), etc.9,10,14,16,27,50 Specifically, based on psoriasis pathogenesis, suppressing M1 and/or shifting M1 towards M2 would be favorable strategies to settle this complaint. Figure 2 shows the mediation of macrophage polarization in psoriasis pathogenesis.
M1 Polarization Exacerbating OS Damage in Psoriasis
ROS are mostly originated from macrophages and crucially serve many inflammatory diseases like psoriasis, vitiligo, SLE, BD, AD, AS, and cancer, psoriasis in particular14,51–53 As the main contributor to OS, ROS are a collective term covering a series of active oxygen-containing compounds, such as superoxide (O2−), hydrogen peroxide (H2O2), hydroxyl radical (OH), lipid hydroperoxide (LOOH), and so on.54 High-level ROS on one side enhance the expression of malondialdehyde (MDA) and inducible nitric oxide synthase (iNOS), on the other side lower the activity of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidases (GSH-px).55 It is confirmed that M1 frequently consumes more nicotinamide adenine dinucleotide phosphate (NADPH) to promote ROS overproduction and OS initiation.51,56 More than that, some proinflammatory factors from the microenvironment could drive M1 polarization that further accelerates the excessive formation of ROS.44 Meanwhile, increasing evidence supports that M1 from psoriatic skin far exceed those from normal skin, thereby producing substantial ROS as well inflammatory cytokines that further initiate OS and switch on a series of pathogenic events in psoriasis.9,11,22,23 The excessive and sustained stimulation of ROS in turn encourage the enhancement of intracellular oxidative metabolites and the impairment of antioxidant defense that contribute to more ROS accumulation, the formation of oxidized lipids and denatured protein, and the destruction of nucleic acids, finally resulting in cytotoxicity and damaging cells/tissues57,58 Moreover, ROS aggressively activate transcription factors in KCs as well as VEGF-independent/VEGF-dependent pathways to facilitate psoriatic KCs hyperproliferation and abnormal angiogenesis in the dermis, contributing to psoriasis progression59,60 Notably, ROS conversely induce and maintain M1 polarization via activating MAPK and NF-κB signals, which constantly stimulate pro-inflammatory genes expression in macrophages and aggravate OS damage to psoriasis individuals.44,61
M1 Polarization Facilitating T Cell Dysregulation in Psoriasis
It has been proved that macrophages impact greatly on the proliferation, phenotype, and subtype of T cells. In general, T cells coexist and interact with macrophages at the site of inflammation, correspondingly the direction of macrophage polarization influencing the activation and differentiation of CD4+ T cells62,63 In the presence of signals from the microenvironment, CD4+ T cells often differentiate into kinds of functional subtypes (eg, Th1/Th17 cells and Th2/Treg cells).64 Usually, M1/M2 keeps a dynamic equilibrium; in psoriasis, however, this balance is disturbed, leading to T cells dysregulation.11 Stimulated by uncontrolled and sustained inflammatory signals from the psoriatic microenvironment, macrophages are heavily skewed towards M1; conversely, M2 activation is relatively suppressed.9 M1 polarization actively induces the differentiation/activation of Th1 or Th17 phenotype rather than Th2/Treg cells through secreting IL-12 or IL-23 respectively.65,66 The activation of Th1 and Th17 cells activation further induces neutrophils/NK cells infiltration, KCs hyperproliferation, and epidermal thickening, thereby inciting the development of psoriasis.67 On the other hand, M1-produced chemokines, eg, CXCL9-11, attract T cells migration to the inflammatory sites and then arouse T cells-mediated inflammatory response.68 In turn, activated Th1/Th17 cells and Th1/Th17-secreted proinflammatory cytokines encourage more M1 polarization to aggravate inflammatory progression and accelerate tissue damage, through releasing diverse inflammatory mediators (IFN-γ, TNF-α, IL-12, IL-17, IL-23, etc) 69–71 These create a vicious circle, thereby accelerating the development of inflammation and the damage of tissue in psoriasis.
