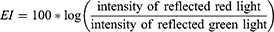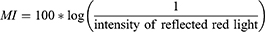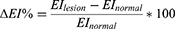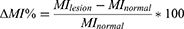Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 14
Narrow-Band Reflectance Spectrophotometry for the Assessment of Erythematous and Hyperpigmented Skin Lesions in Localized Scleroderma: A Preliminary Study
Authors Szczepanek M, Frątczak A, Lis-Święty A
Received 22 March 2021
Accepted for publication 15 April 2021
Published 28 May 2021 Volume 2021:14 Pages 575—580
DOI https://doi.org/10.2147/CCID.S312208
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Jeffrey Weinberg
Michal Szczepanek, Aleksandra Frątczak, Anna Lis-Święty
Department of Dermatology, School of Medicine in Katowice, Medical University of Silesia, Katowice, Poland
Correspondence: Anna Lis-Święty
Department of Dermatology, School of Medicine in Katowice, Medical University of Silesia, Francuska 20/24, Katowice, 40-027, Poland
Tel +48 602720948
Email [email protected]
Background: Localized scleroderma (LoS) is an inflammatory fibrosing disease of the connective tissue, whose esthetic sequelae are atrophic skin lesions with hyperpigmentation. The key element of diagnostic and therapeutic procedures is the assessment of the disease’s severity and damage. The study objective was to analyze the usefulness of narrow-band reflectance spectrophotometry (NBRS) to assess erythema and hyperpigmentation in LoS lesions.
Materials and Methods: Erythema indexes (EI) and melanin indexes (MI) were determined with the use of DermaLab Combo skin colour probe for LoS lesions and symmetrically located areas of normal skin. Then, relative percentage differences were determined for EI and MI, which were compared with the visual assessments of erythema and hyperpigmentation according to the Localized Scleroderma Cutaneous Assessment Tool (LoSCAT).
Results: A total of 84 LoS lesions were studied in 41 patients. The study showed a moderate correlations between the spectrophotometric measurements and clinical assessments of erythema as well as hyperpigmentation (Spearman correlation coefficient, r), r = 0.37; p = 0.00047 and r = 0.55; p=0.0000001, respectively.
Conclusion: NBRS seems to be a useful tool to assess the severity of erythema and hyperpigmentation in LoS lesions. Further studies are required in order to compare the spectrophotometric results with other objective methods.
Keywords: colour, erythema, morphea, postinflammatory hyperpigmentation
Introduction
Localized scleroderma (LoS) is a rare inflammatory fibrosing disease of the connective tissue occurring in children and adults. The disease affects mostly skin, less frequently the subcutaneous tissue, and only certain forms, mainly in children, may affect deeper tissues (fascia, muscles, bones). In adults, the most common LoS subtype is plaque LoS, while the linear and mixed subtypes are rare.1 In the initial, inflammatory stage of the disease, a red or purple macules appear, which gradually become sclerotic in the centre and acquire a wax yellow colour. After several months or years, the lesions become atrophic, hyperpigmented or discoloured. The histological profile also changes depending upon the phase of the disease. Fibrosis forms the main part of the old lesion and there is little inflammation.2 Differentiation of the disease activity and damage is of key importance in making decisions on the implementation or withdrawal of antiinflammatory and immunosuppressive therapies.3 Treatment of aesthetic defects caused by LoS often requires determination of the degree of hyperpigmentation reduction. A tool which is commonly used for this purpose is Localized Scleroderma Cutaneous Assessment Tool (LoSCAT) consisting of two components: a scale classifying activity – modified Localized Scleroderma Skin Severity Index (mLoSSI), and a skin damage scale – Localized Scleroderma Skin Damage Index (LoSDI).4 Visual evaluation, applied e.g., to erythema and dyspigmentation, involves however subjective opinion of the assessing person. Instrumental methods (laser Doppler flowmetry, laser Doppler imaging, high-frequency ultrasound with colour Doppler, infrared thermography, reflectance confocal microscopy), although provide objective results, are much less frequently used, mainly because of complex conditions of taking measurements and high costs.4,5 Therefore, searching for simple and inexpensive techniques to assess severity and damage in LoS is still justified. Technology offers a few solutions for eliminating subjectivity and quantifying skin erythema and hyperpigmentation, such as spectra-based assessment or imaging-based assessment.6 Narrow-band reflectance spectrophotometry (NBRS) was used to determine skin colour, both erythematous and hyperpigmented skin lesions, but so far this method has not been used in LoS.7–14
The objective of this study was to examine the usefulness of NBRS in assessing the severity of erythema and hyperpigmentation in LoS lesions in relation to visual assessment by LoSCAT.
