Back to Journals » International Journal of Nanomedicine » Volume 17
Nanostructured Titanium Implant Surface Facilitating Osseointegration from Protein Adsorption to Osteogenesis: The Example of TiO2 NTAs
Authors Wu B, Tang Y, Wang K, Zhou X, Xiang L
Received 18 February 2022
Accepted for publication 20 April 2022
Published 29 April 2022 Volume 2022:17 Pages 1865—1879
DOI https://doi.org/10.2147/IJN.S362720
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yan Shen
Bingfeng Wu,1,* Yufei Tang,1,2,* Kai Wang,3 Xuemei Zhou,3 Lin Xiang1,4
1State Key Laboratory of Oral Diseases & National Clinical Research Center for Oral Diseases, West China Hospital of Stomatology, Sichuan University, Chengdu, Sichuan, People’s Republic of China; 2Department of Orthodontics, West China Hospital of Stomatology, Sichuan University, Chengdu, Sichuan, People’s Republic of China; 3School of Chemical Engineering, Sichuan University, Chengdu, Sichuan, People’s Republic of China; 4Department of Oral Implantology, West China Hospital of Stomatology, Sichuan University, Chengdu, Sichuan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xuemei Zhou, School of Chemical Engineering, Sichuan University, No. 24th, South Section 1, Yihuan Road, Chengdu, 610065, People’s Republic of China, Email [email protected] Lin Xiang, State Key Laboratory of Oral Diseases & National Clinical Research Center for Oral Diseases Sichuan University, No. 14th, 3rd Section, Renmin South Road, Chengdu, 610041, People’s Republic of China, Tel +86 28 85503579, Email [email protected]
Abstract: Titanium implants have been widely applied in dentistry and orthopedics due to their biocompatibility and resistance to mechanical fatigue. TiO2 nanotube arrays (TiO2 NTAs) on titanium implant surfaces have exhibited excellent biocompatibility, bioactivity, and adjustability, which can significantly promote osseointegration and participate in its entire path. In this review, to give a comprehensive understanding of the osseointegration process, four stages have been divided according to pivotal biological processes, including protein adsorption, inflammatory cell adhesion/inflammatory response, additional relevant cell adhesion and angiogenesis/osteogenesis. The impact of TiO2 NTAs on osseointegration is clarified in detail from the four stages. The nanotubular layer can manipulate the quantity, the species and the conformation of adsorbed protein. For inflammatory cells adhesion and inflammatory response, TiO2 NTAs improve macrophage adhesion on the surface and induce M2-polarization. TiO2 NTAs also facilitate the repairment-related cells adhesion and filopodia formation for additional relevant cells adhesion. In the angiogenesis and osteogenesis stage, TiO2 NTAs show the ability to induce osteogenic differentiation and the potential for blood vessel formation. In the end, we propose the multi-dimensional regulation of TiO2 NTAs on titanium implants to achieve highly efficient manipulation of osseointegration, which may provide views on the rational design and development of titanium implants.
Keywords: nanostructure, TiO2 nanotube arrays, titanium implant, osseointegration, anodization
Introduction
Titanium was first applied as an implant material in the late 1960s by Brånemark.1 The term “osseointegration” was created to describe the direct contact between the implant and the bone, which can be revealed using the light microscope.1 To date, titanium still plays an indispensable role in bone tissue-related diseases and is considered an attractive first-rate metal material based on the following several aspects.2,3 First, it exhibits excellent biocompatibility, one of the essential characteristics of a titanium implant. In a broad sense, biocompatibility can be understood as “the ability of an implant to perform with an appropriate host response in a specific application”.4 Second, titanium performs a superior ability in corrosion resistance. For example, it is resistance to the electrochemical corrosion from the encompassed interstitial fluid. Such resistance may be attributed to the oxide layer (TiO2) naturally formed on the titanium surface with a thickness of several nanometers.5 Third, the surface charge of the titanium implant surface can be manipulated via different techniques. The surface charge has been widely acknowledged to impact protein adsorption and cell behaviors.6,7 Fourth, the appropriate elastic modulus allows the titanium implant to be undeformed under stress. Generally, when the implant has a higher elastic modulus than the surrounding bone tissue, it causes a “stress shielding” effect. That is, the bone tissue suffers less stress than it does, which usually leads to aseptic loosening.3,5,8 Especially, titanium has a comparatively lower elastic modulus than many other biomaterials used in medical implant, so titanium is the most applicable one to use in orthopedics for the suitable mechanical property.3
Besides, titanium can naturally form a TiO2 oxide layer on its surface when exposed to oxygen-containing environments, including the living human body. Although such an oxide layer without specific surface topography shows excellent biocompatibility on titanium implants, its biological activity is inadequate. It is reported to induce a layer of fibrous tissue formation around the implant, preventing osteogenesis-related cells adhere to the implant surface, and further causing implant failure.9,10 Therefore, the additional TiO2 layer with surface topography can be artificially designed on the titanium implant surface to exhibit better biological activity and prevent the formation of fibrous tissue, which is beneficial to osseointegration.11,12
For example, scientific attention has recently been directed to manufacturing and stabilizing the nanostructured TiO2 oxide layer on titanium implants. By fabricating the nanostructured surface, the surface nano-topological pattern is formed simultaneously.13 Studies show that nano-topography can improve osseointegration in several aspects, including protein adsorption,14,15 fibrin clot attachment,16,17 cell behavior18,19 and immune response.20 On one side, the nano-topological oxide layer increases cell adhesion and influences the secretion of cytokines.21 On the other side, it mimics the intrinsic topography of the native bone with great structural complexity, which might be the signal for osteoblast-like cells to recognize the implant surface. Such reorganization by the cells is required in osteogenesis, and it is essential for osteointegration by promoting the osteogenesis on the implant surface to achieve contact osteogenesis.22–24 Therefore, efforts to fabricate appropriate nano-topological TiO2 patterns on the implant surface have been devoted.
Previous studies have suggested that nanotubes, nanorods, nanodots and other nano-techniques can be applied to fabricate surfaces with distinct biological properties. However, the function of the surface mainly contributes to only one specific stage during osteogenesis.16,23,25–30 That is, systematic investigations and discussions of a nano-topological layer that influence each stage of osseointegration for a titanium implant are lacking. Therefore, in this review, we use TiO2 nanotube arrays (TiO2 NTAs) as an example, to discuss the mechanism at each stage during osseointegration, and the rational design of TiO2 NTAs on titanium implants.
TiO2 Nano-Topography
It is found that ordered and partially ordered surface nano-topological patterns contribute to cell adhesion and osteogenic differentiation.31 To modify the titanium surface in a vertical dimension, nano-topography involving nanodots,32–36 nanorods37–39 and nanotubes40–42 can be constructed. TiO2 NTAs have been widely investigated in bone repair. It is confirmed as a promising material with outstanding ability of biocompatibility, corrosion resistance and osseointegration.9 Moreover, its multi-dimensional structure, including length, diameter, wall thickness and spacing, makes it a potential candidate to be regulated for efficient osseointegration.43–45 More importantly, compared with nanodots and nanorods, often prepared by sputter deposition or spray, the anodic TiO2 NTAs strongly adhere to the titanium surface and show an adjustable aspect ratio. The high aspect ratio provides sufficient vertical space for further modification, and provides the nanotubular layer with solid stability. Thus, in this review, we take TiO2 NTAs as an example to clarify how the TiO2 nanostructure facilitates osseointegration.46
TiO2 NTAs equip the titanium surface with different patterns based on the tube-like protrusions. Anodization is the most used technique to prepare a defined TiO2 nanotube layer on the titanium implant.47 As shown in Table 1, under different anodization time, voltage and electrolytes, TiO2 NTAs can be regulated in length, diameter, wall thickness and spacing.48 The nanotube diameter has shown to be an essential factor to impact the bioactivity of TiO2 NTAs, which may be attributed to the high sensitivity of cultured cells to sense on the surface.16,49–51 Other parameters such as spacing and wall thickness need to be further studied. So far, TiO2 NTAs have been suggested to affect protein adsorption, cell behaviors like adhesion, proliferation and differentiation through the recognition between cell and implant surface.23,40–42 In detail, the TiO2 NTAs impact the osteointegration via protein adsorption,52 platelet activation,42 inflammatory response16 and osteogenic property.53 Hence, in this review, the biological role of TiO2 NTAs at each stage in osseointegration will be discussed.
 |
Table 1 Adjust Anodization Parameters to Regulate TiO2 NTAs with Multi-Dimensional Structure, Impacting on the Biological Effect |
The Process of Implant Osseointegration
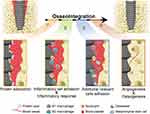
The implant osseointegration is complicated with multiple biological processes, which can be regulated by different implant surface topography.54,55 These surface characteristics impact each stage of interaction between bone tissue and implant. So we first sketch out the process of implant osseointegration (Figure 1).
