Back to Journals » Journal of Hepatocellular Carcinoma » Volume 9
Natural Killer Group 2D Receptor and B1a Cells Crosstalk in Post-Hepatitis C Virus Infection Hepatocellular Carcinoma and Cirrhosis
Authors Hammad R , Eldosoky MA , Mosaad AM, El-Nasser AM, Kotb FM , Elshennawy SI, Eldesoky NAR, Selim MA, Naguib GG , Ahmed OA, Alboraie M , Aglan RB
Received 8 March 2022
Accepted for publication 5 July 2022
Published 18 July 2022 Volume 2022:9 Pages 609—619
DOI https://doi.org/10.2147/JHC.S360886
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmed Kaseb
Reham Hammad,1 Mona A Eldosoky,1 Alshaimaa M Mosaad,2 Asmaa M El-Nasser,3 Fatma M Kotb,4 Salwa I Elshennawy,1 Noha Abdel-Rahman Eldesoky,5 Mohamed A Selim,6 Gina G Naguib,7 Ossama A Ahmed,7 Mohamed Alboraie,8 Reda Badr Aglan9
1Clinical Pathology Department, Faculty of Medicine (for Girls), Al-Azhar University, Cairo, Egypt; 2Hepatogastroenterology and Infectious Diseases Department, Faculty of Medicine for Girls, Al-Azhar University, Cairo, Egypt; 3Medical Microbiology and Immunology Department, Faculty of Medicine (for Girls), Al-Azhar University, Cairo, Egypt; 4Internal Medicine Department, Faculty of Medicine for Girls, Al-Azhar University, Cairo, Egypt; 5Biochemistry and Molecular Biology Department, Faculty of Pharmacy (for Girls), Al-Azhar University, Cairo, Egypt; 6Botany and Microbiology Department, Faculty of Science (for Boys), Al-Azhar University, Cairo, Egypt; 7Internal Medicine, Gastroenterology and Hepatology, Faculty of Medicine, Ain Shams University, Cairo, Egypt; 8Department of internal medicine, Al-Azhar University, Cairo, Egypt; 9Hepatology and Gastroenterology Department, National Liver Institute Menoufia university, Menoufia, Egypt
Correspondence: Mohamed Alboraie, Department of internal medicine, Al-Azhar University, Cairo, Egypt, Tel +20222602687, Fax +20224020184, Email [email protected]
Background: Natural killer (NK) and B1a cells are implicated in innate immune surveillance against chronic hepatitis C virus (CHCV). NK group 2D (NKG2D) receptor is important for B cell differentiation. This study was designed to assess whether B1a cells and NK Cells expressing NKG2D are implicated in post-hepatitis C infection hepatocellular carcinoma (post-HCV HCC) and cirrhosis using flow cytometry and investigate the association between NK-expressing NKG2D and B1a in complications of CHCV infection.
Methods: In this cross-sectional study, 111 participants were included and divided into the post-HCV HCC (n = 50), post-HCV liver cirrhosis (n = 31), and CHCV (n = 30) groups.
Results: The percentage of B1a cells (B1a%) and the mean fluorescence intensity (MFI) of NKG2D (NKG2D MFI) showed a significant increase in the CHCV group compared with those in the post-HCV liver cirrhosis and post-HCV HCC groups (P < 0.05). A positive correlation was observed between NKG2D MFI and B1a% (r = 0.6, P < 0.001). The receiver operating characteristic (ROC) curve revealed that NKG2D MFI and B1a% differentiated between patients with CHCV infection and those with HCC with a sensitivity of 92% and 98%, respectively, and differentiated between patients with CHCV infection and those with liver cirrhosis with a sensitivity of 94% and 90%, respectively.
Conclusion: Downregulation of B1a frequency and NKG2D intensity is implicated in the progression of CHCV infection to cirrhosis and HCC. NKG2D receptor is associated with the frequency of circulating B1a cells. NKG2D intensity and B1a% can be used as indicators of CHCV progression.