All events above contribute to the occurrence and progression of psoriasis. Taken together, M1 polarization serves in psoriasis as a critically promoting factor, thus inhibition of M1 polarization may be a potential target for this disease cure.
Biological Properties and Applications of Loganin
Loganin, subordinate to iridoid glycoside, is the main active ingredient extracted from Cornus officinalis. It exerts a broad range of biological activities, containing immunomodulation, antioxidation, anti-inflammation, neuroprotection, etc.13–15,72 As a traditional Chinese herbal medicine, Cornus officinalis covers more than 300 chemical compounds, particularly loganin being notable.73
The convincing proofs as following have manifested that loganin not only prevents M1 polarization but also provokes M2 polarization, thus laying a theoretical and experimental foundation for treating OS and immunoinflammatory-related diseases,16,74,75 Previous documents proved that loganin effectively inhibited M1 polarization and induced M2 activation, going on to encourage the depletion of ROS, iNOS, IL-6, TNF-α, and IL-1β as well as the elevation of anti-inflammatory cytokines, SOD, CAT, and GSH-Px through blocking the TLR4/NF-κB signaling pathway.74–77 Similar reports also showed that loganin remarkably reduced the expression of the pro-inflammatory chemokines/cytokines (including IL-6, TNF-α, IL-1β, CXCL10, and COX-2) via modulating M1/M2 polarization, further alleviating the chronic inflammatory disease called ulcerative colitis (UC) on mice.16 Apart from that, Cui et al discovered that loganin could prevent BV-2 microglia cells from polarizing to M1 phenotype and cut down the production of IL-6, TNF-α, NO, iNOS, partially through jamming TLR4/TRAF6/NF-κB signals.15,78 Findings from Quah et al showed that the extracts of Cornus officinalis, particularly loganin, were possibly applied in the treatment of AD and could significantly decrease the production of iNOS, NO, and pro-inflammatory cytokines (IL-6, IL-1β, and TNF-α) in LPS-induced macrophage (M1) through down-regulation the activation of NF-κB pathway.14 A recent study, using network pharmacology combined with laboratory verification, has revealed that loganin could availably regulate T cells differentiation and IL-17-related signaling pathway in the M1-dominated inflammatory model.78 Taken together, loganin, as an excellent anti-inflammatory, immunomodulatory, and antioxidant herbal medicine, could effectively regulate macrophage polarization to eliminate ROS, mitigate inflammatory factors and equilibrate immune cells. Thus, based on its multiple pharmacological effects, loganin nowadays has become extensively available for various diseases, especially those associated with OS and immune inflammation; however, reports about loganin application in psoriasis scarcely emerge yet. Therefore, on the basis of above studies, we speculate that loganin would potentially control psoriasis through reducing OS and balancing immunity as well preventing inflammation via inhibition of M1 polarization.
Hypothesis for Loganin in the Treatment of Psoriasis
Considering that M1 polarization-mediated OS and immune imbalance critically contribute to psoriasis and loganin has the ability to prevent OS aggression and balance T cells via curbing M1 polarization, we hypothesized that loganin would be a quite promising agent for psoriasis recovery. Here, a series of proofs are provided as follows to support the potential of loganin in treating psoriasis: (1) Psoriasis is a chronic, immune-mediated inflammatory skin disease.1–3 (2) The pathogenesis of psoriasis mainly involves OS insult and immune inflammation.51,79 (3) M1 polarization triggers OS damage and T cells imbalance in psoriasis.14–16,73 (4) Several studies have confirmed that M1 remarkably goes up in psoriasis and M1-dominated polarization is a crucial player in psoriasis, which contribute to the appearance of elevated ROS and unbalanced T cells.9,21–23 (5) Loganin exerts powerfully immunomodulatory, antioxidant, and anti-inflammatory properties, with few side/toxic effects.13,14,80 (6) Loganin potently prohibits M1 polarization and shifts M1 towards M2, which is beneficial to the differentiation of T cells to Treg cells, the reduction of ROS and IFN-γ, IL-β, TNFα as well as the amelioration of inflammatory state in KCs.14,81 (7) Loganin has been employed in controlling numerous OS-related or immune-inflammatory diseases through decreasing M1/M2 ratio, enhancing antioxidant defense, arresting OS damage, and regulating T cells balance.14,16,27,81
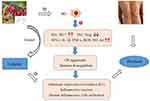
The supposed process of loganin in the management of psoriasis is elaborately framed based on its inhibition of OS, equilibrium of T cells, and suppression of inflammatory factors/pathways via regulating macrophage polarization (shown in Figure 3).
