Back to Journals » International Journal of Nanomedicine » Volume 19
Nanoplatform-Mediated Autophagy Regulation and Combined Anti-Tumor Therapy for Resistant Tumors
Authors Yang C, Ding Y, Mao Z, Wang W
Received 22 October 2023
Accepted for publication 4 January 2024
Published 26 January 2024 Volume 2024:19 Pages 917—944
DOI https://doi.org/10.2147/IJN.S445578
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor R.D.K. Misra
Caixia Yang,1,2,* Yuan Ding,1,2,* Zhengwei Mao,3 Weilin Wang1,2
1Department of Hepatobiliary and Pancreatic Surgery, the Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, People’s Republic of China; 2Key Laboratory of Precision Diagnosis and Treatment for Hepatobiliary and Pancreatic Tumor of Zhejiang Province, Hangzhou, Zhejiang, People’s Republic of China; 3MOE Key Laboratory of Macromolecular Synthesis and Functionalization, Department of Polymer Science and Engineering, Zhejiang University, Hangzhou, Zhejiang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhengwei Mao, Department of Polymer Science and Engineering, Zhejiang University, No. 866 Yuhangtang Road, Hangzhou, Zhejiang, 310058, People’s Republic of China, Tel +86057187783820, Fax +86057187068001, Email [email protected] Weilin Wang, Department of Hepatobiliary and Pancreatic Surgery, the Second Affiliated Hospital, Zhejiang University School of Medicine, No. 88 Jiefang Road, Hangzhou, Zhejiang, 310009, People’s Republic of China, Email [email protected]
Abstract: The overall cancer incidence and death toll have been increasing worldwide. However, the conventional therapies have some obvious limitations, such as non-specific targeting, systemic toxic effects, especially the multidrug resistance (MDR) of tumors, in which, autophagy plays a vital role. Therefore, there is an urgent need for new treatments to reduce adverse reactions, improve the treatment efficacy and expand their therapeutic indications more effectively and accurately. Combination therapy based on autophagy regulators is a very feasible and important method to overcome tumor resistance and sensitize anti-tumor drugs. However, the less improved efficacy, more systemic toxicity and other problems limit its clinical application. Nanotechnology provides a good way to overcome this limitation. Co-delivery of autophagy regulators combined with anti-tumor drugs through nanoplatforms provides a good therapeutic strategy for the treatment of tumors, especially drug-resistant tumors. Notably, the nanomaterials with autophagy regulatory properties have broad therapeutic prospects as carrier platforms, especially in adjuvant therapy. However, further research is still necessary to overcome the difficulties such as the safety, biocompatibility, and side effects of nanomedicine. In addition, clinical research is also indispensable to confirm its application in tumor treatment.
Keywords: autophagy, tumor resistance, nanotechnology, combination therapy, co-delivery
Introduction
Cancer is a global public health problem, especially malignant tumors. According to the American Cancer Society, mortality of cancer has continued to decline by 29% since 1991.1 However, cancer remains one of the leading causes of death in the world. With the combined effects of multiple factors such as the aging of the population, population growth, serious environmental pollution and bad living habits, the overall cancer incidence and death toll have been increasing worldwide. As of 2020, an estimated 19.3 million new cancer cases occurred. Notably, the global cancer burden is expected to increase by 47% compared to 2020.2 The increasing burden of cancer has brought considerable difficulties and challenges to both health care system and social development. Anti-tumor therapy mainly involves surgical treatment, radiotherapy, gene therapy, and other types of treatments. In recent years, with the continuous breakthroughs in medical scientific research, the innovation of medical technology, there are several types of current anti-tumor drugs:3 cytotoxic chemotherapy drugs,4 cell differentiation inducers,5 cell death inducers, antiangiogenic agents,6 hormonal therapy drugs,7 targeted therapy drugs,8 immunotherapy drugs,9 bacteria-based therapy drugs,10,11 photodynamic therapy and photothermal drugs,12 etc.
Although the above conventional therapies have improved the survival rate of tumor patients to a certain extent, they also have obvious limitations, such as non-specific targeting, drug resistance, systemic toxic effects, adverse side effects and limited indications,13 especially the multidrug resistance (MDR) of tumors, which is the main obstacle to tumor treatment, especially metastatic tumors. This MDR reaction not only causes poor anti- tumor efficacy,14 but also is related to the recurrence and metastasis of cancer,15 leading to treatment failure.16 Tumors may develop multidrug resistance to anti-tumor therapy drugs through a variety of mechanisms: 1) changes in the autophagy pathway; 2) overexpression of transmembrane ATP-dependent drug efflux pumps; 3) changes in drug metabolism through glutathione Glypeptide-S-transferase or cytochrome P450 pathway; 4) DNA repair mechanisms; 5) modification of apoptotic signal transduction,17 et cetera. As shown in Figure 1.
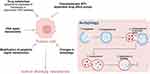 |
Figure 1 The mechanisms of multi-drug resistance in tumor especially the autophagy mechanism. |
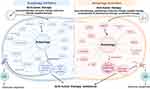 |
Figure 2 The different role of autophagy in tumor drug resistance. |
Among them autophagy, as an important and most commonly studied drug resistance mechanism, is a cellular degradation process and an important protective mechanism to maintain or restore cellular homeostasis under physiological and pathological conditions by selectively clearing damaged or redundant peroxisomes, endoplasmic reticulum, mitochondria or DNA, which can reduce the accumulation of abnormal proteins and organelles and maintain cell homeostasis,18 playing an important role in drug resistance.19 Studies have shown that activated autophagy may lead to two opposite outcomes, exerting pro-death or pro-survival effects. Depending on the different cell fate, activation or inhibition of autophagy is differentially exploited to enhance tumor therapy. On the one hand, if autophagy is inhibited, autophagy activators can be used to kill tumor cells or enhance the sensitivity of drug-resistant tumor cells to anti-tumor drugs. On the other hand, if protective autophagy is activated for cell survival, autophagy inhibitors can be used to synergistically enhance the killing effect of anti-tumor drugs,20 as shown in Figure 1.
At present, the combination therapeutic methods of autophagy regulators and anti-tumor drugs are commonly used to improve anti-tumor efficacy, which have received increasing attention.21 However, despite various attempts to improve the anti-tumor efficacy, the increased adverse reactions and minimal improvement in survival limit the combination therapy’s clinical applications.22 In addition, for patients, the non-specific systemic toxic side effects of anti-tumor drugs are also a huge physical burden. Therefore, there is an urgent need for new anti-tumor drugs or treatment methods to reduce adverse reactions, improve the treatment efficacy and expand their therapeutic indications more effectively and accurately.
The emergence of nanotechnology provides a new perspective. Nanotechnology can take full advantages of combination therapy,23 exert synergistic anti-tumor effect, especially on drug-resistant tumors,24 enhancing efficacy while reducing toxic and side effects.25 Continuous innovation and development in nanomedicine have greatly improved the effectiveness and accuracy of tumor treatment,26 making tumor nanooncology a very popular field.27,28
Therefore, this review will emphasize the applications of nanotechnology-based combination therapy with autophagy regulatory drugs and anti-tumor drugs in anti-tumor treatment, and will elaborate from the following aspects: (1) co-delivery of autophagy inhibitors combined with anti-tumor drugs based on nanoplatforms; (2) co-delivery of autophagy activators combined with anti-tumor drugs based on nanoplatforms; (3) co-delivery of dual autophagy regulators based on nanoplatforms; (4) delivery of anti-tumor drugs through nano-autophagy platforms; (5) Adjuvant anti-tumor therapy through the nano-autophagy platform. We expect to provide a clearer foundation and direction for future research on tumor treatment, especially the treatment of drug resistant tumors, through the elaboration and discussion of these aspects.
The Important Role of Autophagy in Tumor Resistance
There are multiple pathways contributed to tumor drug resistance, and the most commonly studied pathway is autophagy. Studies have shown that autophagy plays a dual role in tumor progression, particularly in tumor drug resistance, whether activation or inhibition can promote tumor progression.29 Autophagy has the ability to effectively inhibit malignant transformation, but it can also enhance tumor progression and drug resistance.30 The current research progress of autophagy in tumor drug resistance will be discussed in this section from two different angles.
Activation of Autophagy Promotes Tumor Resistance
When tumor cells are killed, a large amount of damaged organelles, abnormal proteins and other harmful components will be produced, which will activate autophagy to clear harmful subsequently, making autophagy become one of the reasons of tumor drug resistance.31
Autophagy Activation Promotes Conventional Treatment Resistance
Autophagy activation by various pathways in tumor cells after chemotherapy plays a key role in resistance to chemotherapy drugs during tumor treatment. Studies have shown that after chemotherapy treatment, autophagy is activated by up-regulated TXNDC17 and COPS3-FOXO3, which promotes resistance to paclitaxel32 and cisplatin.33 In addition, studies using clinical samples also found that in drug resistant patients, increased autophagy levels were positively correlated with their resistance to 5-fluorouracil34 and doxorubicin, and the anti-tumor efficacy of epirubicin could be significantly enhanced when combined with autophagy inhibitor.35
Besides, immunotherapy has remained popular in recent years, but many tumors have developed drug resistance to it, according to research. Further research found that the autophagy pathway is activated in immunotherapy resistant tumor cells, and the up-regulated autophagic flux can degrade MHC-I and promote tumor immune escape. When the autophagy inhibitor chloroquine is combined to inhibit autophagy, the efficacy of dual ICB therapy (anti-PD1 and anti-CTLA4 antibodies) is significantly enhanced to inhibit tumor cell proliferation with stronger anti-tumor immune response.36
It is worth noting that targeted therapy is another effective method to treat tumors. However, during the treatment, a significant number of patients exhibit resistance to targeted therapy drugs. A lot of researchers have conducted studies and suggested that activation of autophagy plays an important role. For example, researchers pointed out that EGFR inhibitor can activate autophagy by up-regulating the AXL signaling pathway to promote resistance to EGFR inhibitors.37 Besides, Yu Z et al found that LOC8500938 and miR-56739 derived from drug responsive cells can inhibit autophagy to reverse docetaxel and trastuzumab resistance, which reveals that differences in autophagy between cells can affect cell fate. Similarly, researchers also found that autophagy can mediate resistance to PARP inhibitors,40 sorafenib,41 BRAF inhibitors,42 histone deacetylase inhibitors and proteasome inhibitors,43 etc. Hence, the combination use of autophagy inhibitors is a vital strategy to reverse drug resistance and regain sensitivity to targeted drugs.
What’s more, radiotherapy is a classic method of tumor treatment, which has effectively improved the prognosis and survival of many tumors, especially metastatic tumors. However, due to the protective autophagy induced by X-ray irradiation and the powerful ability of damaged DNA repair, radiotherapy resistance remains the main cause of radiotherapy failure44 in glioblastoma, ovarian cancer45 and other tumors.
For some hormone-sensitive tumors, endocrine therapy is the first choice, but it is prone to ineffective outcomes due to drug resistance. To this end, researchers have conducted studies and found that in estrogen receptor-positive (ER+) breast cancer patients with low VDR expression, the activation of the autophagy pathway promotes resistance to tamoxifen.46 Based on the previous studies, researchers discovered that the inhibition of autophagy can significantly enhance the sensitivity to enzalutamide47 and glucocorticoid.48
Autophagy Activation Promotes Resistance to Non-Conventional Treatment
Due to the limitations of commonly used treatment methods and unsatisfactory efficacy, many new cancer treatment methods have emerged. Among them, photodynamic therapy and photothermal therapy have received the most attention. However, long-term treatment often fails to achieve the expected level of effects. It is because of the activation of protective autophagy promotes tumor resistance to photodynamic therapy,49 limiting the effectiveness of monotherapy. Considering the limitations and drug resistance of mono-therapy, researchers have proposed various combination therapies with multiple drugs to treat tumors. However, as treatment progresses, researchers have observed that certain patients still exhibit high levels of resistance to combination therapy due to the crucial role of protective autophagy. For example, Zanotto-Filho A et al found that combination therapy with temozolomide and curcumin upregulates ERK1/2 and activates autophagy, leading to the resistance of glioblastoma to combination therapy.50
The activation of protective autophagy maintains cell homeostasis while providing benefits to tumor cells by clearing harmful components caused by drug damage and re-degrading them into usable substances, as evidenced by the work of researchers, making it an important pathway for tumor resistance (Figure 2). This is reflected in both monotherapy and combination therapy. Therefore, it is feasible and important to targeting autophagy for drug resistance treatment, especially with the regulation of immune response, which can help overcome tumor resistance.
Inhibition of Autophagy Promotes Tumor Resistance
Generally speaking, it is common that the activation of protective autophagy can promote tumor drug resistance. However, many researchers have discovered that the inhibition of autophagy can also promote tumor drug resistance. Thus, this section will specifically elaborate on the important role of autophagy inhibition in resistance to different treatments and explore its reasons.
Autophagy Inhibition Promotes Conventional Treatment Resistance
Researchers observed that down-regulated autophagy levels can promote the survival of drug-resistant cells.
Chemotherapy can inhibit autophagy by regulating microRNA mediated downstream signaling pathways, and promote tumor resistance, such as microRNA-199a-5p51 and miR-519a.52 Similar phenomenon was also observed in resistance to cisplatin, 5-fluorouracil and doxorubicin, by activating PI3K-Akt-mTOR signaling to inhibit autophagy,53–55 suggesting that inhibition of autophagy is a key factor.
Furthermore, with the advancement of immunotherapy in recent years, Li ZL et al found that during the immunotherapy treatment, TNC accumulation caused by impaired autophagy can inhibit the T cell-mediated tumor killing effect and promote resistance to PD-1/PD-L1 in triple-negative breast cancer patients with autophagy defects,56 which shows that autophagy inhibition can promote tumor resistance by affecting the interaction between tumor cells and cells in the tumor microenvironment.
As for targeted therapy, inhibition of autophagy also plays an important role in tumor resistance. Researchers using clinical samples showed that autophagy was inhibited in patients with high expression of G6PD and ADRB2, or low expression of PTEN and FAT4, contributing to the resistance to lapatinib,57 sorafenib,58 trastuzumab59 and ceritinib,60 through degradation of HIF1α or promoting epithelial–mesenchymal transition. Additionally, experimental studies also observed that inhibiting autophagy can promote tumor resistance to diphtheria toxin-EGF61 and sorafenib,62 by activating caspase and PGE 2 signaling pathways or miR-21 mediated Akt/PTEN signaling pathway. And inhibiting the Akt pathway can activate autophagy to exert autophagy-dependent cell death, thus reversing the acquired resistance.63 In this case, using a variety of methods activating autophagy can reverse resistance by up-regulating autophagy-dependent death pathway.
Identically, researchers pointed out that miR-221/222-mediated inhibition of autophagy promotes dexamethasone resistance in multiple myeloma,64 suggesting autophagy inhibition contributing to the tumor resistance to endocrine therapy.
Autophagy Inhibition Promotes Resistance to Non-Conventional Treatment
In addition to the above-mentioned mainstream treatments, researchers also demonstrated that Beclin-1- deficient glioma can promote resistance to adenovirus mediated oncolytic therapy by inhibiting autophagy,65 which indicating that the inhibition of autophagy has an important impact on tumor resistance to various treatments.
From the studies mentioned above, we can find that autophagy inhibition may promote tumor drug resistance through various pathways: (1) Autophagy inhibition is a concomitant result of changes in pro-survival mechanisms, such as microRNA-mediated pathways, meanwhile activating other pro-survival pathways and making them the main survival pathways, independent of autophagy, leading to tumor drug resistance; (2) Autophagy inhibition can inhibit the degradation of pro-survival pathway proteins, such as antioxidant pathways and EMT, promoting tumor resistance; (3) Autophagy inhibition can promote tumor resistance by regulating the interaction between tumors and cells in tumor microenvironment, such as the anti-tumor immune cells. For this reason, researchers outlined that activating autophagy can activate autophagy-dependent cell death, rather than adaptive cell survival, to reverse tumor resistance. For example, the new autophagy activator pomiferin can inhibit SERCA and activate CaMKKβ-AMPK-mTOR signaling to trigger autophagy-dependent cell death and overcome tumor multidrug resistance,66 as shown in Figure 2.