Keywords: B1a cells, chronic hepatitis C, hepatocellular carcinoma, NKG2D
Introduction
Hepatitis C virus (HCV) infection is a serious health concern and the leading cause of liver disease worldwide. Although present medications are helpful, they are costly and have unfavorable side effects. Understanding how HCV interacts with host cells could lead to the discovery of new cellular targets for developing innovative treatments.1
Liver cirrhosis and hepatocellular cancer (HCC) develop in approximately 20% of patients with persistent viremia. During acute HCV infection, innate and adaptive immune systems work together to resolve the infection. In contrast, HCV has sophisticated systems that allow it to evade both host defenses. As a result, the immune response in chronic HCV infection changes over time accompanied by disease progression.2
Natural killer (NK) cells are involved in all stages of HCV infection. Decreased NK cell count, activation marker expression, altered NK cell subset distribution, and functional polarization headed for a cytolytic phenotype are stamps of chronic HCV infection.3 One of the main activating receptors needed for recognizing target cells is the NK group 2D (NKG2D) receptor.4
NKG2D ligands (NKG2DLs) are remotely related to major histocompatibility complex (MHC) class I molecules. They bind to the NKG2D receptor in response to cellular stress, viral infection, or malignant transformation, marking stressed cells for removal by NKG2D+ lymphocytes.5 Hence, the NKG2D receptor acts as a sensor that decodes cellular stress into activation signals for immune cells.6
Mature B cells are classified into B1 and B2 cells according to their function and phenotype. Antibodies (Abs) created by B1 cells contribute to T cell-independent Abs responses.6 B1 cells, named “innate B cells”, could be subdivided into CD5+ B1a cells and CD5− B1b cells, where they differ in their responses toward certain antigens (Ags). The involvement of B1a cells in controlling several bacterial infections was proven.7
Chronic hepatitis C virus (CHCV) infection is known for sustained B cell activation, increased differentiation, and diminished proliferative potential.8 B1a cells are immunoglobulin M-positive B (IgM+ B) cells and involved in viral infection pathogenesis.9
Even though the NKG2D receptor is not expressed in all B cell subsets, it has been observed that the absence of this receptor in hematological precursors affects peripheral B1a cell counts in cases of bacterial infections.4 However, the association between host NKG2D receptor and B1a cell subsets in patients with HCV infection and its complications has not been elucidated.
Hence, this study was designed to assess whether B1a cells and NK-expressing NKG2D are involved in post-HCV HCC and liver cirrhosis using flow cytometry. Furthermore, the relationship between NK-expressing NKG2D and B1a cells in CHCV and its related complications regarding HCC transformation and liver cirrhosis were explored.
Patients and Methods
In this cross-sectional comparative study, 111 participants were included and divided into the post-HCV HCC (n = 50), CHCV + liver cirrhosis (n = 31), and CHCV (n = 30) groups. Patients were recruited from National Liver Institute, Menoufia University, Alzahraa University Hospital, and Ain Shams University Viral Hepatitis Treatment and Research Unit. Patients were recruited from May of the year 2019 to October of the year 2021. All participants provided informed consents.
The study was conducted according to the Declaration of Helsinki, and the Ethics Committee of the Faculty of Medicine, Al-Azhar University, approve the study protocol. The Institutional Review Board number was 202105844.
The inclusion criteria were as follows: (i) adults aged more than 18 years with positive HCV antibodies (ii) HCV RNA levels of more than 2000 IU/mL in the past 6 months for CHCV to confirm the absence of antiviral therapy or interferons intake, to avoid their impact on the peripheral blood B1a and NKG2D expression level (iii) naïve early diagnosed HCC patients confirmed with ultrasound findings and pathology (iv) post HCV cirrhosis patients included were diagnosed by clinical, histological, laboratory, and/or ultrasound findings. The exclusion criteria were as follows: (i) acutely or chronically HBV-infected patients (determined by serology) (ii) patients with a history of alcoholism or autoimmune diseases (iii) HCC or cirrhosis not associated with HCV (iv) treated HCC patients with any of the treatment options eg, chemotherapy, curative resection radiofrequency ablation and systemic targeted agents (v) cirrhosis patients with the presence of positive blood or ascitic cultures, leukocyte counts equal or greater than 250/μL to avoid and (vi) pregnant women.