Firstly, when the orthopedic implant is inserted into the aimed position, the water molecules adsorb on the implant surface in several nanoseconds.56 The hydrated titanium surface provides a favorable condition for the adsorption of proteins from blood, forming a “protein layer” which involves proteins for host inflammatory response and cell adhesion.23,55–59
Afterward, the blood platelets attach to the titanium implant surface, secreting inner contents to form the fibrin clots, which facilitate the migration of cells towards the implant surface.23 Blood is the main route for cell migration, in which angiogenesis plays a vital role and runs through the overall osseointegration process. Besides, vascular invasion supplies nutrients, oxygen, cytokines, growth factors, osteoblasts, osteoclast precursors and mesenchymal stem cells (MSCs) for osteogenesis. The close relationship between osteogenesis and vascularization is called “angiogenic–osteogenic coupling”.60
During cell migration, neutrophils and macrophages are considered as first arrivals to initiate an inflammatory response, and clean the wound site and the necrotic tissue.16,61 The most recent work suggests that neutrophils are essential in recruiting and orchestrating innate and adaptive immunocytes, especially recruiting MSCs at the initial stage of bone regeneration.62 For macrophages, its polarization determines the fate of bone regeneration. Although many investigations demonstrate diversity in macrophage polarization, which expanded M1/M2 phenotypes, M1 and M2 macrophages are considered the main phenotypes in peri-implant immune response.63–65 Our preliminary results suggest that M1/M2-related gene expression participates in bone metabolism around the implant; thus, our following discussions will be focused on M1 and M2 macrophages. Macrophages can be polarized to proinflammatory M1 macrophages and anti-inflammatory M2 macrophages in response to the local microenvironment.64 The former secrets proinflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-1 (IL-1) to intensify the inflammatory response.30 Inversely, the M2 phenotype participates in alleviating inflammatory response and promoting tissue repair by releasing cytokines like interleukin-4 (IL-4) and interleukin-10 (IL-10).66 These two phenotypes exhibit entirely different bioactivity. Long-persisting M1 macrophages may cause the formation of fibrotic encapsulation which brings implant failure.16,67 However, M2 macrophages release chemokines and growth factors involving transforming growth factor-β (TGF-β) and platelet-derived growth factor (PDGF) to facilitate the migration, homing and osteogenic differentiation of MSCs.25,68
When MSCs from bone marrow arrive at the implant surface, they accelerate tissue healing and osteogenesis under the existence of inflammatory cytokines and growth factors. They differentiate into different cell types including osteoblasts, chondrocytes and fibroblasts depending on the biological microenvironment which is affected by the surface topological characteristics.23,30,42,59 The nanostructure on titanium implants that mimics the intrinsic topography of the native bone makes MSCs and osteoblast-like cells adhesion on the surface, achieving contact osteogenesis, and promoting osseointegration.22,23
The above-mentioned biological processes are strongly influenced by the topography of the titanium implant surface. Previous studies show that the nano-topological characteristics of TiO2 NTAs on titanium implants provide favorable conditions for osseointegration.16,30,42,58,69 To understand how TiO2 NTAs participate in the whole processes in osseointegration, according to different pivotal biological processes, we define osseointegration into four stages: protein adsorption, inflammatory cell adhesion/inflammatory response, additional relevant cells adhesion, and angiogenesis/osteogenesis. Although the stage “angiogenesis” is categorized as the last stage, it actually runs through the entire osseointegration process. In the following, the function of TiO2 NTAs will be discussed at each of the four stages, respectively.
The Impact of TiO2 NTAs on Titanium Implants on Osseointegration
Protein Adsorption
Protein adsorption on the titanium surface occurs immediately after forming a hydrated surface. Such a “protein layer” is fundamental for the subsequent biological processes. For example, previous studies show that vitronectin accelerates the attachment of osteoblast, and the fibrin functions for the recruitment of cells.70,71 It is also suggested that the adsorbed vitronectin or fibronectin influences the initial adhesion and spreading of osteoblast-like cells and other cells.72,73 These proteins at the interface between the implant surface and cells work as extracellular signals for the organization of cell cytoskeleton.21 Therefore, the capability of the titanium implant to adsorb such proteins from blood fundamentally determines the subsequent cell attachment and spreading.74
It is suggested that protein adsorption ability is closely related to the surface properties of titanium implants, such as wettability and surface charge.14,21,75–78 Surface wettability is an essential factor for protein adsorption. In comparison with a hydrophilic surface, previous studies show hydrophobic one can adsorb more protein.14,79 However, the quantity of adsorbed proteins hardly determines the biological effect of the implant. Instead, the type of adsorbed proteins is essential. For example, it is suggested that an enhanced quantity of adsorption of albumin on a hydrophilic surface, results in anti-inflammatory cytokines expressed by macrophages. On the contrary, hydrophobic surface adsorbs more IgG2, which results in more pro-inflammatory cytokines expressed by macrophages.80 Thus, the hydrophilic surface may induce a better reparative effect through its adsorbed anti-inflammatory cytokines.
Taking TiO2 NTAs on titanium implant as an example, the protein adsorption is mainly attributed to its hydrophilic surface and high surface area.14 For instance, TiO2 NTAs of 30–130 nm inner diameter exhibit more hydrophilic than bare titanium, but such hydrophilicity weakens after being aged in the air for three months.49,78 In addition, TiO2 NTAs of 90 nm diameter with hydrophilic surface adsorbs more vitronectin and fibrinogen than bare titanium.16 Moreover, Gong et al report that hydrophilic TiO2 NTAs can selectively adsorb proteins, such as promoting bovine serum albumin adsorption, and decreasing fibrinogen adsorption. The phenomenon attributes to the surface charge of different proteins and the hydrophilicity, surface area and surface charge of TiO2 NTAs.49
The conformation of adsorbed proteins is impacted by the surface of TiO2 NTAs as well. For instance, it is demonstrated that TiO2 NTAs of 30 nm diameter show noticeably weakened pro-inflammatory properties on macrophage polarization, since the adsorbed fibronectin, which is reported to be involved in the manipulation of integrin-induced macrophage behavior on biomaterial, displayed different conformations as the nanotube size changed, specific in the changing of exposed Arg-Gly-Asp (RGD) domain.52,81 TiO2 NTAs of 30 nm diameter allow the maximum exposure of RGD domain in fibronectin.52 Hence, TiO2 NTAs can regulate the protein conformation, which is also significant in protein adsorption, even more critical than the adsorbed concentration.