Materials and Methods
Patients
This cross-sectional study included adult LoS patients of the Department of Dermatology at the Medical University of Silesia in Katowice, Poland. The research was conducted in accordance with the Declaration of Helsinki Ethical Principles and Good Clinical Practices and was approved by the Ethics Committee of Medical University of Silesia in Katowice, Poland, approval No KNW/0022/KB1/68/1/19. Figure 1 shows the study design. Each participant received information about the study and gave his/her informed consent to take part in it. LoS diagnosis was established clinically on the basis of criteria proposed by Kreuter et al, and in doubtful cases the diagnosis was verified by a histopathological examination.15 Only LoS patients of phototype III (typical of the Polish population) were qualified for the study if they had an active stage (erythematous macules, lilac ring) or inactive stage (hyperpigmented lesions) of the disease. Subjects excluded from the study were those with only sclerotic, hypopigmented lesions or deep LoS. Exclusion criteria consisted also of other pathologies or inflammations in the region of interest, artificial UV radiation (i.e., phototherapy) or sunbathing 30 days before the examination, any febrile illness, intake of any medication affecting the cardiovascular system.
 |
Figure 1 Flowchart of study design. Research design for the evaluation of erythema and hyperpigmentation in localized scleroderma lesions. |
Clinical and Narrow-Band Reflectance Spectrophotometry Assessment
LoSCAT was used to assess skin lesions, as described in the previous study.16 All assessments were conducted by two examiners (MS, AF) who had a few years’ experience in the treatment of LoS. Before the beginning of the study, both investigators completed an intensive training course carried out by an expert in LoS (ALŚ). The colour measurement of LoS lesions and normal skin was conducted with NRBS using DermaLab Combo with a skin colour probe (Cortex Technologies, Hadsund, Denmark). The device emits and then measures the light reflected from the skin at two range of wavelengths 550 nm ± 30 nm (green) and 660 nm ± 60 nm (red) in order to measure haemoglobin (erythema) and melanin (hyperpigmentation). The intensity of the reflected light is the basis for the calculation of erythema index (EI) and melanin index (MI).13,17
Before the measurements, the accuracy of the instrument was secured by calibration using the specific white and black calibration standards supplied by the manufacturer. The patients were asked to resign from physical activity, vasoactive substances (e.g., alcohol, nicotine, spicy food) and local treatment on the day before the measurements.18 All the assessments were performed in the same, air-conditioned room at a temperature of 20°C, the patients were in the horizontal position and the lesions were uncovered for at least 15 minutes before the probe was applied to the skin. A round area with a 2 cm diameter was labelled with a marker pen in the lesion and in a corresponding area of normal skin on the other side of the body (or, if this was not possible, at a distance of 5 cm from the LoS lesion). For each of these areas three repeated NBRS measurements, using the lowest possible compression, were performed and the results were recorded with an automatically determined mean value of the EI and MI.
Moreover, relative percentage differences were determined for the EI and MI. The relative percentage difference in the EI (ΔEI%) was defined as the difference in EI for a lesion (EIlesion) and normal skin (EInormal), divided by the (EInormal) multiplied by 100.