All participants were subjected to the following: full history taking; complete blood count (CBC); routine biochemical tests (eg, creatinine, alanine aminotransferase [ALT], and total and direct bilirubin using Cobas automated biochemical analyzer); alpha fetoprotein in HCC; coagulation assay of the international normalized ratio; abdominal ultrasonography; and flow cytometry assay for identifying the percentage of B1a and NK cells before measuring the mood of expression of the NKG2D receptor.
Sample Collection
Six milliliters of blood were drawn from each subject. The first 2-mL blood was transferred into an ethylenediamine tetraacetic acid tube for CBC (Sysmex KX-21, Japan) and flow cytometry assay. The rest of the sample was transferred into a serum gel separator tube for assessing the biochemical markers using a chemistry autoanalyzer device (Cobas Integra 400 Plus, Roche Diagnostics, Germany).
Flow Cytometry Assay
Flow cytometry was performed at the Clinical Pathology Department, Al-Azhar University, using FACSCalibur (BD, Biosciences, San Jose, USA). The compensation setting was established before acquiring the samples using CaliBRITE color beads (lot no. 5093879; BD, Biosciences, San Jose, USA) for unlabeled fluorescein isothiocyanate (FITC)/phycoerythrin (PE) color compensation and (lot no. 70689; BD, Biosciences, San Jose, USA) for allophycocyanin (APC) color compensation. Fifty µL of fresh sample after adjusting the cell count (1 × 106 peripheral blood mononuclear cells) was added to three falcon tubes.
The first tube was loaded with 5 uL of each of the following fluorochrome-conjugated antibodies; FITC-conjugated anti-human surface IgM (catalog no. 555782; lot no. 5022938), APC-conjugated anti-human CD19 (catalog no. 555415; lot no. 0111592), and PE-conjugated anti-human CD5 (catalog no. 555353; lot no. 4248536) (all from BD Biosciences, USA).
The second tube was loaded with a cocktail of fluorochrome-conjugated antibodies: FITC-conjugated anti-human CD3/PE-conjugated anti-human CD16+CD56 (catalog no. 95131; lot no. 6012680) with APC-conjugated anti-human NKG2D (catalog no. 562064; lot no. 8142790).
The third tube was loaded with isotype control of FITC, PE, and APC to determine nonspecific binding (all from BD Biosciences, USA) and incubated for 10 min at room temperature.
Then, a lysis reagent for destroying erythrocytes was added 5 min before cells were washed with FACS buffer and centrifuged at 500 xg. Furthermore, 700,000 events (number of cells counted using flow cytometry) were acquired for analysis to ensure the evaluation of rare populations in the peripheral blood.
For the gating strategy, initial gating was performed using forward/sideway scatter on mature lymphocytes and then the percentage B1a cells was evaluated as percentage of total lymphocytes according to the combination of surface marker expression of CD19+ CD5+ IgM. NK cells were identified from initial gating as CD56+16-positive and CD3-negative population before measuring the expression of the NKG2D receptor. The gating strategy is illustrated in Figure 1.
 |
Figure 1 Gating strategy for B1a% and NKG2D+NK% and the detection of the NKG2D MFI. The figure illustrates the gating strategy for NKG2D+NK%, B1a%, and NKG2D MFI in the area under the M1 marker. |
Statistical Analysis
Data were analyzed using Statistical Package for the Social Sciences (version 26; IBM Corp., Armonk, NY, USA). Nonparametric quantitative data were expressed as medians (interquartile ranges). Parametric data were expressed as means ± standard deviations, in addition to frequency and percentage for categorical data. Nonparametric data between more than two groups were compared using the Kruskal–Wallis test, while analysis of variance was used for comparing parametric data, followed by the post-hoc test. Moreover, nonparametric data between two independent groups were compared using the Mann–Whitney test, while Student’s t-test was used for comparing parametric data between two independent groups. Categorical data were compared using the chi-square (χ2) test. The correlations between quantitative variables were determined using Spearman correlation coefficients. Receiver operating characteristic (ROC) curves were constructed to detect the best cutoff value of significant markers for detecting the progression of CHCV to either HCC or liver cirrhosis. Differences with p-values of less than 0.05 were considered statistically significant.