In summary, autophagy activation or inhibition can induce tumors resistance to multiple therapies. The changes in different pathways and the dependence on different survival pathways are key factors contributing to the effect of different autophagy level on the proliferation of resistant tumor cells. Furthermore, the functional changes of non-tumor cells in the tumor microenvironment due to autophagy are also crucial factors of tumor resistance. Therefore, the combined use of autophagy regulators, taking into account multiple changes, is an important research direction. Besides, in addition to inhibiting or activating autophagy solely to overcome tumor resistance which may lead to adaptive survival and proliferation in tumor cells. Researchers have also demonstrated that regulating different stages of autophagy to coexist activation and inhibition of autophagy can also effectively reverse tumor resistance (Figure 2). Yan J et al pointed out that the early stage autophagy inhibitor 3-MA can promote colorectal cancer survival, while the late stage autophagy inhibitor CQ increase the apoptosis, suggesting that the blockage of autophagy-lysosome contributes to the efficacy.67
Although combining autophagy regulators can achieve significant benefits in anti-tumor therapy, their clinical efficacy is often disappointing and sometimes leads to higher systemic toxicity.68 The reason why is that after the drug enters the body, differences in spatiotemporal delivery, physical and chemical properties, metabolic efficiency, payload, pharmacokinetics and biodistribution restrict the efficacy, and due to complex dosage regimens, patient’s poor compliance and enhanced toxicity, the clinical applicability of the combination therapy is also limited.15 At present, there is still a lot of room for improvement in this combination therapy.69 Therefore, how to maintain the spatial and temporal unity of the optimal dosage, pharmacokinetics and biodistribution of combined drugs, and how to ensure that the therapeutic dose of combination drugs correctly arrives,21 meanwhile minimizing the systemic adverse reactions is main difficulty of combination therapies.
Nanotechnology Based Delivery System to Precisely Treat Tumors
Nanomedicine is a field that applies nanotechnology in medicine which has made significant contributions to clinical treatment after decades of development and is receiving increasing attention.16 Nanoparticles based drug delivery systems have shown strong advantages and can be used in many aspects of tumor diagnosis or treatment, such as tumor detection, tumor treatment, biomarker identification, progression evaluation and new diagnostic imaging agents, et cetera.14 The emergence of the field can effectively treat some refractory tumors which has brought great breakthroughs in tumor treatment, especially in advanced tumors, metastatic tumors70 and multidrug resistant tumors.71
Nanomedicine uses nanoparticles as carriers, which can deliver anti-tumor drugs or combination drugs with different functional pathways.72 Through the enhanced permeability and retention (EPR) effect73,74 and the functional modification, nanoparticles can acquire the abilities of active targeting, bio-stability, increased circulation, stimulus response and in situ release, et cetera, leading to reduced systemic side effects and amplified efficacy and synergistic anti-tumor functions. Researchers have confirmed that nanomedicine had the excellent drug loading capacity and stability, longer blood circulation time, better targeting ability and tumor aggregation ability, more powerful anti-tumor activity and cytotoxicity and less toxic effects.75
Nanoparticles can not only protect drugs from degradation, but also prevent these anti-tumor drugs agglomerated at normal tissues while highly accumulated in tumors, profit by the EPR effect.76 Studies have demonstrated that compared with free drugs, the efficacy of anti-tumor nanomedicine is much higher and the toxicity to other healthy tissues is also greatly reduced. Moreover, it is also effective in reducing the renal clearance of drugs and extending their half-life in the blood. Besides, thanks to nanocarriers and functional modification, nanomedicine not only enhances active targeting to specific tumor tissues, increases the payload of anti-tumor drugs, improves the solubility of insoluble drugs, but also controls the drug release.77 At present, frequently used therapeutic nanocarriers including liposomes, albumin, polymeric micelles, dendrimers, carbon nanoparticles, polymer-drug conjugates, PLGA,78 polyaspartic acid nanoparticles79 and inorganic nanoparticles,80 especially the biocompatible nanocarriers, such as liposomes and polymeric micelles, can deliver a variety of drugs more safely and effectively.16 Based on these advantages, nanoparticles provide an important solution for co-delivery of combined drugs to tumors simultaneously and efficiently while reducing systemic side effects. Furthermore, a number of preclinical studies have pointed out that nanomedicine has unique advantages in combination therapy, with more powerful anti-tumor efficacy and less systemic side effects. As shown in Table 1, we enumerate the commonly used combination therapies based on nanomedicine.
 |
Table 1 The Combination Therapy Co-Delivered by Nanoparticles |
There are various methods to deliver multiple drugs (Figure 3A): (1) free drug combined with nanodrug; (2) nanodrugs delivered via separate nanocarriers; (3) nanodrugs delivered simultaneously by co-delivery nanosystem,22 which is the most common approach to combat complex tumors and achieve better therapeutic effects,106 such as the example in Figure 3B.106 It can simultaneously deliver anti-tumor drugs with different physical and chemical properties and different pharmacological properties,107 such as chemotherapy drugs, immunotherapy drugs and autophagy regulators, with excellent advantages of sequential and precise release.108 Besides, it has good performance in maintaining the payload rate of each drug, reducing drug dosage, minimizing adverse cytotoxic effects and improving efficacy,80 which makes the nanodelivery system effective in reversing multidrug resistance.109
 |
Figure 3 Nanotechnology based co-delivery system to deliver different treatment drugs. (A) The model of the co-delivery system; (B) The example of co-delivery system based on nanotechnology. Notes: (A) Is created with BioRender.com. (B) Is reproduced with permission from Cheng K, Ding Y, Zhao Y, et al. Sequentially Responsive Therapeutic Peptide Assembling Nanoparticles for Dual-Targeted Cancer Immunotherapy. Nano letters. 2018;18(5):3250–3258.106 Copyright 2018 American Chemical Society. |
For example, researchers found that co-delivery nanosystems not only increases the sensitivity of tumor to chemotherapy drugs,110 but also solves the following problems in treatment: (1) Improve drug solubility, especially water-insoluble drugs. For example, albumin-based nanomedicine improves solubility of drugs with better safety;111 (2) Increase drug permeability in tumor tissue; (3) Improve the spatiotemporal release and control the release sequence of combined drugs;112 (4) Enhance the stability of combined drugs at non-target sites; (5) Improve the pharmacokinetics, release kinetics and bioavailability;113 (6) Increases the possibility of drug combinations with overlapping mechanisms and toxicities to prevent the side effects outside the tumor and generate synergistic effect within the tumor;69 (7) Enhance targeting specificity,114 et cetera.
In summary, the emergence of nanomedicine can solve the problems in combination therapy more effectively. In particular, co-delivery nanosystem has become a promising combination anti-cancer treatment strategy, effectively overcoming tumor multidrug resistance and synergistically improving efficacy. The next, this review will focus on the application of nanotechnology in combination therapy of anti-tumor drugs with autophagy regulators in the treatment of tumor, especially in resistant tumors.
Application of Nanomedicine Combined with Autophagy Regulator in Tumor Drug Resistance Treatment
Autophagy regulators combined with anticancer drugs is a feasible strategy to overcome drug resistance.115 Research observed that the combined regimen significantly improves the therapeutic effect,116 which has synergistic anti-tumor effects and reverses tumor drug resistance,117 enabling patients to obtain better therapeutic response and longer survival.118 As for the clinical difficulties in combination therapy mentioned above, nanoparticles based drug delivery system is a good solution, which can specifically deliver therapeutic drugs to the target site, as well as reducing the absorption of non-specific targets to alleviate systemic toxicity.14 Currently, most nanotechnology-mediated autophagy regulator combination therapies are under research, mainly including the following regimens:
Co-Delivery of Autophagy Inhibitors and Anti-Tumor Drugs
When tumor cells are killed, a large amount of damaged organelles, abnormal proteins and other harmful substances will be produced. At this time, cell protective autophagy is activated, which can remove harmful substances in time, making it one of the crucial reasons of tumor drug resistance.31 In order to overcome this problem, researchers have utilized nanotechnology to create new combination regimens with autophagy inhibitors and various anti-tumor drugs.
Co-Delivery of Autophagy Inhibitors Combined with Chemotherapy Drugs
The activation of protective autophagy is one of the most common reasons why tumors become resistant to chemotherapy. Currently, the combination of cytotoxic chemotherapy drugs and autophagy inhibitors to treat tumors has been widely studied.31,87 For example, studies have shown that the combining docetaxel nanoparticles with free autophagy inhibitor chloroquine (CQ) can significantly improve the therapeutic efficacy of chemotherapy in breast cancer,87,119 but the non-specific release of chemotherapy drugs and free autophagy inhibitors may cause more systemic toxic side effects.
The co-delivery nanotechnology is an effective method to reduce toxic side effects. Researchers co-delivered si-Beclin1 or CQ with doxorubicin120–122 or paclitaxel123 through one or two delivery nanosystems, with more effective and more durable inhibitory effect on drug-resistant tumor cells,124 while avoiding possible adverse effects.125 Besides, chemotherapy prodrugs also provide new methods to effectively reduce systemic adverse reactions. For instance, Lin YX et al synthesized siBec1@PPN nanoparticles to co-deliver cisplatin prodrug Pt(IV)-peptide-bis(pyrene) and autophagy inhibitor Beclin1 siRNA. The nanoparticles decomposed the prodrug complex to Pt(II) by high intracellular glutathione for chemotherapy At the same time, Beclin1 siRNA inhibited Beclin 1 mediated autophagy pathway, significantly reversing cisplatin resistance in lung cancer with excellent in vivo safety.126
Co-Delivery of Autophagy Inhibitors Combined with Immunotherapy Drugs
Autophagy causes MHC-I deficiency by degrading MHC-I, leading to adaptive T cell immune dysfunction, together with the existence of immunosuppressive tumor-associated immune cells, jointly contributing to ineffectiveness of immunotherapy. Therefore, regulating the surface antigen of tumor cells by regulating autophagy is a considerable way to activate anti-tumor immune responses. Zhao Xet al co-delivered CQ and TLR9 agonist through FNC@NFT nanoparticles, to promote cell immune response while inhibits autophagy, significantly improving the therapeutic efficacy in pancreatic cancer.127 Similarly, Zuo L et al also synthesized HAL/3MA@X-MP nanoparticles, co-delivered the sonosensitizer PpIX and autophagy inhibitor 3-methyladenine (3-MA), stimulated P pIX to generate a large amount of ROS via ultrasound, while 3MA inhibited the protective autophagy of tumor cells, synergistically promoted immunogenic cell death (ICD). Meanwhile, 3-MA inhibited the NF-κB pathway in tumor cells, downregulated the expression of PD-L1, and jointly activated the anti-tumor immune response, significantly improving the therapeutic efficacy in breast cancer.128
In addition, many researchers have discovered that inhibiting autophagy can not only regulate anti-tumor immune responses by affecting tumor cells, but also affect immune cells in the tumor microenvironment to promote anti-tumor immune response, combining with anti-tumor drugs can synergistically significantly enhance immunotherapy efficacy: (1) Combination with chemotherapy prodrug: Yang X et al co-delivered the chemotherapy prodrug Pt(IV) and CQ through Pt(IV)/CQ/PFH NPs-DPPA-1 nanoparticles,129 to promote cell apoptosis; (2) Combination with chemokinetic therapeutic drugs: researchers co-delivered iron, artemisinin (ART)130 or Fe, MIL88 with CQ,131 through L-FHM@A-CQ or CQ-dual MOF nanoparticles, generating excessive ROS through the chemokinetic drugs, while inhibiting protective autophagy; (3) Combination with photoimmunotherapy drugs: Chen M et al synthesized CQ/IR780-Mil nanoparticles to co-deliver the photoimmunogenic death inducer IR 780 and CQ. IR780 is activated by light to produce phototoxicity and induce ICD while inhibiting the protective autophagy subsequently.132 Meanwhile, CQ can activate the NF-κB pathway in macrophages to reset its phenotype, increasing the proportion of pro-inflammatory macrophages, synergistically activating the anti-tumor immune response to enhance the immunotherapy efficacy in breast cancer, lung metastasis cancer and melanoma.
On this basis, if combined with immunotherapy drugs, a stronger anti-tumor immune response will be activated, synergistically treating tumors through multiple pathways and significantly improving prognosis. For example, Luo Y et al synthesized D/B/CQ@ZIF-8@CS nanoparticles to co-deliver the anti-glycolytic agent 2-deoxy-d-glucose (2-DG), the GLUT1 inhibitor BAY-876 and the CQ. By inhibiting aerobic glycolysis through 2-DG, inhibiting glucose uptake by BAY-876 while inhibiting protective autophagy, the nanoparticles synergistically cut off the energy source of cells and enhance the effect of starvation therapy. In addition, this process could increase glucose levels and reduce lactate levels in the tumor environment, reversing the immunosuppressive microenvironment. When combined with immunotherapy, it could significantly improve the immunotherapy efficacy of anti-CTLA-4 in breast cancer.133 Similarly, Ruan S et al co-delivered D&H-AA&C nanoparticles and anti-PD-L1 antibodies, significantly enhancing the therapeutic efficacy and prevent recurrence in glioma.134
Co-Delivery of Autophagy Inhibitors Combined with Novel Treatments
Protective autophagy induced by X-ray irradiation and the strong ability of repairing damaged DNA contribute to radioresistance and radiotherapy failure, leading to tumor recurrence and metastasis. Xu Q et al synthesized Au@Cu 2-x Se nanoparticles to co-deliver copper selenide and gold, inhibiting autophagy by alkalinizing lysosomes, significantly improved the sensitivity of glioblastoma to radiotherapy.44 Similarly, Li Y et al also synthesized HCQ-HMSN nanoparticles to co-deliver hydroxychloroquine (HCQ) and silica, enhancing the response of colorectal cancer.135 These studies indicate the vital role of autophagy activation in tumor radiotherapy. Thus, combining radiotherapy sensitizers with autophagy inhibitors based on nanotechnology is an effective method for radiotherapy sensitization.
In photodynamic therapy (PDT), elevated reactive oxygen species (ROS) activate protective autophagy in tumor cells, thereby weakening the anti-tumor function of the treatment and promoting drug resistance. Thus, inhibiting protective autophagy can improve the anti-tumor efficacy of PDT. Ma Z et al synthesized Pheophorbide a (PA)-Bisaminoquinoline (BAQ) Conjugate (PBC) nanoparticles to co-deliver the photosensitizer PA and autophagy inhibitor BAQ. It damaged lysosomal function through protonated and intelligently transforming into nanofibers (NFs) in the lysosome to inhibit autophagy. At the same time, PA was activated to exert phototoxicity function, significantly overcoming the therapeutic resistance in oral cancer cells.136 Similarly, Zhang X et al co-delivered photosensitizer aggregation induced emission (AIE) and autophagy inhibitor triptolide (TP) through (TP+A) @TkPEG nanoparticles to generate ROS by laser irradiation, and then TP inhibited the antioxidant pathway mediated by Nrf2, while inhibiting the protective autophagy, significantly enhancing the therapeutic efficacy in breast cancer.137 Besides, Li N et al co-delivered photosensitizer CONs and autophagy inhibitor pTRPM2 through CONs/pTRPM2 nanoparticles, inhibiting autophagy by TRPM2-mediated Ca2+ influx, significantly improving the efficacy of ROS mediated apoptosis in prostate cancer.138 In addition, researchers pointed out that autophagy inhibition can significantly improve the efficiency of photothermal cancer therapy. For instance, Wang L et al screened autophagy inhibitors that best cooperate with photothermal therapy, and then synthesized PD/I nanoparticles to co-deliver the ICG and Daurisoline (DAS), inhibiting the fusion of autophagosomes and lysosomes, sensitizing the ICG-mediated photothermal therapy in breast cancer.139
In addition, ultrasound-triggered sonodynamic therapy (SDT) is an emerging cancer treatment method. Assisted by ultrasound, on the one hand, it can generate a large amount of ROS through sonosensitize to induce tumor cell apoptosis; on the other hand, shock waves and shear stress will be generated causing mechanical damage to tumor cells. The two aspects synergize to good anti-tumor efficacy,140 but this ROS-based treatment is limited by the activation of protective autophagy.141 To solve this problem, Zhou L et al synthesized PpIX/3-MA@Lip nanoparticles to co-deliver autophagy inhibitor and sonosensitizer protoporphyrin IX (PpIX) to promote oxidative stress with the assistance of ultrasound while simultaneously inhibiting protective autophagy, significantly improving the therapeutic efficacy in ROS resistant breast cancer.142
Co-Delivery of Autophagy Inhibitors Combined with Other Treatments
Starvation therapy is an effective method to inhibit tumor growth and survival by blocking blood flow or depriving it of essential nutrients/oxygen supply. However, tumor drug resistance limit the applications of the method.143 Researchers have noticed that protective autophagy has a great impact on tumor drug resistance through promoting the survival of tumor cells under harsh conditions such as starvation.144 In order to overcome this problem, researchers combined this therapy with autophagy inhibitors. Liu X et al synthesized CQ@ZIF-GOx nanoparticles to co-deliver glucose oxidase GOx and CQ, increased oxidative stress level and promoted tumor cell apoptosis, which significantly enhanced the therapeutic efficacy in breast cancer and liver cancer.145 Similarly, Deng Y et al also co-delivered Ca@GOx nanoparticles and CQ-NPs nanoparticles to tumor tissues. While Ca@GOx induced mitochondrial Ca2+ overload to generate ROS, CQ-NPs inhibited cell protection autophagy to aggravate cell death and significantly enhance the therapeutic efficacy in breast cancer cells.146 In addition, combining autophagy inhibitors with other stress-inducing drugs also shows excellent anti-tumor properties. Wang T et al synthesized TeDNBs-HSA/OME (TeDNBs-HO) nanoparticles to co-deliver endoplasmic reticulum stress activator TeO3 2− and autophagy inhibitor omeprazole (OME), leading to autophagy dysfunction, which significantly improved the therapeutic efficacy in breast cancer.147 These studies mentioned above further proved that the activation of protective autophagy played a key role in promoting drug resistance to a variety of anti-tumor drugs, whether classic or new. For this reason, the combination of autophagy inhibitors based on nanotechnology to overcome drug resistance is an interesting research direction.