Similarly, the relative percentage difference in the MI (ΔMI%) was determined.
Statistical Analysis
Statistical calculations were performed with the use of Statistica v. 12 (StatSoft Inc., Tulsa, Oklahoma, USA). Descriptive statistics (mean, median, minimum, maximum, lower quartile, upper quartile, standard deviation) were generated. Data distribution was assessed with the use of the Shapiro–Wilk test. Interrater reliability of LoSCAT findings was tested with the use of Cohen’s kappa statistic and interclass correlation coefficient. The Spearman’s rank coefficient was used to examine the correlation between clinical evaluations and measurements with the use of NBRS.12,19 A p value of <0.05 was considered statistically significant.
Results
Forty-one patients (9 males, 32 females, aged 18–72 years, mean age 52 years) with LoS were enrolled in the study. The disease duration was between 0 and 37 years (median 4 years), and the median time between the onset of the disease and LoS diagnosis was 6 months. The most common subtype was plaque LoS (25 patients), while a generalized subtype was observed in 14 women, and a linear subtype in 2 patients. Median clinical scores for particular domains were: 3 (IQR: 2–6) for dyspigmentation, 2 (IQR: 1–4) for skin atrophy, 0 (IQR: 0–2) for skin thickness, 1 (IQR: 0–3) for erythema, 0 (IQR: 0–2) for subcutaneous tissue atrophy. Median for mLoSSI was 4 (IQR: 1–10), and for LoSDI 6 (IQR: 4–11). The Cohen’s kappa statistics and interclass correlation coefficients for components of the LoSCAT were >0.8, which reflects almost perfect reliability.
A total of 84 LoS lesions were examined with the use of NBRS. They were most commonly located on the abdomen (n = 23), and lower extremities (n = 22), then the back (n = 14), upper extremities (n = 13), chest (n = 11), and face (n= 1). Figure 2 shows the measurement results and correlations between spectrophotometric measurements and visual assessments of erythema and hyperpigmentation. A moderately strong positive and statistically significant correlations were observed between erythema assessments by mLoSSI and relative difference in EI (r = 0.37; p = 0.00047) as well as between the hyperpigmentation assessments by LoSDI and relative difference in MI (r = 0.55; p=0.0000001).
Discussion
The present study has provided preliminary results of the use of NBRS in the assessment of erythema and hyperpigmentation in LoS lesions. A moderately strong positive correlation was observed between the relative percentage difference in EI and visual assessment of erythema, and a moderately strong positive correlation between the relative percentage difference in MI and clinical evaluation of hyperpigmentation. The usefulness of NBRS to assess the severity of erythematous skin lesions was indicated in previous reports, but only three studies showed that a correlation between spectrophotometric measurements and visual assessment was good or very good.7–9 Lahti et al7 used NBRS to assess contact skin reactions and erythema induced by ultraviolet B (UVB) radiation. Spectrophotometric measurements, aside to good correlation with visual assessments, showed a good correlation with the measurements obtained using colorimetry and laser Doppler flowmetry.7 Similar results were obtained by Baquié et al using NBRS to examine erythema severity after exposure to UVB, as well as after the use of vasodilators.8 A very good correlation between spectrophotometric measurements and clinical evaluations was observed by Troilius et al,9 who examined a decrease in port wine stain severity after treatment with pulsed dye laser. In other studies focused on the analysis of erythema and vascular lesions, a correlation between NRBS and visual assessments was characterized by moderate strength, and the results were similar to the ones shown in the present study.10–13
Literature data also suggested a possibility of using NBRS to assess skin hyperpigmentation. Baquié et al8 and Thibodeau et al14 assessed MI in various skin phototypes, and the spectrophotometric findings were compatible with visual perception. In studies concerning burn scars, however, the strength of correlation between hyperpigmentation assessments done by NBRS and visual assessments was only moderate.10,13 Our study confirms these observations.