To sum up, many studies on TiO2 NTAs have proved their favorable ability to adsorb proteins due to the hydrophilic surface and tunable diameters.82 However, as the wettability of the surface is just one of the key factors that influence protein adsorption, it is hard to conclude precisely the best degree of contact angle for protein adsorption. But according to previous studies, the contact angle of TiO2 NTAs below 50 degrees shows better biological activity compared with materials with larger contact angle.16,42,49,80 In addition to the surface wettability, the nanotube diameter also impacts protein adsorption. Considering that protein conformation plays a more significant role in protein function, we thus believe that a diameter of about 30 nm is more suitable for the function of relevant protein.52
Inflammatory Cell Adhesion/Inflammatory Response
Along with protein adsorption, platelets from blood adhere to the titanium implant surface, secreting endogenous substances to recruit more platelets to assemble irreversibly, forming blood clots with polymerized fibrin, and resulting in the formation of peri-implant hematoma.83 It is suggested that TiO2 NTAs are able to accelerate platelets adhering, aggregating, transforming, and spreading, but TiO2 NTAs of 50–100 nm diameter decrease platelets adhesion and activation.26,84 In order to clarify the function of TiO2 NTAs in platelet adsorption in detail, Bai et al culture platelets on TiO2 NTAs of different diameters. In comparison with platelets cultured on a bare titanium surface, platelets cultured on TiO2 NTAs stretch more lamellipodia and filopodia, and more platelets are activated, releasing more growth factors (PDGF-AB and TGF-β).42 The growth factors can further influence subsequent biological processes such as cell recruitment, cell differentiation and macrophage polarization.85 These bioactive factors are essential in tissue regeneration and the healing process, regarded as regulators of cell behaviors.64,85
Under the cytokines in the microenvironment, neutrophils and macrophages are recruited to clean the wound site, considered the first arrival to the peri-implant.61 Neutrophils are activated by the interaction between their integrin and platelets, phagocytosing foreign body and necrotic tissue.86,87 The latest study also shows the critical role of neutrophils in bone regeneration, indicating their significant effect in recruiting and regulating immunocytes and MSCs at the initial bone regeneration.62 So far, the impact of TiO2 NTAs on neutrophils behaviors is less studied. Macrophages also arrive around the implant at an early time, and initiate the host body response.61 They are polarized to different phenotypes according to the diverse local microenvironment, including blood clot conditions, reinforcing the inflammatory response (M1 macrophages) or accelerating tissue repair (M2 macrophages) by secreting different cytokines.64,88 M1 polarization during the early stage of bone repairing determines the cytokines released by M2 macrophages, which means prolonged M1 polarization results in M2 macrophages releasing fibrosis-related cytokines, leading to the formation of fibrous encapsulation, even to the implant failure.89
To verify how TiO2 NTAs influence macrophages, macrophages are co-cultured with blood clots on TiO2 NTAs. More macrophages tend to be polarized to the M2 phenotype, which is beneficial to tissue repair. RNA sequence analysis in vivo shows a decrease in inflammatory-related signaling pathways and an increase in metabolism-related signaling pathways in TiO2 nanotubes groups, corresponding to in vitro experiments.42 Besides, this study also finds that different nanotube diameters impacted macrophages polarization to a different degree, and 15 nm is the optimal one for osteogenesis.42 Similarly, investigation on TiO2 NTAs of 90–5000 nm diameter suggests the sample of 90 nm diameter remarkably allows the macrophages to extend more filopodia from the cell body and induce more macrophages M2-polarization, decreasing the inflammatory factors production and facilitating osteogenesis.16 In the above two works, we noticed that two different optimal diameters of TiO2 NTAs were reported. The latter proposes that 90 nm diameter is optimal for M2-polarization, while the former reports 15 nm is optimal. We speculate that the reason is connected with the different purposes of the study. The latter study aims to clarify that nanoscale-topography is more efficient on M2-polarization than microscale-topography by comparing 90 nm diameter and 5000 nm diameter, while the former study further focuses on the optimal nanoscale-topography for M2-polarization and compares the diameter of 15 nm, 60 nm and 120 nm.16,42 Thus, for facilitating M2-polarization, we propose that the diameter of 15 nm in the nanoscale investigation is a more precise nanotube parameter than 90 nm.42
As a biophysical signal, the effect of TiO2 NTAs on macrophages polarization relates to the change of relevant signaling pathways. Peroxisome proliferator-activated receptor (PPAR) signaling pathway and RhoA/ROCK signaling pathway have been confirmed as M2-polarization related pathway.90,91 After culture macrophages on 90 nm diameter TiO2 NTAs, the PPAR signaling pathway and RhoA/ROCK signaling pathway are up-regulated. Meanwhile, pathways related to M1-polarization, including mitogen-activated protein kinase (MAPK), Adenosine monophosphate-activated protein kinase (AMPK) signaling pathway, TNF, nuclear factor light chain enhancer of activated B cells, and nucleotide‐binding oligomerization domain (NOD)–like receptor signaling pathways, are down-regulated after culture macrophages on 90 nm diameter TiO2 NTAs.16,92–97 Therefore, TiO2 NTAs with suitable size can first regulate the formation of stable inflammatory cell adhesion, and then regulate the bioactive factors’ secretion at the cellular level and signaling pathway expression at the molecular level.
These studies exhibit a significant connection between innate immunity and TiO2 NTAs. Besides, the important role of TiO2 NTAs in adaptive immunity was discovered recently. TiO2 NTAs can activate T lymphocytes and induce the expression of fibroblast growth factor-2 (FGF-2) by blocking key MAPK signaling pathways; however, the optimal nanotube diameter is 105 nm, rather than the optimal diameter of 15 nm for macrophage M2-polarization.42,98 M2-polarization facilitates osseointegration, while T lymphocyte plays a vital role in fibrosis.99 Therefore, we can deeply investigate the difference in the optimal TiO2 NTAs diameters of the two cells, and try to increase M2-polarization and decrease T lymphocyte activation, to avoid fibrotic encapsulation forming around the implant and facilitate osseointegration.
To sum up, we propose that TiO2 NTAs of about 15 nm in diameter are more suitable for inflammatory regulation. They can facilitate M2-polarization as well as prevent fibrous tissue formation.16,42,98 Figure 2 shows how TiO2 NTAs manipulate inflammatory cell adhesion and inflammatory response.
Additional Relevant Cells Adhesion
The hematoma formed in initial inflammation is also regarded as a fibrillar scaffold for recruiting repairment-related cells including MSCs, osteoblasts and fibroblasts under the effect of released cytokines and chemokines.42,68,87 The different cells adhere to the titanium implant surface and play their specific roles, as discussed below.
It is widely accepted that cells can perceive and respond to the extracellular matrix (ECM) biochemical environment and the ECM biophysical environment such as the nano-topological surface. After migrating to the biomaterial surface, MSCs and other repairment-related cells are able to adapt to the nano-topography, and recognize the ECM proteins adsorbed on the implant surface, such as collagens, vitronectins, fibronectins, and laminins, via a kind of cell transmembrane receptor proteins that are referred as integrins.55 Integrins are responsible for cell-matrix adhesion, connecting the intracellular and extracellular environment through their globular head domain. They can be combined with specific domains on ECM proteins such as RGD domain.74,100 After integrins bind with the targeted ECM proteins, intracellular signaling pathways induce integrins to assemble at the plasma membrane and change their conformation to influence the cytoskeletal organization.101 The integrin assembling accelerates hundreds of cytoplasmic proteins and signaling molecules to move to the attachment site, enhancing the adhesion strength and forming focal adhesions. The connection between the cell actomyosin system with ECM is formed by focal adhesions that form a “gear box” to perceive mechanical forces of ECM and achieve mechanotransduction.102 Subsequently, the cascade reaction influences cell behaviors like migration and spreading.74
Studies have proved that TiO2 NTAs have a favorable function for cell adhesion by culturing different cells on TiO2 NTAs, and have shown that the nanostructure of TiO2 NTAs has a positive role in accelerating adhesion of migrated MSCs and osteoblasts.103 However, MSCs show diverse optimal diameters for cell adhesion and osteogenic differentiation, respectively, after culturing on TiO2 NTAs of different diameters. For example, MSCs, cultured on TiO2 NTAs of 30 nm diameter, exhibit promoted cell adhesion and proliferation without noticeable osteogenic differentiation, but on TiO2 NTAs of 200 nm diameter, MSCs show promoted osteogenic differentiation but impaired cell adhesion.104 Considering that cell adhesion mainly relies on the biophysical signal and ECM proteins, studies confirm the synergistic effect of TiO2 NTAs topological signal and pre-adsorbed proteins (fibronectin, vitronectin, and laminin) to promote MSCs’ adhesion.82 In addition, TiO2 NTAs, solely as a kind of biophysical signal, can affect MSCs’ adhesion without adsorbed proteins, which may be attributed to the hollow structure of TiO2 NTAs, providing anchoring sites for cell attachment.105 Such configuration makes focal adhesion complex and F-actin stronger and more stable, compared to that on bare titanium.53 Besides, the size and complexity of focal adhesion complex further grow as adhesion time increases.106
Similar to MSCs, osteoblasts’ adhesion is intensified on TiO2 NTAs as well.45,107 Immunofluorescence and SEM analysis show more extensive focal adhesion and wider filopodia after culturing osteoblasts on TiO2 NTAs of 15 nm diameter, compared to that on 20–100 nm diameter and bare titanium.45 It has been proved that the osteoblast adhesion is related to the PI3K-Akt-mTOR pathway, Ras-MAPK-ERK1/2 pathway and p130Cas-RhoA GTPase pathway,107 which are mainly functioning in response to extracellular biophysical signal through integrins. In addition to diameter, the lateral spacing of TiO2 NTAs also influences osteoblast behaviors. Osteoblasts cultured on nanotubes of 80 nm spacing display slightly less spreading and focal adhesion, compared with nanotubes of 18 nm spacing.44 Such phenomenon can be attributed to the reduced surface area for cell attachment on the top wall surface as the spacing increases.44
Besides, macrophages, adhered to TiO2 NTAs of 15 nm diameter, stretch a high density of filopodia since the topological signal up-regulated RhoA family protein expression in macrophages.42,107,108
To conclude, TiO2 NTAs of 15–30 nm diameter have a positive effect on repairment-related cell adhesion.42,45,104 In detail, cells cultured on TiO2 NTAs form intensified focal adhesion and stretch filopodia from the cell body.45,53 Such structures intensify cell adhesion, and further lay a foundation for subsequent contact osteogenesis on the implant surface, facilitating osseointegration.