Significantly, at present, the combination of anti-tumor drugs is commonly used in clinical to improve the therapeutic efficacy, and research on combination therapies has been more attractive.21 However, their unpredictable adverse reactions and minimal improvement in survival rate limit their clinical application.22 Researchers have conducted in-depth studies and found that autophagy plays a key role in resistance to combination therapy.50 For this reason, Wang C et al synthesized PP/siRNA/HA nanoparticles to co-deliver polyethyleneimine (PEI), paclitaxel (PTX) and Mdr1 siRNA. It could inhibit autophagy through alkalinization of lysosomes by PEI, with combination of chemotherapy drug PTX while downregulating multidrug resistance genes mediated by siRNA, significantly improving the therapeutic efficacy in multidrug resistant lung cancer.148 Similarly, Wang X et al co-delivered glucose oxidase GOx, curcumin and autophagy inhibitor Obatoclax. Through the consumption of glucose by GOx, curcumin promotes mitochondrial Ca2+ overload and cell hunger, while Obatoclax inhibits autophagy and synergistically cuts off cellular energy sources, significantly improving the therapeutic efficacy in breast cancer.149
In addition, Zhang H et al synthesized BP-AS@D nanoparticles to co-deliver photothermal drug BPNS, chemotherapeutic drug DOX and autophagy inhibitor siClC-3. Inhibition of lysosomal acidification by siClC-3 not only inhibits protective autophagy, but also avoids the sequestration by lysosomes, significantly enhancing the therapeutic effect of chemotherapy and photothermal therapy on cervical cancer.150 A similar phenomenon was observed in the combination therapy of sonodynamic therapy, Gao C et al co-delivered sonosensitizer hematoporphyrin (HP), hypoxia improving agent chlorella (Chl) and autophagy inhibitor CQ, through MChl-CQ-HP-NP nanoparticles, synergistically reversing the tumor suppressor immune micro-environment and improving the efficacy in melanoma.151
This section summarizes the research on tumor treatment based on nanotechnology-based co-delivery of autophagy inhibitors combined with various anti-tumor drugs (Figure 4A), such as an example of which shown in Figure 4B.136 On the one hand, autophagy inhibitors can inhibit harmful substances such as damaged organelles and abnormal proteins by inhibiting protective autophagy. On the other hand, it can inhibit the drug from degradation by lysosomes, increase the intracellular concentration, sensitize tumor cells to anti-tumor drugs, and overcome drug resistance. In addition, autophagy inhibitors can not only inhibit autophagy mediated MHC-I degradation, but also inhibit NF-κB mediated signaling pathways in tumor cells to down-regulate PDL1, changing tumor antigens and improving the anti-tumor immune responses. Moreover, it can sensitize anti-tumor drugs to promote the ICD, promote the release of DMAP, promote the infiltration of CD8+T cells, and activate anti-tumor immune responses. On this basis, combining with immunotherapy drugs can not only enhance the anti-tumor effect, but also effectively prevent recurrence. Additionally, many researchers have proved that autophagy inhibitors can affect immune cells in the tumor microenvironment to promote anti-tumor efficacy. For example, CQ can reprogram TAM by activating NF-κB signaling pathways in macrophages, resetting them to the M1 phenotype, activating the anti-tumor immune response, and significantly enhancing the efficacy of anti-tumor drugs subsequently.
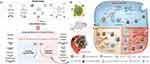 |
Figure 4 Co-delivery of autophagy regulators and anti-tumor therapies based on nanotechnology. (A) The model of the co-delivery method; (B) The example of nanotechnology based co-delivery of autophagy inhibitors and anti-tumor therapies. Notes: (A) Is created with BioRender.com. (B) Is reproduced from Ma Z, Lin K, Tang M, et al. A pH-Driven Small-Molecule Nanotransformer Hijacks Lysosomes and Overcomes Autophagy-Induced Resistance in Cancer. Angew Chem Int Ed Engl. 2022;61(35):e202204567.136 © 2022 Wiley-VCH GmbH. |
This subsection reveals that for drug-resistant tumor cells that use activated autophagy as their primary means of survival, the delivery of autophagy inhibitors by nanotechnology can not only inhibit the original survival pathway, but also inhibit the removal and degradation of damaged organelles and harmful substances caused by co-delivered combination antitumor drugs, which strengthen the toxic effect of the combination drugs, and synergistically promote the death of drug-resistant cells. In addition, the nanodrugs released outside tumor cells can also influence the non-tumor cells in the tumor microenvironment such as immune cells with activated anti-tumor immune responses and synergistically reverse drug resistance. Therefore, when using the co-delivery system mentioned above, it is worth nothing that the combination regimen may play an exciting role in the cells in TME which can synergistically improve the efficacy through different pathways such as the activated immune response.
Co-Delivery of Autophagy Activators and Anti-Tumor Drugs
Excessive activation of autophagy can induce programmed death of tumor cells, and can be used to induce apoptosis in tumor cells with anti-tumor effects. Researchers have demonstrated that this method is more effective for tumor cells with high metabolic rate.152 Besides, for tumor cells that lack autophagy tendency, such as ovarian cancer, breast cancer, and prostate cancer, activating autophagy can also improve the sensitivity of tumors to anti-tumor drugs, effectively overcoming drug resistance.153 Moreover, studies have shown that the enhancement of autophagy in cells with less activated autophagy may inhibit the uptake of external nutrients, leading to the inhibition of tumor proliferation.154 In addition, researchers also discovered that the activation of autophagy can increase drug uptake. The uptake rate of anti-tumor drugs with activated autophagy is significantly higher than that without activated autophagy.155 Based on these findings, researchers used nanotechnology to combine a variety of anti-tumor drugs with autophagy activators to treat tumors, showing excellent performance, especially in reversing tumor drug resistance, as follows:
Co-Delivery of Autophagy Activators Combined with Conventional Treatment
Autophagy activators can increase the sensitivity of tumor cells to chemotherapy drugs and enhance anti-tumor efficacy. Yan J et al synthesized CPAH/CPTAH nanoparticles to co-deliver autophagy activator RAPA and chemotherapy drug 9-NC. Through RAPA pre-induction of autophagy of ovarian cancer cells, ovarian cancer is more sensitive to chemotherapy drugs, and then secondary nucleus-targeting micelles directly delivered 9-NC to the nucleus, which induces excessive autophagy and activates the apoptosis pathway, significantly enhancing the therapeutic efficacy in ovarian cancer.156 Similarly, Mohammed SA et al co-delivered chemotherapy drug bortezomib (BTZ) and autophagy activator siSHARP1 through Lipo-siRNA-BTZ-PEG-cRGD nanoparticles to promote autophagy-dependent apoptosis, synergistically enhancing the therapeutic efficacy in acute myeloid leukemia.157 Which means that combination of autophagy activators affects cell fate from another perspective and promotes tumor sensitivity to chemotherapy.
Besides, researchers demonstrated that the efficacy of immunotherapy is affected by the immune-suppressive microenvironment of the tumor, due to the limitation of insufficient antigen presentation in antigen presenting cell (APC) such as dendritic cells, the acidic microenvironment, insufficient DAMP et al. Notably, inhibition of autophagy in DC can limit the antigen processing and presentation efficiency.158 In order to solve this problem, An J et al co-delivered the acidity regulator CaCO3 and autophagy activator ovalbumin (OVA) through HOCN nanoparticles to promote the cell viability of DC by reducing the acidity in tumors. Meanwhile, OVA activated autophagy by upregulating Ca2+ levels, while promoting the release of adenosine triphosphate (ATP) by calcium overload to enhance DAMP level, which synergistically improved DC antigen presentation efficiency and therapeutic efficacy in colon cancer.158 Similarly, Yu Z et al and Li TF et al also synthesized PLGA-PEG-AEAA NP nanoparticles159 and Nano-DOX nanoparticles,160 co-activate autophagy dependent apoptosis to induce ICD, subsequently activate DC, reshaping the immunosuppressive tumor microenvironment and significantly enhancing the therapeutic efficacy in liver cancer and glioblastoma.
In addition, vaccines based on nanotechnology combined with autophagy activators play an essential role in tumor immunotherapy with better efficacy of activating DC in the body. Therefore, Wang Y et al synthesized NP-B-OVA nanoparticles as a vaccine to co-deliver the autophagy activator beclin1 peptide Bec1 and the antigen peptide OVA, by activating autophagy and delivering antigen peptides in DC, which synergistically improved the antigen presentation efficiency, and significantly strengthened cytotoxic T lymphocytes (CTL) mediated immunotherapy effect on melanoma.161 Similarly, Yue H et al developed GO-OVA nanoparticles as another vaccine to co-deliver autophagy activator GO and antigenic peptide OVA to treat thymoma.162
In short, the studies mentioned above suggest that autophagy activators can regulate antigen presentation in immune cells. For tumors that are sensitive to autophagy activators, delivering them can not only activate autophagy dependent death, but also activate immune cells to exert anti-tumor functions, collaboratively treating tumors; For tumors that are insensitive to autophagy activators, delivering them as tumor vaccine is a very feasible option, which can inhibit tumors and prevent the recurrence effectively.
Co-Delivery of Autophagy Activators Combined with Novel Treatment
Researchers pointed out that autophagy triggered by ROS during photodynamic therapy (PDT) usually exhibits anti-apoptotic effects and promotes cell survival. However, excess autophagy promotes tumor cell apoptosis, transforming autophagy from pro-survival effects to pro-death. Deng Y et al synthesized CD-Ce6-3BP nanoparticles, co-delivered the respiratory inhibitor 3-bromopyruvate (3BP) and the photosensitizer chlorin e6 (Ce6). Autophagy was activated by 3BP, while HK-II and GAPDH were simultaneously downregulated to suppress respiration, with reduced intracellular oxygen consumption rate to enhance Ce6-mediated photodynamic therapy, further activating autophagy, significantly enhancing the therapeutic effect of PDT on oral epidermal cancer.163 In addition, Sun M et al co-delivered the metabolic inhibitors lonidamine, ferrocene and glucose oxidase through FG/T-Nanoprodrug nanoparticles, synergistically activating autophagy dependent cell death and significantly enhancing the therapeutic efficacy against cisplatin-resistant lung cancer.164
In addition to the combination of some classic anti-tumor drugs, many researchers have explored new combination drug regimens. For example, Liu R et al synthesized HA-TPGS-STZ nanoparticles to co-delivery TPGS and autophagy activator Sertaconazole (STZ), generating ROS through TPGS to induce mitochondria-related apoptosis, while activating autophagy dependent apoptosis, significantly enhancing the therapeutic effect on lung cancer.165 Besides, Kavya KV et al co-delivered curcumin and Bcl 2 siRNA through Poly@Cur-FA nanoparticles, which significantly enhanced the therapeutic efficacy of curcumin in cervical cancer by inhibiting Bcl2 to activate autophagy dependent cell death.166 Similarly, Hanafy NAN et al synthesized Curcumin-Niacin NPs nanoparticles to co-deliver niacin and curcumin, activating the GPR109A/AMPK/NRF-2 signaling pathway to induce excess autophagy collaborated with curcumin, significantly improving the therapeutic efficacy in liver cancer.167 These studies indicated that autophagy activators exhibit excellent therapeutic sensitization and synergistic effects in combination therapy with classic or new anti-tumor drugs.
For tumors that lack an autophagic tendency, using autophagy activators to promote autophagy-dependent cell death can improve their antitumor efficacy. For example, Chen J et al synthesized 5-Fu/Cur-P@HMPB nanoparticles co-deliver curcumin (Cur), 5-fluorouracil (5-Fu) and hollow mesoporous Prussian blue (HMPB). Through the HMPB mediated transformation from H2S to Prussian white (PW), excess ROS were generated to activate autophagy, triggering autophagy dependent cell death together with Cur, which significantly improved the therapeutic efficacy of 5-Fu in colorectal cancer.168 Similarly, Zhang R et al synthesized PVP-Fe-Cu-Ni-S nanoparticles to co-deliver photothermal agents, photosensitizers and chemokinetic drugs, which synergistically activated autophagy dependent ferroptosis, significantly enhancing the therapeutic effect on breast cancer.169
This section summarizes studies on tumor treatment through nanotechnology-based co-delivery nanosystem of autophagy activators combined with various anti-tumor drugs (Figure 4A). Excessive activation of autophagy can degrade cellular components or protective factors, leading to the higher degree of cell damage than the level of nutrients produced by degradation or uptake from the outside. Cell death dependent on autophagy, such as ferroptosis, is caused by the fact that cells cannot obtain survival materials in time to repair damage, particularly in tumors with high metabolic rates or lack of autophagy tendency.
In addition, autophagy activation can not only increase drug uptake which significantly improve the therapeutic efficacy, but also affect the function of tumor-related immune cells. On the one hand, they cooperate with anti-tumor drugs to promote ICD and enhance DAMP. On the other hand, they can activate autophagy in DC to improve its antigen presentation efficiency. This method reshapes the immunosuppressive tumor microenvironment and activates anti-tumor immune responses. Except for these, vaccines based on nanotechnology with autophagy activators also have a significant impact on immunotherapy, which can improve the antigen presentation efficiency of APC to strengthen CTL, with effective inhibition of tumor progression. Therefore, for the combination of autophagy activators through nanotechnology, more attention can be paid to the stimulation of tumor immune responses in the future research, which can not only promote ICD of tumor cells, but also directly affect immune cells, synergistically activate anti-tumor immune responses, and even play an important role as immune vaccines.
Moreover, we classified the researches in another way by categorizing different co-delivery approaches based on the characteristics of nanoplatforms, as shown in Table 2. From Table 2, it is evident that organic nanomaterials as delivery platforms are currently the most commonly used, possibly due to their excellent biocompatibility, improved safety, and lower toxicity. Among these, liposomes are the most commonly used materials which are approved by FDA with outstanding biocompatibility. This greatly facilitated their role as co-delivery nanoplatforms. As for inorganic materials, the most commonly used platform is metal-organic frameworks, especially due to their porous structure, which can optimize drug loading capacity and expand the possibilities for drug combinations. However, it is important to note that their application may lead to potential adverse reactions in future research. We hope that Table 2 can serve as a research foundation and guide for more combinations therapies research based on nanotechnology in the future.
 |
Table 2 The Combination Therapy with Autophagy Regulators Through Different Nanoplatforms |
Dual Regulation of Autophagy by Co-Delivery System Based on Nanotechnology
Generally, drug-induced protective autophagy is often regarded as a troublesome problem, which leads to tumor drug resistance. However, studies have indicated that utilization of drugs with the properties of activating protective autophagy could make tumors rely on this survival pathway. Subsequently, inhibiting this pro-survival pathway could lead to the accumulation of autophagosomes, which can effectively enhance the sensitivity of tumor cells to autophagy inhibitors and overcome the limitations caused by mono-therapy of autophagy regulation, as shown in Figure 5A.
