Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Nasal Tip Cutaneous Metastasis of Hepatocellular Carcinoma: A Case Report
Authors Li S , Cai X, Yu K, Pan W
Received 31 July 2023
Accepted for publication 3 October 2023
Published 17 October 2023 Volume 2023:16 Pages 2893—2897
DOI https://doi.org/10.2147/CCID.S429480
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Songting Li,1,2 Xiaolan Cai,3 Keyao Yu,1,2 Weihua Pan1,2
1Department of Dermatology, Second Affiliated Hospital of Naval Medical University, Shanghai, People’s Republic of China; 2Shanghai Key Laboratory of Molecular Medical Mycology, Shanghai, People’s Republic of China; 3Department of Otorhinolaryngology, Qilu Hospital of Shandong University, Jinan, People’s Republic of China
Correspondence: Weihua Pan, Department of Dermatology, Second Affiliated Hospital of Naval Medical University, Shanghai, People’s Republic of China, Tel +86-2188485495, Email [email protected]
Background: Cutaneous metastasis is rare in clinical practice, especially that from primary hepatocellular carcinoma (HCC), which is even rarer.
Case Presentation: This report describes a male patient with HCC with cutaneous metastases to the nasal tip. The patient developed a raised nodule at the nasal tip 5 years after surgery for HCC, with surface ulceration and crusting and no obvious symptoms. Abdominal computed tomography (CT) showed an obvious mass in the liver. The skin lesions on the nasal tip were confirmed to be cutaneous metastasis of HCC by histopathological and immunohistochemical examinations.
Conclusion: The incidence of cutaneous metastasis of HCC is extremely low, and nasal tip cutaneous metastasis of HCC has no specific clinical manifestations; therefore, it needs to be distinguished from rosacea rhinophyma, fungal and atypical mycobacterial infections, tumours of vascular origin, and tumours of skin appendages that occur in the nasal tip and is prone to misdiagnosis and missed diagnosis, thus requiring clinical dermatologists and otolaryngologists to be aware of such metastasis.
Keywords: primary, hepatocellular carcinoma, metastatic cancer, skin, nasal tip
Introduction
Primary hepatocellular carcinoma (HCC) is a malignant tumour originating from hepatocytes in the liver. It is the sixth most prevalent malignant tumour in the world and often metastasizes in later stages. The common metastasis routes of HCC include haematogenous metastasis, lymphatic metastasis, implantation metastasis and direct infiltration, with haematogenous intrahepatic metastasis being the earliest and most common. HCC cells can enter the systemic circulation after invading the hepatic vein and cause distant metastasis outside the liver. The incidence of lung metastasis is highest, followed by metastasis to the adrenal glands, bone, and ovaries. Cutaneous metastasis is relatively rare,1 and occurs in about 0.8–2.7% of HCC cases.2 This report describes a case of HCC with cutaneous metastases on the nasal tip. The patient was diagnosed with HCC 3 years ago and underwent surgery. Recently, a raised nodule appeared at the nasal tip, accompanied by surface ulceration and crusting, which was confirmed by a pathological examination to be cutaneous metastasis of HCC. Cutaneous metastases often do not have typical clinical manifestations. Cutaneous metastases at the nasal tip have various manifestations and need to be differentiated from various skin diseases. Misdiagnosis and missed diagnosis are common in clinical practice. Therefore, clinical dermatologists and otolaryngologists should be aware of this type of metastasis.
Case Presentation
A 61-year-old male patient sought treatment for an infiltrating erythematous plaque on the nasal tip, which gradually enlarged with ulceration and haemorrhage over the prior 2 months. The patient developed a red papule on the nasal tip 2 months prior. The skin lesion gradually enlarged to the size of a broad bean. In the past 2 weeks, a plaque developed, with ulcerations and bleeding, and a black crust formed. The patient had a 40-year history of hepatitis B and had not received standardized antiviral therapy. Three years ago, a 2.0×1.5 cm space-occupying mass in the left lobe of the liver was found through a physical examination. Local surgical resection was performed. The patient was diagnosed with well-differentiated hepatocellular carcinoma and nodular cirrhosis based on postoperative histopathological and immunohistochemical examinations, and further treatment was not administered.