Clinical Significance
As a chronic, recurrent, inflammatory disease that seriously bears upon both people’s mental and physical health, psoriasis has become a highlight in the field of dermatology. Although various therapeutic measures are employed in psoriasis, some disadvantages confine themselves to a long-time application, eg, transient efficacy, easy recurrence, high costs, severe side effects, etc. Hence, a safer, more effective, and economical antipsoriatic drug is urgently required. Loganin, a naturally active compound with diverse bioactivities and few adverse reactions, has been widely used in various immune-mediated inflammatory diseases;13,15–19 consequently, it is infinitely preferable to psoriasis treatment owing to its antioxidant, immunoregulatory, and anti-inflammatory activities that would be likely to prevent OS damage and equilibrate T cells through inhibiting M1 polarization and shifting M1 towards M2.10–14
Future Research
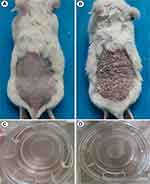
To prove this hypothesis and clarify the role of loganin in psoriasis, future research in vivo and in vitro will be performed on animal models and three-dimensional skin models. Psoriasis-associated indicators, meanwhile, need to be inspected containing clinical symptoms/lesions, histopathological changes, cytological, and serological alterations like cutaneous erythema/scales, epidermal parakeratosis/hyperkeratosis, granular layer deficiency, spinous layer hypertrophy, telangiectasia, inflammatory cells infiltration in the dermis, T cells/macrophages quantities and classification, and inflammatory/OS-related indexes levels, regardless of loganin presence or absence. In vivo studies, a well-established psoriasis-like mouse model, as successfully built in our previous study (shown in Figure 4), will be employed to evaluate the effects of loganin; while in vitro experiments, based on psoriasis-like three-dimensional skin models and M1/M2 polarization models, provide a guarantee for exploring the specific mechanism of loganin against psoriasis. The psoriasis-like three-dimensional skin models have also been successfully established in our previous experiments.82 Beyond that, randomized placebo-controlled clinical trials may offer scientific proofs for the clinical application of loganin.
Conclusions
In conclusion, psoriasis as a complex inflammatory disease tends to recur and impair skin, joints, and other organs, negatively influencing individual health. At present, it has been confirmed that the pathogenesis of psoriasis involves M1-dominated macrophage polarization.20 Hence, a great hope of cure is unfolded for psoriasis by targeting these key points. Currently, the therapies aiming at some crucial pathways or factors or cells have displayed a positive efficacy on psoriasis in practice, but their side effects become obstacles to different extent;5–7 especially, the recent-year developed biologics are outstanding for psoriasis treatment; for example, the main four-class inhibitors comprising TNF inhibitors (infliximab, etanercept, adalimumab, and certolizumab), IL-17 inhibitors (secukinumab, ixekizumab, brodalumab), IL-23 inhibitors (guselkumab, tildrakizumab, risankizumab), and IL-12/23 inhibitor (ustekinumab) are most frequently applied in psoriasis.5 Despite the fine effect of biologics, they prevent individuals from using due to their high expenditure, uncertain long-term efficacy and obvious adverse effects. Consequently, safe and cost-effective pharmaceutical products are required. Herein, we elaborate on the pathogenesis of psoriasis and exhibit the activities and applications of loganin. The available cited evidence indicate that loganin would be a suitable alternative for psoriasis treatment and could be applied along with other drugs. Actually, further experiments in vivo and in vitro will be needed to attest the recommended pathogenesis in psoriasis and the improvements in psoriasis parameters following loganin therapy.