It seems that the results of spectrophotometric measurements in skin inflammatory lesions may be influenced by frequent coexistence of erythema and hyperpigmentation. Melanin shows absorption in a wide range of light wavelength (400–700 nm), increasing together with a decrease in the wavelength. Thus, an increase in melanin concentration increases absorption both with regard to green and red light. Therefore, EI may be revalued at the skin pigmentation increase, and severe erythema may cause a decrease in the MI. Such a relationship was observed during NRBS examination of erythema and hyperpigmentation in burn scars, and psoriasis treated with psoralen ultraviolet A (PUVA).10,12 Due to the course of LoS – regression of erythematous lesions with hyperpigmentation – it must be assumed that the effect of melanin in this study is similar, which affects the spectrophotometric assessments of erythematous lesions. The results of NBRS may be also affected by various accompanying factors influencing blood flow in the vessels. During the present study, proper environmental conditions were organized, the patient was prepared for the NBRS measurements, and measurements were made with a maximum reduction of probe compression. The major limitation of our study is a small sample size and no comparison of NBRS with other objective tools. However, it should be emphasized that visual assessment of skin color remains the “gold standard” in clinical practice and research.20 Moreover, the NBRS seems be the preferred instrument, as tristimulus colorimetric devices are limited in their ability to differentiate metameric colors, which are colors with identical perceived appearance but different spectral features.21 And hyperspectral imaging, even though it enables contact less investigation and is globally looking for the entire region of interest (more precise tool for in vivo skin study), it takes time for data acquisition, is more expensive, and requires training for accurate performance.22
Conclusions
NBRS seems to be a useful tool to assess the severity of erythema and hyperpigmentation in LoS lesions. This technique has numerous advantages, such as the short time needed for performing measurements, the inexpensiveness and availability of required equipment, and the simplicity of operation. Conducting further studies on the use of NBRS in this disease is justified.
Abbreviations
EI, erythema index; LoS, localized scleroderma; LoSCAT, localized scleroderma cutaneous assessment tool; LoSDI, localized scleroderma skin damage index; MI, melanin index; mLoSSI, modified localized scleroderma skin severity index; NBRS, narrow-band reflectance spectrophotometry; PUVA, psoralen ultraviolet A; UVB, ultraviolet B; ΔEIr%, relative percentage difference in the erythema index; ΔMIr%, relative percentage difference in the melanin index.
Acknowledgments
This research was supported by Medical University of Silesia: KNW-1-158/N/9/K and PCN-1-197/N/0/K.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lis-Święty A, Skrzypek-Salamon A, Ranosz-Janicka I, Brzezińska-Wcisło L. Localized scleroderma: clinical and epidemiological features with emphasis on adulthood- versus childhood-onset disease differences. J Eur Acad Dermatol Venereol. 2017;31(10):
2. Asano Y, Fujimoto M, Ishikawa O, et al. Diagnostic criteria, severity classification and guidelines of localized scleroderma. J Dermatol. 2018;45(7):755–780. doi:10.1111/1346-8138.14161
3. Careta MF, Romiti R. Localized scleroderma: clinical spectrum and therapeutic update. An Bras Dermatol. 2015;90(1):62–73. doi:10.1590/abd1806-4841.20152890