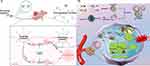 |
Figure 5 Dual regulation of autophagy based on nanotechnology. (A) The model of the dual regulatory function; (B) The example of dual regulation of autophagy in starvation therapy based on nanotechnology. Notes: (A) Is created with BioRender.com. (B) Is reproduced from Li F, Chen T, Wang F, et al. Enhanced Cancer Starvation Therapy Enabled by an Autophagy Inhibitors-Encapsulated Biomimetic ZIF-8 Nanodrug: Disrupting and Harnessing Dual Pro-Survival Autophagic Responses. ACS Appl Mater Interfaces. 2022;14(19):21860-21871.170 Copyright 2022 American Chemical Society. |
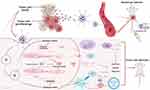 |
Figure 6 The applications of nanotechnology in combination therapy with autophagy regulator in tumor drug resistance treatment. |
Based on the thesis, researchers have clarified the excellent effect of this method on tumor treatment in various anti-tumor treatments: (1) Chemotherapy: researchers synthesized CD133-DOX nanoparticles,171 ER-NPs nanoparticles172 and Nano-Pt nanoparticles,173 delivered DOX, 5-FU or cisplatin into tumor cells, damaging cells and activating high-level protective autophagy, which made tumor cells more sensitive to autophagy inhibitors. At the same time, the combination with autophagy inhibitors promoted cell apoptosis, significantly improving the therapeutic efficacy on liver cancer, cervical cancer, or non-small cell lung cancer; (2) Photothermal therapy: Zhang Y et al synthesized Cu-Pd based TNP-1 nanoparticles, which improved the susceptibility of cancer cells to photothermal therapy mediated killing, with significant therapeutic effect against multidrug resistant breast cancer;20 (3) Starvation therapy: Li F et al synthesized mCG@ZIF nanoparticles, co-delivered CQ and GOx based on the ZIF-8 platform. Through the dual protective autophagy activated by ROS and starvation, cancer cells were more sensitive to autophagy inhibitors. Subsequently, CQ in the nanoparticles is released to inhibit protective autophagy, significantly enhancing the therapeutic efficacy in breast cancer, as a classic example of this method, shown in Figure 5B;170 (4) Autophagy activators: researchers synthesized C6-ceramide nanoliposome nanoparticles174 and CNL nanoparticles,175 combined with the autophagy inhibitor vinblastine or CQ. Autophagy is activated with the formation of autophagosomes, while autophagy inhibitors are used to inhibit the degradation of autophagosomes, resulting in autophagosome accumulation, preventing damaged organelle repair, promoting apoptosis, and significantly enhancing the therapeutic effect on liver cancer, colorectal cancer or head and neck squamous cell carcinoma.
This subsection suggests that for drug-resistant tumor cells in which autophagy does not play a dominant role, the delivery of autophagy activators by nanotechnology can disturb the homeostasis in cells, disrupt their original signaling pathways, and make autophagy becoming the main survival pathway. Subsequently, the autophagy inhibitors co-delivered by nanoplatform spatially can block this autophagy-dependent protective effect, promote apoptosis of drug-resistant tumor cells, and reverse tumor drug resistance. This approach significantly expands the indications of combination drugs in different tumors, which can be an important combination treatment option when encountering refractory tumors in future studies.
Delivery of Anti-Tumor Drugs Based on Autophagy Regulatory Nanoplatform
The unpredictable non-selective effect of each drug is a significant factor in the unavoidable systemic adverse reactions that occur when combined drugs are taken. Researchers have pointed out that many nanomaterials have the characteristics of regulating autophagy. Making good use of this characteristic can not only enhance the classic anti-tumor drugs’ effects, but also regulate autophagy. While using the nanotechnology to deliver, they can exert a dual synergistic anti-tumor effect, reverse tumor drug resistance and reduce systemic adverse reactions. Based on this, researchers conducted a series of studies utilizing nanomaterials with autophagy regulatory characteristic to deliver mono anti-tumor drug while performed synergistic effects, as follows:
Delivery of Anti-Tumor Drugs Based on Autophagy Inhibitory Nanoplatform
Researchers have proposed a variety of nano-autophagy-inhibiting materials to deliver anti-tumor drugs, all of which have significantly enhanced the original therapies: (1) Chemotherapy: Li N et al synthesized mPEG-bP (DPA-b -DMAEMA)/EPI nanoparticles to deliver epirubicin (EPI) through the polycationic nanomicelles (PEDD-Ms) platform. PEDD-Ms alkalinizes lysosomes, leading to lysosome damage and inhibiting autophagy, which significantly enhanced the response of liver cancer to EPI;176 (2) Chemokinetic therapy: Xie Y et al synthesized Fe2O3@ DMSA nanoparticles to deliver Fe2O3 through DMSA platform, which inhibited protective autophagy to sensitize Fe-mediated Fenton-like reactions, significantly enhancing the therapeutic effect on liver cancer;177 (3) Delivery of metabolic inhibitors: Zhang P et al synthesized MOND nanoparticles to deliver cholesterol oxidase (COD) through molybdenum oxide, which activated the AKT/mTOR pathway to inhibit protective autophagy, significantly enhancing the therapeutic effect of COD on bladder cancer.178
Delivery of Anti-Tumor Drugs Based on Autophagy Activator Nanoplatform
According to the previous studies, autophagy activator nanomaterials have excellent performance in delivering various anti-tumor drug for combination therapy: (1) Chemotherapy: Lu HY et al delivered the chemotherapy drug CPT based on the GCMSN platform, inducing aggravated oxidative stress by activating autophagy through GCMSN, significantly improving the therapeutic efficacy on lung cancer;179 (2) Chemokinetic therapy: Wang XS et al synthesized Fe(CO)5@Au nanoparticles to produce CO and iron mediated by near-infrared light. CO damaged mitochondria and induced autophagy, promoting the aggregation of nanomedicines in lysosomes. At the same time, iron destroyed lysosomes, significantly enhancing the killing effect against breast cancer;180 (3) Photothermal therapy: Lv C et al co-delivered Zn2+ and the photothermal agent rGO based on the ZIF-8 NPs platform. Autophagy-dependent cell death was activated by excess Zn2+, with the photothermal effects of rGO induced by light, significantly enhancing the therapeutic efficacy in tongue squamous cell carcinoma.181
Dual Regulation of Autophagy Through Delivery Nanosystem Based on Autophagy Regulatory Nanoplatform
Studies have illustrated that activating autophagy can increase the uptake of anti-tumor drugs by cells, but the late stage of autophagy (autophagolysosomes) will sequester drugs including nanodelivery systems,182,183 sorafenib,184 cisplatin185 and doxorubicin (DOX),186 keeping them away from the target site, leading to drug resistance. Therefore, maximizing the therapeutic ability of drugs by allowing them to escape from lysosomal degradation or sequestration is a long-term challenge for nanodelivery.
Researchers believe that if late autophagy is blocked, drug metabolism can be inhibited and intratumoral concentration of drugs will be elevated (Figure 5A). Based on this thesis, researchers found that the activation of autophagy based on nanotechnology allows drugs to remain in lysosomes. Then, destroying lysosomes or inhibiting lysosomal metabolism can greatly increase drug concentrations and promote apoptosis. For this reason, researchers have conducted various studies in combination with different anti-tumor therapies: (1) Chemotherapy: Researchers have synthesized PEGylated MoS2/DOX NSs nanoparticles,187 PLGA-lysoGM1/DOX nanoparticles,188 PTX- PTX-MSNs-PDA nanoparticles189 and Nab-PTX nanoparticles.190 They can enhance the cellular uptake rate by the nanoplatform mediated autophagy-lysosome pathway, while combined with lysosomes blockers, significantly increased the accumulation of DOX and PTX, with better therapeutic effect on cervical cancer, breast cancer, glioma, lung cancer and colorectal cancer. Besides, Kong C et al also discovered that the delivery of triptolide prodrug based on the UPSM platform can significantly enhance the release of triptolide and its therapeutic efficacy in lung cancer;191 (2) Immunotherapy: Li X et al. Synthesized HCQ@aPDL1-Viro nanoparticles to co-deliver anti PDL1 and CQ based on JEV platform. Lysosomal escape through JEV promoted the drug aggregation in tumors, inducing autophagy while inhibiting autophagolysosome by HCQ, which significantly enhanced the therapeutic effect on breast cancer.192
These studies provide a good research basis for future therapeutic applications of nanomaterials with autophagy regulatory properties to deliver mono drug or combination drugs while reducing systemic toxicity. In addition, a lot of studies have suggested a variety of nanoplatforms that can be used to load drugs in this way. For example, Jiang L et al pointed out that LA-nBSA nanomaterials can activate autophagy through nBSA while LA promoting the escape of nanocapsules, protecting it from degradation,193 indicating the potential role as a nanoplatform in increasing the intracellular concentration of drugs.
In this subsection, we can find that in addition to improving the efficacy of delivered drugs, the nanoplatforms with autophagy regulatory properties can also enhance the efficacy synergistically by promoting the death of drug-resistant tumor cells. However, combined with the contents of subsection 4.1 and subsection 4.2, which indicate that autophagy plays an essential role in non-tumor cells, but there is still a lack of research on the role of nano-autophagy materials in the tumor microenvironment, especially in tumor immunity, which, in the future, is a highly worthwhile research direction.
Autophagy Regulatory Nanomedicine as Adjuvants for Adjuvant Therapy
It is worth noting that studies have demonstrated that nanomaterials with autophagy regulatory functions can not only serve as delivery platforms, but also function as adjuvant treatments for anti-tumor treatments, as follows:
Adjuvant Different Tumor Therapies
Zhang Q and Xiong Q et al discovered fullerene C60 (Nano-C60)194 and CONP nanoparticles195 can upregulate ATG 5 or activate the ROS/ERK signaling pathway to activate autophagy and enhance the sensitivity of tumors to DOX and gemcitabine, respectively. Similarly, the bio-ATTEC nanoparticles synthesized by Liu M et al, which induce autophagy-dependent cell death by degrading mitochondria, can significantly enhance the sensitivity of metastasis melanoma to chemotherapy prodrugs.196 These studies suggest the potential application of autophagy regulatory nanomaterials in adjuvant chemotherapy.
Furthermore, nanomaterials with autophagy regulatory properties are more commonly used in sensitized radiotherapy. For example, researchers have synthesized nano-Se nanoparticles,197 AgNPs nanoparticles,198,199 FePt/GO NSs nanoparticles,200 Fe3O4 @Ag nanoparticles201 and GON nanoparticles,202 which can improving the killing effect of oxidative stress by regulating autophagy, significantly enhancing the therapeutic effect of radiotherapy on breast cancer, glioma and non-small cell lung cancer. Moreover, a similar phenomenon also occurs in the adjuvant treatment of magnetothermal therapy. The SPIO nanoparticles synthesized by Sadhukha T et al can induce oxidative stress through magnetothermal conversion to activate temperature-dependent autophagy and promote autophagy-dependent cell death. At the same time, the elevated temperature promotes acute cell membrane damage and effectively inhibits lung cancer.203 These studies indicate that autophagy regulatory nanomaterials have good auxiliary therapeutic potential in both radiotherapy and magnetothermal therapy.
Notably, the efficacy of tumor immunotherapy is largely limited by the inhibitory tumor immune microenvironment (TIME). Zhang L et al synthesized LDH NP nanoparticles to inhibit autophagy by exerting acid neutralization in TIME and increase the proportion of tumor-associated macrophages and T cells with anti-tumor functions. At the same time, it can capture the tumor antigens in the tumor microenvironment, synergistically activating anti-tumor immune responses. Combined with Toll-like receptor 9 agonist CpG can significantly improve the therapeutic effect on melanoma and colon cancer.204 In addition, metal immunotherapy by supplementing metal ions into the tumor microenvironment is also a new strategy to activate anti-tumor immune responses. Based on this, Jia Y et al synthesized NanoAlum nanoparticles, which activates T cells in the tumor microenvironment through neutralizing the acidic tumor microenvironment and releasing Mg2+, while inhibiting the protective autophagy to induce apoptosis. When combined with methotrexate or CpG, it shows excellent therapeutic effects on melanoma and colon cancer.205 These findings suggest the potential application of autophagy regulatory nanomaterials as immunoregulators and adjuvants in adjuvant immunotherapy.
Adjuvant Dual Regulation of Autophagy
Similar to what was mentioned above, nanomaterials with autophagy activator properties can also enhance the sensitivity to autophagy inhibitors and improve the anti-tumor efficacy. For example, researchers synthesized Nano-C60 nanoparticles206 and Nano-Nd2O3 nanoparticles,207 which induced autophagy through nanomaterials, and then inhibited the late autophagy with KN93 or Bafilomycin A1, leading to excessive accumulation of autophagosomes and inhibition of degradation, significantly enhancing the therapeutic effect of nanomedicines on osteosarcoma and non-small cell lung cancer.
It is clear from this perspective that nanomaterials with autophagy regulatory characteristics will have a wide range of applications in the future, especially as adjuvant treatments, which can exert excellent functions of sensitizing drugs. Currently, many studies have elaborated a variety of nanomaterials with autophagy regulatory ability, such as Bi2S3 NPs nanoparticles208 and PN-CeO2 nanoparticles,209 which can act as protective autophagy inhibitors to promote apoptosis of liver cancer and skin squamous cell carcinoma cells. On the contrary, NIC-NCT nanoparticles,210 MnO-MS nanoparticles,211 nano-ZnO nanoparticles,212,213 nano-CuO nanoparticles,214 USIONP nanoparticles,215 PEG-CS-FA-Sb nanoparticles,216 nano-Et3PAuCl nano Particles,217 α-NTP-LNs nanoparticles218 and Bowl 6 nanoparticles,219 etc can promote autophagy-dependent cell death by inhibiting mTOR, ERK and AKT pathways, activating TRPC6 pathway and other pathways, effectively inhibiting the proliferation of lung cancer, liver cancer, neuroblastoma, prostate Cancer, colorectal, nasopharyngeal and gastric cancer.
This subsection mainly introduces the essential role of nanotechnology in the combination of anti-tumor drugs and autophagy regulators, especially those autophagy regulatory nanoplatforms, which not only can exert autophagy regulatory functions, but also deliver anti-tumor drugs to exert a synergistic therapeutic effect, particularly in adjuvant treatments, including radiochemotherapy, magnetic heat therapy, autophagy inhibitors and immunotherapy treatment, etc, with excellent performance and potential role. This further indicates that nano-autophagy materials play an essential role in the future combination research, and are important nanoplatforms worthy of being used for drug delivery or adjuvant therapy, which can be extended to a variety of diseases.
In conclusion, for tumors that develop protective autophagy resulting in drug resistance after anti-tumor treatment, delivering autophagy inhibitors in combination with anti-tumor therapeutic drugs through nanoplatforms can significantly sensitize the drug, provide synergistic efficacy and reverse drug resistance. For tumors with high metabolic rate or lack of autophagy tendency, nanoplatforms can be used to deliver autophagy activators combined with anti-tumor drugs, inducing programmed death of tumor cells through excessive activation of autophagy, overcoming drug resistance with better anti-tumor effect. These two combination therapies are worthy of being studied when combined with the regulation of non-tumor cells in the tumor microenvironment especially the immune cells to exert a great antitumor immune effect in the future research (Figure 6).
However, for different tumors, different intracellular signaling pathways or sensitivities to the same function way may lead to different responses to the same nanomedicine with opposite effects in different tumors. For example, the Fe3O4 nanoparticles mentioned earlier were found that they activated protective autophagy by activating the Beclin l/Bcl-2/VPS34 complex, promoting tumor proliferation and drug resistance.220
What’s more, research also indicated that the same signaling pathway can play opposite roles in different tumor-related cells. Inhibiting the NF-κB signaling pathway in tumor cells through 3-MA can downregulate PDL1 expression,128 improving the efficacy of immunotherapy, while CQ reprograms TAM by activating the NF-κB signaling pathway in macrophages and resets them to a pro-inflammatory phenotype.132 It’s possible because the same protein mediates different signaling pathways in tumor cells or immune cells, and the changes in cellular responses caused by inhibiting different stages of autophagy may also play a role. These studies suggest that it is necessary to select appropriate autophagy regulators for combination therapy according to tumor type, cell type, cell response differences, signaling pathway differences, drug sensitivity and the combination of antitumor drug pathways in future research.