Disclosure
Xiaofeng Chen, Qiyan Deng, Xiaolong Li and Li Xian are the co-first authors for this study. The authors declare that there is no conflict of interest regarding the publication of this paper.
References
1. Su W, Wei Y, Huang B, Ji J. Identification of hub genes and immune infiltration in psoriasis by bioinformatics method. Front Genet. 2021;12:606065. doi:10.3389/fgene.2021.606065
2. Branisteanu DE, Cojocaru C, Diaconu R, et al. Update on the etiopathogenesis of psoriasis (review). Exp Ther Med. 2022;23(3):201. doi:10.3892/etm.2022.11124
3. Puig L, Costanzo A, Muñoz-Elías EJ, et al. The biological basis of disease recurrence in psoriasis: a historical perspective and current models. Br J Dermatol. 2022;186(5):773–781. doi:10.1111/bjd.20963
4. Parab S, Doshi G. An update on emerging immunological targets and their inhibitors in the treatment of psoriasis. Int Immunopharmacol. 2022;113(Pt A):109341. doi:10.1016/j.intimp.2022.109341
5. Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323(19):1945–1960. doi:10.1007/BF03325637
6. Mascarenhas-Melo F, Carvalho A, Gonçalves MBS, Paiva-Santos AC, Veiga F. Nanocarriers for the topical treatment of psoriasis - pathophysiology, conventional treatments, nanotechnology, regulatory and toxicology. Eur J Pharm Biopharm. 2022;176:95–107. doi:10.1016/j.ejpb.2022.05.012
7. Nogueira S, Rodrigues MA, Vender R, Torres T. Tapinarof for the treatment of psoriasis. Dermatol Ther. 2022;35(12):e15931. doi:10.1111/dth.15931
8. Wang PW, Lin TY, Yang PM, Fang JY, Li WT, Pan TL. Therapeutic efficacy of Scutellaria baicalensis Georgi against psoriasis-like lesions via regulating the responses of keratinocyte and macrophage. Biomed Pharmacother. 2022;155:113798. doi:10.1016/j.biopha.2022.113798
9. Li L, Zhang HY, Zhong XQ, et al. PSORI-CM02 formula alleviates imiquimod –induced psoriasis via affecting macrophage infiltration and polarization. Life Sci. 2020;243:117231. doi:10.1016/j.lfs.2019.117231
10. Hou Y, Zhu L, Tian H, et al. IL-23-induced macrophage polarization and its pathological roles in mice with imiquimod-induced psoriasis. Protein Cell. 2018;9(12):1027–1038. doi:10.1007/s13238-018-0505-z
11. Lin SH, Chuang HY, Ho JC, Lee CH, Hsiao CC. Treatment with TNF-α inhibitor rectifies M1 macrophage polarization from blood CD14+ monocytes in patients with psoriasis independent of STAT1 and IRF-1 activation. J Dermatol Sci. 2018;91(3):276–284. doi:10.1016/j.jdermsci.2018.05.009
12. Alalaiwe A, Chen CY, Chang ZY, Sung JT, Chuang SY, Fang JY. Psoriasiform inflammation is associated with mitochondrial fission/GDAP1L1 signaling in macrophages. Int J Mol Sci. 2021;22(19):10410. doi:10.3390/ijms221910410
13. Kim MJ, Bae GS, Jo IJ, et al. Loganin protects against pancreatitis by inhibiting NF-κB activation. Eur J Pharmacol. 2015;765:541–550. doi:10.1016/j.ejphar
14. Quah Y, Lee SJ, Lee EB, et al. Cornus officinalis ethanolic extract with potential anti-allergic, anti-inflammatory, and antioxidant activities. Nutrients. 2020;12(11):3317. doi:10.3390/nu12113317
15. Cui Y, Wang Y, Zhao D, Feng X, Zhang L, Liu C. Loganin prevents BV-2 microglia cells from Aβ1-42 -induced inflammation via regulating TLR4/TRAF6/NF-κB axis. Cell Biol Int. 2018;42(12):1632–1642. doi:10.1002/cbin