Angiogenesis/Osteogenesis
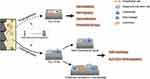
Protein adsorption, inflammatory response and cell adhesion are indispensable during osseointegration, which can be considered as the foreshadowing of angiogenesis and osteogenic differentiation. Angiogenesis and osteogenesis can also be impacted by TiO2 NTAs (Figure 3).
Angiogenesis starts from inflammatory responses, providing a healing site with nutrients, cytokines, growth factors and chemokines, removing waste products and setting up access for cell recruitment.60 New blood vessel formation is essential in osteogenesis, and runs through the whole process.109 However, the process is complicated, and involves various bioactive factors and biological reactions.110 The TiO2 NTAs are applied to cardiovascular stents, where excellent endothelial cell activity is required. Though little research on the angiogenic application of TiO2 NTAs in bone implants, it is found that the TiO2 NTAs can obviously promote endothelial cell spreading and migration in the application to vascular stents, through which we can speculate the angiogenesis potent of TiO2 NTAs.111 Culturing bovine aortic endothelial cells (BAECs) on TiO2 NTAs, in comparison with cells on flat titanium, the cells appear prominently more elongated morphology, larger spreading area and increased cell migration ability, with more protrusions forming.112,113 Noticeably, these protrusions from the cell body form broad cellular interconnections, suggesting an active state for cell function and intercellular signal delivery, which contributes to angiogenesis.114 An essential process in angiogenesis is the capability of viable endothelial cells to grow and proliferate in response to different biomaterial surfaces. Thus, the increased endothelial activity on TiO2 NTAs may promote new blood vessel formation, and further accelerate various biological mediator transportation and cell recruitment in the peri-implant microenvironment, promoting osteogenesis indirectly.115
Osteogenesis is the crucial stage in osseointegration. Osteogenic associated cells such as MSCs and osteoblasts are recruited to the implant position and adhesion on the implant surface, secreting osteoid and mineralizing. The key to osseointegration is to promote osteogenesis on the implant surface, achieving contact osteogenesis and avoiding the formation of fibrous encapsulation.23,24 TiO2 NTAs can directly affect osteoblasts as an extracellular mechanical signal, transmitting into intracellular signals and regulating cell behaviors. For example, TiO2 NTAs made osteoblasts stretch well with a high amount of filopodia, and the filopodia could grow into nanotube pores.116,117 It suggested that the surface nano-pattern plays a guiding role in cell adhesion and spreading. An excellent cell stretching and spreading condition indicates a good condition for cell function. Thus, the stretched osteoblasts morphology hinted at better osteogenic ability on TiO2 NTAs, with enhanced alkaline phosphatase (ALP) activity, mineral deposition, and osteogenesis-related gene expression.45,50,117,118
Furthermore, researchers make use of the adjustability of TiO2 NTAs, to explore the most suitable diameter for cell adhesion and osteogenic differentiation. When culturing MSCs and osteoblasts on TiO2 NTAs of different diameters, respectively, Park et al found that they both performed better osteogenic differentiation on TiO2 NTAs of 15 nm diameter compared with cell culture on 100 nm TiO2 NTAs.45,50 However, Oh et al report that MSCs cultured on 100 nm diameter TiO2 NTAs exhibit an elongated morphology with better osteogenic differentiation ability compared with MSCs cultured on 30 nm diameter nanotubes. They further perform quantitative real-time PCR analysis and immunofluorescent staining of osteopontin (OPN) and osteocalcin (OCN), connecting the elongated morphology with osteogenic differentiation. The result confirms the better osteogenic differentiation guiding function of 100 nm diameter TiO2 NTAs than 30 nm diameter TiO2 NTAs and bare titanium.118 The results of the two studies on the optimal diameter for osteogenesis seem to be contradictory. Regarding the different opinions on the optimal diameter, from our perspective, in Park’s research, the high mineralization ability on nanotubes of 15 nm diameter is much related to its high quantity of MSCs, according to the cell adhesion, proliferation and migration in the study. Besides, Park et al used rat MSCs for the experiment, while Oh et al used human MSCs. Previous studies confirm that the same cell from different species has distinct cell behaviors.119,120 Hence, the MSCs derived from humans and rats also affect the results due to the different osteogenic differentiation abilities.
Additionally, the lateral spacing of TiO2 NTAs can regulate osteogenic differentiation. Osteoblasts cultured on TiO2 NTAs of 80 nm spacing show a significant increase of ALP, OPN and OCN expression than TiO2 NTAs of 18 nm spacing, suggesting remarkable high osteogenic activity.44
In addition to the direct physical signal, macrophages co-cultured on TiO2 NTAs can also affect MSCs indirectly. To compare MSCs differentiation in different conditions, MSCs are first cultured with TiO2 NTAs directly, and only found a slightly increased ALP activity. Subsequently, cytokines collected from macrophages cultured on the same TiO2 NTAs are added to the cultured MSCs. And ALP activity, osteogenic gene expression and mineralization remarkably increase, compared to the same process on bare titanium.16 According to the study, we speculate that TiO2 NTAs also accelerate osteogenic differentiation through cytokine modulation, as we reviewed above in inflammatory cell adhesion and inflammatory response.42,89
According to the direct and cytokines-induced indirect effect of TiO2 NTAs on osteogenesis, we propose that TiO2 NTAs of about 100 nm diameter are suitable for osteogenesis.16,118
Conclusion and Future Perspectives
In conclusion, we have summarized the impact of TiO2 NTAs on osseointegration at four different stages, respectively. In the first stage, TiO2 NTAs not only impact the type of adsorbed proteins, but change the conformation of adsorbed protein. In the second stage, TiO2 NTAs mainly manipulate inflammatory response by regulating platelet behaviors, macrophage polarization and T lymphocyte behaviors. In the third stage, the repairment-related cells, including MSCs and osteoblasts, adhere to TiO2 NTAs surface, stretch filopodia from the cell body, and form intensified focal adhesion. The last stage includes angiogenesis and osteogenesis. Although angiogenesis is closely linked with osteogenesis, it indeed begins from the inflammatory stage, playing an essential role in cell recruitment and biological mediator transportation. For osteogenesis, osteogenic differentiation is manipulated by TiO2 NTAs, with promoted osteogenesis-related gene expression, ALP activity and mineralization. More importantly, the above-mentioned biological processes can be controlled as nanotube diameter and spacing change.
Based on this, we speculate that, in addition to diameter and spacing, other parameters of nanotube such as length and wall thickness also possess the potential to regulate biological processes on TiO2 NTAs, and we call it “multi-dimensional regulation.”