4. Lis-Święty A, Janicka I, Skrzypek‐Salamon A, Brzezińska‐Wcisło L. A systematic review of tools for determining activity of localized scleroderma in paediatric and adult patients. J Eur Acad Dermatol Venereol. 2017;31(1):30–37. doi:10.1111/jdv.13790
5. Mazzilli S, Vollono L, Cosio T, et al. Reflectance confocal microscopy applied to linear (en coup de sabre) morphea. Skin Appendage Disord. 2020;6(3):171–174. doi:10.1159/000506748
6. Abdlaty R, Hayward J, Farrell T, Fang Q. Skin erythema and pigmentation: a review of optical assessment techniques. Photodiagnosis Photodyn Ther. 2021;33:102127. doi:10.1016/j.pdpdt.2020.102127
7. Lahti A, Kopola H, Harila A, et al. Assessment of skin erythema by eye, laser Doppler flowmeter, spectroradiometer, two-channel erythema meter and Minolta chroma meter. Arch Dermatol Res. 1993;285(5):278–282. doi:10.1007/BF00371596
8. Baquié M, Kasraee B. Discrimination between cutaneous pigmentation and erythema: comparison of the skin colorimeters Dermacatch and Mexameter. Ski Res Technol. 2014;20(2):218–227. doi:10.1111/srt.12109
9. Troilius A, Ljunggren B. Reflectance spectrophotometry in the objective assessment of dye laser‐treated port‐wine stains. Br J Dermatol. 1995;132(2):245–250. doi:10.1111/j.1365-2133.1995.tb05020.x
10. Gankande TU, Duke JM, Wood FM, Wallace HJ. Interpretation of the DermaLab Combo® pigmentation and vascularity measurements in burn scar assessment: an exploratory analysis. Burns. 2015;41(6):
11. Haedersdal M, Efsen J, Gniadecka M, Fogh H, Keiding J, Wulf H. Changes in skin redness, pigmentation, echostructure, thickness, and surface contour after 1 pulsed dye laser treatment of port-wine stains in children. Arch Dermatol. 1998;134(2):
12. Suh DH, Kwon TE, Kim SD, et al. Changes of skin blood flow and color on lesional and control sites during PUVA therapy for psoriasis. J Am Acad Dermatol. 2001;44(6):
13. Draaijers L, Tempelman F, Botman Y, Kreis R, Middelkoop E, van Zuijlen P. Colour evaluation in scars: tristimulus colorimeter, narrow-band simple reflectance meter or subjective evaluation? Burns. 2004;30(2):
14. Thibodeau E, D’Ambrosio J. Measurement of lip and skin pigmentation using reflectance spectrophotometry. Eur J Oral Sci. 1997;105(4):
15. Kreuter A, Krieg T, Worm M, et al. German guidelines for the diagnosis and therapy of localized scleroderma. J Dtsch Dermatol Ges. 2016;14(2):
16. Skrzypek-Salamon A, Lis-Święty A, Ranosz-Janicka I, Brzezińska-Wcisło L. Localized scleroderma cutaneous assessment tool (LoSCAT) adapted for use in adult patients: report from an initial validation study. Health Qual Life Outcomes. 2018;16(1):185. doi:10.1186/s12955-018-1010-z
17. Abdlaty R, Doerwald-Munoz L, Madooei A, et al. Hyperspectral imaging and classification for grading skin erythema. Front Phys. 2018;6:1–10. doi:10.3389/fphy.2018.00072
18. Amalu W, Block J, Chaudhry A et al. International academy of clinical thermology quality assurance guidelines. Standards and protocols in clinical thermographic imaging. Current Revision July 2015; July 2018. https://www.researchgate.net/publication/326331585.
19. Zou K, Tuncali K, Silverman S. Correlation and simple linear regression. Radiology. 2003;227(3):617–622. doi:10.1148/radiol.2273011499
20. Taylor S, Westerhof W, Im S, Lim J. Noninvasive techniques for the evaluation of skin color. J Am Acad Dermatol. 2006;54(5 Suppl 2):S282–90. doi:10.1016/j.jaad.2005.12.041
21. Ly B, Dyer E, Feig J, Chien A, Del Bino S. Research techniques made simple: cutaneous colorimetry: a reliable technique for objective skin color measurement. J Invest Dermatol. 2020;140(1):3–12.e1. doi:10.1016/j.jid.2019.11.003
22. Abdlaty R, Doerwald L, Hayward J, Fang Q Spectral assessment of radiation therapy-induced skin erythema.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.