16. Liu S, Shen H, Li J, et al. Loganin inhibits macrophage M1 polarization and modulates sirt1/NF-κB signaling pathway to attenuate ulcerative colitis. Bioengineered. 2020;11(1):628–639. doi:10.1080/21655979.2020.1774992
17. Yang Y, Gu Y, Zhao H, Zhang S. Loganin attenuates osteoarthritis in rats by inhibiting il-1β-induced catabolism and apoptosis in chondrocytes via regulation of phosphatidylinositol 3-kinases (PI3K)/Akt. Med Sci Monit. 2019;25:4159–4168. doi:10.12659/MSM.915064
18. Mo FF, Liu HX, Zhang Y, et al. Anti-diabetic effect of loganin by inhibiting FOXO1 nuclear translocation via PI3K/Akt signaling pathway in INS-1 cell. Iran J Basic Med Sci. 2019;22(3):262–266. doi:10.22038/ijbms.2019.30246.7294
19. Wang JW, Pan YB, Cao YQ, et al. Loganin alleviates LPS-activated intestinal epithelial inflammation by regulating TLR4/NF-κB and JAK/STAT3 signaling pathways. Kaohsiung J Med Sci. 2020;36(4):257–264. doi:10.1002/kjm2.12160
20. Vičić M, Kaštelan M, Brajac I, Sotošek V, Massari LP. Current concepts of psoriasis immunopathogenesis. Int J Mol Sci. 2021;22(21):11574. doi:10.3390/ijms222111574
21. Miki H, Han KH, Scott D, Croft M, Kang YJ. 4-1BBL regulates the polarization of macrophages, and inhibition of 4-1BBL signaling alleviates imiquimod-induced psoriasis. J Immunol. 2020;204(7):1892–1903. doi:10.4049/jimmunol.1900983
22. Wang H, Peters T, Sindrilaru A, Scharffetter-Kochanek K. Key role of macrophages in the pathogenesis of CD18 hypomorphic murine model of psoriasis. J Invest Dermatol. 2009;129(5):1100–1114. doi:10.1038/jid.2009.43
23. Fuentes-Duculan J, Suárez-Fariñas M, Zaba LC, et al. A subpopulation of CD163-positive macrophages is classically activated in psoriasis. J Invest Dermatol. 2010;130(10):2412–2422. doi:10.1038/jid.2010.165
24. Thapa B, Lee K. Metabolic influence on macrophage polarization and pathogenesis. BMB Rep. 2019;52(6):360–372. doi:10.5483/BMBRep.2019.52.6.140
25. Zhou D, Huang C, Lin Z, et al. Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cell Signal. 2014;26(2):192–197. doi:10.1016/j.cellsig.2013.11.004
26. Vergadi E, Ieronymaki E, Lyroni K, Vaporidi K, Tsatsanis C. Akt signaling pathway in macrophage activation and M1/M2 polarization. J Immunol. 2017;198(3):1006–1014. doi:10.4049/jimmunol.1601515
27. Funes SC, Rios M, Escobar-Vera J, Kalergis AM. Implications of macrophage polarization in autoimmunity. Immunology. 2018;154(2):186–195. doi:10.1111/imm
28. Cao J, Dong R, Jiang L, et al. LncRNA-MM2P identified as a modulator of macrophage M2 polarization. Cancer Immunol Res. 2019;7(2):292–305. doi:10.1158/2326-6066.CIR-18-0145
29. Shapouri-Moghaddam A, Mohammadian S, Vazini H, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233(9):6425–6440. doi:10.1002/jcp.26429
30. Hu X, Herrero C, Li WP, et al. Sensitization of IFN-gamma Jak-STAT signaling during macrophage activation. Nat Immunol. 2002;3(9):859–866. doi:10.1038/ni828
31. Zhu W, Xu R, Du J, et al. Zoledronic acid promotes TLR-4-mediated M1 macrophage polarization in bisphosphonate-related osteonecrosis of the jaw. FASEB J. 2019;33(4):5208–5219. doi:10.1096/fj.201801791RR
32. Ryan AE, Colleran A, O’Gorman A, et al. Targeting colon cancer cell NF-κB promotes an anti-tumour M1-like macrophage phenotype and inhibits peritoneal metastasis. Oncogene. 2015;34(12):1563–1574. doi:10.1038/onc.2014.86