Although many experiments are performed to investigate TiO2 NTAs on titanium implants, from our perspective, these studies have some limitations. Firstly, they mainly focused on a particular stage during osseointegration, instead of regulating the entire process of osseointegration. Secondly, as a nanomaterial with multi-dimensional regulation potential, TiO2 NTAs possess numerous adjustable parameters. Before we take advantage of the nanotube parameters, we need to figure out the corresponding biological effect using different TiO2 NTAs. However, present studies focus the majority on the diameter, and do not pay enough attention to the other parameters such as length, wall thickness and spacing. From another perspective, most in vivo experiments in current studies grow TiO2 NTAs on the smooth surface of a titanium plate or rod. However, the clinically used implants with thread are more challenging to be modified with TiO2 NTs. These shortcomings limit the clinical application of TiO2 NTAs in implant surgery.
To achieve highly efficient regulation, we address the assumption to manipulate the entire osseointegration by multi-dimensional regulation. Several aspects need to be noted in the following studies.
Firstly, when we focus on diverse stages of osseointegration, we notice that there is no single biological process, since every biological process in osseointegration affects mutually. Therefore, we need to keep an integrated perspective to achieve one-to-multiphase efficient regulation.
Then, how to achieve one-to-multiphase regulation in osseointegration becomes a significant challenge. In our opinion, immunoregulation may be a suitable way for such highly efficient osseointegration adjustment. Researchers have proved that immunology is an indispensable factor in bone regeneration, and importantly, the immunology microenvironment seems to be the common regulatory factor in the almost whole process of osseointegration.121 On this basis, we can adopt a new idea to design TiO2 NTAs on titanium implants. Instead of directly facilitating MSCs or osteoblast-like cell adhesion and differentiation, it is more balancing to adjust the initial inflammatory response to establish an appropriate microenvironment around the implant, and subsequently, promote angiogenesis and osteogenesis indirectly.
Thus, the immunomodulation function of TiO2 NTAs may become a significant research direction for designing a new generation of implant biomaterial with outstanding osseointegration properties. As the anodization technique develops, we can even customize personalized TiO2 NTAs parameters on titanium implant surfaces in different cases, to achieve more efficient osseointegration. Such efficient regulation needs to be reached by further investigations on multi-dimensional regulation. Specifically, more studies are required to investigate the coordinating combination of TiO2 NTAs parameters including diameter, spacing, wall thickness and length. The TiO2 NTAs in this review are taken as an example to illustrate the function of nanostructures on the titanium implant. We hope the underlying mechanism discussed here can be applied to other surfaces.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No.82170997), Sichuan Science and Technology Program (No.2021YJ0422), Project of Chengdu Science and Technology Bureau (No.2021-YF05-02054-SN), and Research Funding from West China School/Hospital of Stomatology Sichuan University (No. RCDWJS2020-6). Bingfeng Wu and Yufei Tang are co-first authors for this study. Xuemei Zhou and Lin Xiang are co-correspondence authors for this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Brånemark PI, Hansson BO, Adell R, et al. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand J Plast Reconstr Surg Suppl. 1977;16:1–132.
2. Zhou Z, Shi Q, Wang J, et al. The unfavorable role of titanium particles released from dental implants. Nanotheranostics. 2021;5(3):321–332. doi:10.7150/ntno.56401
3. Quinn J, McFadden R, Chan CW, Carson L. Titanium for orthopedic applications: an overview of surface modification to improve biocompatibility and prevent bacterial biofilm formation. iScience. 2020;23(11):101745. doi:10.1016/j.isci.2020.101745
4. Nair LS, Laurencin CT. Biodegradable polymers as biomaterials. Prog Polym Sci. 2007;32(8–9):762–798. doi:10.1016/j.progpolymsci.2007.05.017
5. Eliaz N. Corrosion of metallic biomaterials: a review. Materials. 2019;12(3):407. doi:10.3390/ma12030407
6. Jager M, Jennissen HP, Dittrich F, Fischer A, Kohling HL. Antimicrobial and osseointegration properties of nanostructured titanium orthopaedic implants. Materials. 2017;10(11):1302. doi:10.3390/ma10111302
7. Kusakawa Y, Yoshida E, Hayakawa T. Protein adsorption to titanium and zirconia using a quartz crystal microbalance method. Biomed Res Int. 2017;2017:1521593. doi:10.1155/2017/1521593
8. Arabnejad S, Johnston B, Tanzer M, Pasini D. Fully porous 3D printed titanium femoral stem to reduce stress-shielding following total hip arthroplasty. J Orthop Res. 2017;35(8):1774–1783. doi:10.1002/jor.23445
9. Nair M, Elizabeth E. Applications of titania nanotubes in bone biology. J Nanosci Nanotechnol. 2015;15(2):939–955. doi:10.1166/jnn.2015.9771
10. Ahn TK, Lee DH, Kim TS, et al. Modification of titanium implant and titanium dioxide for bone tissue engineering. Adv Exp Med Biol. 2018;1077:355–368.
11. Awad NK, Edwards SL, Morsi YS. A review of TiO(2) NTs on Ti metal: electrochemical synthesis, functionalization and potential use as bone implants. Mater Sci Eng C Mater Biol Appl. 2017;76:1401–1412. doi:10.1016/j.msec.2017.02.150
12. Jafari S, Mahyad B, Hashemzadeh H, Janfaza S, Gholikhani T, Tayebi L. Biomedical applications of TiO(2) nanostructures: recent advances. Int J Nanomedicine. 2020;15:3447–3470. doi:10.2147/IJN.S249441
13. Chopra D, Gulati K, Ivanovski S. Understanding and optimizing the antibacterial functions of anodized nano-engineered titanium implants. Acta Biomater. 2021;127:80–101. doi:10.1016/j.actbio.2021.03.027
14. Barberi J, Spriano S. Titanium and protein adsorption: an overview of mechanisms and effects of surface features. Materials. 2021;14(7):1590. doi:10.3390/ma14071590
15. Li Y, Xiao Y, Liu C. The horizon of materiobiology: a perspective on material-guided cell behaviors and tissue engineering. Chem Rev. 2017;117(5):4376–4421. doi:10.1021/acs.chemrev.6b00654
16. Zhu Y, Liang H, Liu X, et al. Regulation of macrophage polarization through surface topography design to facilitate implant-to-bone osteointegration. Sci Adv. 2021;7(14):eabf6654. doi:10.1126/sciadv.abf6654
17. Yang D, Lü X, Hong Y, Xi T, Zhang D. The molecular mechanism of mediation of adsorbed serum proteins to endothelial cells adhesion and growth on biomaterials. Biomaterials. 2013;34(23):5747–5758. doi:10.1016/j.biomaterials.2013.04.028
18. Nobles KP, Janorkar AV, Williamson RS. Surface modifications to enhance osseointegration-resulting material properties and biological responses. J Biomed Mater Res B Appl Biomater. 2021;109(11):1909–1923. doi:10.1002/jbm.b.34835
19. Bauer S, Schmuki P, von der Mark K, Park J. Engineering biocompatible implant surfaces: part I: materials and surfaces. Prog Mater Sci. 2013;58(3):261–326.
20. Xu AT, Xie YW, Xu JG, Li J, Wang H, He FM. Effects of strontium-incorporated micro/nano rough titanium surfaces on osseointegration via modulating polarization of macrophages. Colloids Surf B Biointerfaces. 2021;207:111992. doi:10.1016/j.colsurfb.2021.111992
21. Ngandu Mpoyi E, Cantini M, Reynolds PM, Gadegaard N, Dalby MJ, Salmerón-Sánchez M. Protein adsorption as a key mediator in the nanotopographical control of cell behavior. ACS Nano. 2016;10(7):6638–6647. doi:10.1021/acsnano.6b01649
22. Kane R, Ma PX. Mimicking the nanostructure of bone matrix to regenerate bone. Mater Today. 2013;16(11):418–423. doi:10.1016/j.mattod.2013.11.001
23. Gittens RA, Olivares-Navarrete R, Schwartz Z, Boyan BD. Implant osseointegration and the role of microroughness and nanostructures: lessons for spine implants. Acta Biomater. 2014;10(8):3363–3371. doi:10.1016/j.actbio.2014.03.037