Moreover, some studies have shown that drug-free nanomaterials may cause disturbances in the intracellular environment, leading to the activation of protection autophagy, promoting drug degradation, and limiting the therapeutic effect of nanomedicines in tumors.221 Therefore, choosing the appropriate nanoplatform to deliver drugs or exert autophagy regulatory functions is an essential step. A comprehensive consideration of the effects of drugs and nanomaterials on autophagy regulation is of great reference value for selecting nanoplatforms.
Dual regulation of autophagy through nanoplatforms appears to be a novel and effective solution to these problems. On the one hand, drugs activate protective autophagy and make it the main survival pathway of cells. At the same time, inhibiting this pathway can lead to the accumulation of autophagosomes and effectively enhance the sensitivity to autophagy inhibitors, with improved functions assisted by nanotechnology. On the other hand, activating autophagy through drugs can improve drugs uptake, and then destroy lysosomes or inhibit lysosomal metabolism can greatly increase drug concentration and improve the efficacy of tumor treatment. And most of the related research is based on nanotechnology. The two aspects mentioned above suggest that the dual autophagy regulation scheme based on nanoplatforms is a vital research direction in the future to overcome drug resistance and expand the indications of treatment.
In addition, the use of nanomaterials with autophagy regulatory properties to deliver mono drug or combination drugs is also a very effective combination method. It can not only promote cell apoptosis synergistically, but also exert dual regulatory functions of autophagy, with reduced systemic adverse reactions caused by the administration of multiple drugs. It suggests the broad application prospects of this nanomaterials with autophagy regulatory characteristics in the future, especially its potential role as an adjuvant treatment.
Summary and Outlook
Delivering autophagy regulators and anti-tumor drugs based on nanoplatforms provides a good solution for the problems of combination therapy mentioned above, with simultaneous delivery of drug, increased drug combination possibilities and targeting specificity, not only effectively inhibiting multi-drug resistance, but also synergistically improving the efficacy and reducing adverse reactions.21
In conclusion, delivering autophagy inhibitors in combination with anti-tumor drugs through nanoplatforms can significantly sensitize the drugs, provide synergistic efficacy, and reverse drug resistance in tumors with protective autophagy. In opposite aspect, nanoplatforms can be used to deliver autophagy activators with anti-tumor drugs to tumors that have a high metabolic rate or lack autophagy tendency which can lead the tumor cells to death by excessive autophagy activation, to overcome the drug resistance and improve the anti-tumor effect. It is worth nothing that these two combination regimens may play an exciting role in the cells in tumor microenvironment which can synergistically improve the efficacy through different pathways such as the activated immune response, and even play an important role as immune vaccines.
However, different cancers may respond differently to the same nanomedicine with opposing effects due to variations in intracellular signaling pathways or sensitivities to the same function. Furthermore, different tumor-related cells may exhibit contrasting functions within the same signaling pathway. These imply that future study must choose the right autophagy regulators for combination therapy based on factors such as tumor kind, cell type, variations in cell response, variations in signaling pathways, drug sensitivity, and the combination of cell pathways.
Furthermore, nanomaterials with autophagy regulating properties has been proven to deliver anti-tumor drugs in combined therapy, making it another key research field potentials, particularly in the adjuvant treatment of anti-tumor therapies, which can be extended to a variety of diseases. However, there is still a lack of the function of nano-autophagy materials in the tumor microenvironment, particularly in tumor immunology, making it an important area for future research.
However, the weakening of EPR effect, the lack of accurate measurement of pharmacokinetics and pharmacodynamics, the unknown side effects caused by nanoplatforms and combination therapies, off-target nanomedicine, payload limitations,222 difficulty in measuring physical and chemical properties and high cost223 limits the combination therapy base on nanotechnology. Therefore, it is necessary to conduct more research on the drug properties of nanomedicines in the future, in order to develop more precise methods for evaluating the pharmacokinetics, biodistribution, functional mechanisms and off-target, achieving clinical transformation and improving patients’ quality of life.
In summary, the delivery of autophagy regulators through nanoplatforms combined with anti-tumor drugs provides a good therapeutic strategy for the treatment of tumors, especially drug resistant tumors, with considerable therapeutic and promising research prospects. For this reason, it is vital to conduct more research on the safety, biocompatibility, toxic and side effects, especially clinical research. In a word, our belief is that with the development of technology and the advancement of experimental research and clinical trials, more specific nano-combination systems will be developed for tumor treatment. There will be more nanotechnology-based combination therapies approved for clinical use, with more precise and powerful anti-tumor efficacy and more significant therapeutic benefits to patients, particularly for individuals with drug resistance in the future.
Acknowledgments
We would like to express our gratitude to Biorender for its images used in our figures.
Funding
We gratefully acknowledge the financial support by the Key Research and Development Program of Zhejiang Province (No. 2021C03121), National Natural Science Foundation of China (No. 82072650, 82001673, and 82272860), Zhejiang University Basic Research Fund (No. 226-2022-00037), Fundamental Research Funds for the Central Universities (No. 226-2023-00038), Chen Xiao-Ping Foundation for the Development of Science and Technology of Hubei Province (No. CXPJJH12000009-07), Key Laboratory of Intelligent Preventive Medicine of Zhejiang Province (No. 2020E10004), Postdoctoral Research Funding Project of Zhejiang Province (No. ZJ2022077).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. Ca A Cancer J Clinicians. 2020;70(1):7–30. doi:10.3322/caac.21590
2. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
3. Dembic Z. Antitumor Drugs and Their Targets. Molecules. 2020;25(23):5776. doi:10.3390/molecules25235776
4. Sun J, Wei Q, Zhou Y, Wang J, Liu Q, Xu H. A systematic analysis of FDA-approved anticancer drugs. BMC Syst. Biol. 2017;11(Suppl 5):87. doi:10.1186/s12918-017-0464-7
5. Sato R, Semba T, Saya H, Arima Y. Concise Review: stem Cells and Epithelial-Mesenchymal Transition in Cancer: biological Implications and Therapeutic Targets. Stem Cells (Dayton, Ohio). 2016;34(8):1997–2007. doi:10.1002/stem.2406
6. Zhang Y, Sun M, Huang G, et al. Maintenance of antiangiogenic and antitumor effects by orally active low-dose capecitabine for long-term cancer therapy. Proc Natl Acad Sci USA. 2017;114(26):E5226–E5235. doi:10.1073/pnas.1705066114
7. Fairchild A, Tirumani SH, Rosenthal MH, et al. Hormonal therapy in oncology: a primer for the radiologist. AJR. 2015;204(6):W620–W630 . doi:10.2214/AJR.14.13604
8. Beck A, Wurch T, Bailly C, Corvaia N. Strategies and challenges for the next generation of therapeutic antibodies. Immunology. 2010;10(5):345–352.
9. Dobosz P, Dzieciątkowski T. The Intriguing History of Cancer Immunotherapy. Front Immunol. 2019;10:2965.
10. Duong MT, Qin Y, You SH, Min JJ. Bacteria-cancer interactions: bacteria-based cancer therapy. Exp. Mol. Med. 2019;51(12):1–15. doi:10.1038/s12276-019-0297-0
11. Gopalakrishnan V, Helmink BA, Spencer CN, Reuben A, Wargo JA. The Influence of the Gut Microbiome on Cancer, Immunity, and Cancer Immunotherapy. Cancer Cell. 2018;33(4):570–580. doi:10.1016/j.ccell.2018.03.015
12. Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Cancer. 2003;3(5):380–387. doi:10.1038/nrc1071
13. Nguyen PL, Gu X, Lipsitz SR, et al. Cost implications of the rapid adoption of newer technologies for treating prostate cancer. J Clin Oncol. 2011;29(12):1517–1524. doi:10.1200/JCO.2010.31.1217
14. Mignani S, Bryszewska M, Klajnert-Maculewicz B, Zablocka M, Majoral JP. Advances in combination therapies based on nanoparticles for efficacious cancer treatment: an analytical report. Biomacromolecules. 2015;16(1):1–27. doi:10.1021/bm501285t
15. Hu CM, Zhang L. Nanoparticle- based combination therapy toward overcoming drug resistance in cancer. Biochem Pharmacol. 2012;83(8):1104–1111. doi:10.1016/j.bcp.2012.01.008
16. Gurunathan S, Kang MH, Qasim M, Kim JH. Nanoparticle-Mediated Combination Therapy: two-in-One Approach for Cancer. Int J Mol Sci. 2018;19(10):3264. doi:10.3390/ijms19103264
17. Assaraf YG, Brozovic A, Gonçalves AC, et al. The multi-factorial nature of clinical multidrug resistance in cancer. Drug Resist Updat. 2019;46:100645. doi:10.1016/j.drup.2019.100645
18. Jin S, White E. Role of autophagy in cancer: management of metabolic stress. Autophagy. 2007;3(1):28–31. doi:10.4161/auto.3269
19. Dalby KN, Tekedereli I, Lopez-Berestein G, Ozpolat B. Targeting the prodeath and prosurvival functions of autophagy as novel therapeutic strategies in cancer. Autophagy. 2010;6(3):322–329. doi:10.4161/auto.6.3.11625
20. Zhang Y, Sha R, Zhang L, et al. Harnessing copper-palladium alloy tetrapod nanoparticle-induced pro-survival autophagy for optimized photothermal therapy of drug-resistant cancer. Nat Commun. 2018;9(1):4236. doi:10.1038/s41467-018-06529-y
21. Ge Y, Ma Y, Li L. The application of prodrug-based nano -drug delivery strategy in cancer combination therapy. Colloids Surf B Biointerfaces. 2016;146:482–489. doi:10.1016/j.colsurfb.2016.06.051
22. Olov N, Bagheri-Khoulenjani S, Mirzadeh H. Combinational drug delivery using nanocarriers for breast cancer treatments: a review. J Biomed Mater Res A. 2018;106(8):2272–2283. doi:10.1002/jbm.a.36410
23. Kankala RK, Liu CG, Chen AZ, et al. Overcoming Multidrug Resistance through the Synergistic Effects of Hierarchical pH-Sensitive, ROS-Generating Nanoreactors. ACS Biomater. Sci. Eng. 2017;3(10):2431–2442. doi:10.1021/acsbiomaterials.7b00569
24. Kankala K, Liu C-G, Yang D-Y, et al. Ultrasmall platinum nanoparticles enable deep tumor penetration and synergistic therapeutic abilities through free radical species-assisted catalysis to combat cancer multidrug resistance [J]. Chem Eng J. 2020;383:123138. doi:10.1016/j.cej.2019.123138
25. Duan C, Yu M, Xu J, Li BY, Zhao Y, Kankala RK. Overcoming Cancer Multi-drug Resistance (MDR): reasons, mechanisms, nanotherapeutic solutions, and challenges. Biomed Pharmacother. 2023;162:114643. doi:10.1016/j.biopha.2023.114643
26. Li T, Li J, Chen Z, et al. Glioma diagnosis and therapy: current challenges and nanomaterial-based solutions. J Controlled Release. 2022;352:338–370. doi:10.1016/j.jconrel.2022.09.065
27. Liu CG, Han YH, Kankala RK, Wang SB, Chen AZ. Subcellular Performance of Nanoparticles in Cancer Therapy. Int j Nanomed. 2020;15:675–704. doi:10.2147/IJN.S226186
28. Bayda S, Adeel M, Tuccinardi T, Cordani M, Rizzolio F. The History of Nanoscience and Nanotechnology: from Chemical-Physical Applications to Nanomedicine. Molecules. 2019;25(1):112. doi:10.3390/molecules25010112
29. Ashrafizadeh M, Zhang W, Zou R, Sethi G, Klionsky DJ, Zhang X. A bioinformatics analysis, pre-clinical and clinical conception of autophagy in pancreatic cancer: complexity and simplicity in crosstalk. Pharmacol Res. 2023;194:106822. doi:10.1016/j.phrs.2023.106822
30. Pietrocola F, Bravo-San Pedro JM, Galluzzi L, Kroemer G. Autophagy in natural and therapy-driven anticancer immunosurveillance. Autophagy. 2017;13(12):2163–2170. doi:10.1080/15548627.2017.1310356
31. Wang C, Hu Q, Shen HM. Pharmacological inhibitors of autophagy as novel cancer therapeutic agents. Pharmacol Res. 2016;105:164–175. doi:10.1016/j.phrs.2016.01.028
32. Zhang SF, Wang XY, Fu ZQ, et al. TXNDC17 promotes paclitaxel resistance via inducing autophagy in ovarian cancer. Autophagy. 2015;11(2):225–238. doi:10.1080/15548627.2014.998931
33. Niu J, Yan T, Guo W, et al. The COPS3-FOXO3 positive feedback loop regulates autophagy to promote cisplatin resistance in osteosarcoma. Autophagy. 2023;19(6):1693–1710. doi:10.1080/15548627.2022.2150003