Results
This study included 111 participants, who were divided into the post-HCV HCC (n = 50), CHCV + liver cirrhosis (n = 31), and CHCV (n = 30) groups.
The demographic characteristics of the subjects under study are shown in Table 1.
 |
Table 1 Demographic and Clinical Data of the Participants Under Study |
Table 2 illustrates the results of the comparison of the groups under study regarding age and biochemical parameters.
 |
Table 2 Laboratory and Flow Cytometry Parameters of the Groups Under Study |
The correlations between the percentage of B1a, the mean fluorescence intensity (MFI) of the NKG2D receptor (NKG2D MFI) and NKG2D+NK%, with other study variables are shown in Table 3.
 |
Table 3 Correlation of B1a%, NKG2D+NK%, and NKG2D MFI with Other Variables Under Study in All Patients (n = 111) |
The correlation between the B1a% and the NKG2D MFI in all patients (n=11) is shown in Figure 2.
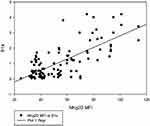 |
Figure 2 Correlation between NKG2D MFI and B1a%. A positive correlation is observed between NKG2D MFI and B1a% (r = 0.6, P < 0.001). |
The post-HCV HCC group was divided into two groups according to the number of lesions: post-HCV HCC with a single liver lesion (n = 24) and post-HCV-HCC with multiple lesions (n = 26). Comparison between both groups regarding NKG2D+NK%, NKG2D MFI, and B1a% revealed no significant differences in the three markers (P = 0.14, P = 0.57, and P = 0.69, respectively). Moreover, the post-HCV HCC group was divided into two groups according to the infiltration pattern of the tumor: post-HCV HCC with focal liver lesions (n = 40) and post-HCV HCC with heterogeneous liver lesions (n = 10). Comparison between both groups regarding NKG2D+NK%, NKG2D MFI, and B1a% revealed no significant differences in the three markers (P = 0.35, P = 0.24, and P = 0.17, respectively).
The output data of the ROC curves of NKG2D+NK%, NKG2D MFI, and B1a% for differentiating between CHCV and liver cirrhosis are presented in (Table 4, Figure 3A) and between CHCV and post-HCV HCC are presented in (Table 4, Figure 3B)
 |
Table 4 The Output Data of the ROC Curve for Discriminative Power of NKG2D+NK%, NKG2D MFI, and B1a% Between CHCV and Post-HCV Liver Cirrhosis and Between CHCV and Post-HCV HCC |
Discussion
HCV infection leads to an inflamed cirrhotic liver with extensive immune infiltration, a key contributor to HCC development and progression.10 HCC is one of the leading causes of cancer-related deaths worldwide with limited treatment options.11 This justifies the need for opening the channels of targeted immunotherapy. Hence, whether circulating B1a and NKG2D+NK cells contribute to HCV progression to liver cirrhosis and HCC and whether the frequency of B1a cells is linked to NKG2D expression were explored.
This study included 111 participants, who were divided into the post-HCV HCC (n = 50), CHCV + liver cirrhosis (n = 31), and CHCV (n = 30) groups. B1a% was significantly different between the groups under study (P < 0.05), with the HCC and CHCV groups having the lowest and highest B1a% values, respectively (Table 2).