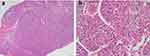
The patient’s systemic examination showed no obvious abnormalities. A dermatological examination showed that no obvious yellow staining in the skin and mucous membranes of the whole body. A red infiltrating nodule was seen on the nasal tip, without peduncles, with a hard and tough texture; the nodule was raised, ulcerated, and covered with a black crust (Figure 1). No similar lesions were found on the skin of any other area of the body. The alpha-fetoprotein level in peripheral blood was 260 μg/L (normal level ≤ 25 μg/L). Whole-body computed tomography (CT) showed irregular liver margins, an enlarged left hepatic lobe, a slightly low-density mass in the left hepatic lobe, an enlarged spleen, and a widened portal vein (Figure 2), findings that were consistent with liver cirrhosis and liver cancer. No space-occupying lesion was observed in other organs. Skin biopsy was performed after local anaesthesia. Histopathology showed epidermal necrosis with ulceration, a dense tumour cell mass in the dermis, with a sinus-like, nodular and cord-like distribution, and some atypical tumour cells (Figure 3). Immunohistochemistry of intradermal tumour cells revealed the following: HepPar-1(+), Arg-1(+), CK8(+), Villin(+), CK7(-), CK20(-), and Ki-67 positive (approximately 90%) (Figure 4). Based on the clinical manifestations, medical history, imaging results, and histopathology results, the patient was diagnosed with cutaneous metastases of hepatocellular carcinoma. The patient gave up systemic treatment and subsequently lost the visit.
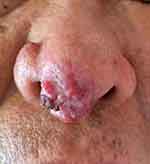 |
Figure 1 Clinical presentation of cutaneous metastasis of HCC. Red infiltrative plaques with ulceration and scab on the nasal tip. |
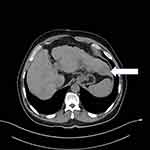 |
Figure 2 CT scan. There is a hypodense mass in the left hepatic lobe. |
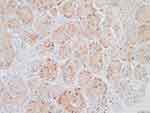 |
Figure 4 Immunohistochemical analysis. Arg-1(+). |
Discussion
Cutaneous carcinoma is the most common malignant tumour of the skin and is a collective term for malignant tumours that occur in exposed parts of the body. Cutaneous carcinoma can be classified by source, ie, primary cutaneous carcinoma or metastatic cutaneous carcinoma. Cutaneous metastases refer to the metastasis of malignant tumours in other parts to the skin through haematogenous or lymphatic pathways or secondary to the skin through interstitial spread or surgical implantation. Cutaneous metastases have various clinical manifestations, including papules, plaques, nodular infiltrative erythema, etc., which are often solitary, may be accompanied by bleeding and ulcers, and often occur in the chest wall, umbilicus, and head and face.3 Melanoma is the most common source of cutaneous metastases, accounting for approximately 45% of metastatic skin tumours. Other malignant cutaneous metastases are most common in lung cancer and breast cancer.4 In addition, cutaneous metastases can also be seen in gastric cancer, colorectal cancer, ovarian cancer, bile duct cancer, oesophageal cancer and other sources.
The sites of extrahepatic metastasis of primary HCC are lung, bone, lymph node and adrenal gland, in descending order based on incidence rate,5 and cutaneous metastasis is rare. Cutaneous metastases may be caused by haematogenous, lymph node or direct tissue invasion. In addition, radiofrequency ablation and ethanol ablation,6 transcatheter arterial chemoembolization,7 and postoperative liver transplantation8 can lead to cutaneous metastasis of HCC. Our patient had completely a different cause of metastasis with iatrogenic implantation. Saeed et al9 reviewed a total of 100,453 patients with cutaneous carcinoma who underwent surgery from 1993 to 2003, of whom 77 were diagnosed with metastatic lesions; however, no cutaneous metastases from HCC were reported. Queen reported that cutaneous metastases of HCC accounted for only 2.7–3.4% of all cutaneous metastases.8 A previous study reported 12 patients with primary HCC with non-iatrogenic cutaneous metastasis and implantation,10 of whom 7 died within 2–19 months after cutaneous metastasis. The aforementioned studies indicate that the incidence of cutaneous metastasis of HCC is low and that the prognosis is poor.
The diagnosis of cutaneous metastases of HCC is challenging because of its lack of specificity in clinical manifestations. Therefore, histopathological diagnosis is very important, not only to differentiate from primary skin tumours but also to exclude cutaneous metastases from other malignant tumours. The clinical manifestations of cutaneous metastases of HCC include single or multiple hard, painless, nonulcerative, red nodules, often similar to pyogenic granulomas, abscesses, and haemangiomas.11 For patients with a history of HCC, painless nodules on the skin should be assessed for the possibility of cutaneous metastasis; histopathological examination is necessary for diagnosis. Histopathologically, tumour cells are often polygonal, with abundant granular eosinophilic cytoplasm, a high nuclear/cytoplasmic ratio, large and round nuclei, and prominent nucleoli.12 Diagnostic clues include the detection of trabecular bone, bile, pseudocast, sarcomatoid, or solid changes. The use of specific immunohistochemical staining, such as for HepPar-1, Arg-1 and mannan-3, is of great value in the diagnosis and identification of cutaneous metastases of HCC.8
Cutaneous metastasis of HCC often represents tumour progression. In most cases, it is accompanied by distant metastasis from other sites, which is closely related to a poor prognosis. The median survival time is only 19 months.13 For HCC patients with cutaneous metastases, treatment of the primary tumour is the priority; the local skin can be treated by complete surgical excision, freezing, and laser cautery. Sorafenib is the first small molecule drug approved by the US Food and Drug Administration (FDA) for advanced HCC. In addition, lenvatinib,14 atezolizumab and bevacizumab13 are effective for the treatment of advanced HCC.