34. Zheng Y, Wu J, Chen H, et al. KLF4 targets RAB26 and decreases 5-FU resistance through inhibiting autophagy in colon cancer. Cancer Biol Ther. 2023;24(1):2226353. doi:10.1080/15384047.2023.2226353
35. Chittaranjan S, Bortnik S, Dragowska WH, et al. Autophagy inhibition augments the anticancer effects of epirubicin treatment in anthracycline-sensitive and -resistant triple-negative breast cancer. Clin Cancer Res. 2014;20(12):3159–3173. doi:10.1158/1078-0432.CCR-13-2060
36. Yamamoto K, Venida A, Yano J, et al. Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I. Nature. 2020;581(7806):100–105. doi:10.1038/s41586-020-2229-5
37. Lotsberg ML, Wnuk-Lipinska K, Terry S, et al. AXL Targeting Abrogates Autophagic Flux and Induces Immunogenic Cell Death in Drug-Resistant Cancer Cells. J Thorac Oncol. 2020;15(6):973–999. doi:10.1016/j.jtho.2020.01.015
38. Yu Z, Tang H, Chen S, et al. Exosomal LOC85009 inhibits docetaxel resistance in lung adenocarcinoma through regulating ATG5-induced autophagy. Drug Resist Updat. 2023;67:100915. doi:10.1016/j.drup.2022.100915
39. Han M, Hu J, Lu P, et al. Exosome-transmitted reverses trastuzumab resistance by inhibiting ATG5 in breast cancer. Cell Death Dis. 2020;11(1):43. doi:10.1038/s41419-020-2250-5
40. Pai Bellare G, Saha B, Patro BS. Targeting autophagy reverses de novo resistance in homologous recombination repair proficient breast cancers to PARP inhibition. Br J Cancer. 2021;124(7):1260–1274. doi:10.1038/s41416-020-01238-0
41. Xu WP, Liu JP, Feng JF, et al. miR-541 potentiates the response of human hepatocellular carcinoma to sorafenib treatment by inhibiting autophagy. Gut. 2020;69(7):1309–1321. doi:10.1136/gutjnl-2019-318830
42. Zahedi S, Fitzwalter BE, Morin A, et al. Effect of early-stage autophagy inhibition in BRAFV600E autophagy -dependent brain tumor cells. Cell Death Dis. 2019;10(9):679. doi:10.1038/s41419-019-1880-y
43. Bi J, Zhang Y, Malmrose PK, et al. Blocking autophagy overcomes resistance to dual histone deacetylase and proteasome inhibition in gynecologic cancer. Cell Death Dis. 2022;13(1):59. doi:10.1038/s41419-022-04508-2
44. Xu Q, Zhang H, Liu H, Han Y, Qiu W, Li Z. Inhibiting autophagy flux and DNA repair of tumor cells to boost radiotherapy of orthotopic glioblastoma. Biomaterials. 2022;280:121287. doi:10.1016/j.biomaterials.2021.121287
45. Liang B, Kong D, Liu Y, et al. Autophagy inhibition plays the synergetic killing roles with radiation in the multi-drug resistant SKVCR ovarian cancer cells. Radiat Oncol. 2012;7:213. doi:10.1186/1748-717X-7-213
46. Li Y, Cook KL, Yu W, et al. Inhibition of Antiestrogen-Promoted Pro-Survival Autophagy and Tamoxifen Resistance in Breast Cancer through Vitamin D Receptor. Nutrients. 2021;13(5):1715. doi:10.3390/nu13051715
47. Nguyen HG, Yang JC, Kung HJ, et al. Targeting autophagy overcomes Enzalutamide resistance in castration-resistant prostate cancer cells and improves therapeutic response in a xenograft model. Oncogene. 2014;33(36):4521–4530. doi:10.1038/onc.2014.25
48. Jiang L, Xu L, Xie J, et al. Inhibition of autophagy overcomes glucocorticoid resistance in lymphoid malignant cells. Cancer Biol Ther. 2015;16(3):466–476. doi:10.1080/15384047.2015.1016658
49. Wei MF, Chen MW, Chen KC, et al. Autophagy promotes resistance to photodynamic therapy-induced apoptosis selectively in colorectal cancer stem-like cells. Autophagy. 2014;10(7):1179–1192. doi:10.4161/auto.28679
50. Zanotto-Filho A, Braganhol E, Klafke K, et al. Autophagy inhibition improves the efficacy of curcumin/temozolomide combination therapy in glioblastomas. Cancer Lett. 2015;358(2):220–231. doi:10.1016/j.canlet.2014.12.044
51. Zeng T, Xu M, Zhang W, et al. Autophagy inhibition and microRNA‑199a‑5p upregulation in paclitaxel‑resistant A549/T lung cancer cells. Oncol Rep. 2021;46(1):149. doi:10.3892/or.2021.8100
52. Li H, Chen L, Li JJ, et al. miR-519a enhances chemosensitivity and promotes autophagy in glioblastoma by targeting STAT3/Bcl2 signaling pathway. J Hematol Oncol. 2018;11(1):70. doi:10.1186/s13045-018-0618-0
53. Xu W, Wei Q, Han M, et al. CCL2-SQSTM1 positive feedback loop suppresses autophagy to promote chemoresistance in gastric cancer. Int J Biol Sci. 2018;14(9):1054–1066. doi:10.7150/ijbs.25349
54. Zitkute V, Kukcinaviciute E, Jonusiene V, Starkuviene V, Sasnauskiene A. Differential effects of 5-fluorouracil and oxaliplatin on autophagy in chemoresistant colorectal cancer cells. J Cell Biochem. 2022;123(6):1103–1115. doi:10.1002/jcb.30267
55. Chen Y, Jia Y, Mao M, et al. PLAC8 promotes Adriamycin resistance via blocking autophagy in breast cancer. J Cell Mol Med. 2021;25(14):6948–6962. doi:10.1111/jcmm.16706
56. Li ZL, Zhang HL, Huang Y, et al. Autophagy deficiency promotes triple-negative breast cancer resistance to T cell-mediated cytotoxicity by blocking tenascin-C degradation. Nat Commun. 2020;11(1):3806. doi:10.1038/s41467-020-17395-y
57. Mele L, la Noce M, Paino F, et al. Glucose-6-phosphate dehydrogenase blockade potentiates tyrosine kinase inhibitor effect on breast cancer cells through autophagy perturbation. J Exp Clin Cancer Res. 2019;38(1):160. doi:10.1186/s13046-019-1164-5
58. Wu FQ, Fang T, Yu LX, et al. ADRB2 signaling promotes HCC progression and sorafenib resistance by inhibiting autophagic degradation of HIF1α. J Hepatol. 2016;65(2):314–324. doi:10.1016/j.jhep.2016.04.019
59. Ning L, Guo-Chun Z, Sheng-Li A, et al. Inhibition of autophagy induced by PTEN loss promotes intrinsic breast cancer resistance to trastuzumab therapy. Tumour Biol. 2016;37(4):5445–5454. doi:10.1007/s13277-015-4392-0
60. Yang Y, Li Y, Yang Q, et al. FAT4 activation inhibits epithelial-mesenchymal transition (EMT) by promoting autophagy in H2228/Cer cells. Med Oncol. 2022;40(1):64. doi:10.1007/s12032-022-01934-2
61. Thorburn J, Staskiewicz L, Goodall ML, et al. Non- cell-autonomous Effects of Autophagy Inhibition in Tumor Cells Promote Growth of Drug-resistant Cells. Mol Pharmacol. 2017;91(1):58–64. doi:10.1124/mol.116.106070
62. He C, Dong X, Zhai B, et al. MiR-21 mediates sorafenib resistance of hepatocellular carcinoma cells by inhibiting autophagy via the PTEN/Akt pathway. Oncotarget. 2015;6(30):28867–28881. doi:10.18632/oncotarget.4814
63. Zhai B, Hu F, Jiang X, et al. Inhibition of Akt reverses the acquired resistance to sorafenib by switching protective autophagy to autophagic cell death in hepatocellular carcinoma. Mol Cancer Ther. 2014;13(6):1589–1598. doi:10.1158/1535-7163.MCT-13-1043
64. Xu J, Su Y, Xu A, et al. miR-221/222-Mediated Inhibition of Autophagy Promotes Dexamethasone Resistance in Multiple Myeloma. Mol Ther. 2019;27(3):559–570. doi:10.1016/j.ymthe.2019.01.012
65. Kaverina NV, Kadagidze ZG, Borovjagin AV, et al. Tamoxifen overrides autophagy inhibition in Beclin-1-deficient glioma cells and their resistance to adenovirus-mediated oncolysis via upregulation of PUMA and BAX. Oncogene. 2018;37(46):6069–6082. doi:10.1038/s41388-018-0395-9
66. Qu YQ, Song LL, Xu SW, et al. Pomiferin targets SERCA, mTOR, and P-gp to induce autophagic cell death in apoptosis-resistant cancer cells, and reverses the MDR phenotype in cisplatin-resistant tumors in vivo. Pharmacol Res. 2023;191:106769. doi:10.1016/j.phrs.2023.106769
67. Yan J, Dou X, Zhou J, et al. Tubeimoside-I sensitizes colorectal cancer cells to chemotherapy by inducing ROS-mediated impaired autophagolysosomes accumulation. J Exp Clin Cancer Res. 2019;38(1):353. doi:10.1186/s13046-019-1355-0
68. Di Maio M, Chiodini P, Georgoulias V, et al. Meta-analysis of single-agent chemotherapy compared with combination chemotherapy as second-line treatment of advanced non-small-cell lung cancer. J Clin Oncol. 2009;27(11):1836–1843. doi:10.1200/JCO.2008.17.5844
69. Zhang RX, Wong HL, Xue HY, Eoh JY, Wu XY. Nanomedicine of synergistic drug combinations for cancer therapy - Strategies and perspectives. J Control Release. 2016;240:489–503. doi:10.1016/j.jconrel.2016.06.012
70. Landesman-Milo D, Ramishetti S, Peer D. Nanomedicine as an emerging platform for metastatic lung cancer therapy. Cancer Metastasis Rev. 2015;34(2):291–301. doi:10.1007/s10555-015-9554-4
71. Iyer AK, Singh A, Ganta S, Amiji MM. Role of integrated cancer nanomedicine in overcoming drug resistance. Adv. Drug Delivery Rev. 2013;65(13–14):1784–1802. doi:10.1016/j.addr.2013.07.012
72. Sun T, Zhang YS, Pang B, Hyun DC, Yang M, Xia Y. Engineered nanoparticles for drug delivery in cancer therapy. Angew Chem Int Ed Engl. 2014;53(46):12320–12364. doi:10.1002/anie.201403036
73. Banik BL, Fattahi P, Brown JL. Polymeric nanoparticles: the future of nanomedicine. Wiley interdisciplinary reviews. Nanomed Nanobiotechnol. 2016;8(2):271–299. doi:10.1002/wnan.1364
74. Brown PD, Patel PR. Nanomedicine: a pharma perspective. Wiley interdisciplinary reviews. Nanomed Nanobiotechnol. 2015;7(2):125–130. doi:10.1002/wnan.1288
75. Danhier F, Lecouturier N, Vroman B, et al. Paclitaxel-loaded PEGylated PLGA-based nanoparticles: in vitro and in vivo evaluation. J Controlled Release. 2009;133(1):11–17. doi:10.1016/j.jconrel.2008.09.086
76. Jin C, Bai L, Wu H, Song W, Guo G, Dou K. Cytotoxicity of paclitaxel incorporated in PLGA nanoparticles on hypoxic human tumor cells. Pharm res. 2009;26(7):1776–1784. doi:10.1007/s11095-009-9889-z
77. Pérez-Herrero E, Fernández-Medarde A. Advanced targeted therapies in cancer: drug nanocarriers, the future of chemotherapy. Eur j Pharm. 2015;93:52–79.
78. Jadia R, Scandore C, Rai P. Nanoparticles for Effective Combination Therapy of Cancer. Int J Nanotechnol Nanomed. 2016;1(1):565.
79. Zhang DY, Shen XZ, Wang JY, Dong L, Zheng YL, Wu LL. Preparation of chitosan-polyaspartic acid-5-fluorouracil nanoparticles and its anti-carcinoma effect on tumor growth in nude mice. World J Gastroenterol. 2008;14(22):3554–3562. doi:10.3748/wjg.14.3554
80. Kamaly N, Xiao Z, Valencia PM, Radovic-Moreno AF, Farokhzad OC. Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem Soc Rev. 2012;41(7):2971–3010.
81. Lv S, Tang Z, Li M, et al. Co-delivery of doxorubicin and paclitaxel by PEG-polypeptide nanovehicle for the treatment of non-small cell lung cancer. Biomaterials. 2014;35(23):6118–6129. doi:10.1016/j.biomaterials.2014.04.034
82. Zucker D, Andriyanov AV, Steiner A, Raviv U, Barenholz Y. Characterization of PEGylated nanoliposomes co-remotely loaded with topotecan and vincristine: relating structure and pharmacokinetics to therapeutic efficacy. J Control Release. 2012;160(2):281–289.
83. Misra R, Sahoo SK. Coformulation of doxorubicin and curcumin in poly(D,L-lactide-co-glycolide) nanoparticles suppresses the development of multidrug resistance in K562 cells. Mol Pharm. 2011;8(3):852–866. doi:10.1021/mp100455h
84. Sarisozen C, Abouzeid AH, Torchilin VP. The effect of co-delivery of paclitaxel and curcumin by transferrin-targeted PEG-PE-based mixed micelles on resistant ovarian cancer in 3-D spheroids and in vivo tumors. Eur J Pharm Biopharm. 2014;88(2):539–550. doi:10.1016/j.ejpb.2014.07.001
85. Chiang CS, Hu SH, Liao BJ, Chang YC, Chen SY. Enhancement of cancer therapy efficacy by trastuzumab-conjugated and pH-sensitive nanocapsules with the simultaneous encapsulation of hydrophilic and hydrophobic compounds. Nanomedicine. 2014;10(1):99–107. doi:10.1016/j.nano.2013.07.009
86. Tang J, Zhang L, Gao H, et al. Co-delivery of doxorubicin and P-gp inhibitor by a reduction-sensitive liposome to overcome multidrug resistance, enhance anti-tumor efficiency and reduce toxicity. Drug Deliv. 2016;23(4):1130–1143. doi:10.3109/10717544.2014.990651
87. Zhang X, Zeng X, Liang X, et al. The chemotherapeutic potential of PEG-b-PLGA copolymer micelles that combine chloroquine as autophagy inhibitor and docetaxel as an anti-cancer drug. Biomaterials. 2014;35(33):9144–9154. doi:10.1016/j.biomaterials.2014.07.028
88. Song Q, Tan S, Zhuang X, et al. Nitric oxide releasing d-α-tocopheryl polyethylene glycol succinate for enhancing antitumor activity of doxorubicin. Mol Pharm. 2014;11(11):4118–4129.
89. Wan WJ, Qu CX, Zhou YJ, et al. Doxorubicin and siRNA-PD-L1 co-delivery with T7 modified ROS-sensitive nanoparticles for tumor chemoimmunotherapy. Int J Pharm. 2019;566:731–744. doi:10.1016/j.ijpharm.2019.06.030
90. Zhang F, Li M, Su Y, Zhou J, Wang W. A dual-targeting drug co-delivery system for tumor chemo- and gene combined therapy. Mater Sci Eng C Mater Biol Appl. 2016;64:208–218. doi:10.1016/j.msec.2016.03.083
91. Kang Y, Lu L, Lan J, et al. Redox-responsive polymeric micelles formed by conjugating gambogic acid with bioreducible poly(amido amine)s for the co-delivery of docetaxel and MMP-9 shRNA. Acta Biomater. 2018;68:137–153. doi:10.1016/j.actbio.2017.12.028
92. Ren Q, Liang Z, Jiang X, et al. Enzyme and pH dual-responsive hyaluronic acid nanoparticles mediated combination of photodynamic therapy and chemotherapy. Int J Biol Macromol. 2019;130:845–852.