The protective role of IgM + B1a previously revealed by Ehrenstein and Notley (2010), who indicated that the Abs produced by B1 cells are polyreactive and may recognize pathogen-associated structures, can explain our findings. Moreover, B1 cells contribute to T cell-independent Ab reactions, in which Abs are produced without prior contact with Ags. Pathogens that have penetrated the peritoneum are attacked by B1a-produced Abs, which act as the initial line of defense.11 In contrast, Zhang et al have argued that B cells reduce antitumor immunity and increase tumor growth by inhibiting antitumor immunity.12
Note that the decrease in B1a% in the HCC group compared with that in the liver cirrhosis group was not attributed to age as no statistically significant difference in age was observed between both groups, ensuring that the decline in B1a% was not related to old age. However, this comes in contrary with the results reported by Rodriguez-Zhurbenko et al, who reported that the decrease in B1a% was related to age.13
According to Kong et al, patients with CHCV infection had a greater frequency of circulating IgM+B subsets in their peripheral blood.9 The discrepancy can be explained as the former study compared patients with CHCV infection with healthy controls.
When comparing the HCC and CHCV groups, the NKG2D MFI was significantly lower in the HCC group. Compared with the CHCV group, the cirrhosis group showed a considerable drop in the NKG2D MFI. However, no significant difference was observed between the HCC and liver cirrhosis groups (Table 2). Our findings agreed with those reported by Szereday et al, who reported that increased NKG2D expression in patients with HCV infection highlights a key protective role of NK cells against HCV progression.14
Major histocompatibility complex (MHC) class I-related chain A and B (MICA/B) and UL16-binding protein 1, 2, 3, 4 (ULBP1, 2, 3, 4) are recognized NKG2D ligands in humans.15 In microbe-infected, altered, or stressed cells, the expression of these NKG2D ligands is frequently elevated. These ligands bind with NKG2D receptors on NK cells, causing them to activate, and play an important role in host defense. In this study, however, reduced NKG2D interferes with the host’s defense mechanisms, resulting in the development of liver cancer.16 This supports with our theory that inducing strong NK cell activity can help prevent post-HCV complications.
In HCV infection, NK cells have been implicated in nonspecific immune clearance, as well as the initiation and modulation of specific antiviral immune responses. NKG2D controls the worsening of liver inflammation by activating NK cells in hepatitis B virus (HBV) infection, according to Wang et al.17 This finding supports the idea that NKG2D has a protective effect that extends beyond NK cell-mediated cytotoxicity and cytokine generation. According to Lenartić et al, NKG2D is found on the earliest progenitors of the NK cell lineage and plays an important role in NK cell development by causing changes in NK cell subsets and inducing faster division of immature NK cells in the bone marrow. Moreover, NKG2D stimulates the immune system by promoting the development of innate-like B cells.6
This study revealed a significant decrease in NKG2D+NK% in the HCC group compared with those in the CHCV and liver cirrhosis groups. Meanwhile, NKG2D+NK% showed no significant difference between the CHCV and liver cirrhosis groups. Liu et al have reported that NK cells are involved in the first line of immune defense against viral infections and tumors.18 Wang et al have specifically highlighted the role of NKG2D+NK cells in tumor transformation and HCC, stating that the NKG2D gene’s signal of activation is transmitted mainly by the adaptor protein (DAP). NKG2DLs can be widely expressed in hepatoma cells. After the combination of NKG2D and DAP10, the motif in the cytoplasm is phosphorylated, and then, signaling pathways are activated, such as PI3K and PLCγ2. Activated NK cells can specifically dissolve hepatomas by releasing various cytokines and perforin and highly expressing FasL, CD16, and TRAIL.19 Previous data explain that decreases in NKG2D, specifically in NK cells, including NKG2D+NK cells, play a critical anti-hepatoma role.