33. Muraille E, Leo O, Moser M. TH1/TH2 paradigm extended: macrophage polarization as an unappreciated pathogen-driven escape mechanism? Front Immunol. 2014;5:603. doi:10.3389/fimmu.2014.00603
34. Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11(11):723–737. doi:10.1038/nri3073
35. Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011;11(11):750–761. doi:10.1038/nri3088
36. Fitzgerald KA, Rowe DC, Barnes BJ, et al. LPS-TLR4 signaling to IRF-3/7 and NF-kappaB involves the toll adapters TRAM and TRIF. J Exp Med. 2003;198(7):1043–1055. doi:10.1084/jem.20031023
37. Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 2013;229(2):176–185. doi:10.1002/path.4133
38. Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122(3):787–795. doi:10.1172/JCI59643
39. Koh YC, Yang G, Lai CS, Weerawatanakorn M, Pan MH. Chemopreventive effects of phytochemicals and medicines on M1/M2 polarized macrophage role in inflammation-related diseases. Int J Mol Sci. 2018;19(8):2208. doi:10.3390/ijms19082208
40. Tsai CF, Chen GW, Chen YC, et al. Regulatory effects of quercetin on M1/M2 macrophage polarization and oxidative/antioxidative balance. Nutrients. 2021;14(1):67. doi:10.3390/nu14010067
41. Lu CH, Lai CY, Yeh DW, et al. Involvement of M1 macrophage polarization in endosomal toll-like receptors activated psoriatic inflammation. Mediators Inflamm. 2018;2018:3523642. doi:10.1155/2018/3523642
42. Fenyo IM, Gafencu AV. The involvement of the monocytes/macrophages in chronic inflammation associated with atherosclerosis. Immunobiology. 2013;218(11):1376–1384. doi:10.1016/j.imbio.2013.06.005
43. Allen JE, Wynn TA. Evolution of Th2 immunity: a rapid repair response to tissue destructive pathogens. PLoS Pathog. 2011;7(5):e1002003. doi:10.1371/journalppat.1002003
44. Rendra E, Riabov V, Mossel DM, Sevastyanova T, Harmsen MC, Kzhyshkowska J. Reactive oxygen species (ROS) in macrophage activation and function in diabetes. Immunobiology. 2019;224(2):242–253. doi:10.1016/j.imbio.2018.11.010
45. Kou X, Qi S, Dai W, Luo L, Yin Z. Arctigenin inhibits lipopolysaccharide-induced iNOS expression in RAW264.7 cells through suppressing JAK-STAT signal pathway. Int Immunopharmacol. 2011;11(8):1095–1102. doi:10.1016/j.intimp.2011.03.005
46. Comte D, Karampetsou MP, Tsokos GC. T cells as a therapeutic target in SLE. Lupus. 2015;24(4–5):351–363. doi:10.1177/0961203314556139
47. Emmi G, Silvestri E, Bella CD, et al. Cytotoxic Th1 and Th17 cells infiltrate the intestinal mucosa of Behcet patients and exhibit high levels of TNF-α in early phases of the disease. Medicine. 2016;95(49):e5516. doi:10.1097/MD
48. Murray PJ. Macrophage Polarization. Annu Rev Physiol. 2017;79:541–566. doi:10.1146/annurev-physiol-022516-034339
49. Biswas SK, Chittezhath M, Shalova IN, Lim JY. Macrophage polarization and plasticity in health and disease. Immunol Res. 2012;53(1–3):11–24. doi:10.1007/s12026-012-8291-9
50. Peng H, Xian D, Liu J, Pan S, Tang R, Zhong J. Regulating the polarization of macrophages: a promising approach to vascular dermatosis. J Immunol Res. 2020;2020:8148272. doi:10.1155/2020/8148272