24. Davies JE. Mechanisms of endosseous integration. Int J Prosthodont. 1998;11(5):391–401.
25. Ma QL, Zhao LZ, Liu RR, et al. Improved implant osseointegration of a nanostructured titanium surface via mediation of macrophage polarization. Biomaterials. 2014;35(37):9853–9867. doi:10.1016/j.biomaterials.2014.08.025
26. Zhang L, Liao X, Fok A, Ning C, Ng P, Wang Y. Effect of crystalline phase changes in titania (TiO(2)) nanotube coatings on platelet adhesion and activation. Mater Sci Eng C Mater Biol Appl. 2018;82:91–101. doi:10.1016/j.msec.2017.08.024
27. Wang W, Zhao L, Ma Q, Wang Q, Chu PK, Zhang Y. The role of the Wnt/β-catenin pathway in the effect of implant topography on MG63 differentiation. Biomaterials. 2012;33(32):7993–8002. doi:10.1016/j.biomaterials.2012.07.064
28. Li Y, Wang S, Dong Y, et al. Effect of size and crystalline phase of TiO(2) nanotubes on cell behaviors: a high throughput study using gradient TiO(2) nanotubes. Bioact Mater. 2020;5(4):1062–1070. doi:10.1016/j.bioactmat.2020.07.005
29. von Wilmowsky C, Bauer S, Roedl S, Neukam FW, Schmuki P, Schlegel KA. The diameter of anodic TiO2 nanotubes affects bone formation and correlates with the bone morphogenetic protein-2 expression in vivo. Clin Oral Implants Res. 2012;23(3):359–366. doi:10.1111/j.1600-0501.2010.02139.x
30. Ma QL, Fang L, Jiang N, et al. Bone mesenchymal stem cell secretion of sRANKL/OPG/M-CSF in response to macrophage-mediated inflammatory response influences osteogenesis on nanostructured Ti surfaces. Biomaterials. 2018;154:234–247. doi:10.1016/j.biomaterials.2017.11.003
31. Gui N, Xu W, Myers DE, Shukla R, Tang HP, Qian M. The effect of ordered and partially ordered surface topography on bone cell responses: a review. Biomater Sci. 2018;6(2):250–264. doi:10.1039/C7BM01016H
32. Sjöström T, Dalby MJ, Hart A, Tare R, Oreffo RO, Su B. Fabrication of pillar-like titania nanostructures on titanium and their interactions with human skeletal stem cells. Acta Biomater. 2009;5(5):1433–1441. doi:10.1016/j.actbio.2009.01.007
33. Sjostrom T, Lalev G, Mansell JP, Su B. Initial attachment and spreading of MG63 cells on nanopatterned titanium surfaces via through-mask anodization. Appl Surf Sci. 2011;257(10):4552–4558. doi:10.1016/j.apsusc.2010.11.064
34. Pan HA, Hung YC, Chiou JC, Tai SM, Chen HH, Huang GS. Nanosurface design of dental implants for improved cell growth and function. Nanotechnology. 2012;23(33):335703. doi:10.1088/0957-4484/23/33/335703
35. Silverwood RK, Fairhurst PG, Sjöström T, et al. Analysis of osteoclastogenesis/osteoblastogenesis on nanotopographical titania surfaces. Adv Healthc Mater. 2016;5(8):947–955. doi:10.1002/adhm.201500664
36. Sahlin H, Contreras R, Gaskill DF, Bjursten LM, Frangos JA. Anti-inflammatory properties of micropatterned titanium coatings. J Biomed Mater Res A. 2006;77(1):43–49. doi:10.1002/jbm.a.30642
37. Zhou J, Li B, Han Y, Zhao L. The osteogenic capacity of biomimetic hierarchical micropore/nanorod-patterned Sr-HA coatings with different interrod spacings. Nanomedicine. 2016;12(5):1161–1173. doi:10.1016/j.nano.2016.01.011
38. Zhang G, Yang Y, Shi J, et al. Near-infrared light II - assisted rapid biofilm elimination platform for bone implants at mild temperature. Biomaterials. 2021;269:120634. doi:10.1016/j.biomaterials.2020.120634
39. Yang H, Yu M, Wang R, et al. Hydrothermally grown TiO(2)-nanorods on surface mechanical attrition treated Ti: improved corrosion fatigue and osteogenesis. Acta Biomater. 2020;116:400–414. doi:10.1016/j.actbio.2020.09.005
40. Su EP, Justin DF, Pratt CR, et al. Effects of titanium nanotubes on the osseointegration, cell differentiation, mineralisation and antibacterial properties of orthopaedic implant surfaces. Bone Joint J. 2018;100-b(1 Supple A):9–16. doi:10.1302/0301-620X.100B1.BJJ-2017-0551.R1
41. Lu R, Wang C, Wang X, et al. Effects of hydrogenated TiO(2) nanotube arrays on protein adsorption and compatibility with osteoblast-like cells. Int J Nanomedicine. 2018;13:2037–2049. doi:10.2147/IJN.S155532
42. Bai L, Zhao Y, Chen P, et al. Targeting early healing phase with titania nanotube arrays on tunable diameters to accelerate bone regeneration and osseointegration. Small. 2021;17(4):e2006287. doi:10.1002/smll.202006287
43. Lee K, Mazare A, Schmuki P. One-dimensional titanium dioxide nanomaterials: nanotubes. Chem Rev. 2014;114(19):9385–9454. doi:10.1021/cr500061m
44. Necula MG, Mazare A, Ion RN, et al. Lateral spacing of TiO(2) nanotubes modulates osteoblast behavior. Materials. 2019;12(18):2956. doi:10.3390/ma12182956
45. Park J, Bauer S, Schlegel KA, Neukam FW, von der Mark K, Schmuki P. TiO2 nanotube surfaces: 15 nm–an optimal length scale of surface topography for cell adhesion and differentiation. Small. 2009;5(6):666–671. doi:10.1002/smll.200801476
46. Ahmed F, Pervez SA, Aljaafari A, et al. Fabrication of TiO(2)-nanotube-array-based supercapacitors. Micromachines. 2019;10(11):742. doi:10.3390/mi10110742
47. Spriano S, Yamaguchi S, Baino F, Ferraris S. A critical review of multifunctional titanium surfaces: new frontiers for improving osseointegration and host response, avoiding bacteria contamination. Acta Biomater. 2018;79:1–22. doi:10.1016/j.actbio.2018.08.013
48. Wang Q, Zhou P, Liu S, et al. Multi-scale surface treatments of titanium implants for rapid osseointegration: a review. Nanomaterials. 2020;10(6):1244.