MHC class I polypeptide-related sequence A (MICA) was identified by Goto and Kato (2015) as a susceptibility gene for post-HCV HCC. MICA is an NKG2DL that interacts with NKG2D to activate NK cell-mediated cytotoxicity against target cells. NKG2D is a critical molecule in tumor immune surveillance because its expression is raised on stressed cells, such as transformed tumor cells, to allow NK cells to recognize them.20
The comparison between the post-HCV HCC group with a single liver lesion (n = 24) and the post-HCV HCC group with multiple lesions (n = 26) regarding NKG2D+NK%, NKG2D MFI, and B1a% revealed no significant differences (P = 0.14, P = 0.57, and P = 0.69, respectively). Moreover, the comparison between the post-HCV HCC group with focal liver lesions (n = 40) and the post-HCV HCC group with heterogenous liver lesions (n = 10) regarding NKG2D+NK%, NKG2D MFI, and B1a% revealed no significant differences (P = 0.35, P = 0.24, and P = 0.17, respectively).
This disagreed with the findings of Wang et al, who reported that NKG2D+NK cells play a critical anti-hepatoma role.19 Another study has found that NKG2D+NK cells are greatly abundant in intrahepatic compartments in patients with HCC and are linked to tumor damage.21 Regarding B1a cells, Shen et al have reported that tumor-infiltrating IgM+ B cells were linked to a better prognosis in solid tumors.22 A study by Faggioli et al has revealed that high infiltration of CD20+ B cells was associated with poor survival in HCC.23 This disparity could be attributed to changes in the pattern of B1a cells under study as we only addressed circulating B1a cells, not the infiltrating ones.
A positive correlation was found between the NKG2D MFI and B1a% (r = 0.6, P < 0.001) Table 3, Figure 2. This finding agrees with the results of the study by Lenartić et al, who reported the role of NKG2D in regulating B1a cell development.6 The role of NKG2D as a stress receptor may explain why it is linked to the control of B1a cells in infection development. B1a cells are generated during the neonatal period when the gastrointestinal tract is colonized by commensal bacteria from food, resulting in a massive boost of the mucosal immune system and the formation of the B cell compartment. The innate B cell response must consequently limit bacterial proliferation at this site to prevent opportunistic infection of the peritoneum.24 Immune cells triggered by infection return to the bone marrow and redirect hematopoietic development via the excretion of soluble factors. It is tempting to believe that activated immune cells recirculate to the bone marrow during perinatal immune system development to encourage the production of B1a cells.25
The output data of the ROC curve of B1a frequency as a predictor of CHCV progression to liver cirrhosis at a cutoff of ≥1.7% and area under the ROC curve (AUC) of 0.91 revealed a sensitivity of 90% and specificity of 70% (P < 0.001) (Table 4, Figure 3A) Meanwhile, its frequency as a predictor of CHCV progression to HCC at a cutoff of ≥1.3% and AUC of 0.99 revealed a sensitivity of 98% and specificity of 87% (P < 0.001) (Table 4, Figure 3B).
The output data of the ROC curve of the NKG2D MFI as a predictor of CHCV progression to liver cirrhosis at a cutoff of ≥68.2 and AUC of 0.96 revealed a sensitivity of 94% and specificity of 77% (P < 0.001) (Table 4, Figure 3A). Meanwhile, its frequency as a predictor of CHCV progression to HCC at a cutoff of ≥60.4 and AUC of 0.98 revealed a sensitivity of 92% and specificity of 90% (P < 0.001) (Table 4, Figure 3B).
The output data of the ROC curve of the NKG2D+NK% as a predictor of CHCV progression to liver cirrhosis has shown no significance (Table 4), while, NKG2D+NK% as a predictor of CHCV progression to HCC at a cutoff of ≥80.5 and AUC of 0.83 revealed a sensitivity of 78% and specificity of 63% (P < 0.001). (Table 4, Figure 3B). Previous findings indicate lower progression discriminative ability of NKG2D+NK% than NKG2D MFI. Also, these findings indicate that both the NKG2D MFI and B1a% can be used as sensitive indicators of CHCV progression.
Our findings support the existence of a protective role for the polyreactive IgM producing B1a and the NKG2D receptor concerned with the cytotoxic activity of NK cells, both biomarkers are involved in the progression of CHCV inflammatory sequela seen in cirrhosis, suggesting that both markers could be used as targets for personalised medicine in CHCV induced cirrhosis. Furthermore, previous data relate NKG2D and B1a cells to antitumor activity and provide evidence that both biomarkers are key molecules in tumor immune surveillance, making them both targets for future cancer immunotherapy.