51. de Medeiros MCS, Medeiros JCA, de Medeiros HJ, Leitão JCGC, Knackfuss MI. Dietary intervention and health in patients with systemic lupus erythematosus: a systematic review of the evidence. Crit Rev Food Sci Nutr. 2019;59(16):2666–2673. doi:10.1080/10408398.2018.1463966
52. Lee DY, Chiu JJ. Atherosclerosis and flow: roles of epigenetic modulation in vascular endothelium. J Biomed Sci. 2019;26(1):56. doi:10.1186/s12929-019-0551-8
53. Vannini F, Kashfi K, Nath N. The dual role of iNOS in cancer. Redox Biol. 2015;6:334–343. doi:10.1016/j.redox.2015.08.009
54. Cheung EC, Vousden KH. The role of ROS in tumour development and progression. Nat Rev Cancer. 2022;22(5):280–297. doi:10.1038/s41568-021-00435-0
55. Xu F, Xu J, Xiong X, Deng Y. Salidroside inhibits MAPK, NF-κB, and STAT3 pathways in psoriasis-associated oxidative stress via SIRT1 activation. Redox Rep. 2019;24(1):70–74. doi:10.1080/13510002.2019.1658377
56. Holmström KM, Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol. 2014;15(6):411–421. doi:10.1038/nrm3801
57. Sahu PK, Jayalakshmi K, Tilgam J, et al. ROS generated from biotic stress: effects on plants and alleviation by endophytic microbes. Front Plant Sci. 2022;13:1042936. doi:10.3389/fpls.2022.1042936
58. Deng Z, Shi F, Zhou Z, et al. M1 macrophage mediated increased reactive oxygen species (ROS) influence wound healing via the MAPK signaling in vitro and in vivo. Toxicol Appl Pharmacol. 2019;366:83–95. doi:10.1016/j.taap.2019.01.022
59. Bito T, Nishigori C. Impact of reactive oxygen species on keratinocyte signaling pathways. J Dermatol Sci. 2012;68(1):3–8. doi:10.1016/j.jdermsci.2012.06.006
60. Lin X, Huang T. Oxidative stress in psoriasis and potential therapeutic use of antioxidants. Free Radic Res. 2016;50(6):585–595. doi:10.3109/10715762.2016
61. Takuathung MN, Potikanond S, Sookkhee S, et al. Anti-psoriatic and anti-inflammatory effects of Kaempferia parviflora in keratinocytes and macrophage cells. Biomed Pharmacother. 2021;143:112229. doi:10.1016/j.biopha.2021.112229
62. Li X, Liu Y, Yang L, Jiang Y, Qian Q. TIM-3 shuttled by MV3 cells-secreted exosomes inhibits CD4+ T cell immune function and induces macrophage M2 polarization to promote the growth and metastasis of melanoma cells. Transl Oncol. 2022;18:101334. doi:10.1016/j.tranon.2021.101334
63. Yuan Q, Niu K, Sun L, Zhao B, Wang XY, Wang B. BAP31 affects macrophage polarization through regulating helper T cells activation. J Mol Histol. 2022;53(5):843–855. doi:10.1007/s10735-022-10095-5
64. Li P, Spolski R, Liao W, Leonard WJ. Complex interactions of transcription factors in mediating cytokine biology in T cells. Immunol Rev. 2014;261(1):141–156. doi:10.1111/imr.12199
65. Krausgruber T, Blazek K, Smallie T, et al. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat Immunol. 2011;12(3):231–238. doi:10.1038/ni.1990
66. Luque-Martin R, Angell DC, Kalxdorf M, et al. IFN-γ drives human monocyte differentiation into highly proinflammatory macrophages that resemble a phenotype relevant to psoriasis. J Immunol. 2021;207(2):555–568. doi:10.4049/jimmunol.2001310
67. Aghamajidi A, Raoufi E, Parsamanesh G, et al. The attentive focus on T cell-mediated autoimmune pathogenesis of psoriasis, lichen planus and vitiligo. Scand J Immunol. 2021;93(4):e13000. doi:10.1111/sji.13000