Conclusion
In summary, cutaneous metastasis of HCC is very rare, especially when it is the only HCC metastasis. For patients with a history of HCC who develop fast-growing hard, painless red nodules, clinicians be alert to the possibility of cutaneous metastasis from HCC. Histopathology is essential for a definitive diagnosis.
Abbreviations
CT, computed tomography; HCC, hepatocellular carcinoma.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for Publication
The patient had given written informed consent for the publication of his clinical details. Institutional approval is not required for this case study.
Funding
This work was supported by the Science and Technology Commission of Shanghai Municipality (20DZ2272000).
Disclosure
All authors declare that they have no conflicts of interest in this work.
References
1. Hu SC, Chen GS, Wu CS, et al. Rates of cutaneous metastases from different internal malignancies: experience from a Taiwanese medical center. J Am Acad Dermatol. 2009;60(3):379–387. doi:10.1016/j.jaad.2008.10.007
2. Patel N, Sheehan-Dare G, Weir J, et al. Cutaneous metastasis as the first presentation of hepatocellular carcinoma. Hepatology. 2018;67(4):1631–1633. doi:10.1002/hep.29615
3. Sariya D, Ruth K, Adams-McDonnell R, et al. Clinicopathologic correlation of cutaneous metastases: experience from a cancer center. Arch Dermatol. 2007;143(5):613–620. doi:10.1001/archderm.143.5.613
4. Hussein MR. Skin metastasis: a pathologist’s perspective. J Cutan Pathol. 2010;37(9):e1–e20. doi:10.1111/j.1600-0560.2009.01469.x
5. Natsuizaka M, Omura T, Akaike T, et al. Clinical features of hepatocellular carcinoma with extrahepatic metastases. J Gastroenterol Hepatol. 2005;20(11):1781–1787. doi:10.1111/j.1440-1746.2005.03919.x
6. de León FJ, Blanes MM, Albares MP, Berbegal L. Cutaneous metastasis from hepatocellular carcinoma after a percutaneous interventional procedure. Actas Dermosifiliogr. 2015;106(5):440–441. doi:10.1016/j.ad.2014.10.014
7. Cho E, Kim HS, Park YM, Kim HO, Lee JY. Cutaneous metastasis of hepatocellular carcinoma following skin injury after transcatheter arterial chemoembolization. Ann Dermatol. 2013;25(1):107–109. doi:10.5021/ad.2013.25.1.107
8. Queen D, Fisher J, Husain S, et al. Cutaneous metastasis of hepatocellular carcinoma following liver transplantation. J Cutan Pathol. 2020;47(1):47–51. doi:10.1111/cup.13555
9. Saeed S, Keehn CA, Morgan MB. Cutaneous metastasis: a clinical, pathological, and immunohistochemical appraisal. J Cutan Pathol. 2004;31(6):419–430. doi:10.1111/j.0303-6987.2004.00207.x
10. Jia J, Luo J, Zou B, et al. Non-iatrogenic implantation of cutaneous metastasis from hepatocellular carcinoma. J Cancer Res Clin Oncol. 2023;149(4):1513–1519. doi:10.1007/s00432-022-04030-0
11. Reuben S, Owen D, Lee P, Weiss A. Hepatocellular carcinoma with cutaneous metastases. Can J Gastroenterol. 2009;23(1):23–25. doi:10.1155/2009/274357
12. Habermehl G, Ko J. Cutaneous metastases: a review and diagnostic approach to tumors of unknown origin. Arch Pathol Lab Med. 2019;143(8):943–957. doi:10.5858/arpa.2018-0051-RA
13. Galle PR, Finn RS, Qin S, et al. Patient-reported outcomes with atezolizumab plus bevacizumab versus sorafenib in patients with unresectable hepatocellular carcinoma (IMbrave150): an open-label, randomised, Phase 3 trial. Lancet Oncol. 2021;22(7):991–1001. doi:10.1016/S1470-2045(21)00151-0
14. Qin HN, Ning Z, Sun R, et al. Lenvatinib as second-line treatment in patients with unresectable hepatocellular carcinoma: a retrospective analysis. Front Oncol. 2022;12:1003426. doi:10.3389/fonc.2022.1003426
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.