Furthermore, the median value (1st quartile–3rd quartile) of AFP level in blood of HCC cases was 64.15 ng/mL (12.25–507.25) (Table 2), indicating that AFP elevation as a diagnostic of HCC is unreliable. Luo et al reported similar finding,26 emphasising the importance of identifying more reliable biomarkers for noninvasive and sensitive screening of early HCC transformation, and both B1a cell percentage and NKG2D receptor can be proposed as future candidates for early HCC diagnosis, potentially improving patient outcomes.
Study Limitations
We could not address NKG2DLs to study the axis. Moreover, NKG2D expression on NK cell subsets needs more attention to determine whether specific NK subsets are implicated in the progression of HCV to liver cirrhosis or HCC. Moreover, the associations of NKG2D expression and B1a cells percentage with HCC subclassifications according to Barcelona Clinic Liver Cancer (BCLC) staging system were not evaluated and recommended to be addressed in future studies.
Conclusion
Downregulation of IgM+B1a cells is linked to the progression of CHCV infection to liver cirrhosis and HCC. Immunotherapeutic treatment for post-HCV HCC and liver cirrhosis may include the activation of the NKG2D receptor. Host NKG2D receptor is associated with the frequency of circulating B1a cells. Downregulation of NKG2D intensity and the percentage of B1a cells in the blood can be used as indicators of CHCV infection progression. In the pathogeneses of complications, the expression level of NKG2D on NK cells is more relevant than the percentage of circulating NK-expressing NKG2D cells. NKG2D intensity and the percentage of B1a cells are proposed to aid in early diagnosis and screening in HCC patients with low AFP.
Disclosure
The authors declare no potential conflicts of interest regarding the research, authorship, and/or publication of this article.
References
1. Ferreira AR, Ramos B, Nunes A, Ribeiro D. Hepatitis C virus: evading the intracellular innate immunity. J Clin Med. 2020;9(3):
2. Baskic D, Vukovic V, Popovic S, et al. Chronic hepatitis C: conspectus of immunological events in the course of fibrosis evolution. PLoS One. 2019;14(7):e0219508. doi:10.1371/journal.pone.0219508
3. Rosen HR, Golden-Mason L. Control of HCV infection by natural killer cells and macrophages. Cold Spring Harb Perspect Med. 2019;23:
4. Prinz D, Klein K, List J, et al. Loss of NKG2D in murine NK cells leads to increased perforin production upon long-term stimulation with IL-2. Eur J Immunol. 2020;50(6):880–890. doi:10.1002/eji.201948222
5. Lazarova M, Steinle A. The NKG2D axis: an emerging target in cancer immunotherapy. Expert Opin Ther Targets. 2019;23(4):281–294. doi:10.1080/14728222.2019.1580693
6. Lenartić M, Jelenčić V, Zafirova B, et al. NKG2D promotes B1a cell development and protection against bacterial infection. J Immunol. 2017;198(4):1531–1542. doi:10.4049/jimmunol.1600461
7. Gil-Borras R, García-Ballesteros C, Benet-Campos C, et al. B1a lymphocytes (CD19+CD5+) deficiency in patients with Crohn’s disease and its relation with disease severity. Dig Dis. 2018;36(3):194–201. doi:10.1159/000486893
8. Oliviero B, Mantovani S, Ludovisi S, et al. Skewed B cells in chronic hepatitis C virus infection maintain their ability to respond to virus-induced activation. J Viral Hepat. 2015;22(4):391–398. doi:10.1111/jvh.12336