93. Wang D, Xu Z, Yu H, et al. Treatment of metastatic breast cancer by combination of chemotherapy and photothermal ablation using doxorubicin-loaded DNA wrapped gold nanorods. Biomaterials. 2014;35(29):8374–8384. doi:10.1016/j.biomaterials.2014.05.094
94. Ren Y, Zhang H, Chen B, et al. Multifunctional magnetic Fe3O4 nanoparticles combined with chemotherapy and hyperthermia to overcome multidrug resistance. Int J Nanomed. 2012;7:2261–2269. doi:10.2147/IJN.S29357
95. Zhang W, Li C, Shen C, et al. Prodrug-based nano-drug delivery system for co-encapsulate paclitaxel and carboplatin for lung cancer treatment. Drug Deliv. 2016;23(7):2575–2580. doi:10.3109/10717544.2015.1035466
96. Chauhan G, Chopra V, Tyagi A, Rath G, Sharma RK, Goyal AK. “Gold nanoparticles composite-folic acid conjugated graphene oxide nanohybrids” for targeted chemo-thermal cancer ablation: in vitro screening and in vivo studies. Eur J Pharm Sci. 2017;96:351–361. doi:10.1016/j.ejps.2016.10.011
97. Mir Y, Elrington SA, Hasan T. A new nanoconstruct for epidermal growth factor receptor-targeted photo-immunotherapy of ovarian cancer. Nanomedicine. 2013;9(7):1114–1122. doi:10.1016/j.nano.2013.02.005
98. Min H, Wang J, Qi Y, et al. Biomimetic Metal-Organic Framework Nanoparticles for Cooperative Combination of Antiangiogenesis and Photodynamic Therapy for Enhanced Efficacy. Adv Mater. 2019;31(15):e1808200. doi:10.1002/adma.201808200
99. Yue W, Chen L, Yu L, et al. Checkpoint blockade and nanosonosensitizer-augmented noninvasive sonodynamic therapy combination reduces tumour growth and metastases in mice. Nat Commun. 2019;10(1):2025. doi:10.1038/s41467-019-09760-3
100. Xiao H, Song H, Yang Q, et al. A prodrug strategy to deliver cisplatin(IV) and paclitaxel in nanomicelles to improve efficacy and tolerance. Biomaterials. 2012;33(27):6507–6519. doi:10.1016/j.biomaterials.2012.05.049
101. Wang Y, Xie Y, Li J, et al. Tumor-Penetrating Nanoparticles for Enhanced Anticancer Activity of Combined Photodynamic and Hypoxia-Activated Therapy. ACS Nano. 2017;11(2):2227–2238. doi:10.1021/acsnano.6b08731
102. Lu X, Wang QQ, Xu FJ, Tang GP, Yang WT. A cationic prodrug/therapeutic gene nanocomplex for the synergistic treatment of tumors. Biomaterials. 2011;32(21):4849–4856. doi:10.1016/j.biomaterials.2011.03.022
103. Li Y, Liu R, Yang J, Ma G, Zhang Z, Zhang X. Dual sensitive and temporally controlled camptothecin prodrug liposomes codelivery of siRNA for high efficiency tumor therapy. Biomaterials. 2014;35(36):9731–9745. doi:10.1016/j.biomaterials.2014.08.022
104. Galstyan A, Markman JL, Shatalova ES, et al. Blood-brain barrier permeable nano immunoconjugates induce local immune responses for glioma therapy. Nat Commun. 2019;10(1):3850. doi:10.1038/s41467-019-11719-3
105. Park J, Wrzesinski SH, Stern E, et al. Combination delivery of TGF-β inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nat Mater. 2012;11(10):895–905. doi:10.1038/nmat3355
106. Cheng K, Ding Y, Zhao Y, et al. Sequentially Responsive Therapeutic Peptide Assembling Nanoparticles for Dual-Targeted Cancer Immunotherapy. Nano Lett. 2018;18(5):3250–3258. doi:10.1021/acs.nanolett.8b01071
107. Teo PY, Cheng W, Hedrick JL, Yang YY. Co-delivery of drugs and plasmid DNA for cancer therapy. Adv Drug Deliv Rev. 2016;98:41–63. doi:10.1016/j.addr.2015.10.014
108. He C, Tang Z, Tian H, Chen X. Co-delivery of chemotherapeutics and proteins for synergistic therapy. Adv Drug Deliv Rev. 2016;98:64–76. doi:10.1016/j.addr.2015.10.021
109. Shapira A, Livney YD, Broxterman HJ, Assaraf YG. Nanomedicine for targeted cancer therapy: towards the overcoming of drug resistance. Drug Resist Updat. 2011;14(3):150–163. doi:10.1016/j.drup.2011.01.003
110. Abeylath SC, Ganta S, Iyer AK, Amiji M. Combinatorial-designed multifunctional polymeric nanosystems for tumor-targeted therapeutic delivery. Acc Chem Res. 2011;44(10):1009–1017. doi:10.1021/ar2000106
111. Parekh G, Shi Y, Zheng J, Zhang X, Leporatti S. Nano-carriers for targeted delivery and biomedical imaging enhancement. Ther Deliv. 2018;9(6):451–468. doi:10.4155/tde-2018-0013
112. Palanikumar L, Jeena MT, Kim K, et al. Spatiotemporally and Sequentially-Controlled Drug Release from Polymer Gatekeeper-Hollow Silica Nanoparticles. Sci Rep. 2017;7:46540. doi:10.1038/srep46540
113. Moss DM, Siccardi M. Optimizing nanomedicine pharmacokinetics using physiologically based pharmacokinetics modeling. Br J Pharmacol. 2014;171(17):3963–3979. doi:10.1111/bph.12604
114. Rawal S, Patel MM. Threatening cancer with nanoparticle aided combination oncotherapy. J Control Release. 2019;301:76–109. doi:10.1016/j.jconrel.2019.03.015
115. Greco F, Vicent MJ. Combination therapy: opportunities and challenges for polymer-drug conjugates as anticancer nanomedicines. Adv Drug Deliv Rev. 2009;61(13):1203–1213. doi:10.1016/j.addr.2009.05.006
116. Feng T, Tian H, Xu C, et al. Synergistic co- delivery of doxorubicin and paclitaxel by porous PLGA microspheres for pulmonary inhalation treatment. Eur J Pharm Biopharm. 2014;88(3):1086–1093. doi:10.1016/j.ejpb.2014.09.012
117. Al-Lazikani B, Banerji U, Workman P. Combinatorial drug therapy for cancer in the post-genomic era. Nat Biotechnol. 2012;30(7):679–692. doi:10.1038/nbt.2284
118. Xu JL, Jin B, Ren ZH, et al. Chemotherapy plus Erlotinib versus Chemotherapy Alone for Treating Advanced Non-Small Cell Lung Cancer: a Meta-Analysis. PLoS One. 2015;10(7):e0131278. doi:10.1371/journal.pone.0131278
119. Zhang X, Yang Y, Liang X, et al. Enhancing therapeutic effects of docetaxel-loaded dendritic copolymer nanoparticles by co-treatment with autophagy inhibitor on breast cancer. Theranostics. 2014;4(11):1085–1095. doi:10.7150/thno.9933
120. Hu C, Gu F, Gong C, Xia Q, Gao Y, Gao S. Co-delivery of the autophagy inhibitor si-Beclin1 and the doxorubicin nano-delivery system for advanced prostate cancer treatment. J Biomater Appl. 2022;36(7):1317–1331. doi:10.1177/08853282211060252
121. Jia HZ, Zhang W, Zhu JY, et al. Hyperbranched-hyperbranched polymeric nanoassembly to mediate controllable co-delivery of siRNA and drug for synergistic tumor therapy. J Control Release. 2015;216:17.
122. Chen L, Qian M, Zhang L, et al. Co-delivery of doxorubicin and shRNA of Beclin1 by folate receptor targeted pullulan-based multifunctional nanomicelles for combinational cancer therapy. RSC Adv. 2018;8(32):17722.
123. Gao M, Xu Y, Qiu L. Enhanced combination therapy effect on paclitaxel-resistant carcinoma by chloroquine co -delivery via liposomes. Int J Nanomed. 2015;10:6615–6632. doi:10.2147/IJN.S91463
124. Schlinkert P, Casals E, Boyles M, et al. The oxidative potential of differently charged silver and gold nanoparticles on three human lung epithelial cell types. J Nanobiotechnology. 2015;13:1. doi:10.1186/s12951-014-0062-4
125. Xu J, Zhu X, Qiu L. Polyphosphazene vesicles for co-delivery of doxorubicin and chloroquine with enhanced anticancer efficacy by drug resistance reversal. Int J Pharm. 2016;498(1–2):70–81. doi:10.1016/j.ijpharm.2015.12.003
126. Lin YX, Wang Y, An HW, et al. Peptide-Based Autophagic Gene and Cisplatin Co-delivery Systems Enable Improved Chemotherapy Resistance. Nano Lett. 2019;19(5):2968–2978. doi:10.1021/acs.nanolett.9b00083
127. Zhao X, Dong Y, Zhang J, et al. Reversing immune evasion using a DNA nano-orchestrator for pancreatic cancer immunotherapy. Acta Biomater. 2023;166(512):523. doi:10.1016/j.actbio.2023.05.001
128. Zuo L, Nie W, Yu S, et al. Biomimetic Nanovesicle with Mitochondria-Synthesized Sonosensitizer and Mitophagy Inhibition for Cancer Sono-Immunotherapy. Nano Lett. 2023;23(7):3005–3013. doi:10.1021/acs.nanolett.3c00383
129. Yang X, Zhao M, Wu Z, et al. Nano-ultrasonic Contrast Agent for Chemoimmunotherapy of Breast Cancer by Immune Metabolism Reprogramming and Tumor Autophagy. ACS Nano. 2022;16(2):3417–3431. doi:10.1021/acsnano.2c00462
130. Zhu H, Gao X, Wang B, et al. A biodegradable hollow nanoagent enables a boosted chemodynamic therapy by simultaneous autophagy inhibition and macrophage reeducation. Int J Pharm. 2023;643:123248. doi:10.1016/j.ijpharm.2023.123248
131. Nirosha Yalamandala B, Chen PH, Moorthy T, Huynh TMH, Chiang WH, Hu SH. Programmed Catalytic Therapy- Mediated ROS Generation and T-Cell Infiltration in Lung Metastasis by a Dual Metal-Organic Framework (MOF) Nanoagent. Pharmaceutics. 2022;14(3):527. doi:10.3390/pharmaceutics14030527
132. Chen M, Yang D, Sun Y, et al. In Situ Self-Assembly Nanomicelle Microneedles for Enhanced Photoimmunotherapy via Autophagy Regulation Strategy. ACS Nano. 2021;15(2):3387–3401. doi:10.1021/acsnano.0c10396
133. Luo Y, Li Y, Huang Z, et al. A Nanounit Strategy Disrupts Energy Metabolism and Alleviates Immunosuppression for Cancer Therapy. Nano Lett. 2022;22(15):6418–6427. doi:10.1021/acs.nanolett.2c02475
134. Ruan S, Xie R, Qin L, et al. Aggregable Nanoparticles-Enabled Chemotherapy and Autophagy Inhibition Combined with Anti-PD-L1 Antibody for Improved Glioma Treatment. Nano Lett. 2019;19(11):8332. doi:10.1021/acs.nanolett.9b03968
135. Li Y, Cho MH, Lee SS, Lee DE, Cheong H, Choi Y. Hydroxychloroquine-loaded hollow mesoporous silica nanoparticles for enhanced autophagy inhibition and radiation therapy. J Control Release. 2020;325:100–110. doi:10.1016/j.jconrel.2020.06.025
136. Ma Z, Lin K, Tang M, et al. A pH-Driven Small-Molecule Nanotransformer Hijacks Lysosomes and Overcomes Autophagy-Induced Resistance in Cancer. Angew Chem Int Ed Engl. 2022;61(35):e202204567. doi:10.1002/anie.202204567
137. Zhang X, Gao H, Wei D, et al. ROS Responsive Nanoparticles Encapsulated with Natural Medicine Remodel Autophagy Homeostasis in Breast Cancer. ACS Appl Mater Interfaces. 2023;15(25):29827–29840. doi:10.1021/acsami.3c03068
138. Li N, Gao Y, Li B, et al. Remote Manipulation of ROS-Sensitive Calcium Channel Using Near-Infrared-Responsive Conjugated Oligomer Nanoparticles for Enhanced Tumor Therapy In Vivo. Nano Lett. 2022;22(13):5427–5433. doi:10.1021/acs.nanolett.2c01472
139. Wang L, Wang Y, Zhao W, et al. Library Screening to Identify Highly-Effective Autophagy Inhibitors for Improving Photothermal Cancer Therapy. Nano Lett. 2021;21(22):9476–9484. doi:10.1021/acs.nanolett.1c02825
140. Yang Y, Huang J, Liu M, et al. Emerging Sonodynamic Therapy-Based Nanomedicines for Cancer Immunotherapy. Adv Sci (Weinh). 2023;10(2):e2204365. doi:10.1002/advs.202204365
141. Zhou L, Huo M, Qian MA. ROS-Responsive Nanomedicine: towards Targeting the Breast Tumor Microenvironment. Curr Med Chem. 2021;28(28):5674–5698. doi:10.2174/0929867328666201209100659
142. Zhou L, Huo M, Qian X, et al. Autophagy blockade synergistically enhances nanosonosensitizer-enabled sonodynamic cancer nanotherapeutics. J Nanobiotechnology. 2021;19(1):112. doi:10.1186/s12951-021-00855-y
143. Yu S, Chen Z, Zeng X, Chen X, Gu Z. Advances in nanomedicine for cancer starvation therapy. Theranostics. 2019;9(26):8026–8047. doi:10.7150/thno.38261
144. Yang B, Ding L, Chen Y, Shi J. Augmenting Tumor-Starvation Therapy by Cancer Cell Autophagy Inhibition. Adv Sci (Weinh). 2020;7(6):1902847. doi:10.1002/advs.201902847
145. Liu X, Gao P, Shi M, et al. An autophagy-inhibitory MOF nanoreactor for tumor-targeted synergistic therapy. Biomater Sci. 2022;10(12):3088–3091. doi:10.1039/D2BM00579D
146. Deng Y, Jia F, Jiang P, et al. Biomimetic nanoparticle synchronizing pyroptosis induction and mitophagy inhibition for anti-tumor therapy. Biomaterials. 2023;301:122293. doi:10.1016/j.biomaterials.2023.122293
147. Wang T, Xiao G, Lu Q, et al. Synergistic Lysosomal Impairment and ER Stress Activation for Boosted Autophagy Dysfunction Based on Te Double-Headed Nano-Bullets. Small. 2022;18(27):e2201585. doi:10.1002/smll.202201585
148. Wang C, Li Z, Xu P, Xu L, Han S, Sun Y. Combination of polythyleneimine regulating autophagy prodrug and Mdr1 siRNA for tumor multidrug resistance. J Nanobiotechnology. 2022;20(1):476. doi:10.1186/s12951-022-01689-y
149. Wang X, Li Y, Jia F, Cui X, Pan Z, Wu Y. Boosting nutrient starvation -dominated cancer therapy through curcumin-augmented mitochondrial Ca2+ overload and obatoclax-mediated autophagy inhibition as supported by a novel nano-regulator GO-Alg@CaP/CO. J Nanobiotechnology. 2022;20(1):225. doi:10.1186/s12951-022-01439-0
150. Zhang H, Meng L, Yin L, et al. ClC-3 silencing mediates lysosomal acidification arrest and autophagy inhibition to sensitize chemo-photothermal therapy. Int J Pharm. 2022;628:122297. doi:10.1016/j.ijpharm.2022.122297
151. Gao C, Kwong CHT, Wang Q, et al. Conjugation of Macrophage-Mimetic Microalgae and Liposome for Antitumor Sonodynamic Immunotherapy via Hypoxia Alleviation and Autophagy Inhibition. ACS Nano. 2023;17(4):4034–4049. doi:10.1021/acsnano.3c00041
152. Kondo Y, Kanzawa T, Sawaya R, Kondo S. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer. 2005;5(9):726–734. doi:10.1038/nrc1692
153. Mele L, Del Vecchio V, Liccardo D, et al. The role of autophagy in resistance to targeted therapies. Cancer Treat Rev. 2020;88:102043. doi:10.1016/j.ctrv.2020.102043
154. Moscat J, Diaz-Meco MT. p62 at the crossroads of autophagy, apoptosis, and cancer. Cell. 2009;137(6):1001–1004. doi:10.1016/j.cell.2009.05.023
155. Zhang X, Dong Y, Zeng X, et al. The effect of autophagy inhibitors on drug delivery using biodegradable polymer nanoparticles in cancer treatment. Biomaterials. 2014;35(6):1932–1943. doi:10.1016/j.biomaterials.2013.10.034
156. Yan J, Shan C, Zhang Z, et al. Autophagy-induced intracellular signaling fractional nano-drug system for synergistic anti-tumor therapy. J Colloid Interface Sci. 2023;645:986–996. doi:10.1016/j.jcis.2023.05.031
157. Mohammed SA, Ju Y. Multifunctional liposomal nanostructure-mediated siRNA/bortezomib co-delivery for SHARP1 knockdown in MLL-AF6 acute myeloid leukemia. Biomater Adv. 2022;134:112663. doi:10.1016/j.msec.2022.112663
158. An J, Zhang K, Wang B, et al. Nanoenabled Disruption of Multiple Barriers in Antigen Cross-Presentation of Dendritic Cells via Calcium Interference for Enhanced Chemo-Immunotherapy. ACS Nano. 2020;14(6):7639–7650. doi:10.1021/acsnano.0c03881
159. Yu Z, Guo J, Hu M, Gao Y, Huang L. Icaritin Exacerbates Mitophagy and Synergizes with Doxorubicin to Induce Immunogenic Cell Death in Hepatocellular Carcinoma. ACS Nano. 2020;14(4):4816–4828. doi:10.1021/acsnano.0c00708