49. Gong Z, Hu Y, Gao F, et al. Effects of diameters and crystals of titanium dioxide nanotube arrays on blood compatibility and endothelial cell behaviors. Colloids Surf B Biointerfaces. 2019;184:110521. doi:10.1016/j.colsurfb.2019.110521
50. Park J, Bauer S, von der Mark K, Schmuki P. Nanosize and vitality: TiO2 nanotube diameter directs cell fate. Nano Lett. 2007;7(6):1686–1691. doi:10.1021/nl070678d
51. Brammer KS, Oh S, Cobb CJ, Bjursten LM, Heyde Hvd JS, Jin S. Improved bone-forming functionality on diameter-controlled TiO2 nanotube surface. Acta Biomater. 2009;5(8):3215–3223. doi:10.1016/j.actbio.2009.05.008
52. Qi H, Shi M, Ni Y, et al. Size-confined effects of nanostructures on fibronectin-induced macrophage inflammation on titanium implants. Adv Healthc Mater. 2021;10:e2100994. doi:10.1002/adhm.202100994
53. Tong Z, Liu Y, Xia R, et al. F-actin regulates osteoblastic differentiation of mesenchymal stem cells on TiO2 nanotubes through MKL1 and YAP/TAZ. Nanoscale Res Lett. 2020;15(1):183. doi:10.1186/s11671-020-03415-9
54. Smeets R, Stadlinger B, Schwarz F, et al. Impact of dental implant surface modifications on osseointegration. Biomed Res Int. 2016;2016:6285620. doi:10.1155/2016/6285620
55. Skoog SA, Kumar G, Narayan RJ, Goering PL. Biological responses to immobilized microscale and nanoscale surface topographies. Pharmacol Ther. 2018;182:33–55. doi:10.1016/j.pharmthera.2017.07.009
56. Roach P, Eglin D, Rohde K, Perry CC. Modern biomaterials: a review - bulk properties and implications of surface modifications. J Mater Sci Mater Med. 2007;18(7):1263–1277. doi:10.1007/s10856-006-0064-3
57. Liu X, Lim JY, Donahue HJ, Dhurjati R, Mastro AM, Vogler EA. Influence of substratum surface chemistry/energy and topography on the human fetal osteoblastic cell line hFOB 1.19: phenotypic and genotypic responses observed in vitro. Biomaterials. 2007;28(31):4535–4550. doi:10.1016/j.biomaterials.2007.06.016
58. Lin DJ, Fuh LJ, Chen WC. Nano-morphology, crystallinity and surface potential of anatase on micro-arc oxidized titanium affect its protein adsorption, cell proliferation and cell differentiation. Mater Sci Eng C Mater Biol Appl. 2020;107:110204. doi:10.1016/j.msec.2019.110204
59. Hidalgo-Bastida LA, Cartmell SH. Mesenchymal stem cells, osteoblasts and extracellular matrix proteins: enhancing cell adhesion and differentiation for bone tissue engineering. Tissue Eng Part B Rev. 2010;16(4):405–412. doi:10.1089/ten.teb.2009.0714
60. Saran U, Gemini Piperni S, Chatterjee S. Role of angiogenesis in bone repair. Arch Biochem Biophys. 2014;561:109–117. doi:10.1016/j.abb.2014.07.006
61. McWhorter FY, Davis CT, Liu WF. Physical and mechanical regulation of macrophage phenotype and function. Cell Mol Life Sci. 2015;72(7):1303–1316. doi:10.1007/s00018-014-1796-8
62. Cai B, Lin D, Li Y, et al. N2-polarized neutrophils guide bone mesenchymal stem cell recruitment and initiate bone regeneration: a missing piece of the bone regeneration puzzle. Adv Sci. 2021;8(19):e2100584. doi:10.1002/advs.202100584
63. Murray PJ, Allen JE, Biswas SK, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41(1):14–20. doi:10.1016/j.immuni.2014.06.008
64. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–969. doi:10.1038/nri2448
65. Lee J, Byun H, Madhurakkat Perikamana SK, Lee S, Shin H. Current advances in immunomodulatory biomaterials for bone regeneration. Adv Healthc Mater. 2019;8(4):e1801106. doi:10.1002/adhm.201801106
66. Mahon OR, Browe DC, Gonzalez-Fernandez T, et al. Nano-particle mediated M2 macrophage polarization enhances bone formation and MSC osteogenesis in an IL-10 dependent manner. Biomaterials. 2020;239:119833. doi:10.1016/j.biomaterials.2020.119833
67. Dondossola E, Holzapfel BM, Alexander S, Filippini S, Hutmacher DW, Friedl P. Examination of the foreign body response to biomaterials by nonlinear intravital microscopy. Nat Biomed Eng. 2016;1:0007. doi:10.1038/s41551-016-0007
68. Hotchkiss KM, Clark NM, Olivares-Navarrete R. Macrophage response to hydrophilic biomaterials regulates MSC recruitment and T-helper cell populations. Biomaterials. 2018;182:202–215. doi:10.1016/j.biomaterials.2018.08.029
69. Nuhn H, Blanco CE, Desai TA. Nanoengineered stent surface to reduce in-stent restenosis in vivo. ACS Appl Mater Interfaces. 2017;9(23):19677–19686. doi:10.1021/acsami.7b04626
70. Kilpadi KL, Chang PL, Bellis SL. Hydroxylapatite binds more serum proteins, purified integrins, and osteoblast precursor cells than titanium or steel. J Biomed Mater Res. 2001;57(2):258–267. doi:10.1002/1097-4636(200111)57:2<258::AID-JBM1166>3.0.CO;2-R
71. Szaba FM, Smiley ST. Roles for thrombin and fibrin(ogen) in cytokine/chemokine production and macrophage adhesion in vivo. Blood. 2002;99(3):1053–1059. doi:10.1182/blood.V99.3.1053
72. Maciel J, Oliveira MI, Gonçalves RM, Barbosa MA. The effect of adsorbed fibronectin and osteopontin on macrophage adhesion and morphology on hydrophilic and hydrophobic model surfaces. Acta Biomater. 2012;8(10):3669–3677. doi:10.1016/j.actbio.2012.06.010
73. Gongadze E, Kabaso D, Bauer S, Park J, Schmuki P, Iglič A. Adhesion of osteoblasts to a vertically aligned TiO2 nanotube surface. Mini Rev Med Chem. 2013;13(2):194–200.
74. Wilson CJ, Clegg RE, Leavesley DI, Pearcy MJ. Mediation of biomaterial-cell interactions by adsorbed proteins: a review. Tissue Eng. 2005;11(1–2):1–18. doi:10.1089/ten.2005.11.1
75. Zhang K, Xing J, Chen J, et al. A spatially varying charge model for regulating site-selective protein adsorption and cell behaviors. Biomater Sci. 2019;7(3):876–888. doi:10.1039/C8BM01158C
76. Rupp F, Gittens RA, Scheideler L, et al. A review on the wettability of dental implant surfaces I: theoretical and experimental aspects. Acta Biomater. 2014;10(7):2894–2906. doi:10.1016/j.actbio.2014.02.040
77. Khudhair D, Bhatti A, Li Y, et al. Anodization parameters influencing the morphology and electrical properties of TiO2 nanotubes for living cell interfacing and investigations. Mater Sci Eng C Mater Biol Appl. 2016;59:1125–1142. doi:10.1016/j.msec.2015.10.042
78. Shin DH, Shokuhfar T, Choi CK, Lee SH, Friedrich C. Wettability changes of TiO2 nanotube surfaces. Nanotechnology. 2011;22(31):315704. doi:10.1088/0957-4484/22/31/315704
79. Aiyelabegan HT, Sadroddiny E. Fundamentals of protein and cell interactions in biomaterials. Biomed Pharmacother. 2017;88:956–970. doi:10.1016/j.biopha.2017.01.136
80. Visalakshan RM, MacGregor MN, Sasidharan S, et al. Biomaterial surface hydrophobicity-mediated serum protein adsorption and immune responses. ACS Appl Mater Interfaces. 2019;11(31):27615–27623. doi:10.1021/acsami.9b09900
81. Abitorabi MA, Pachynski RK, Ferrando RE, Tidswell M, Erle DJ. Presentation of integrins on leukocyte microvilli: a role for the extracellular domain in determining membrane localization. J Cell Biol. 1997;139(2):563–571. doi:10.1083/jcb.139.2.563
82. Wu S, Zhang D, Bai J, et al. Adsorption of serum proteins on titania nanotubes and its role on regulating adhesion and migration of mesenchymal stem cells. J Biomed Mater Res A. 2020;108(11):2305–2318. doi:10.1002/jbm.a.36987
83. Smith SA, Travers RJ, Morrissey JH. How it all starts: initiation of the clotting cascade. Crit Rev Biochem Mol Biol. 2015;50(4):326–336. doi:10.3109/10409238.2015.1050550
84. Huang Q, Yang Y, Zheng D, et al. Effect of construction of TiO(2) nanotubes on platelet behaviors: structure-property relationships. Acta Biomater. 2017;51:505–512. doi:10.1016/j.actbio.2017.01.044