9. Kong F, Feng B, Zhang H, et al. Abnormal phenotypic features of IgM+B cell subsets in patients with chronic hepatitis C virus infection. Exp Ther Med. 2017;14(2):1846–1852. doi:10.3892/etm.2017.4682
10. Chen CJ. Global elimination of viral hepatitis and hepatocellular carcinoma: opportunities and challenges. Gut. 2018;67(4):595–598. doi:10.1136/gutjnl-2017-315407
11. Ehrenstein MR, Notley CA. The importance of natural IgM: scavenger, protector and regulator. Nat Rev Immunol. 2010;10(11):778–786. doi:10.1038/nri2849
12. Zhang Z, Ma L, Goswami S, et al. Landscape of infiltrating B cells and their clinical significance in human hepatocellular carcinoma. Oncoimmunology. 2019;8(4):e1571388. doi:10.1080/2162402X.2019.1571388
13. Rodriguez-Zhurbenko N, Quach TD, Hopkins TJ, Rothstein TL, Hernandez AM. Front human B-1 cells and B-1 cell antibodies change with advancing age. Front Immunol. 2019;10:483. doi:10.3389/fimmu.2019.00483
14. Szereday L, Meggyes M, Halasz M, Szekeres-Bartho J, Par A, Par G. Immunological changes in different patient populations with chronic hepatitis C virus infection. World J Gastroenterol. 2016;22(20):4848–4859. doi:10.3748/wjg.v22.i20.4848
15. Mou X, Zhou Y, Jiang P, et al. The regulatory effect of UL-16 binding protein-3 expression on the cytotoxicity of NK cells in cancer patients. Sci Rep. 2014;20(4):6138.
16. Gao B. NKG2D, its ligands, and liver disease - good or bad? Hepatology. 2010;51(1):8–11. doi:10.1002/hep.23320
17. Wang Y, Wang W, Shen C, et al. NKG2D modulates aggravation of liver inflammation by activating NK cells in HBV infection. Sci Rep. 2017;7(1):88. doi:10.1038/s41598-017-00221-9
18. Liu P, Chen L, Zhang HJ. Natural killer cells in liver disease and hepatocellular carcinoma and the NK cell-based immunotherapy. J Immunol Res. 2018;2018:1206737. doi:10.1155/2018/1206737
19. Wang J, Li CD, Sun L. Recent advances in molecular mechanisms of the NKG2D pathway in hepatocellular carcinoma. Biomolecules. 2020;10(2):301. doi:10.3390/biom10020301
20. Goto K, Kato NJ. MICA SNPs and the NKG2D system in virus-induced HCC. J Gastroenterol. 2015;50(3):261–272. doi:10.1007/s00535-014-1000-9
21. Oliviero B, Varchetta S, Paudice E, et al. Natural killer cell functional dichotomy in chronic hepatitis B and chronic hepatitis C virus infections. Gastroenterology. 2009;137(3):1151–1160.e1. doi:10.1053/j.gastro.2009.05.047
22. Shen M, Wang J, Yu W, et al. A novel MDSC-induced PD-1(−) PD-L1(+) B-cell subset in breast tumor microenvironment possesses immunosuppressive properties. Oncoimmunology. 2018;7(4):e1413520. doi:10.1080/2162402X.2017.1413520
23. Faggioli F, Palagano E, Di Tommaso L, et al. B lymphocytes limit senescence-driven fibrosis resolution and favor hepatocarcinogenesis in mouse liver injury. Hepatology. 2018;67(5):1970–1985. doi:10.1002/hep.29636
24. Wesemann DR, Portuguese AJ, Meyers RM, et al. Microbial colonization influences early B-lineage development in the gut lamina propria. Nature. 2013;501(7465):112–115. doi:10.1038/nature12496
25. de Bruin AM, Voermans C, Nolte MA. Impact of interferon-γ on hematopoiesis. Blood. 2014;124(16):2479–2486. doi:10.1182/blood-2014-04-568451
26. Luo P, Wu S, Yu Y, et al. Current status and perspective biomarkers in AFP negative HCC: towards screening for and diagnosing hepatocellular carcinoma at an earlier stage. Pathol Oncol Res. 2020;26(2):599–603. doi:10.1007/s12253-019-00585-5
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