68. Marshall A, Celentano A, Cirillo N, McCullough M, Porter S. Tissue-specific regulation of CXCL9/10/11 chemokines in keratinocytes: implications for oral inflammatory disease. PLoS One. 2017;12(3):e0172821. doi:10.1371/journal.pone
69. Xie K, Chai YS, Lin SH, Xu F, Wang CJ. Luteolin regulates the differentiation of regulatory T Cells and activates IL-10-dependent macrophage polarization against acute lung injury. J Immunol Res. 2021;2021:8883962. doi:10.1155/2021/8883962
70. Liu X, Jiang S, Zhang Q, et al. Tim-3 regulates tregs’ ability to resolve the inflammation and proliferation of acute lung injury by modulating macrophages polarization. Shock. 2018;50(4):455–464. doi:10.1097/SHK.0000000000001070
71. Zhao Y, Liu B, Wang Y, Xiao B. Effect of fasudil on experimental autoimmune neuritis and its mechanisms of action. Braz J Med Biol Res. 2019;53(1):e8669. doi:10.1590/1414-431X20198669
72. Huang J, Zhang Y, Dong L, et al. Ethnopharmacology, phytochemistry, and pharmacology of Cornus officinalis Sieb. et Zucc. J Ethnopharmacol. 2018;213:280–301. doi:10.1016/j.jep.2017.11.010
73. Xu R, Yuan Y, Qi J, et al. Elucidation of the intestinal absorption mechanism of loganin in the human intestinal Caco-2 cell model. Evid Based Complement Alternat Med. 2018;2018:8340563. doi:10.1155/2018/8340563
74. Wen H, Xing L, Sun K, Xiao C, Meng X, Yang J. Loganin attenuates intestinal injury in severely burned rats by regulating the toll-like receptor 4/NF-κB signaling pathway. Exp Ther Med. 2020;20(1):591–598. doi:10.3892/etm.2020.8725
75. Zhang J, Wang C, Wang H, Li X, Xu J, Yu K. Loganin alleviates sepsis-induced acute lung injury by regulating macrophage polarization and inhibiting NLRP3 inflammasome activation. Int Immunopharmacol. 2021;95:107529. doi:10.1016/j.intimp.2021.107529
76. Zhang C, Xiao C, Dang E, et al. CD100-Plexin-B2 promotes the inflammation in psoriasis by activating NF-κB and the inflammasome in keratinocytes. J Invest Dermatol. 2018;138(2):375–383. doi:10.1016/j.jid.2017.09.005
77. Irrera N, Vaccaro M, Bitto A, et al. BAY 11-7082 inhibits the NF-κB and NLRP3 inflammasome pathways and protects against IMQ-induced psoriasis. Clin Sci. 2017;131(6):487–498. doi:10.1042/CS20160645
78. Xia CY, Xu JK, Li L, et al. Identifying the mechanism underlying antidepressant-like effects of loganin by network pharmacology in combination with experimental validation. J Ethnopharmacol. 2021:114526. doi:10.1016/jjep.2021.114526
79. Cannavò SP, Riso G, Casciaro M, Di Salvo E, Gangemi S. Oxidative stress involvement in psoriasis: a systematic review. Free Radic Res. 2019;53(8):829–840. doi:10.1080/10715762.2019.1648800
80. Yao L, Peng SX, Xu YD, et al. Unexpected neuroprotective effects of loganin on 1-Methyl-4-Phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity and cell death in zebrafish. J Cell Biochem. 2017;118(3):615–628. doi:10.1002/jcb.25749
81. Yang S, Yuan HQ, Hao YM, et al. Macrophage polarization in atherosclerosis. Clin Chim Acta. 2020;501:142–146. doi:10.1016/j.cca.2019.10.034
82. Lai R, Xian D, Xiong X, Yang L, Song J, Zhong J. Proanthocyanidins: novel treatment for psoriasis that reduces oxidative stress and modulates Th17 and Treg cells. Redox Rep. 2018;23(1):130–135. doi:10.1080/13510002.2018.1462027
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.