160. Li TF, Xu YH, Li K, et al. Doxorubicin-polyglycerol-nanodiamond composites stimulate glioblastoma cell immunogenicity through activation of autophagy. Acta Biomater. 2019;86:381–394. doi:10.1016/j.actbio.2019.01.020
161. Wang Y, Lin YX, Wang J, et al. In Situ Manipulation of Dendritic Cells by an Autophagy-Regulative Nanoactivator Enables Effective Cancer Immunotherapy. ACS Nano. 2019;13(7):7568–7577. doi:10.1021/acsnano.9b00143
162. Yue H, Wei W, Gu Z, et al. Exploration of graphene oxide as an intelligent platform for cancer vaccines. Nanoscale. 2015;7(47):19957. doi:10.1039/C5NR04986E
163. Deng Y, Song P, Chen X, et al. 3-Bromopyruvate-Conjugated Nanoplatform-Induced Pro-Death Autophagy for Enhanced Photodynamic Therapy against Hypoxic Tumor. ACS Nano. 2020;14(8):9711–9727. doi:10.1021/acsnano.0c01350
164. Sun M, Wang C, Lv M, Fan Z, Du J. Mitochondrial-targeting nanoprodrugs to mutually reinforce metabolic inhibition and autophagy for combating resistant cancer. Biomaterials. 2021;278:121168. doi:10.1016/j.biomaterials.2021.121168
165. Liu R, Li Q, Qin S, et al. Sertaconazole- repurposed nanoplatform enhances lung cancer therapy via CD44-targeted drug delivery. J Exp Clin Cancer Res. 2023;42(1):188. doi:10.1186/s13046-023-02766-2
166. Kavya KV, Vargheese S, Shukla S, et al. A cationic amino acid polymer nanocarrier synthesized in supercritical CO2 for co-delivery of drug and gene to cervical cancer cells. Colloids Surf B Biointerfaces. 2022;216:112584. doi:10.1016/j.colsurfb.2022.112584
167. Hanafy NAN, Sheashaa RF, Moussa EA, Mahfouz ME. Potential of curcumin and niacin-loaded targeted chitosan coated liposomes to activate autophagy in hepatocellular carcinoma cells: an in vitro evaluation in HePG2 cell line. Int J Biol Macromol. 2023;245:125572. doi:10.1016/j.ijbiomac.2023.125572
168. Chen J, Xue F, Du W, et al. An Endogenous H2S-Activated Nanoplatform for Triple Synergistic Therapy of Colorectal Cancer. Nano Lett. 2022;22(15):6156–6165. doi:10.1021/acs.nanolett.2c01346
169. Zhang R, Xu S, Yuan M, et al. An ultrasmall PVP-Fe-Cu -Ni-S nano-agent for synergistic cancer therapy through triggering ferroptosis and autophagy. Nanoscale. 2023;15(30):12598–12611. doi:10.1039/D3NR02708B
170. Li F, Chen T, Wang F, et al. Enhanced Cancer Starvation Therapy Enabled by an Autophagy Inhibitors-Encapsulated Biomimetic ZIF-8 Nanodrug: disrupting and Harnessing Dual Pro-Survival Autophagic Responses. ACS Appl Mater Interfaces. 2022;14(19):21860–21871. doi:10.1021/acsami.2c00552
171. Yin W, Pham CV, Wang T, et al. Inhibition of Autophagy Promotes the Elimination of Liver Cancer Stem Cells by CD133 Aptamer-Targeted Delivery of Doxorubicin. Biomolecules. 2022;12(11):1623. doi:10.3390/biom12111623
172. Ghosh C, Nandi A, Basu S. Supramolecular self-assembly of triazine-based small molecules: targeting the endoplasmic reticulum in cancer cells. Nanoscale. 2019;11(7):3326–3335. doi:10.1039/C8NR08682F
173. Wu J, Huang X, Xiao Z, et al. Nano-Pt mitochondria induced-dependent apoptosis and cytoprotective autophagy in human NSCLC cells. Colloids Surf B Biointerfaces. 2023;227:113344. doi:10.1016/j.colsurfb.2023.113344
174. Adiseshaiah PP, Clogston JD, McLeland CB, et al. Synergistic combination therapy with nanoliposomal C6-ceramide and vinblastine is associated with autophagy dysfunction in hepatocarcinoma and colorectal cancer models. Cancer Lett. 2013;337(2):254–265. doi:10.1016/j.canlet.2013.04.034
175. Shaw JJP, Boyer TL, Venner E, et al. Inhibition of Lysosomal Function Mitigates Protective Mitophagy and Augments Ceramide Nanoliposome-Induced Cell Death in Head and Neck Squamous Cell Carcinoma. Mol Cancer Ther. 2020;19(12):2621–2633. doi:10.1158/1535-7163.MCT-20-0182
176. Li N, Han S, Ma B, et al. Chemosensitivity enhanced by autophagy inhibition based on a polycationic nano-drug carrier. Nanoscale Adv. 2021;3(6):1656–1673. doi:10.1039/D0NA00990C
177. Xie Y, Jiang J, Tang Q, et al. Iron Oxide Nanoparticles as Autophagy Intervention Agents Suppress Hepatoma Growth by Enhancing Tumoricidal Autophagy. Adv Sci (Weinh). 2020;7(16):1903323. doi:10.1002/advs.201903323
178. Zhang P, Shi Y, Xu Y, et al. A Nano-Autophagy Inhibitor Triggering Reciprocal Feedback Control of Cholesterol Depletion for Solid Tumor Therapy. Adv Healthc Mater;2023. e2302020. doi:10.1002/adhm.202302020
179. Lu HY, Chang YJ, Fan NC, et al. Synergism through combination of chemotherapy and oxidative stress-induced autophagy in A549 Lung cancer cells using redox-responsive nanohybrids: a new strategy for cancer therapy. Biomaterials. 2015;42:30–41. doi:10.1016/j.biomaterials.2014.11.029
180. Wang XS, Zeng JY, Li MJ, Li QR, Gao F, Zhang XZ. Highly Stable Iron Carbonyl Complex Delivery Nanosystem for Improving Cancer Therapy. ACS Nano. 2020;14(8):9848–9860. doi:10.1021/acsnano.0c02516
181. Lv C, Kang W, Liu S, et al. Growth of ZIF-8 Nanoparticles In Situ on Graphene Oxide Nanosheets: a Multifunctional Nanoplatform for Combined Ion-Interference and Photothermal Therapy. ACS Nano. 2022;16(7):11428–11443. doi:10.1021/acsnano.2c05532
182. Zhou Y, Han Y, Li G, Yang S, Xiong F, Chu F. Preparation of Targeted Lignin⁻Based Hollow Nanoparticles for the Delivery of Doxorubicin. Nanomaterials (Basel). 2019;9(2):188. doi:10.3390/nano9020188
183. Andhari SS, Wavhale RD, Dhobale KD, et al. Self-Propelling Targeted Magneto -Nanobots for Deep Tumor Penetration and pH-Responsive Intracellular Drug Delivery. Sci Rep. 2020;10(1):4703. doi:10.1038/s41598-020-61586-y
184. Gavini J, Dommann N, Jakob MO, et al. Verteporfin-induced lysosomal compartment dysregulation potentiates the effect of sorafenib in hepatocellular carcinoma. Cell Death Dis. 2019;10(10):749. doi:10.1038/s41419-019-1989-z
185. Xu Y, Zheng H, Kang JS, et al. 5-Nitro-2-(3-phenylpropylamino) benzoic acid induced drug resistance to cisplatin in human erythroleukemia cell lines. Anat Rec (Hoboken). 2011;294(6):945–952. doi:10.1002/ar.21392
186. Seebacher NA, Richardson DR, Jansson PJ. A mechanism for overcoming P-glycoprotein-mediated drug resistance: novel combination therapy that releases stored doxorubicin from lysosomes via lysosomal permeabilization using Dp44mT or DpC. Cell Death Dis. 2016;7(12):e2510. doi:10.1038/cddis.2016.381
187. Zhu X, Ji X, Kong N, et al. Intracellular Mechanistic Understanding of 2D MoS2 Nanosheets for Anti-Exocytosis-Enhanced Synergistic Cancer Therapy. ACS Nano. 2018;12(3):2922–2938. doi:10.1021/acsnano.8b00516
188. Yin Y, Wang J, Yang M, et al. Penetration of the blood-brain barrier and the anti-tumour effect of a novel PLGA-lysoGM1/DOX micelle drug delivery system. Nanoscale. 2020;12(5):2946–2960. doi:10.1039/C9NR08741A
189. Ding L, Zhu X, Wang Y, et al. Intracellular Fate of Nanoparticles with Polydopamine Surface Engineering and a Novel Strategy for Exocytosis-Inhibiting, Lysosome Impairment-Based Cancer Therapy. Nano Lett. 2017;17(11):6790–6801. doi:10.1021/acs.nanolett.7b03021
190. Lin YW, Lin TT, Chen CH, et al. Enhancing Efficacy of Albumin-Bound Paclitaxel for Human Lung and Colorectal Cancers through Autophagy Receptor Sequestosome 1 (SQSTM1)/p62-Mediated Nanodrug Delivery and Cancer therapy. ACS Nano. 2023. doi:10.1021/acsnano.3c04739
191. Kong C, Li Y, Liu Z, et al. Targeting the Oncogene KRAS Mutant Pancreatic Cancer by Synergistic Blocking of Lysosomal Acidification and Rapid Drug Release. ACS Nano. 2019;13(4):4049–4063. doi:10.1021/acsnano.8b08246
192. Li X, Wang ZG, Zhu H, et al. Inducing Autophagy and Blocking Autophagic Flux via a Virus-Mimicking Nanodrug for Cancer Therapy. Nano Lett. 2022;22(22):9163–9173. doi:10.1021/acs.nanolett.2c04091
193. Jiang L, Liang X, Liu G, et al. The mechanism of lauric acid-modified protein nanocapsules escape from intercellular trafficking vesicles and its implication for drug delivery. Drug Deliv. 2018;25(1):985–994. doi:10.1080/10717544.2018.1461954
194. Zhang Q, Yang W, Man N, et al. Autophagy-mediated chemosensitization in cancer cells by fullerene C60 nanocrystal. Autophagy. 2009;5(8):1107–1117. doi:10.4161/auto.5.8.9842
195. Xiong Q, Liu A, Ren Q, et al. Cuprous oxide nanoparticles trigger reactive oxygen species-induced apoptosis through activation of erk-dependent autophagy in bladder cancer. Cell Death Dis. 2020;11(5):366. doi:10.1038/s41419-020-2554-5
196. Liu M, Liu Z, Qin G, Ren J, Qu X. Bioorthogonally Activatable Autophagy-Tethering Compounds for Aptamer-Guided Mitochondrial Degradation. Nano Lett. 2023;23(11):4965–4973. doi:10.1021/acs.nanolett.3c00798
197. Chen F, Zhang XH, Hu XD, Liu PD, Zhang HQ. The effects of combined selenium nanoparticles and radiation therapy on breast cancer cells in vitro. Artif Cells Nanomed Biotechnol. 2018;46(5):937–948. doi:10.1080/21691401.2017.1347941
198. Liu Z, Tan H, Zhang X, et al. Enhancement of radiotherapy efficacy by silver nanoparticles in hypoxic glioma cells. Artif Cells Nanomed Biotechnol. 2018;46(sup3):S922–S930. doi:10.1080/21691401.2018.1518912
199. Liu P, Jin H, Guo Z, et al. Silver nanoparticles outperform gold nanoparticles in radiosensitizing U251 cells in vitro and in an intracranial mouse model of glioma. Int J Nanomed. 2016;11:5003–5014. doi:10.2147/IJN.S115473
200. Ma S, Miao H, Luo Y, et al. FePt/GO Nanosheets Suppress Proliferation, Enhance Radiosensitization and Induce Autophagy of Human Non-Small Cell Lung Cancer Cells. Int J Biol Sci. 2019;15(5):999–1009. doi:10.7150/ijbs.29805
201. Zhang X, Liu Z, Lou Z, et al. Radiosensitivity enhancement of Fe3O4@Ag nanoparticles on human glioblastoma cells. Artif Cells Nanomed Biotechnol. 2018;46(sup1):975–984. doi:10.1080/21691401.2018.1439843
202. Li F, Li Z, Jin X, et al. Ultra-small gadolinium oxide nanocrystal sensitization of non-small-cell lung cancer cells toward X-ray irradiation by promoting cytostatic autophagy. Int J Nanomed. 2019;14:2415–2431. doi:10.2147/IJN.S193676
203. Sadhukha T, Wiedmann TS, Panyam J. Enhancing therapeutic efficacy through designed aggregation of nanoparticles. Biomaterials. 2014;35(27):7860–7869.
204. Zhang L, Jia Y, Yang J, et al. Efficient Immunotherapy of Drug-Free Layered Double Hydroxide Nanoparticles via Neutralizing Excess Acid and Blocking Tumor Cell Autophagy. ACS Nano. 2022;16(8):12036–12048. doi:10.1021/acsnano.2c02183
205. Jia Y, Hu J, Zhu C, et al. Engineered NanoAlum from aluminum turns cold tumor hot for potentiating cancer metalloimmunotherapy. J Control Release. 2023;354:770–783. doi:10.1016/j.jconrel.2023.01.043
206. Xu J, Wang H, Hu Y, et al. Inhibition of CaMKIIα Activity Enhances Antitumor Effect of Fullerene C60 Nanocrystals by Suppression of Autophagic Degradation. Adv Sci (Weinh). 2019;6(8):1801233.
207. Chen Y, Yang L, Feng C, Wen LP. Nano neodymium oxide induces massive vacuolization and autophagic cell death in non-small cell lung cancer NCI-H460 cells. Biochem Biophys Res Commun. 2005;337(1):52–60.
208. Hao BM, Liu YN, Zhang CY, et al. Autophagic blockage by bismuth sulfide nanoparticles inhibits migration and invasion of HepG2 cells. Nanotechnology. 2020;31(46):465102. doi:10.1088/1361-6528/abadc6
209. Wang Y, Huang Y, Fu Y, et al. Reductive damage induced autophagy inhibition for tumor therapy. Nano Res. 2023;16(4):5226–5236. doi:10.1007/s12274-022-5139-z
210. Ray E, Vaghasiya K, Sharma A, et al. Autophagy-Inducing Inhalable Co-crystal Formulation of Niclosamide-Nicotinamide for Lung Cancer Therapy. AAPS Pharm Sci Tech. 2020;21(7):260.
211. Yang F, Wang X, Sun J, et al. Mesopore-encaged active MnOx in nano-silica selectively suppresses lung cancer cells by inducing autophagy. Biomater Sci. 2023;11(6):2056–2064. doi:10.1039/D2BM01826H
212. Liu Z, Du Z, Li K, Han Y, Ren G, Yang Z. TRPC6-Mediated Ca2+ Entry Essential for the Regulation of Nano-ZnO Induced Autophagy in SH-SY5Y Cells. Neurochem Res. 2020;45(7):1602–1613. doi:10.1007/s11064-020-03025-y
213. Yang R, Wu R, Mei J, Hu FR, Lei CJ. Zinc oxide nanoparticles promotes liver cancer cell apoptosis through inducing autophagy and promoting p53. Eur Rev Med Pharmacol Sci. 2021;25(3):1557–1563.
214. Du Z, Chai X, Li X, Ren G, Yang X, Yang Z. Nano-CuO causes cell damage through activation of dose-dependent autophagy and mitochondrial lncCyt b-AS/ND5-AS/ND6-AS in SH-SY5Y cells. Toxicol Mech Methods. 2022;32(1):37–48. doi:10.1080/15376516.2021.1964665
215. Wen J, Chen H, Ren Z, Zhang P, Chen J, Jiang S. Ultrasmall iron oxide nanoparticles induced ferroptosis via Beclin1/ATG5-dependent autophagy pathway. Nano Converg. 2021;8(1):10. doi:10.1186/s40580-021-00260-z
216. Zamanvaziri A, Meshkat M, Alazmani S, Khaleghi S, Hashemi M. Targeted PEGylated Chitosan Nano-complex for Delivery of Sodium Butyrate to Prostate Cancer: an In Vitro Study. Technol Cancer Res Treat. 2023;22:15330338231159223. doi:10.1177/15330338231159223
217. Menconi A, Marzo T, Massai L, et al. Anticancer effects against colorectal cancer models of chloro(triethylphosphine)gold(I) encapsulated in PLGA-PEG nanoparticles. Biometals. 2021;34(4):867–879. doi:10.1007/s10534-021-00313-0
218. Luo H, Lu L, Yang F, et al. Nasopharyngeal cancer-specific therapy based on fusion peptide-functionalized lipid nanoparticles. ACS Nano. 2014;8(5):4334–4347. doi:10.1021/nn405989n
219. Kim I, Song YH, Singh N, et al. Anticancer activities of self-assembled molecular bowls containing a phenanthrene-based donor and Ru(II) acceptors. Int J Nanomed. 2015;10:143–153. doi:10.2147/IJN.S88287
220. Shi M, Cheng L, Zhang Z, Liu Z, Mao X. Ferroferric oxide nanoparticles induce prosurvival autophagy in human blood cells by modulating the Beclin 1/Bcl-2/VPS34 complex. Int J Nanomed. 2014;10(207):–. doi:10.2147/IJN.S72598
221. Becker AL, Orlotti NI, Folini M, et al. Redox-active polymer microcapsules for the delivery of a survivin-specific siRNA in prostate cancer cells. ACS Nano. 2011;5(2):1335–1344. doi:10.1021/nn103044z
222. Aryal S, Hu CM, Zhang L. Polymeric nanoparticles with precise ratiometric control over drug loading for combination therapy. Mol Pharm. 2011;8(4):1401–1407. doi:10.1021/mp200243k
223. Huang P, Wang D, Su Y, et al. Combination of small Molecule prodrug and nanodrug delivery: amphiphilic drug-drug conjugate for cancer therapy. J Am Chem Soc. 2014;136(33):11748–11756. doi:10.1021/ja505212y
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