85. Anitua E, Sánchez M, Nurden AT, Nurden P, Orive G, Andía I. New insights into and novel applications for platelet-rich fibrin therapies. Trends Biotechnol. 2006;24(5):227–234. doi:10.1016/j.tibtech.2006.02.010
86. Herter JM, Rossaint J, Zarbock A. Platelets in inflammation and immunity. J Thromb Haemost. 2014;12(11):1764–1775. doi:10.1111/jth.12730
87. Burkhardt MA, Gerber I, Moshfegh C, et al. Clot-entrapped blood cells in synergy with human mesenchymal stem cells create a pro-angiogenic healing response. Biomater Sci. 2017;5(10):2009–2023. doi:10.1039/C7BM00276A
88. Alfarsi MA, Hamlet SM, Ivanovski S. The effect of platelet proteins released in response to titanium implant surfaces on macrophage pro-inflammatory cytokine gene expression. Clin Implant Dent Relat Res. 2015;17(6):1036–1047. doi:10.1111/cid.12231
89. Chen Z, Klein T, Murray RZ, et al. Osteoimmunomodulation for the development of advanced bone biomaterials. Mater Today. 2016;19(6):304–321. doi:10.1016/j.mattod.2015.11.004
90. Yao Q, Liu J, Zhang Z, et al. Peroxisome proliferator-activated receptor γ (PPARγ) induces the gene expression of integrin α(V)β(5) to promote macrophage M2 polarization. J Biol Chem. 2018;293(43):16572–16582. doi:10.1074/jbc.RA118.003161
91. Xu Y, Cui K, Li J, et al. Melatonin attenuates choroidal neovascularization by regulating macrophage/microglia polarization via inhibition of RhoA/ROCK signaling pathway. J Pineal Res. 2020;69(1):e12660. doi:10.1111/jpi.12660
92. Yu WP, Ding JL, Liu XL, et al. Titanium dioxide nanotubes promote M2 polarization by inhibiting macrophage glycolysis and ultimately accelerate endothelialization. Immun Inflamm Dis. 2021;9:746–757. doi:10.1002/iid3.429
93. Zhu L, Zhao Q, Yang T, Ding W, Zhao Y. Cellular metabolism and macrophage functional polarization. Int Rev Immunol. 2015;34(1):82–100. doi:10.3109/08830185.2014.969421
94. Deng RH, Zou MZ, Zheng D, et al. Nanoparticles from cuttlefish ink inhibit tumor growth by synergizing immunotherapy and photothermal therapy. ACS Nano. 2019;13(8):8618–8629. doi:10.1021/acsnano.9b02993
95. Zhou H, Coveney AP, Wu M, et al. Activation of both TLR and NOD signaling confers host innate immunity-mediated protection against microbial infection. Front Immunol. 2018;9:3082. doi:10.3389/fimmu.2018.03082
96. Wu TM, Nan FH, Chen KC, Wu YS. Sarcodia suieae acetyl-xylogalactan regulate RAW 264.7 macrophage NF-kappa B activation and IL-1 beta cytokine production in macrophage polarization. Sci Rep. 2019;9(1):19627. doi:10.1038/s41598-019-56246-9
97. Chen D, Xie J, Fiskesund R, et al. Chloroquine modulates antitumor immune response by resetting tumor-associated macrophages toward M1 phenotype. Nat Commun. 2018;9(1):873. doi:10.1038/s41467-018-03225-9
98. Singhatanadgit W, Toso M, Pratheepsawangwong B, Pimpin A, Srituravanich W. Titanium dioxide nanotubes of defined diameter enhance mesenchymal stem cell proliferation via JNK- and ERK-dependent up-regulation of fibroblast growth factor-2 by T lymphocytes. J Biomater Appl. 2019;33(7):997–1010. doi:10.1177/0885328218816565
99. Adusei KM, Ngo TB, Sadtler K. T lymphocytes as critical mediators in tissue regeneration, fibrosis, and the foreign body response. Acta Biomater. 2021;133:17–33. doi:10.1016/j.actbio.2021.04.023
100. Paavolainen O, Peuhu E. Integrin-mediated adhesion and mechanosensing in the mammary gland. Semin Cell Dev Biol. 2021;114:113–125. doi:10.1016/j.semcdb.2020.10.010
101. Boettiger D. Mechanical control of integrin-mediated adhesion and signaling. Curr Opin Cell Biol. 2012;24(5):592–599. doi:10.1016/j.ceb.2012.07.002
102. Sun Z, Costell M, Fässler R. Integrin activation by talin, kindlin and mechanical forces. Nat Cell Biol. 2019;21(1):25–31. doi:10.1038/s41556-018-0234-9
103. Khrunyk YY, Belikov SV, Tsurkan MV, et al. Surface-dependent osteoblasts response to TiO(2) nanotubes of different crystallinity. Nanomaterials. 2020;10(2):320. doi:10.3390/nano10020320
104. Xu Z, Lai Y, Wu D, et al. Increased mesenchymal stem cell response and decreased staphylococcus aureus adhesion on titania nanotubes without pharmaceuticals. Biomed Res Int. 2015;2015:172898. doi:10.1155/2015/172898
105. Mu P, Li Y, Zhang Y, et al. High-throughput screening of rat mesenchymal stem cell behavior on gradient TiO(2) nanotubes. ACS Biomater Sci Eng. 2018;4(8):2804–2814. doi:10.1021/acsbiomaterials.8b00488
106. Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6(1):56–68. doi:10.1038/nrm1549
107. Chen S, Guo Y, Liu R, et al. Tuning surface properties of bone biomaterials to manipulate osteoblastic cell adhesion and the signaling pathways for the enhancement of early osseointegration. Colloids Surf B Biointerfaces. 2018;164:58–69. doi:10.1016/j.colsurfb.2018.01.022
108. Fischer RS, Lam PY, Huttenlocher A, Waterman CM. Filopodia and focal adhesions: an integrated system driving branching morphogenesis in neuronal pathfinding and angiogenesis. Dev Biol. 2019;451(1):86–95. doi:10.1016/j.ydbio.2018.08.015
109. Dai J, Rabie AB. VEGF: an essential mediator of both angiogenesis and endochondral ossification. J Dent Res. 2007;86(10):937–950. doi:10.1177/154405910708601006
110. Raines AL, Olivares-Navarrete R, Wieland M, Cochran DL, Schwartz Z, Boyan BD. Regulation of angiogenesis during osseointegration by titanium surface microstructure and energy. Biomaterials. 2010;31(18):4909–4917. doi:10.1016/j.biomaterials.2010.02.071
111. Lee PP, Cerchiari A, Desai TA. Nitinol-based nanotubular coatings for the modulation of human vascular cell function. Nano Lett. 2014;14(9):5021–5028. doi:10.1021/nl501523v
112. Peng L, Eltgroth ML, LaTempa TJ, Grimes CA, Desai TA. The effect of TiO2 nanotubes on endothelial function and smooth muscle proliferation. Biomaterials. 2009;30(7):1268–1272. doi:10.1016/j.biomaterials.2008.11.012
113. Brammer KS, Oh S, Gallagher JO, Jin S. Enhanced cellular mobility guided by TiO2 nanotube surfaces. Nano Lett. 2008;8(3):786–793. doi:10.1021/nl072572o
114. Beltrán-Partida E, Valdéz-Salas B, Moreno-Ulloa A, et al. Improved in vitro angiogenic behavior on anodized titanium dioxide nanotubes. J Nanobiotechnology. 2017;15(1):10. doi:10.1186/s12951-017-0247-8
115. Shi B, Andrukhov O, Berner S, Schedle A, Rausch-Fan X. The angiogenic behaviors of human umbilical vein endothelial cells (HUVEC) in co-culture with osteoblast-like cells (MG-63) on different titanium surfaces. Dent Mater. 2014;30(8):839–847. doi:10.1016/j.dental.2014.05.005
116. Peng Z, Ni J, Zheng K, et al. Dual effects and mechanism of TiO2 nanotube arrays in reducing bacterial colonization and enhancing C3H10T1/2 cell adhesion. Int J Nanomedicine. 2013;8:3093–3105. doi:10.2147/IJN.S48084
117. Oh S, Daraio C, Chen LH, Pisanic TR, Fiñones RR, Jin S. Significantly accelerated osteoblast cell growth on aligned TiO2 nanotubes. J Biomed Mater Res A. 2006;78(1):97–103. doi:10.1002/jbm.a.30722
118. Oh S, Brammer KS, Li YS, et al. Stem cell fate dictated solely by altered nanotube dimension. Proc Natl Acad Sci U S A. 2009;106(7):2130–2135. doi:10.1073/pnas.0813200106
119. Eyckmans J, Luyten FP. Species specificity of ectopic bone formation using periosteum-derived mesenchymal progenitor cells. Tissue Eng. 2006;12(8):2203–2213. doi:10.1089/ten.2006.12.2203
120. Zhang Y, Polman M, Mohammad AF, et al. Species-independent stimulation of osteogenic differentiation induced by osteoclasts. Biochem Biophys Res Commun. 2022;606:149–155. doi:10.1016/j.bbrc.2022.03.115
121. Caetano-Lopes J, Canhão H, Fonseca JE. Osteoimmunology–the hidden immune regulation of bone. Autoimmun Rev. 2009;8(3):250–255. doi:10.1016/j.autrev.2008.07.038
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.